Abstract
O-glycosylation in the field of carcinogenesis has been a critical topic of concern for several decades. The abnormal function of enzymes catalyzing the first step of this process, named polypeptide N-acetylgalactosaminyltransferases (ppGalNAc-Ts) has been determined to play an important role in cancer development and metastasis. Accordingly, we investigated the expression of GalNAc-T6 in endometrial carcinoma and evaluated the relationship between invasion characteristics and the cellular level of GalNAc-T6. The results suggested that positive GalNAc-T6 expression is significantly associated with histological grade of tumors and myometrial invasion characteristic. In vitro experiments showed that the over expression of GalNAc-T6 had strong association with the decrease of endometrial cell invasiveness. Taken together, our data support the use of GalNAc-T6 as a potential indicator of good prognosis and noninvasive tumor in patients with endometrial carcinoma.
Keywords: O-glycosylation, polypeptide N-acetylgalactosaminyltransferase 6, endometrial carcinoma, cell migration, cell invasion
Introduction
In recent years, an outbreak of research focusing on glycosylation process has provided a growing body of evidence supporting its importance in normal cell and defined how aberrant glycosylation can contribute to the generation of pathological lesions [1,2]. This enzymatic process synthesizes glycoprotein through the attachment of one or more glycans to a polypeptide backbone via nitrogen or oxygen linkages which produces N-glycan and O-glycan respectively [3]. One common type of O-glycosylation which initiated with the attachment of monomeric carbohydrate moieties to serine and threonine residues of mucin protein via the catalysis of polypeptide N-acetylgalatosaminyltransferases (GalNAc-Ts) is mucin-type O-glycosylation. O-glycosylation plays a crucial role in post-translational modification of mucin, given its specificity of individual tissues [4]. Until now, a total of 20 human GALNT gene entries have been reported, named GALNT1 to GALNT20 which encodes for the according enzymes [5]. In order to facilitate for their characterization, GALNT genes were grouped into subfamilies containing paralogs exhibited high similarity in genomic structure and encoded enzymes with related functions. The GALNT3/GALNT6 subfamily is a well characterized example with high similarity in DNA, amino acid sequence and similar kinetic properties [6]. Abnormal expression of GalNAc-T6 has been reported to have prognostic role in several different kinds of cancer such as gastric carcinoma [7,8], lung adenocarcinoma [9,10], renal cell carcinoma [11], and oral squamous cell carcinoma [12]. Among popular types of cancer in females, endometrial carcinoma is the most common gynecological carcinoma in developed countries including Japan [13]. Given the fact that number of cases are still increasing, seeking new biomarkers to predict cancer prognosis and develop therapeutic targets is a priority of current research. Following up on verifying the role of abnormal O-glycosylation in cancers, we investigated expression patterns of GalNAc-T6 in endometrioid endometrial carcinoma, which is the predominant subtype in clinical setting.
In the present study, we found that positive expression of GalNAc-T6 significantly associated with well-differentiated histological grades and non-invasive tumors. In addition, in vitro studies demonstrated that modification of GalNAc-T6 expression significantly changed endometrial carcinoma cells invasion and migration characteristics.
Materials and methods
Cases selection
Subjects of current research were two hundred and eighteen patients who were diagnosed with endometrioid endometrial carcinoma at the hospital of the University of Occupational and Environmental Health (Kitakyushu, Japan) in 20-year period from 1990 to 2009. All of the patients received standard treatment for endometrial carcinoma included hysterectomy and bilateral salpingo-oophorectomy, pelvic lymph node dissection, para-aortic lymph node dissection or biopsy; the following chemotherapy therapy were decided according to surgical stage and classification of risk factors. Histopathological classification was determined according to the 1988 FIGO staging system (International Federation of Gynecology and Obstetrics). Study was established with the agreement of the Hospital Review Board on Ethical Issues.
Immunohistochemistry
Preparation of antibody against GalNAc-T6 was described previously [11,12]. The specificity of antibody was checked by Western Blotting (Figure 4A). Considering that GalNAc-T6 expression in placenta was previously reported [6], we performed preliminary imunohistochemical evaluation using generated antibody on placenta samples. In order to evaluate the expression of GalNAc-T6 in normal endometrium, samples of endometrium in proliferative and secretary phases were also included.
Figure 4.
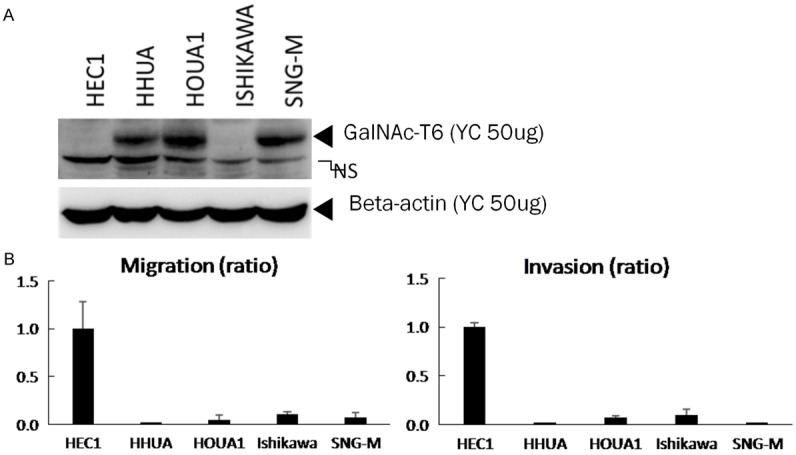
Invasion and migration activity of endometrial carcinoma cells and GalNAc-T6 expression. A. Whole cell lysates (50 µg) of indicated endometrial carcinoma cell lines were subjected to western blot analysis with anti-GalNAc-T6 and anti-beta actin antibodies. B. Each cell line was seeded into control and Matrigel-coated chambers at a density of 2.5 × 104 cells per well. After 22 h, non-migrating and non-invading cells were removed, migrating and invading cells were counted as described in the Materials and Methods. Cell number of HEC1 cell line was set to 1. The data were averaged from three independent experiments.
Immunohistochemical staining was performed by the antibody-linked dextran polymer method as described previously [11] (Envision; DAKO Cytomation, Tokyo, Japan). In brief, formalin-fixed paraffin-embedded tissue blocks were cut, deparaffinized in xylene, then rehydrated through sequential changes of alcohol and distilled water. Antigen retrieval was performed at 121°C for 3 minutes with 0.01 M citrate buffer (pH=6.0). Slides then were incubated with polyclonal antibody against GalNAc-T6 (diluted 1:1600) 1 hour at room temperature. The second antibody-peroxidase-linked polymers (Dako REAL EnVision/HRP, Rabbit/Mouse) were then applied. Finally those slides were counterstained with Meyer’s haematoxylin and all sections were observed under a light microscope. Staining patterns were scored independently by two board-certified pathologists in our department with blinded analysis (observers were blinded to the clinicopathological data). Final score was decided according to the agreement of the two observers; for the cases of disagreement, a consensus score was determined by the third board-certified pathologist in our department. Due to the heterogeneous in endometrial cancer cells within one tumor, score was obtained at the area showed strongest staining intensity. Signal intensity ranged from nul (0), weak (1), moderate (2), and strong (3). To analyze the significance of expression patterns, scores were sorted in to negative (score 0 and 1) and positive groups (score 2 and 3) (Figure 2).
Figure 2.
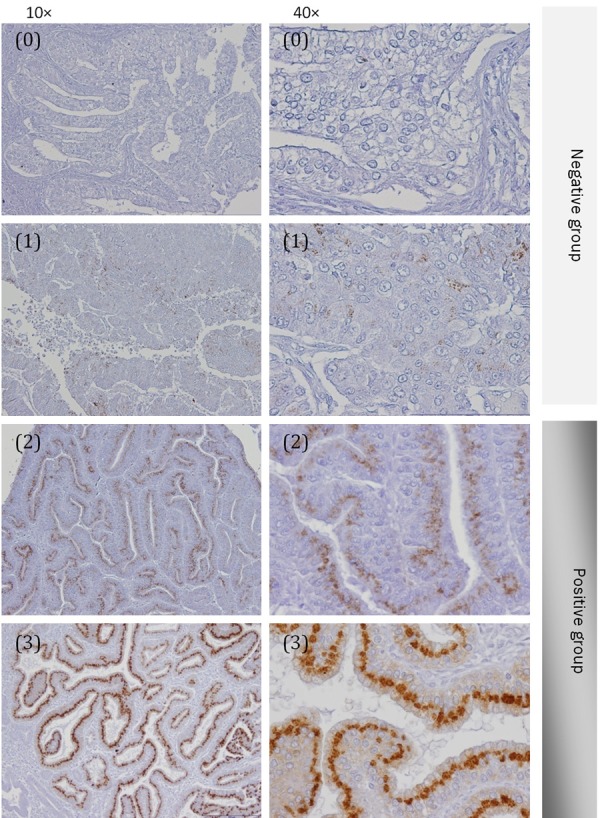
Immunohistochemitry expression of GaNAc-T6 in endometrioid endometrial carcinomas. Score (0) and (1): negative group; score (2) and (3): positive group. Figures are presented at 10 × and 40 × magnification.
Cell culture and antibodies
The following human endometrial cell lines were used: HEC1, HHUA, HOUA1, ISHIKAWA, SNG-M. These cell lines were cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in 5% CO2 atmosphere. The same antibody against GalNAc-T6 used in immunohistochemistry was utilized for the following experiments. Anti β-actin antibody was purchased from Sigma-Aldrich (Saint Louis, Missouri, USA).
Knockdown with GalNAc-T6 siRNA and over expression of GalNAc-T6
For knockdown GalNAc-T6, 25 base pair Stealth™ siRNAs were commercially generated (Invitrogen): 5’-UUUCUCUUGGGCCAUCUUUGCUGCC-3’ and 5’-GGCAGCAAAGAUGGCCCAAGAGAAA-3’ for GalNAc-T6#36; 5’-AAACAU-CUCUGGGUAGACAUUGUGC-3’ and 5’GCACAAUGUCUACCCAGAGAUG-UUU-3’ for GalNAc-T6#37. siRNA transfection was performed as previously described [14]. For overexpression of GalNAc-T6, cDNA of GalNAc-T6 was ligated into the multicloning site of pEB GFP-T2A-Puro expression plasmid. This plasmid stably expresses GFP and Puromycin resistant protein as well as GalNAc-T6 protein. Transfected cells were cultured for 2 weeks with 3 ng/mL puromycin, and over 80% cells were expressed GFP. Knocked down and overexpressed cells were used for western blotting, migration assays and invasion assays.
Western blot
Western blot analysis was performed as described previously [12]. Briefly, 50 µg of whole cell lysates was separated by 8% SDS-PAGE and transferred onto polyvinylidene difluoride membranes and probed with anti-GalNAc-T6 (1:1000) and anti-β-actin (1:5000) antibodies. The bound antibody was visualized using an enhanced chemiluminescence kit (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and the signal intensity was quantified using Multi Gauge software version 3.0 (Fujifilm, Tokyo, Japan).
Invasion assay and migration assay
Cell culture and invasion and migration assay have been described previously [14]. Cells passing through the membrane in the upper chamber were stained with DAPI. Migrated cells and invaded cells were counted in 0.8 mm × 1.1 mm square area for non-transfected cells and 2.0 mm × 2.7 mm square area for transfectants. Cell counts were normalized with total cell number prepared separately.
Statistical analysis
Statistical analyses were carried out using SPSS version 18.0 (SPSS Inc, Chicago, IL, USA). Mann-Whitney U-test (Pearson χ2) was used to assess relationship between clinicopathological variables and GalNAc-T6 expression. Kaplan-Meier method was used to evaluate the prognostic value of GalNAc-T6. P<0.05 was considered to be statistically significant.
Results
Clinicopathological characteristics
Median age was 57.5 year ranged from 22 years old to 86 years old. The majority of patients were diagnosed at early stage (75.2%) and low grade (87.2%). Negative myometrial invasion in which tumor invaded to less than one third of myometrium only rated 24.3%. Vascular invasion was detected in approximately one third of patients (34.9%) but cervical involvement, ovarian and adnexa metastasis, pelvic lymph node metastasis were observed in a small number of patients, rating 11.0%, 6.4% and 7.8% respectively (Table 1).
Table 1.
Clinicopathological characteristics of patients with endometrioid endometrial carcinoma
| Number of patients (%) n=218 | |||
|---|---|---|---|
| Mean age (year, range) | 57.5 (22-86) | ||
| FIGO stage | Early | I | 152 (69.7) |
| II | 12 (5.5) | ||
| Advanced | III | 50 (23.0) | |
| IV | 4 (1.8) | ||
| Grade | Low | 1 | 127 (58.3) |
| 2 | 63 (28.9) | ||
| High | 3 | 28 (12.8) | |
| Myometrial invasion | Negative | 53 (24.3) | |
| Positive | 165 (75.7) | ||
| Vascular invasion | Negative | 142 (65.1) | |
| Positive | 76 (34.9) | ||
| Pelvic LN metastasis | Negative | 201 (92.2) | |
| Positive | 17 (7.8) | ||
| Cervical invasion | Negative | 194 (89.0) | |
| Positive | 24 (11.0) | ||
| Ovarian metastasis | Negative | 204 (93.6) | |
| Positive | 14 (6.4) | ||
All patients underwent surgery for total hysterectomy and bilateral salpingo-oophorectomy and received standard treatment with chemotherapy. Surgical stages were decided based on 1988 FIGO staging system (International Federation of Gynecology and Obstetrics).
Immunohistochemistry staining and prognostic significance
In placenta samples of last semester, GalNAc-T6 immunostaning was positive (Figure 1A). The positive staining was totally abolished after omitting the GalNAc-T6 antibody from the negative control slides. All of the glands in normal endometrium of proliferative phase showed negative staining. The glands in normal endometrium of secretary phase showed scattered weak cytoplasmic staining which was classified as score 1 (negative group) (Figure 1B).
Figure 1.
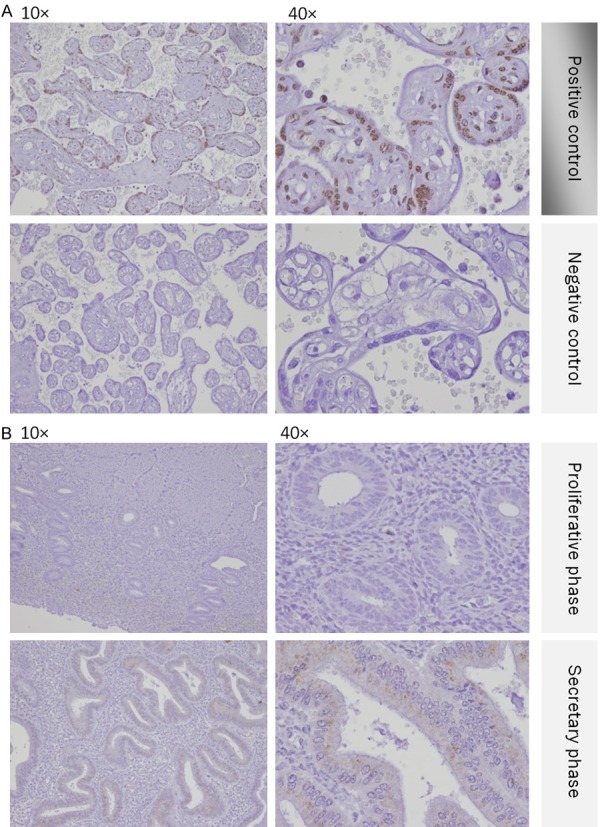
Immunohistochemitry expression of GaNAc-T6 in placenta and normal endometrium. A. Placenta samples were positive. B. Both proliferative and secretary endometrium was negative.
One hundred and fifty-two cases (69.7%) had positive immunostaining with GalNAc-T6 and 66 patients were negative staining (30.3%) (Table 2). All of the positive cases showed glandular staining pattern in the perinuclear region (Figure 2). The expression of GalNAc-T6 has statistical relationship with histological grade of the tumor (P=0.002) and myometrial invasion characteristic (P=0.017). There was not significant correlation between GalNAc-T6 expression and other features such as FIGO stage (P=0.366), vascular invasion (P=0.356), and pelvic lymph nodes metastasis (P=0.118) (Table 2).
Table 2.
Correlation between clinicopathological variables and GalNAc-T6 expression
| GalNAc-T6 | ||||
|---|---|---|---|---|
|
| ||||
| Positive n=152 | Negative n=66 | P value | ||
| FIGO stage | Early (I, II) | 117 | 47 | 0.366 |
| Advanced (III, IV) | 35 | 19 | ||
| Grade | 1 and 2 | 139 | 50 | 0.002 |
| 3 | 13 | 16 | ||
| Myometrial invasion | Negative | 30 | 23 | 0.017 |
| Positive | 122 | 43 | ||
| Vascular invasion | Negative | 102 | 40 | 0.356 |
| Positive | 50 | 26 | ||
| Pelvic LN metastasis | Negative | 143 | 58 | 0.118 |
| Positive | 9 | 8 | ||
| Cervical invasion | Negative | 135 | 59 | 0.901 |
| Positive | 17 | 7 | ||
| Ovarian invasion | Negative | 145 | 59 | 0.098 |
| Positive | 7 | 7 | ||
Possitive expression of GalNAc-T6 was significantly associated with low grade of tumors (1 and 2) (P=0.002) and myometrial invasion negative (P=0.017).
Overall survival rate after cumulative of those patients exhibiting positive GalNAc-T6 staining appeared to be significant higher than the negative staining group (92.1% compared with 80.1%; P=0.030) (Figure 3).
Figure 3.
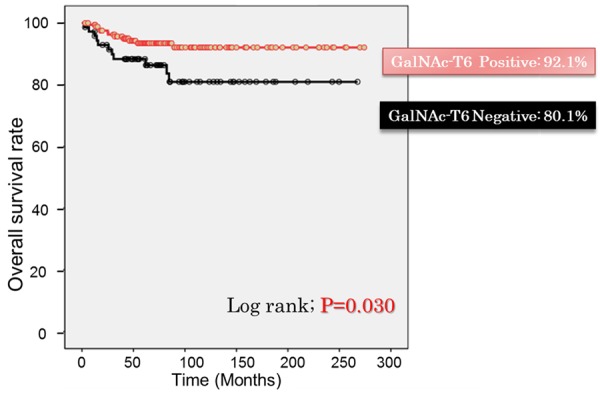
Overall survival rate of patients with endometrioid emdometrial carcinoma according to GalNAc-T6 expression. Patients with positive expression of GalNAc-T6 showed significant higher overall survival rate (92.1% compared with 80.1%) (P=0.030).
Invasion and migration of endometrial cells in association with GalNAc-T6 levels
To evaluate GalNAc-T6 function relating to cell invasion and migration activities, five endometrial cancer cell lines were examined. Western blotting was performed to assess the basal cellular level of GalNAc-T6 protein expression (Figure 4A). GalNAc-T6 was observed as a 25 kDa band in HHUA, HOUA1 and SNG-M cells while very low to non-detectable levels were found in HEC1 and ISHIKAWA cell lines. Accordingly, in invasion and migration assay, ISHIKAWA and especially HEC1 cell lines exhibited high invasion and migration activities compared with the other three GalNAc-T6-expressing cell line (Figure 4B). To evaluate the effect of modifying GalNAc-T6 expression on invasion and migration activities, GalNAc-T6 knockdown by specific siRNA was performed in HHUA and HOUA1 cell lines. The efficacy of the knockdown experiment was confirmed by Western blotting which displayed significant decreases in GalNAc-T6 expression in both cell lines transfected with siGANLT6#36 and siGANLT6#37 compared with the wild type (Figure 5A). Invasion and migration assays showed that knockdown of GANLT6 increased the invasion activity but not the migration activity of both cell lines (Figure 5B). To clarify the contribution of GalNAc-T6 on invasion, we established a HHUA transfectant cell line with overexpression of GalNAc-T6 (Figure 6A). Overexpression of GalNAc-T6 significantly decreased invading cells but not migrating cells (Figure 6B). These results are complementary to the results of knockdown assay.
Figure 5.
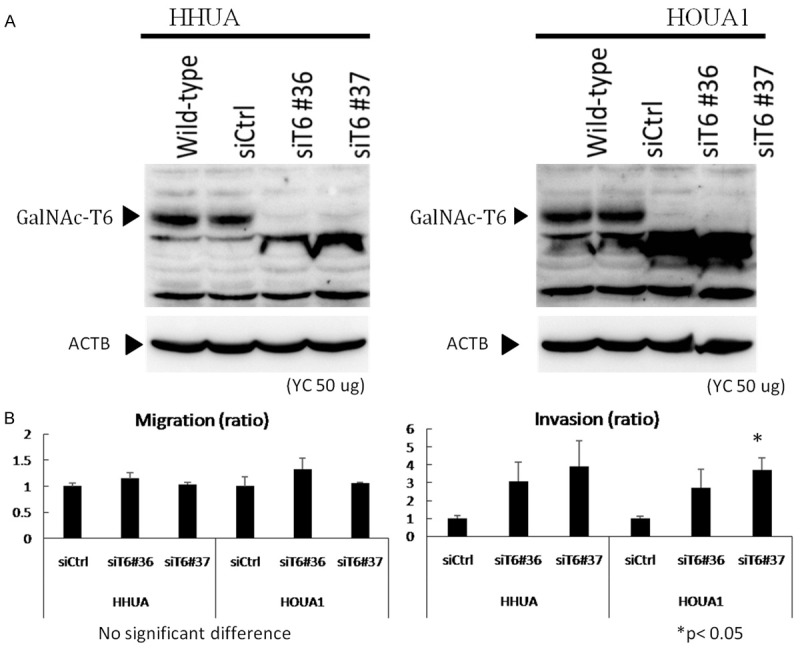
Induction of invasion by knockdown of GalNAc-T6. A. GALNT6 siRNA (siT6#36 and #37) and control siRNA (siCtrl) was transfected into HHUA or HOUA1 cell lines. Whole cell lysates (50 µg) were used for western blotting with anti-GalNAc-T6 and anti-beta actin antibodies. B. siRNA-transfected HHUA and HOUA1 cell lines were seeded into control and Matrigel-coated chambers at a density of 2.5 × 104 cells per well. After 22 h, non-migrating and non-invading cells were removed, migrating and invading cells were counted as described in the Materials and Methods. Cell number of each cell transfected with control siRNA (siCtrl) was set to 1. The data were averaged from two independent experiments.
Figure 6.
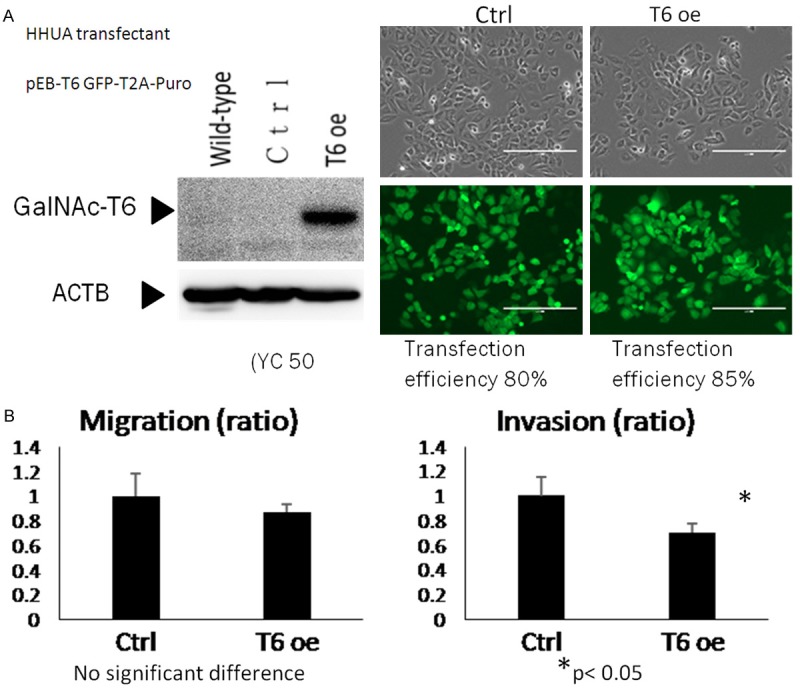
Inhibition of invasion by overexpression of GalNAc-T6. A. GALNT6 expression plasmid and control expression plasmid were transfected into HHUA cell line. Whole cell lysates (50 µg) were used for western blotting with anti-GalNAc-T6 and anti-beta actin antibodies. Exposure time of Western blot with anti-GalNAc-T6 antibody was shorter than that of Figure 3A. B. GalNAc-T6 over expressed-HHUA cell line was seeded into control and Matrigel-coated chambers at a density of 2.5 × 104 cells per well. After 22 h, non-migrating and non-invading cells were removed, and migrating and invading cells were counted as described in the Materials and Methods. Cell number of each cell transfected with control plasmid was set to 1. The data were averaged from two independent experiments.
Discussion
Numerous reports have demonstrated an association between functional abnormalities of GalNAc-Ts family and neoplastic transformation. Among the gynecological cancers, our research is the first one evaluated the role of abnormal GalNAc-T6 expression in endometrial carcinomas. Findings of our study indicated that positive GalNAc-T6 staining predicted better overall survival rate of patients with endometrial carcinoma (P=0.003) (Figure 3). Observations in numerous types of cancer have proved the specific role of GalNAc-T6 in carcinogenesis however the prognostic role was still ambiguous. Indeed, positive expression of GalNAc-T6 was found to correlate with breast carcinoma at low stage [15], and predict good prognosis of patients with pancreatic cancer [16]. On the other hand, patients with renal cell carcinomas exhibiting positive GalNAc-T6 had shorter postoperative median disease-specific survival [11], and increased GalNAc-T6 expression predicted a worse overall survival rate of patients with lung adenocarcinoma [10]. These seemingly conflicting results of different research indicate the complex functional pathway of GalNAc-T6 depending upon the cancer types. Indeed, the O-glycosylation pattern in one specific cancer cell type was said to be determined not by a single GalNAc-T isotype but a repertoire of enzymes [17]. Therefore, we hypothesized that the role of GalNAc-T6 in endometrial carcinogenesis is in tight cooperation with other members of GalNAc-Ts family.
Moreover, the findings of our research showed that positive GalNAc-T6 expression is significantly associated with well-differentiated tumors (P=0.002) and negative myometrial invasion (P=0.017) (Table 2). Further experiments at cellular level revealed the relation between GalNAc-T6 protein level and the motility of endometrial cells. HEC1 and ISHIKAWA cell lines were both observed to have undetectable levels of GalNAc-T6 protein; but these two cell lines were found with huge difference in invasion and migration ratio (Figure 4A). The difference might be due to the natural characteristics of each cell line. The GalNAc-Ts profile of Ishikawa cell line still remains under investigation. Knockdown GalNAc-T6 by siRNA and overexpression of GalNAc-T6 from plasmid transfectant revealed the striking change of invasion character in response to the alteration of GalNAc-T6 protein level (Figure 4B, 4C). Cell migration is defined as the movement of individual cell, or cell clusters from one place to another [18]. Inside the living body, collective migration of cell clusters is the prevalent mode of migration during development, wound healing, and tissue regeneration [19]. Cell invasion refers to 3-dimentional migration of cells as they penetrate extracellular matrix (ECM) through a process involved ECM degradation and proteolysis [20]. In addition, collective cancer cell migration is increasingly regarded as a mode of invasion during metastasis in epithelial cancers [21]. Since there is no clear criteria to discriminate these two cellular motion, cell invasion was generally stated to encompass cell migration [20]. To some extent, the correlation between the modification of GalNAc-T6 protein level and the change in endometrial cancer cells motility detected in our research indicated that the overexpression of GalNAc-T6 in patient with endometrial carcinoma is associated with lower invasion characteristics and therefore better prognosis.
The potential connection between GalNAc-T6 and cell invasion characteristics also led us to the questions about the relationship between GalNAc-T6 and other cell-cell adhesion markers. In order to dissociate, cancer cells tend to downregulate the key members of adhesion junction family through the epithelial to mesenchymal transition process (EMT) [22]. Previous research in both normal and cancerous mammary epithelial cells also provided evidence for the connection between GalNAc-T6 protein level and the EMT process via the change of cell adhesion molecules [23,24]. In spite of the contrary role of GalNAc-T6 in these two types of cancer, we could not rule out the possibility that there are similar substrates or subsequent downstream factors in endometrial carcinomas.
In summary, our research provides evidence to support the expression of GalNAc-T6 as one prognostic factor for patients with endometrioid endometrial carcinomas. The link between GalNAc-T6 expression and cancer cells invasion characteristics is valuable information which shed light on the additional researches investigating functional pathways of GalNAc-T6. Further studies are warrant for thoroughly understanding of aberrant O-glycosylation processes in endometrial carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuster MM, Esko JD. The sweat and sour of cancer: glycans as novel therapeutic targets. Nature. 2005;5:526–42. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 3.Clausen H, Eric BP. A family of UDP-GalNac: polypeptide N-acetylgalactosaminyl-transferase control the initiation of mucin-type O-linked glycosylation. Glycobiology. 1996;6:635–46. doi: 10.1093/glycob/6.6.635. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature. 2004;4:45–54. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 5.Bennett EP, Mandel U, Clausen H, Gerken TA, Timothy FA, Lawrence TA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNac-transferase gene family. Glycobiology. 2012;22:736–56. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, Kessel AG, Olofsson S, Clausen H. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem. 1999;274:25362–70. doi: 10.1074/jbc.274.36.25362. [DOI] [PubMed] [Google Scholar]
- 7.Onitsuka K, Shibao K, Nakayama Y, Minagawa N, Hirata K, Izumi H, Matsuo K, Nagata N, Kitazato K, Kohno K, Itoh H. Prognostic significance of UDP-N-acetyl-α-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-3 (GalNAc-T3) expression in patients with gastric carcinoma. Cancer Sci. 2003;94:32–36. doi: 10.1111/j.1349-7006.2003.tb01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes J, Marcos NT, Berois N, Osinaga E, Magalhaes A, Sousa JP, Almeida R, Gartner F, Reis CA. Expression of UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J Histochem Cytochem. 2009;57:79–86. doi: 10.1369/jhc.2008.952283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu C, Oyama T, Osaki T, Li J, Takenoyama M, Izumi H, Sugio K, Kohno K, Yasumoto K. Low expression of polypeptide GalNAc N-acetylgalactosaminyl transferase-3 in lung adenocarcinoma: impact on poor prognosis and early recurrence. Br J Cancer. 2004;90:436–42. doi: 10.1038/sj.bjc.6601531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Yamada S, Wu Y, Wang KY, Liu YP, Uramoto H, Kohno K, Sasaguri Y. Polypeptide N-acetylgalactosaminyltransferase-6 expression independently predicts poor overall survival in patients with lung adenocarcinoma after curative resection. Oncotarget. 2016;7:54463–54473. doi: 10.18632/oncotarget.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitada S, Yamada S, Kuma A, Ouchi S, Tasaki T, Nabeshima A, Noguchi H, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Matsumoto T, Sasaguri Y. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer. 2013;109:472–81. doi: 10.1038/bjc.2013.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada Y, Izumi H, Noguchi H, Kuma A, Kawatsu Y, Kimura T, Kitada S, Uramoto H, Wang KY, Sasaguri Y, Hijioka H, Miyawaki A, Oya R, Nakayama T, Kohno K, Yamada S. Strong expression of polypeptide N-acetylgalactosaminyltransferase 3 independently predicts shortened disease-free survival in patients with early stage oral squamous cell carcinoma. Tumour Biol. 2016;37:1357–68. doi: 10.1007/s13277-015-3928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamagami W, Aoki D. Annual report of the Committee on Gynecologic Oncology, the Japanese society of obstetrics and gynecology. J Obstet Gynaecol Res. 2015;41:167–77. doi: 10.1111/jog.12596. [DOI] [PubMed] [Google Scholar]
- 14.Izumi H, Takahashi M, Uramoto H, Nakayama Y, Oyama T, Wang KY, Sasaguri Y, Nishizawa S, Kohno K. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011;102:1007–13. doi: 10.1111/j.1349-7006.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 15.Berois N, Mazal D, Ubillos L, Trajtenberg F, Nicolas A, Sastre-Garau X, Magdelenat H, Osinaga E. UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 as a new immunohistochemical breast cancer marker. J Histochem Cytochem. 2006;54:317–28. doi: 10.1369/jhc.5A6783.2005. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Yamada S, Inenaga S, Imamura T, Wu Y, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Sasaguri Y. Polypeptide N-acetylgalactosaminyltransferase 6 expression in pancreatic cancer is an independent prognostic factor indicating better overall survival. Br J Cancer. 2011;104:1882–89. doi: 10.1038/bjc.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, Nielsen PA, Hollingsworth MA, Burchell J, Taylor-Papadimitriou J, Clausen H. Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3. J Biol Chem. 1997;272:23503–14. doi: 10.1074/jbc.272.38.23503. [DOI] [PubMed] [Google Scholar]
- 18.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 19.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–57. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 20.Friedl P, Bröcker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Enomoto A, Asai N, Kato T, Takahashi M. Collective invasion of cancer: perspectives from pathology and development. Pathol Int. 2016;66:183–92. doi: 10.1111/pin.12391. [DOI] [PubMed] [Google Scholar]
- 22.Trepat X, Chen Z, Jacobson K. Cell migration. Compr Physiol. 2012;2:2369–92. doi: 10.1002/cphy.c110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, Hirata K, Nakamura Y, Katagiri T. Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res. 2010;70:2759–69. doi: 10.1158/0008-5472.CAN-09-3911. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Katagiri T, Chung S, Kijima K, Nakamura Y. Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia. 2011;13:320–26. doi: 10.1593/neo.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]


