Abstract
Background:
Ormenis eriolepis Coss (Asteraceae) is an endemic Moroccan subspecies, traditionally named “Hellala” or “Fergoga”. It’s usually used for its hypoglycemic effect as well as for the treatment of stomacal pain. As far as we know, there is no scientific exploration of anti tumoral activity of Ormenis eriolepis extracts.
Materials and Methods:
In this regard, we performed a screening of organic extracts and fractions in a panel of both hematological and solid cancer cell lines, to evaluate the potential in vitro anti tumoral activity and to elucidate the respective mechanisms that may be responsible for growth arrest and cell death induction. The plant was extracted using organic solvents, and four different extracts were screened on Jurkat, Jeko-1, TK-6, LN229, SW620, U2OS, PC-3 and NIH3T3 cells.
Results:
Cell viability assays revealed that, the IC50 values were (11,63±5,37μg/ml) for Jurkat, (13,33±1,67μg/ml) for Jeko-1, (41,67±1,98μg/ml) for LN229 and (19,31±4,88μg/ml) for PC-3 cells upon treatment with Oe-DF and Oe-HE respectively. Both the fraction and extract exhibited no effects on TK6 and NIH3T3. Cytometry analysis accompanied by DNA damage signaling protein levels monitoring (p-H2A.X), showed that both the Dichloromethane Fraction and Hexanic extract induce DNA double stranded breaks (DSBs) accompanied by cell cycle arrest in G1 (Jurkat, Jeko -1 and LN22) and G2/M (PC-3) phases which is agreed with the caspase activity observed. Additional experiments with selective inhibitors of stress and survival pathways (JNK, MAPK, Rho, p53, and JAK3) indicated that none of these pathways was significantly involved in apoptosis induction. The bioactive compound analysis by CG/MS indicated that the major compounds in Oe-DF were: Linoleic Acid (15,89%), Podophyllotoxin (17,89%) and Quercetin (22,95%). For Oe-HE the major molecules were: Linoleic Acid (9,76%), α-curcumene (7,07%), α-bisabolol (5,49%), Campesterol (4,41%), Stigmasterol (14,08%) and β-sitosterol (7,49%).
Conclusion:
Our data suggest that bioactive compounds present in Ormenis eriolepis show significant anti proliferative activity inducing cell cycle arrest and cell death operating through apoptosis pathway.
Keywords: Ormenis eriolepis Coss, cancer cell lines, cytotoxicity, apoptosis, bioactive compounds
Introduction
Plants have been used for medicinal purposes since time immemorial. In recent years, there is strong current interest in discovering new edible natural products with medicinal properties. It has been estimated by WHO (World Health Organization) (2002) that about 90% of the world’s population from developing countries rely mainly on traditional medicines (mostly derived from plants) for their primary health care. In Morocco, the use of traditional medicine is widespread practice, the use of plants in the form of infusions or decoctions is a common practice among people of rural communities and their use is increasing in urban populations (Gonzalez-Tejero et al., 2008). The ethnobotanical and ethnopharmacological surveys conducted in different areas allowed the compilation of an inventory of 360 species and more than 500 prescriptions are recorded (Bellakhdar, 1997).
The genus Ormenis which includes Ormenis eriolepis Coss (Asteraceae) an endemic Moroccan subspecies, traditionally named “Hellala” or “Fergoga”. It’s usually used for its hypoglycemic effect as well as for the treatment of stomacal pain. Traditionally the inflorescences of this plant are mixed with honey and used for the treatment of the cardialgia ulcer and stomacal pain.
The ability of chemotherapeutic agents to induce apoptosis in tumor cells has become a therapeutic approach which may be enhanced by the development of novel approaches during treatment (Gibb et al., 1997). Evidence from various studies suggests that metabolites derived from plants may possess pro-apoptotic properties and have great potential for possible applications in cancer prevention (Moongkarndi et al., 2004; Kwon et al., 2006; Prasanna et al., 2009; Radhika et al., 2010; Choedon et al., 2010).
Apoptosis is characterized by particular morphological changes, including plasma membrane bleb, cell shrinkage, depolarization of mitochondria, chromatin condensation, DNA fragmentation and cell cycle arrest that can be triggered by damage on DNA (Ben-Porath and Weinberg, 2005; Maruyama et al., 2009).
The p-H2AX has been considered the main signaling protein involved in the response to DNA damage playing an important role in DNA repair (Yuan et al., 2010).
The relationship between apoptosis and cell cycle arrest has been a recent focus. Apoptosis provides a number of useful clues when generating effective therapies and many chemotherapeutic agents exert their anticancer effects by inducing apoptosis and/or cell cycle arrest in cancer cells (Schuchmann and Galle, 2004).
As far as we know, there is no scientific exploration of anti tumoral activity of Ormenis eriolepis extracts. In this regard, the purpose of this study was the screening of organic extracts and fractions in a panel of both hematological and solid cancer cell lines, to evaluate the potential anti tumoral activity and to elucidate the respective mechanisms that may be responsible for growth arrest and cell death induction. Finally, we suggest potential bioactive compounds responsible for these effects upon determination of chemical composition of both Oe-DF and Oe-HE by GC/MS.
Materials and Methods
Plant material
The aerial parts of Ormenis eriolepisCoss. (Asteraceae) were collected in 2009 from Ouarzazat (Morocco). The plant was identified at the Scientific Institute of Rabat by Prof. M. Fennane, and the specimen was deposited in the Scientific Institute herbarium.
Preparation of Extracts
The collected parts were air-dried and mechanically ground to produce a fine powder. 120g of plant powder was successively extracted using a Soxhlet apparatus with n-hexane (1.3L) and methanol (1.3L) to obtain hexanic extract (Oe-HE 3,91g) and methanolic extract (Oe-ME 10,94g) the resulting extracts were then evaporated by a Rotavapor to give dried extracts. The methanol concentrated extract was dissolved in distilled water and was successively extracted with dichloromethane (1.3L) and ethyl acetate (1.3L) to obtain dichloromethane fraction (Oe -DF 3,28g) and ethyl acetate fraction (Oe-AF 3,16g). All extracts were evaporated by a Rotavapor and kept at -20°C until use.
Cell culture
Jurkat, Jeko-1 and TK6 cells were maintained in RPMI-1640 with L-Glutamine and HEPES (Sigma-Aldrich, St. Louis, MO). LN229, SW620, U2OS, PC-3 and NIH3T3 cells were maintained in DMEM High Glucose (4.5 g/l) with L-glutamine (Invitrogen, Carlsbad, CA). All cells were grown in a humidified incubator at 37°C with 5% CO2. RPMI and DMEM were supplemented with 10% heat inactivated foetal bovine serum and 100 units/ml penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO). All cell lines were subconfluently grown and passaged, routinely tested for mycoplasma contamination and subjected to frequent morphological tests and growth curve analysis as quality-control assessments. All cell lines were treated at a prophylactic concentration of 5 μg/ml Plasmocin™ (InvivoGen, San Diego, CA).
Drugs and inhibitors
SP600125, U0126, H1152, Pifithrin α and JAK3 inhibitor I (Calbiochem, Darmstadt, Germany) were added directly to the media at the indicated concentration and cells were harvested or analyzed at the time points indicated in the figure legends.
Cell viability assays
The number of viable cells in culture was determined based on quantification of ATP, which signals the presence of metabolically-active cells, using the Cell Titer-Glo luminiscent assay kit (Promega, Madison, WI, USA). Following the manufacturer’s instructions, the cells were plated in 96-well plates, treated 24 h later with extracts dissolved in DMSO for the indicated times and concentrations, followed by addition of Cell Titer-Glo reagent. Luminiscence was detected using a multi-well Synergy Mx scanning spectrophotometer (Biotek, Winooski, VT).
Cell cycle analysis
Cell cycle analysis was performed using propidium iodide staining. Briefly, cells were washed in phosphate-buffered saline (PBS) and fixed in 70% ethanol. Fixed cells were then washed twice in PBS and stained in propidium iodide (50 g/ml) in the presence of 50 g/ml RNase A (Sigma-Aldrich, St Louis, MO, USA), then analysed by flow cytometry using a FACScan (Coulter Epics XL-MSL; Beckman Coulter, Fullerton, CA, USA) and winMDI software.
Annexin V-FITC/propidium iodide flow cytometric analysis
Analysis of phosphatidylserine externalization in apoptotic cells was determined by an ApoTarget Annexin-V-FITC Apoptosis kit (Invitrogen), according to the manufacturer’s instructions. 2 x 105 cells were seeded in 6-well plates and treated with 50μg/ml of Mv-DF for 48h. They were then collected and suspended in 100μl of Annexin V-binding buffer. 5μL of Annexin-V-FITC and 1O-β of propidium iodide were added and incubated 15 min at room temperature in the dark. Flow cytometry analysis was carried out using a FACScan (Coulter Epics XL-MSL; Beckman Coulter, Fullerton, CA, USA) and winMDI software.
Caspase activity analysis
Enzymatic activity of caspases was determined by measurement of caspases-3 and 7 activity by means of the luminometric Caspase-Glo 3/7 assay (Promega) according to the manufacturer’s protocol using a Synergy HT multi-detection microplate reader (Bio-Tek, Winooski, VT, USA).
Gel electrophoresis and immunoblotting
Cells were harvested in a buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA and 1% (v/v) Triton X-100 plus protease and phosphatase inhibitors. Protein content was measured by the Bradford procedure. Cell lysates were electrophoresed in SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred to Immobilon-P strips (Millipore, Billerica, MA) for 2 h at 60 V. The sheets were pre-incubated in TBS (20 mM Tris-HCl pH 7.5, 150 mM NaCl), 0.05% Tween 20 and 5% defatted milk powder for 1 h at room temperature and then incubated for 1 h at room temperature in TBS, 0.05% Tween 20, 1% BSA and 0.5% defatted milk powder containing the appropriate antibodies: pH2A.X (#9718, 1:1000) and β-tubulin (T0198, Sigma-Aldrich, St. Louis, MO, 1:4000). After washing in TBS, 0.05% Tween 20, the sheets were incubated with a peroxidase-coupled secondary antibody (Dako, Glostrup, Denmark, 1/2000 dilution,) for 1 h at room temperature. After incubation, the sheets were washed twice in TBS, 0.05% Tween 20 and once in TBS. The peroxidase reaction was visualized by the enhanced chemiluminiscence detection system (Millipore, Billerica, MA).
Derivatizations for GC/MS analysis
For this purpose, 100μl of the extracts were dried with N2 gas, then 100μl of derivatization agent (N, O-bis (trimethylsilyl) trifluoroacetamide with 1% of trimethylchlorosilane) was added, mixed and heated 10 minutes at 60°C.
Gas chromatography/mass spectrometry (GC/MS) analysis
The GC-MS analyses of Ormenis eriolepis Dichloromethane Fraction (Oe-DF) and Hexanic Extract (Oe-HE) were carried out at the Instrumental Technical Services of the “Estación Experimental del Zaidín” (CSIC, Granada, Spain). Briefly, 1 μl of the derivative solution was injected in a Varian 450GC coupled to 240 Ion Trap Mass Spectrometer as detector. The injection conditions were: splitless mode with 1 minute duration pulse, the injector temperature was 250°C; the He column flow was 1 ml/minute in a capillary column (Varian Factor Four VF-5 ms 30mx0.25mmx0.25 pm). For Mass spectrometry conditions, the EI ionization was 70 eV, the transfer line was at 280°C and the Trap at 240°C, mass range acquisition was from m/z 50 to m/z 500 and cared in Full Scan mode. Qualitative analysis of compounds was based on the comparison of their spectral mass and their relative Retention time with those of NIST08 mass spectra database and Kovats RI on the chromatograms recorded in Full Scan or in SIM mode usin g the characteristics ions. Quantitative analysis was realized by integration of peaks and calculated as percent of total identified area on the TIC chromatograms.
Statistical Analysis
Data are presented as means ± SD of at least three different assays performed in triplicate. IC50 value and the statistical significance of differences by Student’s t test were assessed using GraphPad Prism (GraphPad Software Inc. La Jolla, CA). Statistically significant differences are indicated by ***P <0.001, **P <0.01 and *P <0.05.
Results
Analysis of the cytotoxic activity of Ormenis eriolepis organic extracts against human cancer cell lines.
To investigate the potential effect of Ormenis eriolepis organic extracts against cancer, various hematological and solid cancer cell lines of different origin were screened. Non transformed cell lines TK-6 and NIH3T3 were also tested as control. Interestingly, both the dichloromethane fraction (Oe-DF) and the hexanic extract (Oe-HE) exhibited respectively a dramatic effect against Jurkat and Jeko-1(figure 1A) and LN229 and PC-3 (figure 1B) cells, both extracts had no effect against normal cell lines TK-6 and NIH3T3.
Figure 1.
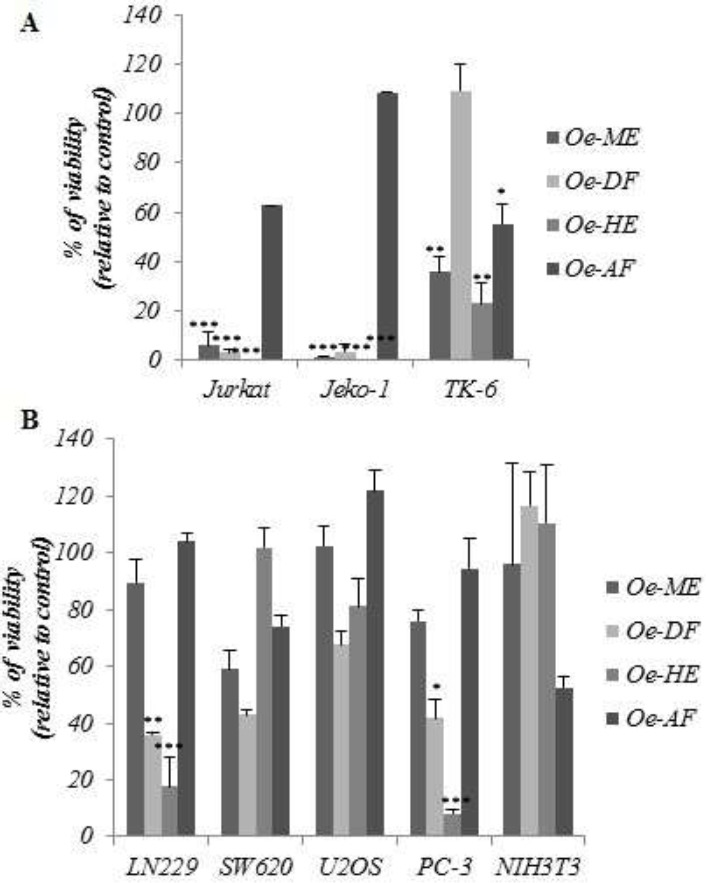
Cytotoxic activity of Ormenis eriolepis organic extracts and fractions in a panel of cancer and nontransformed cell lines. A. suspension cells panel Jurkat, Jeko-1, and TK-6 and B. adherent cells panel LN229, SW620, U2OS, PC-3 and NIH3T3; were incubated for 48 h with 50 μg/ml of each extract and fraction. Results represent the mean ±SD of at least 3 independent experiments indicating the percentage of viable cells relative to vehicle-treated (control) cells. Statistically significant differences are indicated by ***p < 0.005 compared with untreated.
In order to calculate the IC50, we next performed a dose-response (0-50μg/ml) viability assay using TK-6 as control for Jurkat and Jeko-1 and NIH3T3 for LN229 and PC-3 cells. The obtained cell growth curves in Figure 2A and 2B prove that Oe-DF and Oe-HE exerts a specific dose dependant inhibitory effect on cell proliferation. The IC50 values were (11,63±5,37μg/mL) for Jurkat and (13,33±1,67μg/mL) for Jeko-1 under Oe-DF treatment and (41,67±1,98μg/mL) for LN229, (19,31±4,88μg/mL) for PC-3 cells upon treatment with Oe-HE. Both the fraction and extract exhibited no effects on TK6 and NIH3T3 respectively. Doses of 20 μg/ml of Oe-DF and 50 μg/ml of Oe-HE were chosen for further mechanistic studies in (Jurkat; jeko-1) and (LN229; PC-3) cells.
Figure 2.
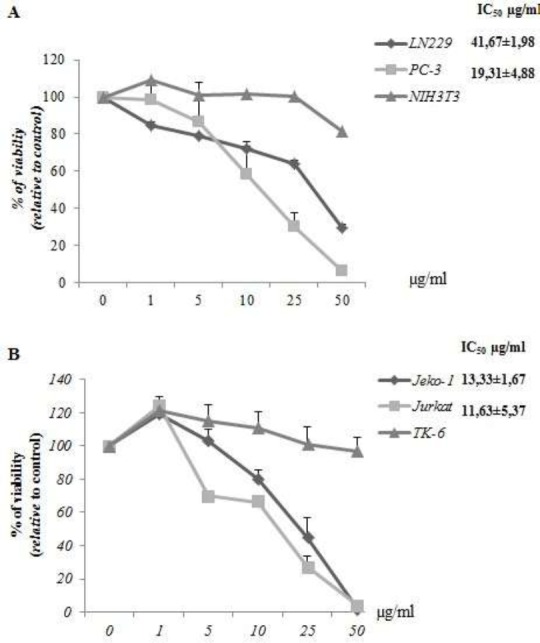
(A) Dose-response analysis of Ormenis eriolepis dichloromethane fraction (Oe-DF) and IC50 determination on Jurkat and Jeko-1 cells. (B) Dose-response analysis of Ormenis eiriolepis hexanic extract (Oe-HE) and IC50 determination on LN229 and PC-3 cells. Cells were treated for 48h at different concentrations ranging from 1 to 50μg/ml; IC50 value (μg/ml) was determined graphically from the viability curves, NIH3T3 and TK6 cells were used as control. Data are expressed as means ±SD of triplicate determinations.
In order to calculate the IC50, we next performed a dose-response (0-50μg/mL) viability assay using TK-6 as control for Jurkat and Jeko-1 and NIH3T3 for LN229 and PC-3 cells. The obtained cell growth curves in Figure 2A and 2B prove that Oe-DF and Oe-HE exerts a specific dose dependant inhibitory effect on cell proliferation. The IC50 values were (11,63±5,37μg/mL) for Jurkat and (13,33±1,67μg/mL) for Jeko-1 under Oe-DF tratement and (41,67±1,98μg/mL) for LN229, (19,31±4,88μg/ml) for PC-3 cells upon treatement with Oe-HE. Both the fraction and extract exhibited no effects on TK6 and NIH3T3 respectively. Doses of 20 μg/ml of Oe-DF and 50 μg/ml of Oe-HE were chosen for further mechanistic studies in (Jurkat; jeko-1) and (LN229; PC-3) cells.
Analysis of cell cycle effect of Ormenis eriolepis organic extracts.
In order to investigate how cell cycle distribution is affected, (jurkat; Jeko-1) were treated with a concentration of 20 μ¦/Μ of Oe-DF and (LN229; PC-3) with 50μg/ml of Oe-HE for 24 and 48h. As shown in figure 3 Oe-DF have effectively reduced the proportion of S-phase cells while strongly increased the proportion of Sub G1 cells (35,62% for jurkat and 64,75% for jeko-1 at 48h). Whereas Oe-HEin LN229 cells mainly exerts a combination of G1 arrest and sub-G1 increase at 24h, with this latter population further increasing at 48h up to 42%. In PC-3 cells Oe-HE induced a clear G2/M arrest which was already detectable at 24h leading at 48h to a G2/M cell population of 44.11%.
Figure 3.
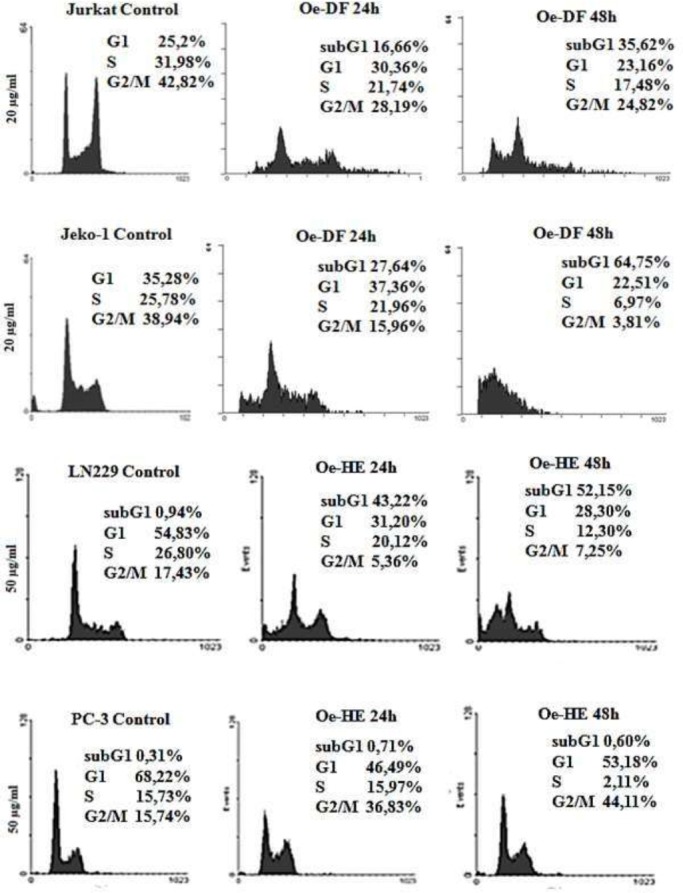
cell cycle distribution analysis of Jurkat, Jeko-1, LN229 and PC-3 cells treated respectively with Ormenis eriolepis Dichloromethane Fraction (Oe-DF) and Hexanic Extract (Oe-HE) by flow cytometry. Jurkat, Jeko-1, LN229 and PC-3 cells were incubated respectively with 20 μg/ml and 50 μg/ml of Oe-DF and Oe-HE for 24h and 48h. Cells were harvested and their DNA content analysed by flow cytometry as described in Materials and Methods. The cell cycle distribution is shown for each experimental condition.
Next, we analyzed the presence of DNA damage by monitoring p-H2A.X levels to investigate the possible molecular mechanism of Ormenis eriolepis. As shown in Figure 4, increased levels of p-H2A.X were detected in Oe-DF treated cells (jurkat and jeko-1) as well as in Oe-HE treated cells (LN229 and PC-3) after 6h to 12h of treatement. These results suggest that both the Dichloromethane Fraction and Hexanic extract induce DNA double stranded breaks (DSBs) accompanied by cell cycle arrest in G1 (Jurkat, Jeko-1 and LN22) and G2/M (PC-3) phases.
Figure 4.
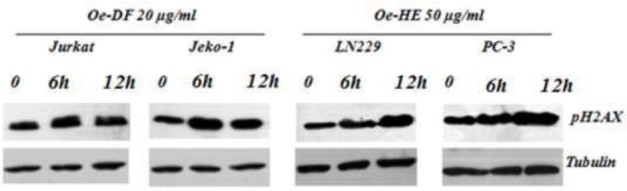
Effect of Oe-DF and Oe-HE on p-H2AX expression levels in Jurkat, Jeko-1, LN229 and PC-3 cells. Cells (4 × 10cells /ml) were treated respectively with 20 μg/ml and 50 μg/ml of Oe-DF and Oe-HE for 0, 6 and 12h. p-H2A.X levels in cellular extracts were detected by immunoblot with specific antibodies. Tubulin was used as an internal control.
Effect of Ormenis eriolepis organic extracts on apoptosis induction and caspases activation.
Double Annexin V/Propidium Iodide staining was performed to analyze and quantify cellular death. Upon exposure to treatment, an increase in the number of Annexin V positive cells was observed for both Oe-DF and Oe-HE as shown in Figure 5. For Jurkat and Jeko-1 cells, a percentage of (11% and 27,9%) was observed at 12h of Oe-DF exposure. where LN229 and PC-3 cells have shown an increase of apoptotic cell population of (24% and 20,4%) as well after 12h of treatment.
Figure 5.
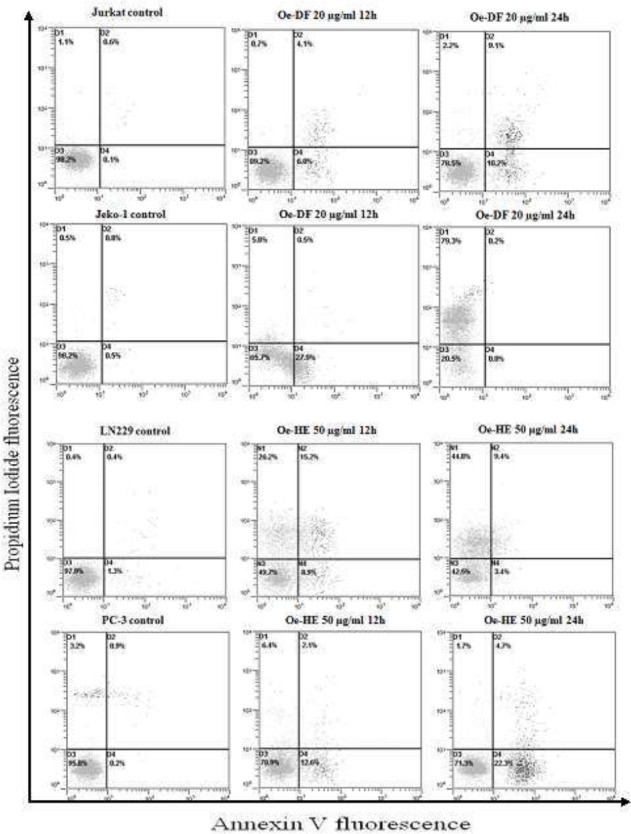
Effect of (Oe-DF) in Jurkat and Jeko-1 cell and (Oe-HE) in LN229 and PC-3 on apoptosis induction analyzed by flow cytometry. Jurkat, Jeko-1, LN229 and PC-3 cells were incubated respectively with 20 μg/ml and 50 μg/ml of Oe-DF and Oe-HE for 12h and 24h. The x-axis shows Annexin V-FITC staining and y-axis indicates Propidium iodide staining; Lower left (LL) quadrant: viable cells; lower right (LR) quadrant: early apoptotic cells; upper left (UL) quadrant: necrotic cells, upper right (UR) quadrant: late apoptotic cells.
Different caspases are activated as a crucial biochemical event during apoptosis. To investigate if Ormenis eriolepis induced apoptosis in Jurkat, Jeko-1, LN229 and PC-3 cell lines is caspases dependent, we performed caspase 3/7 activity assays upon treatment of (Jurkat and Jeko-1) cells with 20μg/ml of Oe-DF and (LN229 and PC-3) cells with 50μg/ml of Oe-HE for 48h. Figure 6 prove that both treatments increased caspase activity to 8 and 14 fold up in jurkat and jeko-1 cells, while Oe-HE increased similarly the caspase activity to 4 fold up in both LN229 and PC-3 cells upon 48h of exposure. Accordingly, this data may suggest that Ormenis eriolepis induced cell death includes caspases activation.
Figure 6.
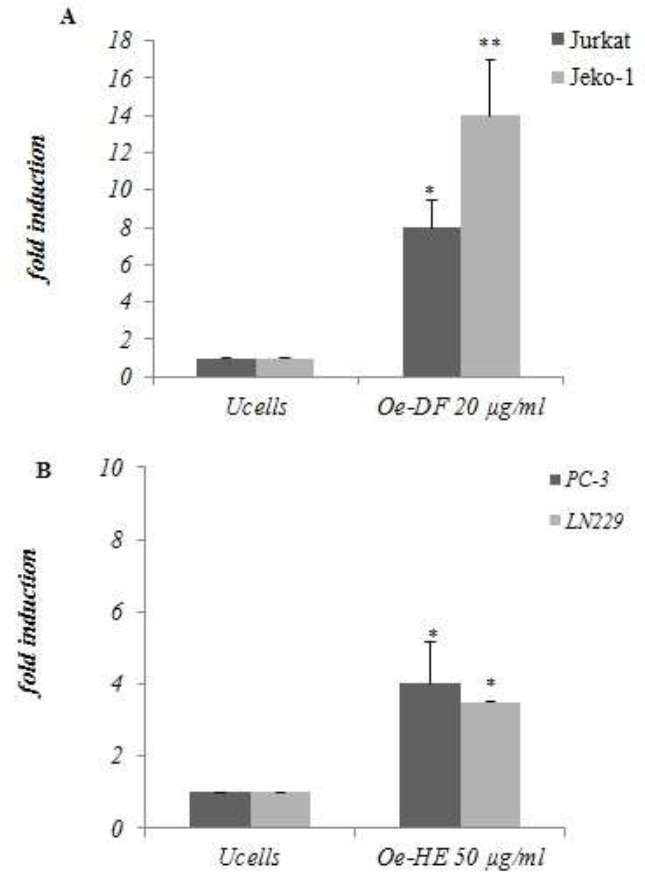
Effect of Ormenis eriolepis Fraction and Extract on caspase activity induction on Jurkat, Jeko-1, LN229 and PC-3 cells. (A) Jurkat and Jeko-1 cells were treated with 20 μg/ml of (Oe-DF) for 48h and caspase activity was measured (B) LN229 and PC-3 cells were treated with 50 μg/ml of (Oe-HE) for 48h and caspase activity was measured. Results indicate the average fold increase ± S.E.M in caspase activity relative to untreated cells from three independent determinations performed in duplicate.
Effect of Ormenis eriolepis organic extracts on stress and survival cell pathways.
As a preliminary investigation of the molecular mechanism of Ormenis eriolepis induced apoptosis, we analyzed additional experiments with a panel of selective inhibitors of stress and survival pathways (SP600125, U0126, H1152, Pifithrin α and JAK3) performed in (Jurkat and Jeko-1) (figure 7A) and (LN229 and PC-3) (Figure 7B) cells upon Oe-DF and Oe-HE treatments at 48h. The results showed that none of these pathways was significantly involved in Ormenis eriolepis induced cell death and that neither JNK, ERK1/2, Rho, p53 nor JAK3 pathways seems involved. Taken together, this results shows that more molecular investigation is needed to figure out the apoptotic signaling regulators responsible of Ormenis eriolepis anti tumoral effect in Jurkat, Jeko-1, LN229 and PC-3 cell lines.
Figure 7.
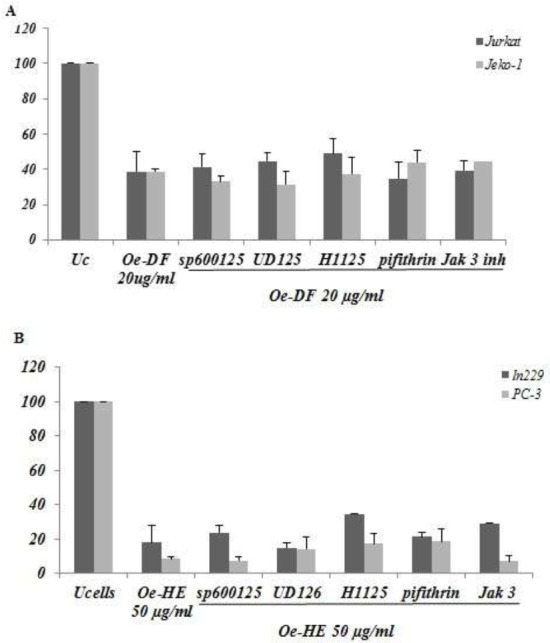
Effect of Ormenis eriolepis Fraction and Extract on the viability of Jurkat, Jeko-1, LN229 and PC-3 cells in presence of a panel of signaling pathways inhibitors. (A) Jurkat and Jeko-1 (B) LN229 and PC-3 cells were untreated or treated respectively with 20 and 50 μg/ml of Oe-DF and Oe-HE in the presence or absence of SP600125, U0126; H1152, Pifithrin or Jak3 inhibitor (all at 10 μM) for 48h. Inhibitors were pre-incubated for 1 hour before the addition of Mv-DF extract. Cell viability is represented as a percentage relative to untreated cells. Data is means ± S.E.M from three independent determinations performed in duplicate.
Characterization of chemical compounds in Ormenis eriolepis Dichloromethane Fraction and Hexane Extract.
In order to recognize the bioactive compounds responsible for these effects, Oe-DF and Oe-HE were subjected to GC-MS analysis and compounds were identified using mass spectrometry (Figure 8A and 8B).
Figure 8.
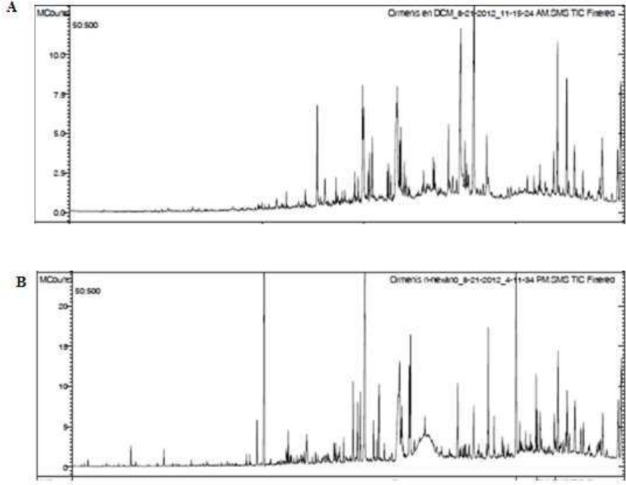
(A) Chromatogram of Ormenis eriolepis Dichloromethane Fraction obtained by CG. Compounds were identified by computer searches in the reference libraries of NIST and Wiley7, and fragmentation patterns were compared with literature Data. Oe-DF constituents are shown in Table 1. (B) Chromatogram of Ormenis eriolepis Hexanic Extract obtained by CG. Oe-HE constituents are shown in Table 2.
Retention time, Cas Numbers and % of identified area are summarized in Table 1 and 2. The mass spectra compounds were matched with computer searches in the references libraries of NIST08 and Wiley 7, and fragmentation patterns were compared with literature and commercially available products data. The major bioactive compounds identified in Oe-DF (Figure 9A) were: Linoleic Acid (15,89%), Podophyllotoxin (17,89%) and Quercetin (22,95%). In Oe-HE (Figure 9B) the major molecules were: Linoleic Acid (9,76%), α-curcumene (7,07%), α-bisabolol (5,49%), Campesterol (4,41%), Stigmasterol (14,08%) and β-sitosterol (7,49%).
Table 1.
Compounds present in dichloromethane fraction of Ormenis eiriolepis identified by CG/MS.
| RT | identified compounds | cas numbers | % Area |
|---|---|---|---|
| 15,877 | Propanoic acid,2-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 17596-96-2 | 0,19604 |
| 16,449 | Glycolic acid, TMS | 33581-77-0 | 0,176436 |
| 16,748 | 2-(Trimethylsiloxy)propenoic acid trimethylsilyl ester | 55191-13-4 | 0,19604 |
| 21,671 | Silanol, 1,1,1 -trimethyl-, 1 -benzoate | 08/12/2078 | 0,470496 |
| 22,147 | Octanoic acid, trimethylsilyl ester | 55494-06-9 | 0,137228 |
| 23,091 | Phenylacetoxytrimethylsilane | 2078-18-4 | 0,352872 |
| 23,531 | Butanedioic acid, bis(trimethylsilyl) ester | 40309-57-7 | 0,431288 |
| 23,877 | Glyceric acid (3TMS) | 38191-87-6 | 0,09802 |
| 24,787 | Nonanoic acid trimethylsilyl ester | 82326-11-2 | 0,176436 |
| 25,247 | Benzaldehyde, p-(trimethylsiloxy)-(7CI,8CI) | 1012-12-0 | 0,058812 |
| 27,294 | Decanoic acid, trimethylsilyl ester | 55494-15-0 | 0,235248 |
| 27,695 | 4’-(Trimethylsilyloxy)acetophenone | 18803-29-7 | 0,470496 |
| 27,988 | malatate | 65143-63-7 | 0,29406 |
| 29,26 | Trimethylsilylvanillin | 6689-43-6 | 0,176436 |
| 29,441 | (S)-dihydroactinidiolide | 17092-92-1 | 0,842972 |
| 30,245 | a-[(Trimethylsilyl)oxy]benzenepropionic acid trimethylsilyl ester | 27750-45-4 | 0,09802 |
| 31,336 | Benzoic acid,4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 2078-13-9 | 0,646932 |
| 31,889 | 4-Methoxy-3-(trimethylsiloxy)benzoic acid methyl ester | 55590-91-5 | 0,548912 |
| 33,343 | Benzoic acid, 3,4-bis[(trimethylsilyl)oxy]-, methyl ester | 27798-58-9 | 1,137032 |
| 35,023 | Nonanedioic acid bis(trimethylsilyl) ester | 17906-08-0 | 2,019212 |
| 35,433 | Protocatechuic acid, di-TMS | 2347-40-2 | 0,999804 |
| 35,098 | Tetradecanoic acid trimethylsilyl ester | 18603-17-3 | 1,097824 |
| 36,994 | 3 -Methoxy-4-[(trimethylsilyl)oxy]benzenepropionic acid trimethylsilyl ester | 56051-49-1 | 0,823368 |
| 37,06 | Benzoic acid,3,5-dimethoxy-4-[(trimethylsilyl)oxy]-, trimethylsilyl ester | 10517-29-0 | 0,9802 |
| 37,919 | 3-[3-[(Trimethylsilyl)oxy]phenyl]propenoic acid trimethylsilyl ester | 32342-01-1 | 0,999804 |
| 38,883 | 1,11-Undecanedioic acid, di(trimethylsilyl) ester | 106450-25-3 | 0,842972 |
| 39,199 | 3-[3,4-Bis(trimethylsilyloxy)phenyl]propenoic acid methyl ester | 22020-29-7 | 2,372084 |
| 41,57 | 2-Propenoic acid, 3-[3,4-bis[(trimethylsilyl)oxy]phenyl]-, trimethylsilyl ester | 10586-03-5 | 1,86238 |
| 42,53 | ALMITIC ACID N-BUTYL ESTER | 111-06-8 | 1,431092 |
| 42,91 | Linoleic acid trimethylsilyl | 56259-07-5 | 15,89884 |
| 43,458 | Octadecanoic acid, trimethylsilyl ester | 18748-91-9 | 4,901 |
| 46,698 | Eicosanoic acid, trimethylsilyl ester | 55530-70-6 | 2,078024 |
| 48,896 | Hexadecanoic acid 2,3-bis[(trimethylsilyl)oxy]propyl ester | 1188-74-5 | 8,586552 |
| 49,699 | Docosanoic acid trimethylsilyl ester | 74367-36-5 | 2,920996 |
| 51,665 | Stearic acid 2,3-bis(trimethylsilyloxy)propyl ester | 1188-75-6 | 4,587336 |
| 55,302 | Podophyllotoxin | 518-28-5 | 17,89845 |
| 56,24 | Quercetin, penta-TMS | 4067-66-7 | 22,95824 |
Table 2.
Compounds present in the hexanic extarct of Ormenis eiriolepis identified by CG/MS
| RT | identified compounds | cas numbers | % Area |
|---|---|---|---|
| 15,879 | 2-(Trimethylsiloxy)propanoic acid trimethylsilyl ester | 17596-96-2 | 0,14554795 |
| 16,377 | Hexanoic acid, trimethylsilyl ester | 14246-15-2 | 0,06849315 |
| 16,453 | Glycolic acid, bis-TMS | 33581-77-0 | 0,08561644 |
| 22,162 | Octanoic acid, trimethylsilyl ester | 55494-06-9 | 0,08561644 |
| 24,802 | Nonanoic acid trimethylsilyl ester | 82326-11-2 | 0,11986301 |
| 27,301 | Decanoic acid, trimethylsilyl ester | 55494-15-0 | 0,65924658 |
| 28,018 | alpha-curcumene | 644-30-4 | 7,07191781 |
| 28,697 | (+) -aromadendrene | 489-39-4 | 0,72773973 |
| 29,368 | (S)-dihydroactinidiolide | 17092-92-1 | 0,10273973 |
| 32,891 | alpha-Bisabolol | 515-69-5 | 5,49657534 |
| 35,032 | Nonanedioic acid bis(trimethylsilyl) ester | 17906-08-0 | 0,39383562 |
| 36,112 | Tetradecanoic acid trimethylsilyl ester | 18603-17-3 | 0,65924658 |
| 39,968 | Hexadecanoic acid, trimethylsilyl ester | 55520-89-3 | 4,29794521 |
| 41,732 | Heptadecanoic acid, trimethylsilyl ester | 55517-58-3 | 0,64212329 |
| 42,728 | 1,2-Hexadecanediol | 6920-24-7 | 0,59075342 |
| 42,949 | Linoleic acid trimethylsilyl | 56259-07-5 | 9,76883562 |
| 43,096 | alpha-Linolenic acid, trimethylsilyl ester | 97844-13-8 | 15,3424658 |
| 43,467 | Octadecanoic acid, trimethylsilyl ester | 18748-91-9 | 2,43150685 |
| 46,707 | Eicosanoic acid, trimethylsilyl ester | 55530-70-6 | 1,27568493 |
| 48,372 | 1-Docosanol | 661-19-8 | 6,59246575 |
| 48,897 | Hexadecanoic acid 2,3-bis[(trimethylsilyl)oxy]propyl ester | 1188-74-5 | 3,3989726 |
| 49,711 | Docosanoic acid trimethylsilyl ester | 74367-36-5 | 2,40582192 |
| 51,26 | 1-TETRACOSANOL | 506-51-4 | 7,32020548 |
| 54,002 | Icosanoic acid 2,3-bis(trimethylsilyloxy)propyl ester | 55517-94-7 | 1,54109589 |
| 54,638 | Hexacosanoic Acid | 506-46-7 | 2,66267123 |
| 57,21 | CAMPESTEROL | 474-62-4 | 4,41780822 |
| 57,567 | Stigmasterol trimethylsilyl ether | 14030-29-6 | 14,0839041 |
| 58,444 | β-Sitosteryl trimethylsilyl ether | 2625-46-9 | 7,49143836 |
Figure 9.
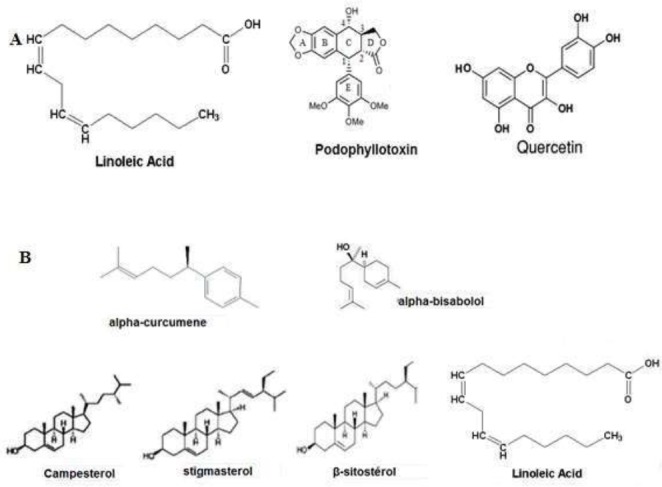
(A) Chemical structures of the major bioactive compounds of Ormenis eriolepis Dichloromethane Fraction. (B) Chemical structures of the major bioactive compounds of Ormenis eriolepis Hexanic Extract.
Discussion
After the rapid expansion of the use of monoclonal antibodies and various synthetic inhibitors directed against matrix metalloproteases or protein kinases, natural products are regaining attention in the oncology field. Due to their wide range of biological activities and low toxicity in animal models, natural products have been used as alternative treatments for cancers (Newman and Cragg 2012). Several drugs currently used in chemotherapy were isolated from plant species. The best known are the Vinca alkaloids, vinblastine and vincristine, isolated from Catharanthus roseus, etoposide and teniposide, which are semi-synthetic derivatives of the natural product epipodophyllotoxin, Paclitaxel isolated from the barkof Taxus brevifolia, the semi-synthetic derivatives of camptothecin, irinotecan and topotecan, isolated from Camptotheca acuminata, among several others (Cragg et al., 1993)
All Cancer cells are characterized by unregulated growth, as well as insufficient and inappropriate vascular supply (Tomida and Tsuruo, 1999).
T-cell lymphoblastic leukemia (Jurkat), Mantle cell lymphoma (Jeko-1), Glioblastoma multiforme (LN229) and Prostate Cancer (PC-3) are cell lines models for aggressive neoplastic disorders; they are known to have alteration in multiple cellular pathways including apoptosis. Deficiencies in this last, contribute to carcinogenesis by creating a permissive environment for genetic instability and accumulation of gene mutations conferring resistance to cytotoxic anticancer drugs and radiation (Irwin et al.;2013).
Ormenis eriolepis Coss (Asteraceae) an endemic Moroccan subspecies, traditionally named “Hellala” or “Fergoga” used for its hypoglycemic effect as well as for the treatment of stomacal pain. As far as we know, there is no scientific exploration of the anti-tumor capacity of Ormenis eriolepis extracts. In this study, we present the first evidence of tumor cell growth inhibitory activity of Oe-DF and Oe-HE by using in vitro experimental models. We show that both organic extract decreased specifically the viability of Jurkat, Jeko-1, LN229 and PC-3 cell lines in a dose specific manner, while it did not affect non-transformed human lymphocyte and mice fibroblasts TK-6 and NIH3T3.
Disturbance of the cancer cell cycle is one of the therapeutic targets for development of new anticancer drugs (Carnero; 2002). Analytical results indicate that Oe-DF have effectively reduced the proportion of S-phase cells while strongly increased the proportion of Sub G1 cells. Whereas Oe-HE in LN229 cells mainly exerts a combination of G1 arrest and sub-G1 increase at 24h, with this latter population further increasing at 48h up. In PC-3 cells Oe-HE induced a clear G2/M arrest which was already detectable at 24h. Therefore, a mechanism inducing cell cycle arrest and senescence can be triggered by damage on DNA (Ben-Porath and Weinberg, 2005; Maruyama et al., 2009). The occurrence of phosphorylation on histone H2AX, namely y-H2AX, has been widely used as a sensitive marker of DNA damage. Both Oe-DF and Oe-HE increased y-H2AX levels after 6h to 12h of treatment. Clearly, more specific studies are needed to investigate how Oe-DF and Oe-HE affect key proteins involved in cell cycle control in the four sensitive cell lines, namely P53, P16, P21, Cyclin dependant kinase and cyclins. Therefore, these findings point to the cycle arrest is being caused by a sophisticated mechanism in which the actions are initiated by DNA double stranded breaks.
The process of programmed cell death, or apoptosis is an important homeostatic mechanism that balances cell division and cell death to maintain appropriate cell number in tissues (Elmore; 2007). Flow cytometry analysis upon Annexin-V/propidium iodide staining detects and quantifies the amount of cells undergoing apoptosis. Up on exposure to treatment, an increase in the number of Annexin V positive cells was observed for both Oe-DF and Oe-HE in Jurkat and Jeko-1 cells, LN229 and PC-3 cells respectively in a time-dependent manner, demonstrating that Ormenis eriolepis induces cell death through the promotion of apoptosis.
Apoptosis signal transduction and execution require the coordinated action of the cascade of caspases (asparate-specific cysteine proteases). Caspases are expressed as inactive proenzymes and become activated by proteolytic processing at internal aspartate residues when cells receive an apoptosis-inducing signal. At present 14 mammalian caspase family members have been described. Some, including caspases-2, -8, -9 and -10 are initiators of cell death (contain large prodomains). Once activated, these initiator caspases in turn activate the executioner caspases such as caspases-3, -6 and -7 (carry small prodomains). Thus, caspases can activate each other (Takahashi, 1998). For this purpose, we determined if the enzymatic activity of caspase 3 and 7 was increased in Jurkat and Jeko-1 under Oe-DF treatment, as well as LN229 and PC-3 under Oe-HE. Data shows, that both treatments increased caspase activity upon 48h of exposure. Which may suggest that Ormenis eriolepis induced cell death may include caspases activation.
Apoptosis can be mediated by several pathways that may involve complex molecular interactions and biochemical regulators. As a very preliminary investigation, we analyzed the effect of a panel of stress and survival pathways inhibitors in Jurkat, Jeko-1, LN229 and PC-3 cell lines under their respective Ormenis eriolepis treatment. For this regard, JNK inhibitor SP600125, the MEK inhibitor U0126, the Rho inhibitor H1152, the p53 inhibitor pifithrin α and the JAK3 inhibitor were used. As shown, none of these inhibitors protected the studied cell lines from Ormenis eriolepis induced cell death. However, further detailed investigations are needed to elucidate the mechanism of action in Jurkat, Jeko-1, LN229 and PC-3 cells in order to obtain definite conclusions.
In this study, GC-MS analysis was conducted on both Oe-DF and Oe-HE in order to recognize the bioactive compounds responsible for these effects. The major bioactive compounds identified in Oe-DF were: Linoleic Acid (15,89%), Podophyllotoxin (17,89%) and Quercetin (22,95%). In Oe-HE the major molecules were: Linoleic Acid (9,76%), α-curcumene (7,07%), α-bisabolol (5,49%), Campesterol (4,41%), Stigmasterol (14,08%) and β-sitosterol (7,49%).
Remarkably, all these identified compounds are known for their cytotoxic activity against cancer cells. Podophyllotoxin, it is effective in the treatment of Wilms tumours, different types of genital tumors (carcinoma verrucosus, for example) and in non-Hodgkin and other lymphomas (Ayres and Loike, 1990) and lung cancer (Utsugi et al., 1996; Subrahmanyam et al., 1998). Three semisynthetic derivatives of podophyllotoxin, etoposide, teniposide and etopophos, were widely used as anticancer drugs and show good clinical effects against several types of neoplasms including testicular and small-cell lung cancers, lymphoma, leukaemia, Kaposi’s sarcoma, etc (Ayres and Loike, 1990).
Numerous studies have described the cancer preventive effects and molecular mechanisms of quercetin, which has been shown to one of the major flavonoids with antiproliferative efficacy on a wide range of cancer cells (Boyer et al., 2004; Kang and Liang, 1997; Suh et al., 2010). For example, quercetin was shown to inhibit the growth of acute lymphoid and myeloid leukemia cells (Kang and Liang, 1997). It was also reported to have growth-inhibitory effects on human gastric and colon cancer cells by inhibiting cell cycle progression at the G1 -S boundary (Suh et al., 2010). These diverse antitumor activities of quercetin make it a lead compound for the development of new effective cancer preventive or therapeutic agents. α-bisabolol is a small oily sesquiterpene alcohol that has been demonstrated to have activity against some malignant adherent human and rat cell lines and against spontaneous mammary tumors in HER-2 transgenic mice as well as in HepG2 and Glioma cells (Costarelli et al. 2010;. Darra et al.; 2008; Chen et al.; 2010).
Campesterol, β-sitosterol and stigmasterol displayed remarkable cytotoxic activity against A549 cells. These in vitro data support findings that a mixture consisting of these three sterols exerted cytotoxic activity against cancer cells (Lai et al., 2010). Notably, β-sitosterol induced G2/M arrest, endoreduplication, and apoptosis on U937 lymphoma cells and HL60 promyelocytic leukemic cells through the Bcl-2 and PI3K/Akt signaling pathways (Moon et al., 2008). Stigmasterol maintained a non-dose-responsive inhibition of growth (around 40-50% inhibition between 1.6 and 50 μg/ml) of HS578T breast cancer cells (Lai et al., 2010).
Conclusions
Our study findings support the notion that Ormenis eriolepis have a combination of several bioactive compounds that can be responsible either alone or together for its outstanding antitumor activity and also the chemoprevention proprieties that can have the plant decoction if used regularly in healthy diet, brings a clear scientific support to the use of Moroccan medicinal plants by traditional healers for the treatment of cancer patients. Fi nally and as preliminary information, it opens the way for further investigations to elucidate the possible molecular mechanisms involved in cancer cell death and cell cycle arrest.
Authors’ contributions
LB performed all experiments and participated in the experimental design, analysis of the data and redaction of the manuscript. CAL participated in the experimental design, analysis of the data and preparation of the manuscript. The plants harvest, identification and extraction were done by NM and YB. SA participated in the analysis of the data and the preparation of the manuscript. SFM participated in the experimental design, data analysis and edited the manuscript. PV directed the research and edited the manuscript. All authors have contributed and approved the manuscript.
Acknowledgements
We are grateful to Dr. Rafael Núñez Gómez (Servicio de Instrumentación Científica, Estación Experimental del Zaidín CSIC, Granada, Spain), for the Gc-MS analysis of Ormenis eriolepis dichloromethane fraction and hexanic extract. Many thanks goes to, Dr. Ruth Villalonga, Mr. Guillem Ramis and Ms. Margalida Serra Sitjar (Cancer Cell Biology Group, Institut Universitari d’Investigació en Ciències de la Salut “IUNICS”) for scientific and technical help.
Footnotes
Competing interest:
The authors declare that they have no competing interests.
References
- 1.Ayres DC, Loike Lignans JD. Chemical, biological and clinical properties. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- 2.Bellakhdar J. La Pharmacopée Marocaine Traditionnelle. Paris: Ed Ibis Press; 1997. [Google Scholar]
- 3.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Boyer J, Brown D, Liu RH. Uptake of quercetin and quercetin 3 -glucoside from whole onion and apple peel extracts by Caco-2 Cell monolayers. J Agric Food Chem. 2004;52:7172–79. doi: 10.1021/jf030733d. [DOI] [PubMed] [Google Scholar]
- 5.Carnero A. Targeting the cell cycle for cancer therapy. Br J Cancer. 2002;87(2):129–133. doi: 10.1038/sj.bjc.6600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Hou J, Yin Y, Jang J, Zheng Z, Fan H, Zou G. alpha-Bisabolol induces dose- and timedependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFkappaB. Biochem Pharmacol. 2010;80:247–54. doi: 10.1016/j.bcp.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Choedon T, Shukla SK, Kumar V. Chemopreventive and anti-cancer properties of the aqueous extract of flowers of Butea monosperma. J Ethnopharmacol. 2010;129:208–13. doi: 10.1016/j.jep.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Costarelli L, Malavolta M, Giacconi R, Cipriano C, Gasparini N, Tesei S, Pierpaoli S, Orlando F, Suzuki H, Perbellini L, Piacenza F, Emanuelli M, Mocchegiani E. In vivo effect of alpha-bisabolol, a nontoxic sesquiterpene alcohol, on the induction of spontaneous mammary tumors in HER-2 / neu transgenic mice. Oncol Res. 2010;18:409–18. doi: 10.3727/096504010x12671222663557. [DOI] [PubMed] [Google Scholar]
- 9.Cragg GMMR, Boyd JH, Cardellina MR, II, Grever SA, Schepartz KM, Snader M. Suffness. Role of plants in the National Cancer Institute Drug Discovery, Development Program. 1993:80–95. [Google Scholar]
- 10.Darra E, Lenaz G, Cavalieri E, Fato R, Mariotto S, Bergamini C, Carcereri de Prati A, Perbellini L, Leoni S, Suzuki H. Alpha-bisabolol: unexpected plantderived weapon in the struggle against tumour survival? Ital J Biochem. 2007;56:323–8. [PubMed] [Google Scholar]
- 11.Elmore S. Apoptosis: A Review of Programmed Cell Death. Toxicologic pathology. 35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibb RK, Taylor DD, Wan T, O’Connor DM, Doering DL, Taylor CG. Apoptosis as a measure of chemosensitivity to cisplatin and taxol therapy in ovarian cancer cell lines. Gynecol Oncol 1997. 2007;65:13–22. doi: 10.1006/gyno.1997.4637. [DOI] [PubMed] [Google Scholar]
- 13.González-Tejero MR, Casares-Porcel M, Sánchez-Rojas CP, Ramiro-Gutiérrez JM, Molero-Mesa J, Pieroni A, Giusti ME, Censorii E, de Pasquale C, Della A, Paraskeva-Hadijchambi D, Hadjichambis A, Houmani Z, El-Demerdash M, El-Zayat M, Hmamouchi M, Eljohrig S. Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J Ethnopharmacol. 2008 Mar 5;116(2):341–57. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 14.Irwin ME, Rivera-Del Valle N, Chandra J. Redox Control of Leukemia: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants & Redox Signaling. 2013;18(11):1349–1383. doi: 10.1089/ars.2011.4258. doi:10.1089/ars.2011.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang TB, Liang MC. Studies on the inhibitory effects of quercetin on the growth of HL460 leukemia cells. Biochem Pharmacol. 1997;54:1013–18. doi: 10.1016/s0006-2952(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HJ, Hong YK, Kim KH, Han CH, Cho SH, Choi JS, Kim BW. Methanolic extracts of Pterocarpus santalinus induces apoptosis in HeLa cells. J Ethnopharmacol. 2006;105:229–34. doi: 10.1016/j.jep.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Lai CS, Mas RH, Nair NK, Mansor SM, Navaratnam V. Chemical constituents and in vitro anticancer activity of Typhoniumflagelliforme (Araceae) J Ethnopharmacol. 2010;127(2):486–494. doi: 10.1016/j.jep.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Maruyama J, Naguro I, Takeda K, Ichijo H. Stress-activated MAP kinase cascades in cellular senescence. Curr Med Chem. 2009;16:1229–1235. doi: 10.2174/092986709787846613. [DOI] [PubMed] [Google Scholar]
- 19.Moon DO, Kim MO, Choi YH, Kim GY. beta-Sitosterol induces G2/M arrest, endoreduplication, and apoptosis through the Bcl-2 and PI3K/Akt signaling pathways. Cancer Lett. 2008 Jun 18;264(2):181–91. doi: 10.1016/j.canlet.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. antioxidation and induction of apoptosis by Garcinia mangostona (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 2004;90:161–6. doi: 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 21.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012 Mar 23;75(3):311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasanna R, Harish CC, Pichai R, Sakthisekaran D, Gunasekaran P. Anti-cancer effect of Cassia auriculata leaf extract in vitro through cell cycle arrest and induction of apoptosis in human breast and larynx cancer cell lines. Cell Biol Int. 2009;33:127–34. doi: 10.1016/j.cellbi.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Radhika NK, Sreejith PS, Asha VV. Cytotoxic and apoptotic activity of Cheilanthes farinose (Forsk.) Kaulf. Against human hepatoma, Hep3B cells. J Ethnopharmacol. 2010;128:166–71. doi: 10.1016/j.jep.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Schttclmtann M, Galle PÄ. Sensitizing to apoptosis-sharpening the medica sword. J. l-lepatol. 2004;40:335–336. doi: 10.1016/j.jhep.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Subrahmanyam D, Renuka B, Rao CV, Sagar PS, Deevi DS, Babu JM, Vyas K. Novel D-ring analogues of podophyllotoxin as potent anti-cancer agents. Bioorg Med Chem Lett. 1998;8:1391–6. doi: 10.1016/s0960-894x(98)00232-7. [DOI] [PubMed] [Google Scholar]
- 26.Suh DK, Lee EJ, Kim HC, Kim JH. Induction of G1/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Arch Pharm Res. 2010;33:781–85. doi: 10.1007/s12272-010-0519-4. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi RQ, Deveraux I, Tamm K, Welsh N, Assamunt GS, Salvesen J, Reed C. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 1998;273:7787–7790. doi: 10.1074/jbc.273.14.7787. [DOI] [PubMed] [Google Scholar]
- 28.Tomida A, Tsuruo T. Drug resistance mediated by cellular stress response to the microenvironment of solid tumors. Anticancer Drug Des. 1999 Apr;14(2):169–77. [PubMed] [Google Scholar]
- 29.Utsugi T, Shibata J, Sugimoto Y, Aoyagi K, Wierzba K, Kobunai T, Terada T, Oh-hara T, Tsuruo T, Yamada Y. Antitumor activity of a novel podophyllotoxin derivative (TOP-53) against lung cancer and lung metastatic cancer. Cancer Res. 1996;56:2809–14. [PubMed] [Google Scholar]
- 30.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010 Feb 5;140(3):384–96. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]


