Abstract
Flotillin-2 (Flot2) is a highly conserved and ubiquitously expressed protein that resides on the cytoplasmic side of the cell membrane within specific cholesterol rich microdomains. Some studies have reported that overexpression of Flot2 is related to cancer progression. However, the role of Flot2 in hepatocellular carcinoma (HCC) remains unclarified. In this study, we aim to explore the correlation between Flot2 expression and HCC progression and the underlying mechanism. In the present study, overexpression of Flot2 in HCC tissues and cell lines was detected, and forced overexpression of Flot2 significantly promoted the proliferation, migration, invasion and metastasis of HCC in vitro and in vivo by modulating cell cycle and inducing EMT, which was mediated via up-regulation of Twist as a result of Raf/MEK/ERK1/2 pathway activation. In contrast, silencing Flot2 expression inhibited these biological processes. Furthermore, high expression of Flot2 was significantly correlated with poor prognosis of HCC patients after curative resection and is an independent risk factor. In conclusion, Flot2 promoted tumor growth and metastasis of HCC through modulating cell cycle and inducing EMT. The expression of Flot2 may play a key role in HCC progression and may be regarded as a potential poor prognostic marker for HCC.
Keywords: Hepatocellular carcinoma, Flot2, EMT, cell cycle, metastasis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer-related death in men worldwide [1]. Although hepatectomy provides long-term survival for HCC patients, the high postoperative recurrence rate is a major problem. HCC metastatic recurrence is the leading cause of high mortality rates after curative resection [2]. Several studies have demonstrated that epithelial-mesenchymal transition (EMT) plays a vital role in the early stage of tumor metastasis [3-11]. EMT refers to a complex process in which epithelial cells transform into mesenchymal cells. In this process, the polarity of epithelial cells disappears and invasive and migratory abilities increase, accompanied by down-regulation of epithelial markers and up-regulation of mesenchymal markers.
Flotillin-2 (Flot2) is a highly conserved and ubiquitously expressed protein that resides on the cytoplasmic side of cell membrane within specific cholesterol rich microdomains. Flotillins have been reported to be involved in a variety of biological process, for instance, axonal growth, membrane trafficking and various signalling pathways [12]. Recent studies have shown that overexpression of Flotillin-2 is related to metastasis in several human cancers. It was reported that overexpression of Flot2 could promote progression and lymph node metastasis of melanoma [13]. Overexpression of Flot2 is also related to poor prognosis of breast and gastric cancer patients, and it is used as a prognostic biomarker [14,15]. Recently, it was found that Flot2 promotes nasopharyngeal carcinoma metastasis via EMT [16].
However, the biological role of Flot2 has not yet been clarified in hepatocellular carcinoma. In the present study, we examined the expression of Flot2 in HCC and explored the relationship between Flot2 and HCC progression. Our results showed that Flot2 significantly promoted the invasion, migration, proliferation and metastasis of HCC in vitro and in vivo through modulating cell cycle and inducing EMT, and this activity was mediated by up-regulation of Twist as a result of Raf/MEK/ERK1/2 pathway activation.
Furthermore, high expression of Flot2 was correlated with poor prognosis in HCC patients after curative resection and was an independent risk factor, indicating that Flot2 may be regarded as a potential poor prognostic marker for HCC.
Methods
Ethics statement
The experiments were approved by the Ethics Committee of Zhongshan Hospital, Fudan University, and informed consent was obtained from each patient. Paraffin-embedded pathological specimens were obtained from 187 HCC patients after curative resection between 2005 and 2012.
Cell culture
Two commonly used human HCC cell lines, MHCC97H and SMMC-7721, were used in this study. The MHCC97H cell line [17] (a human HCC cell line with high metastatic potential, established at Liver Cancer Institute, Fudan University, Shanghai, China) and SMMC-7721 cell line (obtained from the Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin at 37°C in a humidified atmosphere of 5% CO2.
Construction of Flot2 overexpressed and silenced HCC cell lines
To explore the role of Flot2 in HCC, MHCC97H and SMMC-7721 were transfected with four types of lentivirus plasmids (Lv-vector, Lv-Flot2, Lv-shVector and Lv-shFlot2, from Shanghai GeneChem, China) to construct Flot2-overexpressed (MHCC97H-Flot2, SMMC-7721-Flot2) and Flot2-silenced (MHCC97H-shFlot2, SMMC-7721-shFlot2) HCC cell lines. A blank vector (Lv-vector and Lv-shVector) was also transfected into MHCC97H and SMMC-7721 cells to establish the control groups. Briefly, 3×105 cells per well were seeded in 6-well plates the day before transfection. After 24 h, the four types of lentiviruses were added to respective HCC cells with 1 ml of DMEM containing no FBS and 5 μg/ml Polybrene (Sigma, USA). Twelve hours later, the medium was removed and replaced with fresh culture medium containing 10% FBS. Three days later, the cells were collected for subsequent culture. The expression of Flot2 was validated with western blotting. This study used a CRISPR/Cas9-based synergistic activation mediator (SAM) system to amplify the expression of Flot2, and the shRNA sequence targeting Flot-2 was 5’-AAGGCAGAAGCCTACCAGAAA-3’ (lentivirus provided by Shanghai GeneChem, China).
RNA isolation and quantitative RT-PCR
Total RNA was extracted from HCC cells with RNAiso Plus (Takara, Kusatsu, Japan). cDNA was synthesized using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Kusatsu, Japan). Quantitative PCR was performed using SYBR®Premix Ex Taq™ (Takara, Kusatsu, Japan). The primers for amplification were as follows: Flot2, forward 5’-CCCCAGATTGCTGCCAAA-3’ and reverse 5’-TCCACTGAGGACCACAATCTCA-3’; GAPDH, forward 5’-TGACTTCAACAGCGACACCCA-3’ and reverse 5’-CACCCTGTTGCTGTAGCCAAA-3’. All experiments were performed independently three times.
Microarray analysis
For microarray and qRT-PCR analyses, total RNA was isolated from MHCC97H-Flot2 cells and MHCC97H-Vector cells. The 60-mer oligonucleotide probes were designed in microarray (Agilent). Performed according to the instructions. GO analysis and KEGG analysis was adopted according to the up-regulated and down-regulated genes.
Western blotting
Briefly, 30 μg of total protein lysate was subjected to western blotting as previously described [18]. The antibodies used in this study were as follows: monoclonal anti-human Flotillin-2, Twist (1:1000, Santa Cruz Biotechnology, USA), E-cadherin, N-cadherin, Vimentin, β-actin, ERK1/2, P-ERK1/2, MEK, P-MEK, C-Raf, P-C-Raf, cyclin D3, CDK2, CDK4, p27, p21 and p18 (1:1000, Cell Signaling Technology, USA). Then, immunoblots were incubated with HRP-conjugated anti-mouse/rabbit IgG (Cell Signaling Technology, USA) at 1:4000 dilution for 1 h at room temperature.
Cell proliferation, migration, and invasion assays
The proliferation of HCC cells was evaluated using a Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) assay. Cells were plated in a 96-well plate at 2×103 cells per well and allowed to grow for different times. The growth rate was determined by the cell number and was counted in triplicate every day using the CCK-8 assay. DMEM containing 10% of the CCK-8 reagent was added to each well and incubated at 37°C for 1-2 h. Viable cells were counted every day by reading the absorbance at 450 nm using a 96-well plate reader BP800 (Dynex Technologies).
Cell migration was assessed with a wound healing assay. HCC cells were seeded on a 6-well plate (3×105 per well) and incubated overnight. Then, a 200 µl pipette tip was used to scratch the cell monolayer. Wound closure was observed and photographed at 0 and 24 h under an inverted microscope.
Cell invasion assays were performed using Boyden chambers with an 8-μm pore size (Corning, Tewksbury, MA, USA), which were pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA) prior to seeding of HCC cells; the incubation time was 24-48 h, as described previously [19]. All experiments were performed independently three times. The invaded cells were assessed by counting the number of stained cells in four random fields at a magnification of 200×.
Xenograft model in nude mice
Male BALB/c nude mice (6 weeks old, weighting 20-22 g) were obtained from the Shanghai Institute of Materia Medica, Chinese Academy of Science. All studies on mice were conducted in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” and were approved by the Shanghai Medical Experimental Animal Care Committee. HCC cells (1×107 cells) in PBS were injected subcutaneously to obtain subcutaneous tumors. After 4 weeks, the mice were sacrificed to obtain the HCC tissues, which were used in the orthotopic model. The xenograft HCC model was established by orthotopic inoculation of histologically intact tumor tissue (2×2×2 mm) into the livers of nude mice as described previously [20]. Each group contained 5 mice. Seven weeks later, the mice were sacrificed and the tumor volume (largest diameter × perpendicular height2/2) and weight were measured and compared using an independent-sample t-test. Then, the tumors and lungs were placed in a 4% paraformaldehyde solution. The total number of liver and lung metastases were counted as previously described [21].
Tissue microarray (TMA) and immunohistochemistry (IHC)
A tissue microarray (TMA) was constructed as described previously [22]. The TMA sections contained 187 pairs of tumors and matched peritumoral tissues. The immunohistochemistry protocol was described previously [22]. The TMA was incubated with mouse monoclonal antibody anti-Flot2 (1:100, Santa Cruz Biotechnology, USA). An UltraVision Quanto Detection System HRP DAB (Thermo Fisher Scientific, CA, USA) was used to detect Flot2 expression. The integrated optical density was assessed using Image-Pro Plus software [23].
Patients and follow-up
HCC specimens used in the TMA were obtained from patients who received radical resection between 2005 and 2012 at the Liver Cancer Institute and Zhongshan Hospital (Fudan University, Shanghai, China). The criteria for resectability have been described previously. The patients did not receive any preoperative anticancer treatment. This study was approved by the research ethics committee of Zhongshan Hospital. Informed consent was obtained from all patients.
Statistical analysis
Analysis was performed with SPSS 18.0 (SPSS Inc). Pearson’s x2 test or Fisher’s exact test was used to compare qualitative variables, and quantitative variables were analysed by an unpaired two-tailed Student’s t-test or Spearman correlation test. The overall survival (OS) and disease free survival (DFS) were analysed by the Kaplan-Meier method. P < 0.05 was considered statistically significant.
Results
The expression of Flot2 in HCC tissues and cell lines
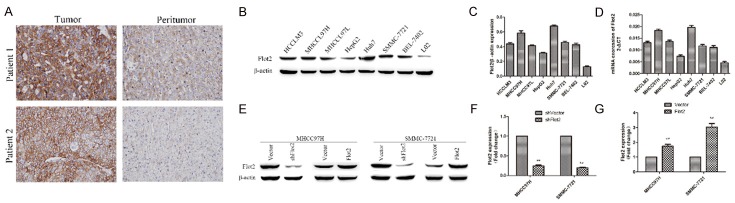
To investigate the role of Flot2 in HCC, we first examined the expression of Flot2 in HCC tissues and cell lines. Immunohistochemistry (IHC) staining of paired tumor and peritumor tissues from HCC patients showed that the expression of Flot2 in HCC tumor tissues was significantly higher than in peritumor tissues (Figure 1A). In addition, qRT-PCR and western blotting revealed that Flot2 was markedly overexpressed in seven HCC cell lines (HCCLM3, MHCC97H, MHCC97L, HepG2, Huh7, SMMC-7721, and BEL-7402) in comparison to a normal hepatic cell line (L02) at the protein (Figure 1B, 1C) and mRNA (Figure 1D) levels. Then, MHCC97H and SMMC-7721 cells were used to construct Flot2 overexpressed and silenced HCC cell lines. As Figure 1E-G shown, the construction of Flot2-overexpressed (MHCC97H-Flot2 and SMMC-7721-Flot2) and Flot2-silenced (MHCC97H-shFlot2 and SMMC-7721-shFlot2) HCC cell lines was confirmed with a western blot.
Figure 1.
Flot2 is overexpressed in HCC tissues and cell lines and construction of Flot2 overexpressed and silenced HCC cell lines. A. Immunohistochemistry staining of paired tumor and peritumor tissues from HCC patients (200×). B-D. Western blotting and qRT-PCR analysis of Flot2 expression at protein and mRNA levels in HCC cell lines (HCCLM3, MHCC97H, MHCC97L, HepG2, Huh7, SMMC-7721, BEL-7402) and a normal hepatic cell line (L02). E. Western blotting analysis of Flot2 expression in Flot2-overexpressed and silenced MHCC97H and SMMC-7721 cells. F. The ratios of Flot2 overexpression in MHCC97H (approximately 1.74 fold) and SMMC-7721 (approximately 3.02 fold). G. The ratios of Flot2 knockdown in MHCC97H (approximately 0.24 fold) and SMMC-7721 (approximately 0.2 fold). All experiments were performed independently three times. The data were analysed by an unpaired two-tailed Student’s t-test. *indicates P < 0.05, **indicates P < 0.01.
Microarray analysis of Flot2-overexpressed HCC cells
To elucidate the molecular mechanisms of Flot2 in HCC, an Agilent cDNA microarray analysis was conducted in the Flot2-overexpressed HCC cell line. The results showed that the expression of 714 genes was up-regulated and the expression of 695 genes was down-regulated in the MHCC97H-Flot2 group compared with the MHCC97H-Vector group. GO analysis showed that these genes were predicted to be involved in many biological processes, such as cell adhesion, EMT regulation, cell proliferation and cell migration. KEGG analysis showed these genes were predicted to be involved in several signalling pathways, such as the MAPK signalling pathway and Ras signalling pathway (Supplementary Figures 1 and 2).
Up-regulating Flot2 expression promoted the migration, invasion and proliferation of HCC cells
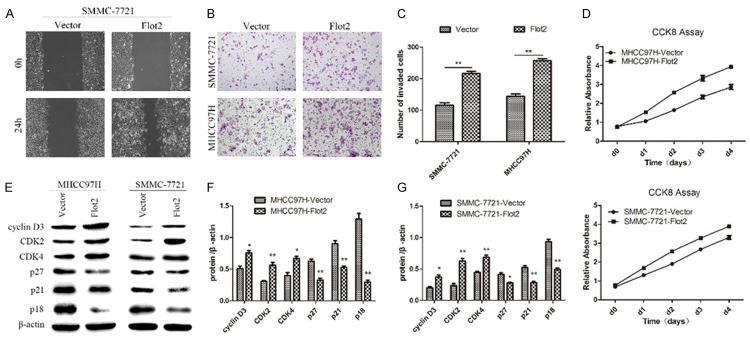
To evaluate the effect of Flot2 overexpression on cell migration, invasion and proliferation of HCC cells, a wound healing assay, Transwell matrix penetration assay, and CCK-8 assay and western blotting were adopted. The wound healing assay showed that closing of the wound was much faster in the Flot2 overexpressed group than in the control group (Figure 2A). In addition, the number of invaded cells in the Flot2 overexpressed group was also increased several-fold compared with the control group in the Transwell matrix penetration assay (Figure 2B, 2C). The CCK-8 assay showed that the proliferation was markedly up-regulated in the Flot2 overexpressed group compared with the control group (Figure 2D). The western blot analysis showed that Flot2 promoted cell proliferation through positive regulation of cell cycle proteins, such as up-regulation of cyclin D3, CDK2 and CDK4 and down-regulation of p27, p21 and p18 (Figure 2E-G). These results indicated that Flot2 overexpression promoted migration, invasion and proliferation of HCC cells.
Figure 2.
The effect of Flot2 overexpression on migration, invasion and proliferation of HCC cells. A. Wound healing assay shown that closing of the wound was much faster in SMMC-7721-Flot2 group than the control group. B, C. Compared with the control group, the invasive ability of Flot2 overexpressed group was significantly increased by Transwell matrix penetration assay (MHCC97H-Flot2 group: 144.6 ± 7.0 vs 256.8 ± 6.9, P < 0.01; SMMC-7721-Flot2 group: 115.8 ± 7.9 vs 216.0 ± 7.5, P < 0.01). D. The proliferation was markedly up-regulated in Flot2 overexpressed group compared with the control group by the CCK8 assay (d0, P > 0.05; d1, d2, d3, d4, P < 0.01). E-G. Western blotting analysis of of cyclin D3, CDK2, CDK4, p27, p21 and p18 expression in Flot2 overexpressed HCC cells. All experiments were performed independently three times. The data were analysed by an unpaired two-tailed Student’s t-test. *indicates P < 0.05, **indicates P < 0.01.
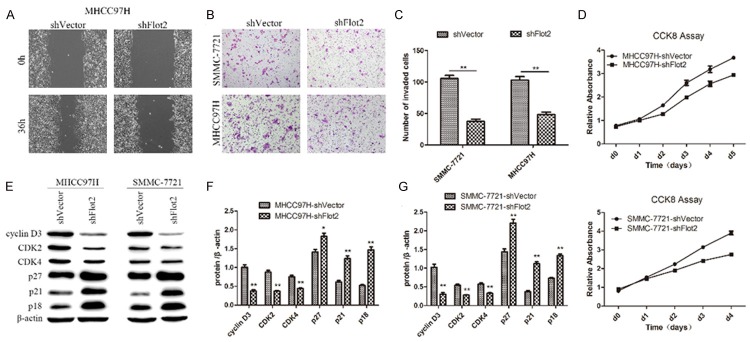
Silencing Flot2 expression inhibited migration, invasion and proliferation of HCC cells
To further investigate whether down-regulation of Flot2 expression in HCC cells could exert a negative effect on cell migratory, invasive and proliferative abilities, the wound healing assay, Transwell matrix penetration assay, CCK-8 assay and western blotting analysis were again conducted. The wound healing assay showed that closing of the wound was much slower in the Flot2 silenced group than in the control group (Figure 3A). In addition, the number of invaded cells in the Flot2 silenced group was also significantly decreased compared with the control group (Figure 3B, 3C) in the Transwell matrix penetration assay. The CCK-8 assay showed that the proliferation was markedly restrained in the Flot2 silenced group (Figure 3D). The western blotting analysis showed that knockdown of Flot2 could inhibit cell proliferation through negative regulation of cell cycle proteins, such as down-regulation of cyclin D3, CDK2 and CDK4 and up-regulation of p27, p21 and p18 (Figure 3E-G). These results indicated that knockdown of Flot2 inhibited the migration, invasion and proliferation of HCC cells.
Figure 3.
The effect of Flot2 knockdown on migration, invasion and proliferation of HCC cells. A. Wound healing assay shown that closing of the wound was much slower in MHCC97H-shFlot2 group than the control group. B, C. Compared with the control group, the invasive ability of Flot2 silenced group was significantly decreased by Transwell matrix penetration assay (MHCC97H-shFlot2 group: 48.3 ± 4.1 vs 102.8 ± 5.8, P < 0.01; SMMC-7721-shFlot2 group: 37.3 ± 3.9 vs 105.5 ± 5.1, P < 0.01). D. The CCK8 assay showed that the proliferation were markedly down-regulated in Flot2 silenced group compared with the control group (d0, d1, P > 0.05; d2, d3, d4, P < 0.01). E-G. Western blotting analysis of the cyclin D3, CDK2, CDK4, p27, p21 and p18 expression in Flot2 silenced HCC cells. All experiments were performed independently three times. The data were analysed by an unpaired two-tailed Student’s t-test. *indicates P < 0.05, **indicates P < 0.01.
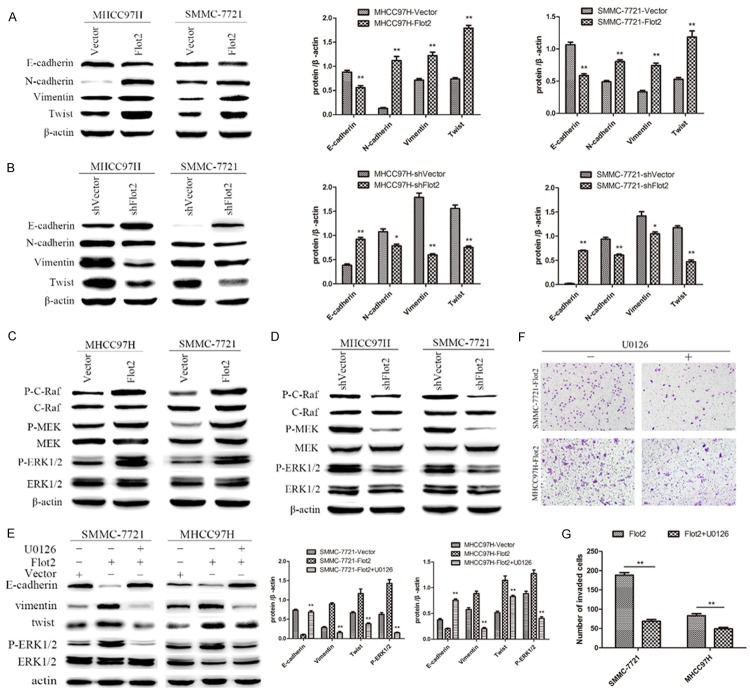
Flot2 induced EMT through modulating Twist expression via the Raf/MEK/ERK1/2 pathway
To further clarify the role of Flot2 in EMT, EMT markers were detected in both the Flot2 overexpressed and silenced groups. Western blotting revealed that Flot2 overexpression significantly inhibited the expression of E-cadherin and enhanced the expression of N-cadherin, Vimentin and Twist (Figure 4A). Moreover, silenced Flot2 expression significantly enhanced the expression of E-cadherin and reduced the expression of N-cadherin, Vimentin and Twist (Figure 4B). As shown in the cDNA microarray analysis, the MAPK signalling pathway was predicted to play a key role in the change of many biological processes caused by Flot2 overexpression. To validate this prediction, phosphorylation of the signalling proteins extracellular signal-regulated kinase (ERK)1/2 in HCC cells was assessed. Compared with the control group, the Flot2 overexpressed group displayed significantly enhanced activation of ERK1/2. In addition, MEK and c-Raf, the upstream activators of the ERK signalling pathway, were also activated (Figure 4C). On the other hand, the Flot2 silenced group displayed significantly reduced activation of ERK1/2, MEK and c-Raf (Figure 4D). U0126, an inhibitor of ERK1/2, significantly inhibited EMT induced by Flot2 overexpression, as shown in Figure 4E. Up-regulation of E-cadherin and down-regulation of N-cadherin, Vimentin and Twist was detected in U0126-treated Flot2-overexpressed HCC cells. Other signalling pathways, such as PI3K/Akt, were not appreciably activated by Flot2 overexpression. Furthermore, the number of invaded cells in the U0126-treated Flot2 overexpressed group was also significantly decreased compared with the untreated Flot2 overexpressed group (Figure 4F, 4G). These results indicated that Flot2-induced EMT was mediated via Twist as a result of Raf/MEK/ERK1/2 pathway activation.
Figure 4.
The effect of Twist and Raf/MEK/ERK1/2 pathway on Flot2 induced EMT. A, B. The expression of Twist and EMT markers E-cadherin, N-cadherin and Vimentin were detected by western blotting in MHCC97H and SMMC-7721 cell lines respectively transfected with Flot2-Vector, shFlot2-Vector and two blank Vectors. C. Western blotting detection of the expression of P-C-Raf, P-MEK and P-ERK1/2 in MHCC97H and SMMC-7721 cells transfected with Flot2-Vector and blank vector respectively, indicating the activity of Raf/MEK/ERK1/2 signalling pathway was enhanced. D. Down-regulation of P-C-Raf, P-MEK and P-ERK1/2 was detected by western blotting in MHCC97H and SMMC-7721 cells transfected with shFlot2-Vector and blank vector respectively. E. Enhanced activity of the Raf/MEK/ERK1/2 signaling pathway significantly inhibited by U0126 (10 μM, 2 h), the inhibitor of ERK1/2, and reversed the Flot2 induced EMT by down-regulating Twist expression. F, G. U0126 treatment significantly suppressed the invasive ability of MHCC97H-Flot2 and SMMC-7721-Flot2 cells. All experiments were performed independently three times. The data were analysed by an unpaired two-tailed Student’s t-test. *indicates P < 0.05, **indicates P < 0.01.
Flot2 promoted tumor growth and metastasis of HCC in vivo
We next determined whether Flot2 influenced the behaviour of tumors in vivo. MHCC97H and SMMC-7721 cells (each cell line included four groups: Lv-Vector, Lv-Flot2, Lv-sh Vector and Lv-shFlot2) were orthotopically implanted into Balb/c nu/nu nude mice livers (n=5 for each group). Seven weeks later, all the mice were sacrificed and metastases in the lung and liver were evaluated by pathological examination.
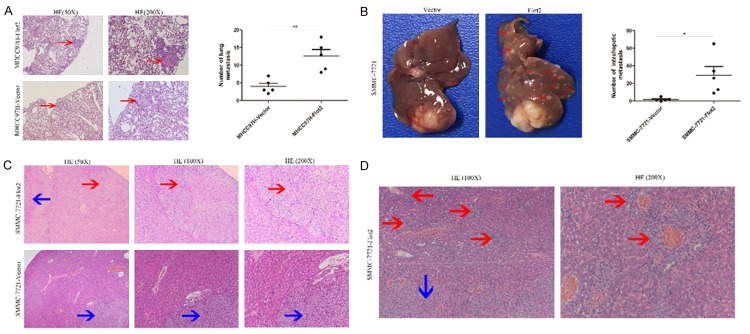
The results showed that the tumors volume of the Flot2 overexpressed group were significantly larger than those of the control group (MHCC97H-Flot2 group P < 0.01, SMMC-7721-Flot2 group P < 0.05) (Figure 5A, 5B); in addition, the tumors volume of the Flot2 silenced group were significantly smaller than those of the control group (MHCC97H-shFlot2 group P < 0.01, SMMC-7721-shFlot2 group P < 0.05) (Figure 5C, 5D). Compared with the MHCC97H-vector group, the mice bearing the MHCC97H-Flot2 group showed more lung metastasis (Figure 6A, P < 0.01), indicating that Flot2 significantly promoted lung metastasis of MHCC97H cell-based tumors. As shown in Figure 6B (P < 0.05), the SMMC-7721-Flot2 group had more intrahepatic metastasis than the SMMC-7721-Vector group, especially, exhibiting many vascular invasions in the liver, indicating that Flot2 significantly promoted intrahepatic metastasis of SMMC-7721 cell-based tumors. All the orthotopic tumors and metastatic nodules in the liver were demonstrated by HE staining (Figure 6C, 6D). Taken together, these results demonstrated that Flot2 promoted tumour growth and metastasis of HCC in vivo.
Figure 5.
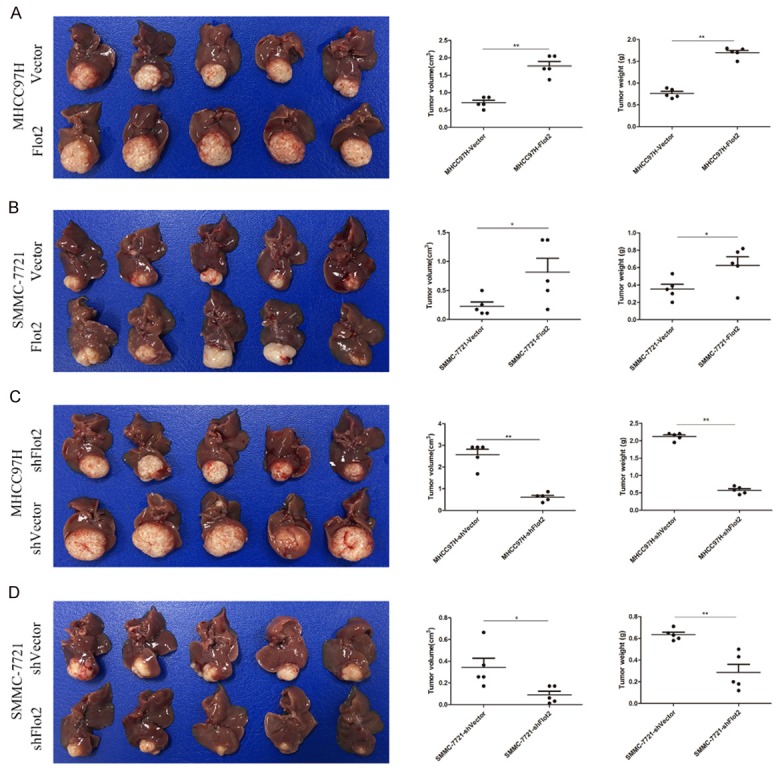
Effects of Flot2 on tumor growth of HCC in vivo. Xenograft tumor model established in nude mice (n=5 for each group). A. The tumors volume and weight of the MHCC97H-Flot2 group were significantly larger and heavier than those of MHCC97H-Vector group (volume, 1.77 ± 0.13 vs 0.71 ± 0.07 cm3, P < 0.01; weight, 1.70 ± 0.12 vs 0.76 ± 0.05 g, P < 0.01). B. The tumors volume and weight of the SMMC-7721-Flot2 group were significantly larger and heavier than those of SMMC-7721-Vector group (volume, 0.82 ± 0.24 vs 0.23 ± 0.07 cm3, P < 0.01; weight, 0.62 ± 0.10 vs 0.35 ± 0.05 g, P < 0.05). C. The tumors volume and weight of the MHCC97H-shFlot2 group were significantly smaller and lighter than those of the MHCC97H-shVector group (volume, 0.61 ± 0.08 vs 2.58 ± 0.54 cm3, P < 0.01; weight, 0.57 ± 0.05 vs 2.12 ± 0.05 g, P < 0.01). D. The tumors volume and weight of the MHCC97H-shFlot2 group were significantly smaller and lighter than those of the SMMC-7721-shVector group (volume, 0.09 ± 0.03 vs 0.34 ± 0.09 cm3, P < 0.05; weight, 0.29 ± 0.08 vs 0.63 ± 0.02 g, P < 0.01). *P < 0.05, **P < 0.01.
Figure 6.
Effects of Flot2 on tumor metastasis of HCC in vivo. A. Representative HE staining of lung metastasis from MHCC97H-Flot2 group and MHCC97H-Vector group, the number of lung metastasis (red arrows) in MHCC97H-Flot2 group were approximately 3-fold than the MHCC97H-Vector group (12.60 ± 1.86 vs 4.00 ± 0.89, P < 0.01). B. Representative pictures of intrahepatic metastasis (red arrows), the number of intrahepatic metastasis in SMMC-7721-Flot2 group were approximately 3-fold than the SMMC-7721-Vector group (29.40 ± 9.96 vs 1.80 ± 0.86, P < 0.05). C. Representative HE staining of intrahepatic metastasis (red arrows represented intrahepatic metastasis; blue arrows represented primary tumor). D. Representative HE staining of vascular invasion in liver of SMMC-7721-Flot2 group (red arrows represented vascular invasion; blue arrow represented primary tumor). *P < 0.05, **P < 0.01.
Correlations between Flot2 expression and clinicopathological features of HCC
To explore the possible correlations between Flot2 expression and clinicopathological features of HCC, immunohistochemistry (IHC) staining was performed using a tissue microarray of 187 HCC patients after curative resection. The patients were divided into low (n=39) and high (n=148) Flot2 expression groups according to expression levels. As Table 1 shows, the expression of Flot2 in HCC tumor tissues was significantly correlated with thrombus (P=0.015) and TNM stage (P=0.027).
Table 1.
Relationship between Flot2 expression and clinicopathologic features
| Relationship between Flot2 expression and clinicopathologic features | |||
|---|---|---|---|
|
| |||
| Flot2 expression | |||
|
| |||
| Clinicopathological features (n) | Low (%) | High (%) | P |
| Age, years | |||
| ≤ 50 (n=79) | 16 (9) | 63 (34) | 0.862 |
| > 50 (n=108) | 23 (12) | 85 (45) | |
| Gender | |||
| Female (n=28) | 8 (4) | 20 (11) | 0.276 |
| Male (n=159) | 31 (17) | 128 (68) | |
| HBsAg | |||
| Negative (n=26) | 6 (3) | 20 (11) | 0.764 |
| Positive (n=161) | 33 (18) | 128 (68) | |
| Cirrhosis | |||
| No (n=143) | 26 (14) | 117 (62) | 0.105 |
| Yes (n=44) | 13 (7) | 31 (17) | |
| AFP | |||
| ≤ 20 ng/ml (n=52) | 11 (6) | 41 (22) | 0.950 |
| > 20 ng/ml (n=135) | 28 (15) | 107 (57) | |
| Size, cm | |||
| ≤ 5 (n=109) | 23 (12) | 86 (46) | 0.922 |
| > 5 (n=78) | 16 (9) | 62 (33) | |
| Number | |||
| Single (n=160) | 33 (18) | 127 (68) | 0.870 |
| Multiple (n=26) | 5 (3) | 21 (11) | |
| Thrombus | |||
| No (n=112) | 30 (16) | 82 (44) | 0.015 |
| Yes (n=75) | 9 (5) | 66 (35) | |
| TNM | |||
| I-II (n=100) | 27 (15) | 73 (39) | 0.027 |
| III-IV (n=87) | 12 (6) | 75 (40) | |
| Encapsulation | |||
| Yes (n=92) | 18 (9) | 74 (40) | 0.669 |
| No (n=95) | 21 (11) | 74 (40) | |
| Grade | |||
| I-II (n=121) | 28 (15) | 93 (50) | 0.298 |
| III-IV (n=66) | 11 (6) | 55 (29) | |
HBeAg: hepatitis B e antigen; AFP: a-fetoprotein; Chi-square test, statistically significant difference (P < 0.05).
Overexpression of Flot2 was correlated with poor prognosis of HCC patients after curative resection
TMA data analysis showed that the 1-, 3-, and 5-year OS rates were 88%, 61%, and 50%, respectively, and the 1-, 3-, and 5-year DFS rates were 68%, 47%, and 39%, respectively. Kaplan-Meier analysis showed that the median OS and DFS times were 66.8 months and 25.5 months for all HCC patients. Patients with high Flot2 expression had shorter overall survival times (P=0.01, HR: 2.18, 95% CI: 1.19-4.01) (Figure 7A) and disease-free survival times (P < 0.01, HR: 2.68, 95% CI: 1.56-4.62) (Figure 7B) compared with patients with low Flot2 expression. The median OS and DFS times were 55.6 months and 18 months, respectively, for patients with high Flot2 expression; and the median OS and DFS times for patients with low Flot2 expression were more than 68.2 months and 66.2 months, respectively.
Figure 7.
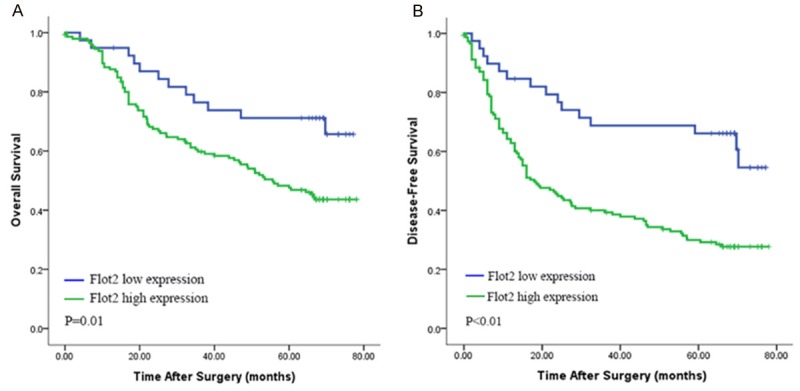
Kaplan-Meier analysis for cumulative overall and disease-free survival curves of patients with high or low Flot2 expression. A. The patients with high Flot2 expression had a shorter overall survival times than the patients with low Flot2 expression (P=0.01, HR: 2.18, 95% CI: 1.19-4.01). B. Patients with high Flot2 expression had a shorter disease-free survival times than the patients with low Flot2 expression (P < 0.01, HR: 2.68, 95% CI: 1.56-4.62).
Furthermore, univariate and multivariate analyses showed that Flot2 expression was also an independent risk factor for both OS (P=0.027, HR: 2.01, 95% CI: 1.085-3.724) and DFS (P < 0.001, HR: 2.701, 95% CI: 1.53-4.768) for HCC patients after curative resection (Table 2).
Table 2.
Univariate and multivariate analysis of risk factors related to OS and DFS of HCC patients
| Univariate and multivariate analyses of factors associated with survival and recurrence | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Factor | Overall Survival | Disease-free Survival | ||||||
|
| ||||||||
| Univariate P | Multivariate | Univariate P | Multivariate | |||||
|
|
|
|||||||
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age: ≤ 50 vs > 50 years | 0.59 | NA | 0.37 | NA | ||||
| Gender: Female vs Male | 0.58 | NA | 0.32 | NA | ||||
| Cirrhosis: No vs Yes | 0.41 | NA | 0.11 | NA | ||||
| HBsAg: Negative vs Positive | 0.47 | NA | 0.85 | NA | ||||
| AFP: ≤ 20 vs > 20 ng/ml | 0.37 | NA | 0.17 | NA | ||||
| Size: ≤ 5 vs > 5 cm | < 0.01 | 2.01 | 1.31-3.07 | < 0.01 | < 0.01 | 1.58 | 1.08-2.30 | 0.02 |
| Number: Single vs Multiple | 0.26 | NA | < 0.01 | NS | ||||
| Thrombus: No vs Yes | < 0.01 | NS | < 0.01 | NS | ||||
| TNM: I vs II vs III | < 0.01 | 1.81 | 1.05-3.13 | 0.03 | < 0.01 | 2.33 | 1.23-4.40 | < 0.01 |
| Encapsulation: Yes vs No | 0.30 | NA | 0.191 | NA | ||||
| Grade: I-II vs III-IV | < 0.01 | 1.65 | 1.07-2.53 | 0.02 | < 0.01 | 1.52 | 1.03-2.24 | 0.04 |
| Flot2: High vs Low | 0.01 | 2.01 | 1.09-3.72 | 0.03 | < 0.01 | 2.70 | 1.53-4.77 | < 0.01 |
HBeAg: hepatitis B e antigen; NA: not adopted; NS: not significant; HR: Hazard Ratio; CI: Confidence Interval; AFP: a-fetoprotein; Flot2: Flotillin-2. Statistically significant difference (P < 0.05).
Discussion
In this study, we first detected the overexpression of Flot2 both in HCC tissues and cell lines. Then, we explored the role of Flot2 in HCC growth and metastasis in vivo and in vitro. In accordance with the studies in other cancers [13,16,24,25], overexpression of Flot2 significantly enhanced the invasion, migration, and proliferation of HCC cells in vitro and promoted tumor growth and metastasis in vivo. Knockdown of Flot2 expression also inhibited the invasive, migratory, and proliferative ability of HCC cells in vitro and decreased tumor growth and metastasis in vivo.
EMT refers to the complex process in which epithelial cells transform into mesenchymal cells. In this process, the polarity of epithelial cells disappears and the invasive and migratory abilities increase, accompanied by down-regulation of epithelial markers and up-regulation of mesenchymal markers. EMT is critical to invasion and metastasis, allowing tumor cells to metastasize and form a secondary tumor in distant organs [26-29]. The complex process is modulated by a series of EMT-inducing transcription factors (EMT-TFs), such as Twist, Snail, Slug, and Zeb1, which have been studied in detail in many experiments [30,31]. In the present study, Flot2 overexpression significantly up-regulated the expression of E-cadherin and down-regulated of the expression of N-cadherin and Vimentin, indicating that Flot2 induces EMT of HCC cells. We observed a strong induction of Twist, as well as Raf/MEK/ERK1/2 pathway activation, in Flot2-overexpressed HCC cells, while U0126, an ERK1/2 inhibitor, significantly inhibited the Flot2-induced down-regulation of E-cadherin and up-regulation of N-cadherin and Vimentin, indicating that Flot2-induced EMT was coordinated by Twist as a result of Raf/MEK/ERK1/2 pathway activation. In addition, we found that Flot2 promoted cell proliferation through positive regulation of the cell cycle, such as up-regulation of cyclin D3, CDK2 and CDK4 and down-regulation of p27, p21 and p18.
Moreover, we found that overexpression of Flot2 promoted growth and metastasis, while knockdown of Flot2 expression inhibited tumor growth and metastasis of HCC in vivo. The SMMC-7721-Flot2 group showed more intrahepatic metastasis in mouse livers, and the MHCC97H-Flot2 group revealed more lung metastasis, both compared with the control group. The tumors volume and weights of the Flot2 overexpressed groups were significantly larger and heavier than those of the control groups, while those of the silenced groups were significantly smaller and lighter than the control groups. According to the above results, we demonstrated that Flot2 promoted tumor growth and metastasis of HCC.
Flot2 expression was up-regulated in several malignant tumors and was suggested to be a prognostic marker for cancers [14-16,25,32-35]. Furthermore, using a tissue microarray analysis, we found that the expression of Flot2 was significantly correlated with thrombus and TNM stage of HCC patients after curative resection. HCC patients with high Flot2 expression had a worse overall survival and disease-free survival compared to patients with low Flot2 expression. Univariate and multivariate analyses showed that Flot2 expression was also an independent risk factor for both OS and DFS of HCC patients. Therefore, in predicting HCC prognosis, Flot2 is very valuable and may help clinicians to distinguish high risk recurrence, and HCC patients with high expression of Flot2 should be closely monitored for early recurrence and metastasis.
In conclusion, this study demonstrated that Flot2 promoted tumor growth and metastasis of HCC through induction of EMT and modulation of cell cycle. The expression of Flot2 correlated with poor prognosis in HCC patients after curative resection and was an independent risk factor, indicating that Flot2 may be regarded as a potential poor prognostic marker for HCC.
Acknowledgements
This study was supported by the National Natural Science Funds of China (No. 81672326 and 81472224).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- 4.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Park MY, Kim KR, Park HS, Park BH, Choi HN, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression of the serum response factor in hepatocellular carcinoma: implications for epithelial-mesenchymal transition. Int J Oncol. 2007;31:1309–1316. [PubMed] [Google Scholar]
- 6.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141. [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu J, Koh Y, Park H, Kim DY, Kim DC, Byun JM, Lee HJ, Yoon SS. Highly expressed integrin-α8 induces epithelial to mesenchymal transition-like features in multiple myeloma with early relapse. Mol Cells. 2016;39:898. doi: 10.14348/molcells.2016.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XL, Liu L, Li DD, He YP, Guo LH, Sun LP, Liu LN, Xu HX, Zhang XP. Integrin β4 promotes cell invasion and epithelial-mesenchymal transition through the modulation of Slug expression in hepatocellular carcinoma. Sci Rep. 2017;7:40464. doi: 10.1038/srep40464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Q, Wei F, Zhang J, Li B. miR-134 suppresses the migration and invasion of nonsmall cell lung cancer by targeting ITGB1. Oncol Rep. 2017;37:823–830. doi: 10.3892/or.2017.5350. [DOI] [PubMed] [Google Scholar]
- 10.Dong ZR, Zhang PF, Wang CH, Zhang C, Cai JB, Shi GM, Ke AW, Sun HC, Qiu SJ, Zhou J. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria: a retrospective analysis. Am J Cancer Res. 2015;5:450. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CH, Guo ZY, Chen ZT, Zhi XT, Li DK, Dong ZR, Chen ZQ, Hu SY, Li T. TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection. Sci Rep. 2015;5:12366. doi: 10.1038/srep12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banning A, Kurrle N, Meister M, Tikkanen R. Flotillins in receptor tyrosine kinase signaling and cancer. Cells. 2014;3:129–149. doi: 10.3390/cells3010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hazarika P, McCarty MF, Prieto VG, George S, Babu D, Koul D, Bar-Eli M, Duvic M. Up-regulation of Flotillin-2 Is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1. Cancer Res. 2004;64:7361–7369. doi: 10.1158/0008-5472.CAN-04-0823. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Yang Q, Guo L, Li XH, Zhao XH, Song LB, Lin HX. Flotillin-2 is associated with breast cancer progression and poor survival outcomes. J Transl Med. 2013;11:190. doi: 10.1186/1479-5876-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Z, Wang J, Sun Z, Sun X, Wang Z, Xu H. Flotillin2 Expression correlates with HER2 levels and poor prognosis in gastric cancer. PLoS One. 2013;8:e62365. doi: 10.1371/journal.pone.0062365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Lin L, Pan C, Shi M, Liao Y, Bin J, Liao W. Flotillin-2 promotes nasopharyngeal carcinoma metastasis and is necessary for the epithelial-mesenchymal transition induced by transforming growth factor-β. Oncotarget. 2015;6:9781–93. doi: 10.18632/oncotarget.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma DN, Chai ZT, Zhu XD, Zhang N, Zhan DH, Ye BG, Wang CH, Qin CD, Zhao YM, Zhu WP, Cao MQ, Gao DM, Sun HC, Tang ZY. MicroRNA-26a suppresses epithelial-mesenchymal transition in human hepatocellular carcinoma by repressing enhancer of zeste homolog 2. J Hematol Oncol. 2016;9:1. doi: 10.1186/s13045-015-0229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye BG, Sun HC, Zhu XD, Chai ZT, Zhang YY, Ao JY, Cai H, Ma DN, Wang CH, Qin CD. Reduced expression of CD109 in tumor-associated endothelial cells promotes tumor progression by paracrine interleukin-8 in hepatocellular carcinoma. Oncotarget. 2016;7:29333. doi: 10.18632/oncotarget.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun FX, Tang ZY, Liu KD, Ye SL, Xue Q, Gao DM, Ma ZC. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66:239–243. doi: 10.1002/(SICI)1097-0215(19960410)66:2<239::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Ye QH, Qin LX, Forgues M, He P, Kim JW, Peng AC, Simon R, Li Y, Robles AI, Chen Y, Ma ZC, Wu ZQ, Ye SL, Liu YK, Tang ZY, Wang XW. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 22.Qian YB, Zhang JB, Wu WZ, Fang HB, Jia WD, Zhuang PY, Zhang BH, Pan Q, Xu Y, Wang L, Tang ZY, Sun HC. P48 is a predictive marker for outcome of postoperative interferon-alpha treatment in patients with hepatitis B virus infection-related hepatocellular carcinoma. Cancer. 2006;107:1562–1569. doi: 10.1002/cncr.22206. [DOI] [PubMed] [Google Scholar]
- 23.Chai ZT, Kong J, Zhu XD, Zhang YY, Lu L, Zhou JM, Wang LR, Zhang KZ, Zhang QB, Ao JY, Wang M, Wu WZ, Wang L, Tang ZY, Sun HC. MicroRNA-26a inhibits angiogenesis by down-regulating VEGFA through the PIK3C2alpha/Akt/HIF-1alpha pathway in hepatocellular carcinoma. PLoS One. 2013;8:e77957. doi: 10.1371/journal.pone.0077957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao K, Xie D, Cao P, Zou Q, Lu C, Xiao S, Zhou J, Peng X. SiRNA-mediated flotillin-2 (Flot2) downregulation inhibits cell proliferation, migration, and invasion in gastric carcinoma cells. Oncol Res. 2014;21:271–279. doi: 10.3727/096504014X13946737557031. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Huang W, Ren C, Wen Q, Liu W, Yang X, Wang L, Zhu B, Zeng L, Feng X, Zhang C, Chen H, Jia W, Zhang L, Xia X, Chen Y. Flotillin-2 promotes metastasis of nasopharyngeal carcinoma by activating NF-kappaB and PI3K/Akt3 signaling pathways. Sci Rep. 2015;5:11614. doi: 10.1038/srep11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946–954. doi: 10.1016/j.surg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Brockhausen J, Tay SS, Grzelak CA, Bertolino P, Bowen DG, d’Avigdor WM, Teoh N, Pok S, Shackel N, Gamble JR. miR-181a mediates TGF-β-induced hepatocyte EMT and is dysregulated in cirrhosis and hepatocellular cancer. Liver Int. 2015;35:240–253. doi: 10.1111/liv.12517. [DOI] [PubMed] [Google Scholar]
- 28.Bae SY, Kim HJ, Lee KJ, Lee K. Translationally controlled tumor protein induces epithelial to mesenchymal transition and promotes cell migration, invasion and metastasis. Sci Rep. 2015;5:8061. doi: 10.1038/srep08061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Li X, Yang H, Chang R, Kong C, Yang L. SIN1 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial-mesenchymal transition. Cancer. 2013;119:2247–2257. doi: 10.1002/cncr.28023. [DOI] [PubMed] [Google Scholar]
- 30.Craene BD, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 31.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty SD, Prieto VG, George S, Hazarika P, Duvic M. High flotillin-2 expression is associated with lymph node metastasis and Breslow depth in melanoma. Melanoma Res. 2006;16:461–463. doi: 10.1097/01.cmr.0000222592.75858.20. [DOI] [PubMed] [Google Scholar]
- 33.Ye GY, Luo Y, Qin SL, Mu YF, Zhong M. High expression of Flotillin-2 is associated with poor prognosis in Chinese patients with colorectal cancer. Int J Clin Exp Med. 2016;9:1752–1757. [Google Scholar]
- 34.Wen Q, Wang W, Chu S, Luo J, Chen L, Xie G, Xu L, Li M, Fan S. Flot-2 expression correlates with EGFR levels and poor prognosis in surgically resected non-small cell lung cancer. PLoS One. 2015;10:e0132190. doi: 10.1371/journal.pone.0132190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen Q, Li J, Wang W, Xie G, Xu L, Luo J, Chu S, She L, Li D, Huang D, Fan S. Increased expression of Flotillin-2 protein as a novel biomarker for lymph node metastasis in nasopharyngeal carcinoma. PLoS One. 2014;9:e101676. doi: 10.1371/journal.pone.0101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.