Abstract
The aim of the present study was to investigate the osteogenic effects of icariside II (ICSII) on canine bone marrow mesenchymal stem cells (BMSCs) and the pathways by which these effects were induced. BMSCs were cultured and expanded to the fourth passage. The proliferative effects of ICSII were assessed using the cell counting kit-8 (CCK-8) assay. The osteogenic response to ICSII in BMSCs in vitro was examined by alkaline phosphatase (ALP) activity assays and Alizarin red staining. Using Western blot assays and real-time PCR (RT-PCR), we examined the expression of osteogenetic proteins/genes. We also evaluated changes in Akt and S6K1 phosphorylation levels, along with changes in the expression of osteogenesis proteins/genes after pretreatment with wortmannin (an inhibitor of phosphatidylinositol 3-kinase; PI3K) or rapamycin [a specific inhibitor of mammalian target of rapamycin (mTOR)] in the presence or absence of ICSII. Our results show that ICSII promotes the proliferation of BMSCs, while inhibiting ALP activity. We also found that calcium nodules form after BMSCs are treated with ICSII and osteogenetic medium for 21 days. ICSII significantly increased the expression of osteogenesis proteins/genes and elevated the phosphorylation levels of Akt and S6K1. Treatment with wortmannin or rapamycin attenuated the expression of p-Akt, p-S6K1, and osteogenesis proteins/genes. These results suggest that ICSII promotes the osteogenic differentiation of canine BMSCs via the PI3K/AKT/mTOR/S6K1 signaling pathways.
Keywords: ICSII, BMSCs, PI3K, AKT, mTOR, S6K1
Introduction
Icariin (ICA) is a major pharmacologically active compound derived from Herba epimedii. As a treatment in traditional Chinese medicine, icariin offers many biological benefits in terms of immunological function modulation, sexual enhancement, neuroprotective effects, cardiovascular protective effects, anti-inflammatory effects, anti-cancer and anti-aging treatment, and antioxidative protective properties [1-3]. Although icariin possesses many biological actions, the osteogenic action of icariin is one of the most promising areas of pharmacological research and development for this agent. Icariin is also reported to have a therapeutic anti-osteoporosis effect [4], and it can regulate/maintain balanced bone remodeling [5], increase core binding factor alpha1 expression [6], stimulate the proliferation of osteoblast-like cells [7], and increase SMAD4 (a marker of bone formation) mRNA levels [8]. Icariin can also enhance bone formation in vivo [9]. Icariin has the potential to become a suitable substitute for some growth factors used in tissue engineering [10]. Icariin exerts its potent osteogenic effects through the activation of BMP signaling and the BMP-2/SMAD4 signal transduction pathway, thus modulating the process of bone formation [11].
Phosphorylation levels of proteins in related signaling pathways are closely related to a series of physiological and biochemical processes, such as proliferation, differentiation, and apoptosis. The PI3K/AKT and Raf/ERK1/2-MAPK/Ras pathways are key signaling pathways that regulate cell proliferation and differentiation. The PI3K/AKT/mTOR pathway can promote stem cell survival [12] and may also be involved in the cell cycle, metabolism, and angiogenesis [13].
Icariside II (ICSII) is the major metabolite generated after oral administration of icariin [14]. A previous study showed that ICSII has the potential to prevent and treat of osteoporosis, in part by suppressing bone resorption of osteoclasts [15]. At the same time, we have demonstrated that ICSII enhances the proliferation of canine BMSCs and facilitates matrix calcification [16]. MSCs can be isolated from bone marrow, umbilical cord blood, and adult sources [17,18]. Canine BMSCs possess a greater ability to differentiate and form bone during the natural bone development process and continue to exhibit a high proliferative potential, even after a long period of cryopreservation. Therefore, canine BMSCs have long been recognized as one of the seed cells with the greatest potential for bone tissue engineering and research [19].
However, no studies to date have been reported that investigate the mechanism by which ISCII exhibits osteogenic effects on canine BMSCs. Moreover, the signaling pathways that confer the osteogenic effects of ICSII remain unknown. Therefore, we investigated the osteogenic effects of ICSII on BMSCs and the related signaling pathways that were affected by ICSII treatment.
Materials and methods
Materials and reagents
ICSII (molecular formula, C27H30O10; molecular weight, 514.52; purity > 98%; Figure 1) was purchased from Tauto Biotech (Shanghai, China). Canine BMSC was purchased from Cyagen Biosciences Inc (Suzhou, China). Dexamethasone (Dex), ascorbic acid (AA), and β-glycerophosphate (β-GP) were purchased from Sigma (Sigma, Aldrich, St Louis, MO, US). CCK-8 (CP002-500) was obtained from SAB (Maryland, US). QuantiChrom Alkaline Phosphatase Assay Kits (DALP-250) were obtained from BioAssay Systems (Hayward, CA, US). Low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS; pH 7.4), penicillin/streptomycin, and trypsin were purchased from Gibco BRL (Grand Island, NY, US). Anti-osteopontin antibody (ab8448), anti-Runx2 antibody (ab23981), anti-osteocalcin (ab13420), anti-FGF basic antibody (ab8880), anti-Sp7/osterix antibody (ab94744), and phospho-anti-S6K1 (ab126818) all were obtained from Abcam (Cambridge, MA, US). Inhibitors, wortmannin and rapamycin, were obtained from Selleck (Houston, TX, US). The phospho-AKT (Ser473) and β-actin antibodies (3700p) were obtained from CST (Beverly, MA, US). Peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG (H+L; 111-035-003) was obtained from Jackson ImmunoResearch (West Grove, PA, US), and peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (H+L; 474-1806) was obtained from KPL (Gaithersburg, Maryland, US). Primers for real-time PCR were obtained from Invitrogen (Carlsbad, CA, US). TBST was purchased from Shanghai Yi Chen Biological Technology Co. Ltd (Shanghai, China).
Figure 1.
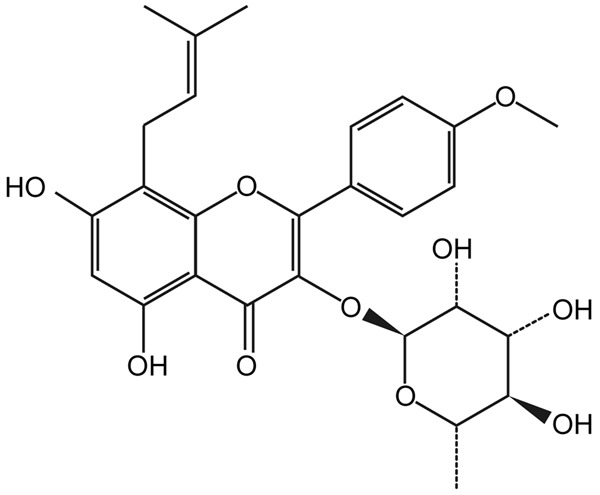
Chemical structure of icariside II (ICSII).
BMSC culture
BMSCs were cultured in complete medium (CM: DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin) and incubated at 37°C in 5% CO2 to form colonies. When the cells were 80% confluent, they were passaged. Cells passaged up to the fourth passage were used in this study.
BMSC viability assays
ICSII was dissolved in dimethyl sulfoxide (DMSO) to obtain a stock solution concentration of 0.1 mol/L, which was then diluted with basic medium to desired concentrations [20].
The effects of ICSII on cell viability were assessed using the CCK-8 assay. BMSCs were seeded in 96-well plates at 3,000 cells per well in growth medium for 24 h. After 24 h, the growth medium was aspirated from the wells, and serum-free medium that was incubated for 24 h was set as the zero value.
BMSCs were treated with the solution containing ICSII at different concentrations (10-9-10-5 mol/L). Cells cultured in the complete medium (CM) served as negative controls (NC). Plates were incubated for an appropriate length of time (1, 3, 5, and 7 days) in the incubator, and the absorbance was measured at an optical density of 450 nm. Each experiment was repeated three times, and the mean value was determined.
ALP activity assays
ALP activities were determined by measuring the amount of ρ-nitrophenol produced using a ρ-nitrophenol phosphate substrate. In total, 5000 cells per well were seeded in six-well culture plates and cultured at 37°C in 5% CO2 in a humidified incubator. After 24 h, the growth medium was aspirated from the wells, and medium containing ICSII at different concentrations (10-9-10-5 mol/L) was added. Cells cultured in the complete medium containing 10% FBS served as negative controls (NCs). Aliquots of supernatants were subjected to ALP activity assays using an ALP activity kit; all results were normalized to the total protein content. The absorbance at 405 nm was read and compared with a ρ-nitrophenol standard curve.
Quantifying mineralization via alizarin red staining
According to the results of the CCK-8 and ALP activity assays, we selected 10-5 mol/L ICSII as the optimal concentration.
Mineralization of the extracellular matrices was demonstrated after 21 days using Alizarin red staining. Passage 4 cells were seeded into six-well plates at an initial density of 3 × 105 cells/ml. Once 60% confluence was reached, all wells were divided into the following three experimental groups: 1) the NC group, which consisted of BMSCs cultured in CM, 2) the OM group (positive control), which consisted of BMSCs cultured in osteogenic induction medium (OM; generated by dissolving dexamethasone 10 nM, ascorbate-2-phosphate 50 µM, and β-glycerophosphate 10 mM in complete medium [21]), and 3) the ICSII group, which consisted of BMSCs cultured in CM containing 10-5 mol/L ICSII. After BMSCs were cultured for 21 days, the cells were washed with PBS, fixed in 10% formaldehyde solution for 30 min, and washed with 1 × PBS. Calcium deposition was determined using Alizarin red staining.
Osteogenesis-related protein/gene expression in BMSCs
The expression of osteogenesis-related proteins was examined by Western blotting. After treating BMSCs for 4, 6, or 8 days with 10-5 Mol/L ICSII, total cellular proteins were prepared by lysing cells in M-PER mammalian protein extraction reagent (PierceBiotechnology, USA) supplemented with a protease and phosphatase inhibitor cocktail that was EDTA-Free (PierceBiotechnology, USA). Following centrifugation at 14.5 × g for 20 min at 4°C to remove cell debris, the protein content of the cell lysate was determined using a bicinchoninic acid (BCA) assay kit (Pierce, USA). Then, 50 μg of protein was denatured, fractionated by electrophoresis on 12% (w/v) sodium dodecyl sulfate polyacrylamide gels (SDS-PAGE), and electrophoretically transferred onto nitrocellulose membranes (Millipore, US). Each membrane was blocked with 5% (w/v) nonfat dry milk in TBST and incubated with one of the following antibodies: Ost, bFGF, OCN, Runx2, OPN, or β-actin. The membranes were washed for 10 minutes each time in TBST for a total of three times, then incubated with the appropriate secondary antibodies. The membranes were again washed three times in TBST, then protein-antibody complexes were detected by enhanced chemiluminescence (ECL) reagents (Amersham Bioscience, Little Chalfont, UK). The mean expression level of the target protein relative to β-actin was determined.
The transcriptional expression of several osteogenic differentiation-related genes was detected by RT-PCR assays. After being treated for 6 days with 10-5 Mol/L ICSII, total RNA was extracted from BMSCs by adding 0.5 mL TRizol reagent. Each 1 μg RNA was subjected to cDNA synthesis with oligo (dT)18 primers. SYBR Green PCR Master Mix (Applied Biosystems) was used to detect the accumulation of PCR products during cycling with the Applied Biosystems 7500 sequence detection system. The thermocycling conditions were predenaturation at 95°C for 30 s, amplification using three-step cycles of denaturation at 95°C for 30 s, annealing and extension at 60°C for 30 s, for a duration of 40 cycles, with a final dissociation cycle at 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. The canine primer sequences are listed in Table 1. Target gene expression was normalised to β-actin. Relative gene expression was calculated with the 2-ΔΔCt formula.
Table 1.
The canine primer sequences
| Gene Bank number | Primers | Primer sequences (5’-3’) | Product size (bp) |
|---|---|---|---|
| NM_001195845.1 | β-actin | S:GCAAGGACCTCTATGCCAACA | 257 |
| A:GAAGCATTTGCGGTGGACG | |||
| XM_844688.3 | Osterix | S:CTGCGGGACTCAACGACACT | 186 |
| A:AGGAGGGTACTCATTGGCATAGC | |||
| XM_003432481.2 | bFGF | S:AAGAGCGATCCCCACGTCAA | 226 |
| A:TTCGTTTCAGTGCCACATACCA | |||
| XM_005627766 | Runx-2 | S:TCCAGACCAGCAGCACTCCATA | 186 |
| A:TTCCATCAGCGTCAACACCATC | |||
| XM_547536.4 | OCN | S:TCACAGACCCAGACAGAACCG | 207 |
| A:AGCCCAGAGTCCAGGTAGCG |
Inhibition of PI3K/AKT/mTOR/S6K1 signaling pathways
The effect of 10-5 mol/L ICSII on the expression levels of p-AKT, p-S6K1, and p-JNK1/2 were determined in BMSCs treated for 0-90 min. Total cell protein extracts were prepared and subjected to Western blot analysis.
The influence of rapamycin and wortmannin on p-AKT and p-S6K1, which are activated by 10-5 mol/L ICSII in BMSCs, was evaluated. Rapamycin and wortmannin were used to pretreat BMSCs for 60 min, and then the cells were co-cultured with 10-5 ICSII for another 30 min. Finally, the phosphorylation levels of S6K1 and Akt were determined by Western blot analysis.
The influence of rapamycin and wortmannin on osteogenic differentiation-related proteins/genes was determined. BMSCs were pretreated with rapamycin and wortmannin for 1 h, then BMSCs were cultured in the presence or absence of 10-5 mol/L ICSII for 6 days. The expression of osteogenic differentiation-related proteins/genes was determined by Western blot analysis and RT-PCR.
Data statistical analysis
All experiments were repeated at least three times, with similar results. All results are presented as the mean ± standard deviation. Data were analyzed using analysis of variance (ANOVA) and Dunnett’s t-test. Differences were considered statistically significant at P < 0.05. All statistical analyses were implemented with SPSS 19.0 software (SPSS Inc., Chicago IL, USA).
Results
Morphology changes in BMSCs after treatment with ICSII
BMSCs were adherent and showed a typical spindle-like, long and elongated fibroblastic shape. The cells possessed high proliferative ability (Figure 2A). BMSCs were treated with ICSII for 10 days, and the cells became stone-like, resembling osteoblasts (Figure 2B), which did not occur in NCs (BMSCs cultured in CM).
Figure 2.
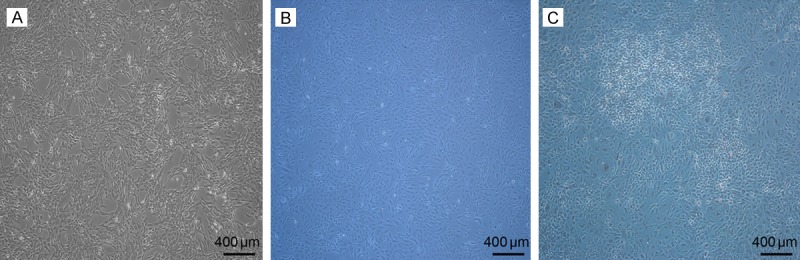
Morphology of BMSCs and treatment with ICSII. A: Morphology of BMSCs in passage 4 (50 × magnification); B: Morphology of BMSCs cultured in the complete medium (50 × magnification); C: Morphological changes in BMSCs treated with ICSII for 10 days (50 × magnification).
Effects of ICSII on BMSC proliferation
The effects of ICSII on BMSC proliferation under a range of concentrations after 1, 3, 5, and 7 days of treatment are shown in Figure 3. Although other ICSII groups did not differ significantly from the negative control (NC) group (P > 0.05), ICSII concentrations of 10-9, 10-8, and 10-7 mol/L significantly enhanced the viability of BMSCs (P < 0.01). ICSII concentrations of 10-6 and 10-5 mol/L slightly inhibited the viability of BMSCs.
Figure 3.
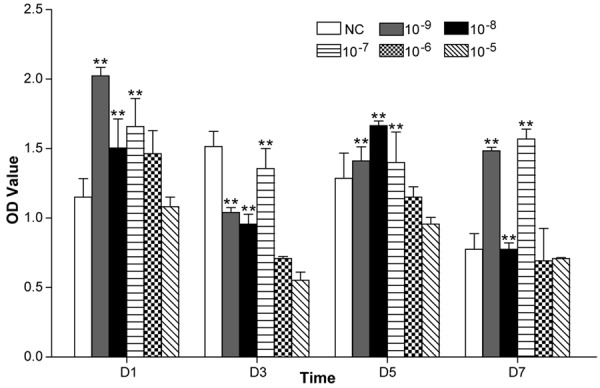
Effect of 10-9 M-10-5 M ICSII on BMSC viability examined by cell counting kit-8 assay. All experiments were carried out in six replicates, and the data were expressed as mean ± SD. *(P < .01) and **(P < 0.05) signify significant differences between the NC and treated groups.
Effects of ICSII on ALP activity
Compared with the NC group, the ALP activity of the group receiving 10-9-10-5 mol/L ICSII was slightly lower after 4, 6, and 8 days of treatment (P < 0.05); a concentration of 10-9-10-5 mol/L ICSII inhibited the ALP activity of BMSCs (Figure 4).
Figure 4.
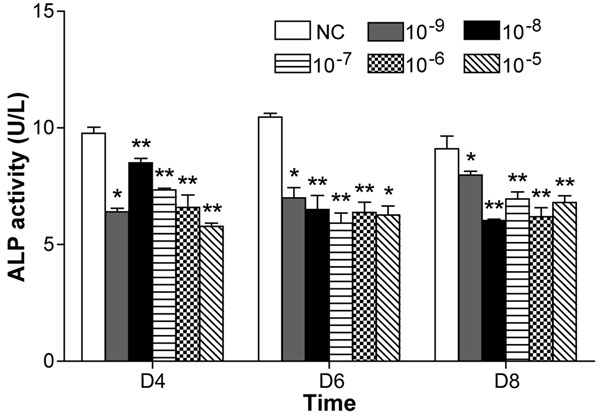
ICSII-induced alkaline phosphatase (ALP) activity assay when BMSCs were treated with ICSII (10-9 M-10-5 M) for 4, 6, and 8 days, respectively. *(P < .01) and **(P < 0.05) signify significant differences between the NC and treated groups.
Calcium nodule formation in response to ICSII treatment
Under our culturing conditions, calcium nodule formation in the ICSII and osteogenic medium (OM) groups, as measured by Alizarin red staining, was comparable with the complete medium (CM) group (Figure 5). BMSCs showed a high level of matrix mineralization when cultured in the presence of 10-5 mol/L ICSII or OM. BMSCs showed no evidence of matrix mineralization when cultured in CM.
Figure 5.
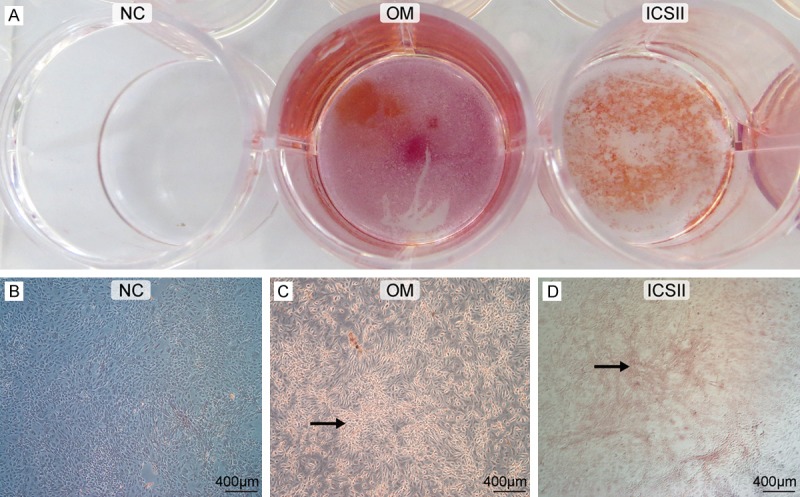
Calcium nodule formation in the NC, OM, and ICSII groups by Alizarin red staining. NC group: BMSCs cultured in CM, OM group: BMSCs cultured in osteogenic medium, ICSII Group: BMSCs cultured in 10-5 mol/L ICSII medium. Magnification 1 × (A), 50 × (B-D). Arrowhead denotes BMSCs (C, D) forming the calcium nodule, which is colored red by Alizarin red staining.
The effect of ICSII on the expression of osteogenesis-related proteins/genes in BMSCs
Treatment with 10-5 mol/L ICSII for 4, 6, or 8 days strongly induced protein expression of osteogenic differentiation-related proteins/genes, such as Osx, Runx2, bFGF, OPN, and OCN (Figure 6). Furthermore, expression of osteogenic differentiation-related protein was most strongly induced with 10-5 mol/L ICSII for 6 days. Based on these results, ICSII could induce the protein expression of osteogenic differentiation-related proteins.
Figure 6.
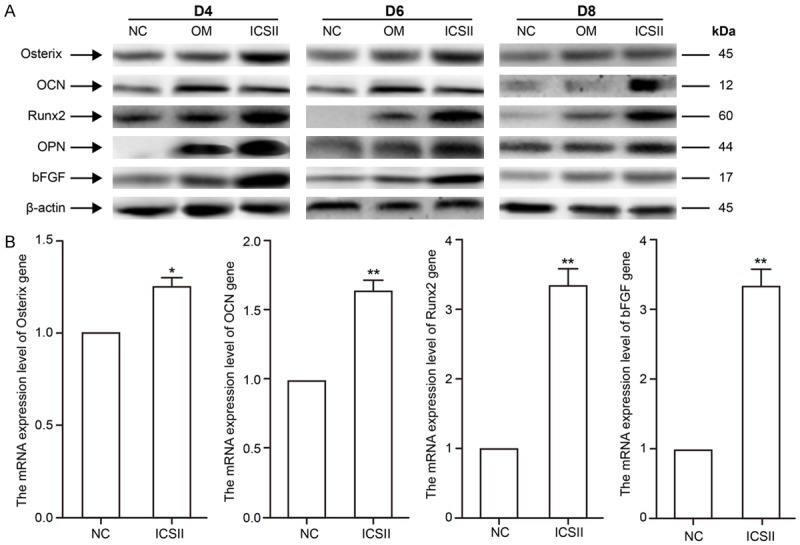
Changes in osteogenic protein/gene expression levels when BMSCs were treated with 10-5 M ICSII. *(P < 0.05) and **(P < 0.01) signify significantly differences between the NC and treated groups. A. BMSCs were treated with ICSII 10-5 M for 4, 6, and 8 days. Total cell extracts were prepared to assess changes in the expression of the osteogenic proteins osterix, OCN, Runx2, OPN, and bFGF by Western blot. B. BMSCs were treated with ICSII 10-5 M for 6 days, and total RNA was extracted for real-time PCR assays to detect the changes in the mRNA expression levels of the osteogenic genes osterix, OCN, Runx2, and bFGF.
PI3K/AKT/mTOR/S6K1 signaling pathway activation in BMSCs mediated by ICSII
As shown in Figure 7A, 10-5 mol/L ICSII activated p-S6K1 and p-Akt. As shown in Figure 7B, rapamycin and wortmannin effectively suppressed the up-regulation of these phosphorylated forms of Akt and S6K1. The expression level of osteogenic differentiation-related proteins/genes was reduced in the presence of rapamycin and wortmannin (Figures 8 and 9).
Figure 7.
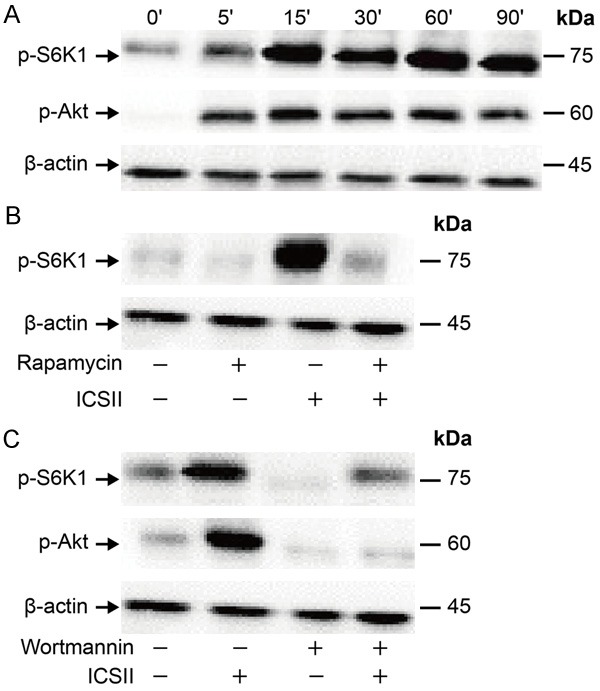
Changes in phosphorylated forms of S6K1 and Akt. (A) BMSCs were treated with 10-5 ICSII for 0-90 min. Total cell protein extracts were subjected to Western blot analysis to detect the active phosphorylated forms of S6K1 and Akt. (B, C) The inhibition status of phosphorylation: BMSCs were cultured with CM containing 100 nM rapamycin (B) or 50 µM wortmannin (C) for 1 h in the presence or absence of 10-5 mol/L ICSII for 30 min. Total cell protein extracts were subjected to Western blot analysis to detect phosphorylated forms of these proteins.
Figure 8.
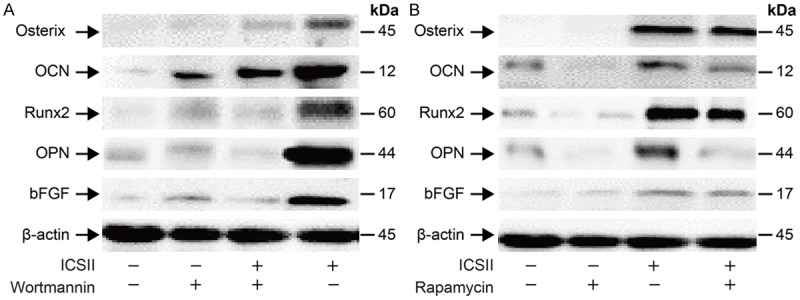
Changes in osteogenic differentiation-related protein expression. Expression was determined by Western blot after BMSCs were pre-treated with 50 µM wortmannin or 100 nM rapamycin for 1 hour, and then cells were cultured for 6 days in the presence or absence of 10-5 mol/L ICSII.
Figure 9.
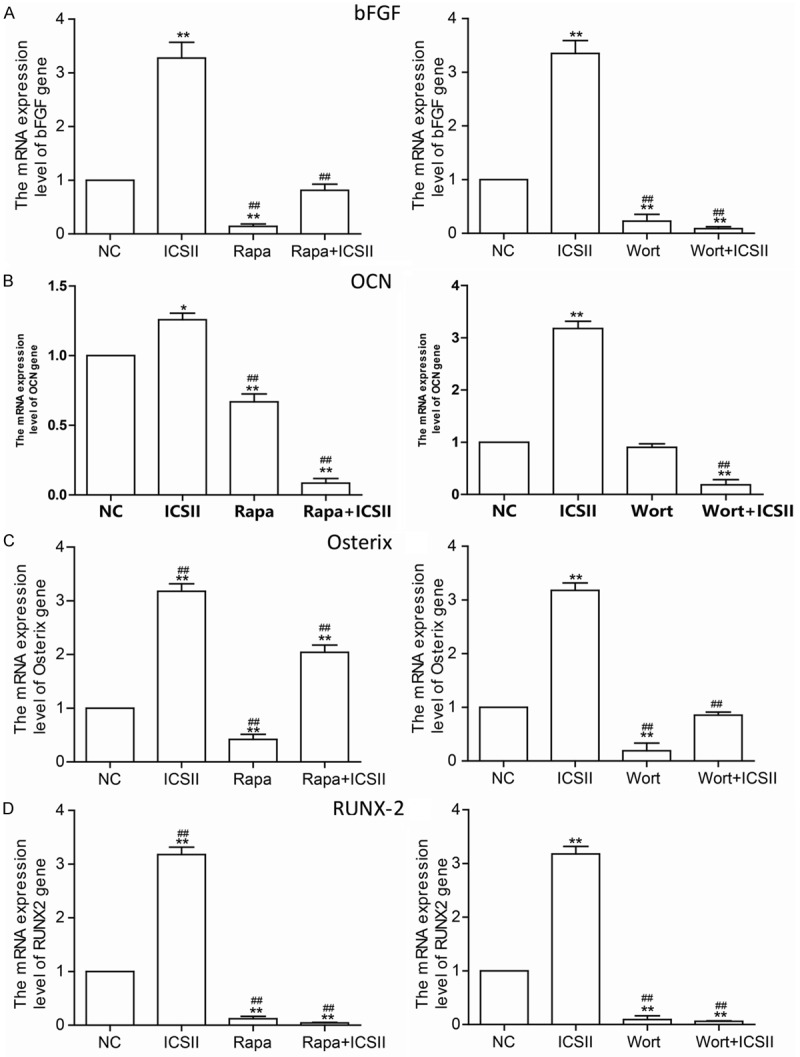
Changes in the expression of the ostegenesis-related genes. BMSCs were pre-treated with 100 nM rapamycin (Rapa) or 50 µM wortmannin (Wort) for 1 h, then cells were cultured for 6 days in the presence or absence of 10-5 mol/L ICSII. *P < 0.05, **P < 0.01 compared with control, #P < 0.05, ##P < 0.01 compared with ICSII group.
Discussion
Canines have been frequently used a model system for regenerative therapy and toxicological safety assessment [22,23]. Therefore, we elected to use canine BMSCs in our study. BMSCs can be differentiated into several possible lineages through specific induction factors contained in defined growth media, such as adipogenic, osteogenic, and chondrogenic differentiation media. Several studies have shown that media containing dexamethasone, arachidonic acid, and α-glycerophosphate induce BMSC differentiation to the osteoblast lineage [23,24].
In our study, we assessed BMSC matrix mineralization and osteoblast differentiation with Alizarin staining after 21 days of treatment with OM or 10-5 mol/L ICSII. BMSCs showed a high level of matrix mineralization when cultured in the presence of OM or 10-5 mol/L ICSII, while BMSCs showed no signs of matrix mineralization when cultured in CM. This indicates that OM and 10-5 mol/L ICSII induce the osteogenic differentiation of BMSCs, as the formation of mineralized nodules is considered a reliable indicator of osteogenic differentiation in vitro [25].
Osteoblastic differentiation is represented by matrix mineralization and ALP expression. ALP is important in bone formation and has the ability to regulate bone matrix mineralization [26]. ALP also is a useful marker to evaluate early osteogenesis [27]. In our experiment, we found that 10-9-10-5 mol/L ICSII inhibited the ALP activity of BMSCs. This may be associated with the ability of ICSII to dramatically promote BMSC viability, because cell differentiation is counteracted by cell proliferation. In other words, when cell proliferation escalates, differentiation levels tend to be reduced [28,29]. Treatment with 10-9, 10-8, and 10-7 mol/L ICSII significantly enhanced the proliferation of BMSCs (P < 0.01) in a concentration-dependent manner, while 10-6-10-5 ICSII slightly inhibited the ALP activity of BMSCs. We detected the expression of osteogenic differentiation-related proteins such as Osx, Runx2, bFGF, OPN, and OCN to demonstrate the osteoblastic differentiation effects of ICSII.
Of the osteogenic differentiation-related proteins, Runx2 is a crucial regulator of osteogenesis [30,31]. OPN plays an important role in promoting bone remodeling [32], while OCN is considered a late and more specific marker of osteogenic differentiation, regulating matrix mineralization [33]. Osterix is a factor of zinc finger transcription and is an important regulator of osteoblast differentiation, maturation, and activity [34]. Furthermore, increased osterix expression is considered an indicator of osteogenic differentiation in canines [35]. bFGF may participate in osteogenic differentiation and the regeneration of the capillary vasculature, inducing bone regeneration [36,37]. After BMSCs were treated with 10-5 mol/L ICSII, expression levels of Osx, Runx2, bFGF, OPN, and OCN were considerably higher than the levels in the NC group on days 4, 6, and 8, suggesting that ICSII improved the osteogenic differentiation of BMSCs.
S6K is important for various cellular processes, including protein synthesis, mRNA processing, glucose homeostasis, cell growth, and survival and proliferation. Moreover, it has been established that S6K possesses important functions in human diseases, including obesity, diabetes, aging, and cancer [38]. PI3K/Akt/mTOR signaling pathways are involved in normal proliferation and differentiation in cells [39]. We explored the signaling pathways induced during ICSII-mediated osteogenic differentiation in BMSCs. 10-5 mol/L ICSII elevated phosphorylation levels of kinase Akt and S6K1. The BMSCs were also pre-treated with wortmannin and rapamycin for 1 h in the presence or absence of 10-5 mol/L ICSII. The expression level of phosphorylated forms Akt and S6K1 and osteogenic differentiation-related protein/genes was reduced. These results indicate that the PI3K/Akt/mTOR/S6K1 signaling pathways help to regulate the ICSII-mediated osteogenic differentiation of BMSCs.
Conclusions
In conclusion, we demonstrate that ICSII, the metabolism product of ICA in the stomach or intestines, pushes BMSCs to commit to osteoblastic differentiation via the PI3K/Akt/mTOR/S6K1 signaling pathway.
Acknowledgements
Current research was financially supported by Shanghai Municipal Science and Technology Commission, the western medicine guide project (Number: 14411963500).
Disclosure of conflict of interest
None.
References
- 1.Li C, Li Q, Mei Q, Lu T. Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii. Life Sci. 2015;126:57–68. doi: 10.1016/j.lfs.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Liang Q, Wei G, Chen J, Wang Y, Huang H. Variation of medicinal components in a unique geographical accession of horny goat weed Epimedium sagittatum Maxim. (Berberidaceae) Molecules. 2012;17:13345–13356. doi: 10.3390/molecules171113345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang ZS, Xiao HJ, Qi T, Hu ZM, Li H, Chen DL, Xu YL, Chen J. Antioxidative protective effect of icariin on the FeSO4/H 2O 2-damaged human sperm based on confocal raman micro-spectroscopy. J Huazhong Univ Sci Technolog Med Sci. 2014;34:755–760. doi: 10.1007/s11596-014-1348-3. [DOI] [PubMed] [Google Scholar]
- 4.Luo Z, Liu M, Sun L, Rui F. Icariin recovers the osteogenic differentiation and bone formation of bone marrow stromal cells from a rat model of estrogen deficiency-induced osteoporosis. Mol Med Rep. 2015;12:382–388. doi: 10.3892/mmr.2015.3369. [DOI] [PubMed] [Google Scholar]
- 5.Ming LG, Chen KM, Xian CJ. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J Cell Physiol. 2013;228:513–521. doi: 10.1002/jcp.24158. [DOI] [PubMed] [Google Scholar]
- 6.Qian G, Zhang X, Lu L, Wu X, Li S, Meng J. Regulation of Cbfa1 expression by total flavonoids of Herba epimedii. Endocr J. 2006;53:87–94. doi: 10.1507/endocrj.53.87. [DOI] [PubMed] [Google Scholar]
- 7.Meng FH, Li YB, Xiong ZL, Jiang ZM, Li FM. Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine. 2005;12:189–193. doi: 10.1016/j.phymed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y, Wang P, Qi Q, Ni H, Ma X, Guo S. The experimental study of the effects of icariin on increasing smad4 mRNA level in MC3T3-E1 cell in vitro. Chin J Orthop. 2005;25:119–123. [Google Scholar]
- 9.Xie X, Pei F, Wang H, Tan Z, Yang Z, Kang P. Icariin: a promising osteoinductive compound for repairing bone defect and osteonecrosis. J Biomater Appl. 2015;30:290–299. doi: 10.1177/0885328215581551. [DOI] [PubMed] [Google Scholar]
- 10.Qin Z, Yin L, Wang K, Liu Q, Cheng W, Gao P, Sun K, Zhong M, Yu Z. [Effects of Icariin promotion on proliferation and osteogenic differentiation of human periodontal ligament stem cells] . Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33:370–376. doi: 10.7518/hxkq.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W, Lin M, Li X, Li C, Gao B, Gan H, Yang Z, Lin X, Liao L, Yang M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med. 2012;30:889–895. doi: 10.3892/ijmm.2012.1079. [DOI] [PubMed] [Google Scholar]
- 12.Bertacchini J, Heidari N, Mediani L, Capitani S, Shahjahani M, Ahmadzadeh A, Saki N. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72:2337–2347. doi: 10.1007/s00018-015-1867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manfredi GI, Dicitore A, Gaudenzi G, Caraglia M, Persani L, Vitale G. PI3K/Akt/mTOR signaling in medullary thyroid cancer: a promising molecular target for cancer therapy. Endocrine. 2015;48:363–370. doi: 10.1007/s12020-014-0380-1. [DOI] [PubMed] [Google Scholar]
- 14.Cao YF, He RR, Cao J, Chen JX, Huang T, Liu Y. Drug-drug interactions potential of icariin and its intestinal metabolites via inhibition of intestinal udp-glucuronosyltransferases. Evid Based Complement Alternat Med. 2012;2012:395912. doi: 10.1155/2012/395912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Zhang J, Fong C, Yao X, Yang M. Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie. 2012;94:2514–2522. doi: 10.1016/j.biochi.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Gu F, Zhang Y, Liu T, Guo P, Huang Y. Icariside II promotes osteogenic differentiation of bone marrow stromal cells in beagle canine. Int J Clin Exp Pathol. 2015;8:4367–4377. [PMC free article] [PubMed] [Google Scholar]
- 17.Luan Y, Ding W, Ju ZY, Zhang ZH, Zhang X, Kong F. Bone marrow-derived mesenchymal stem cells protect against lung injury in a mouse model of bronchopulmonary dysplasia. Mol Med Rep. 2015;11:1945–1950. doi: 10.3892/mmr.2014.2959. [DOI] [PubMed] [Google Scholar]
- 18.You HJ, Namgoong S, Han SK, Jeong SH, Dhong ES, Kim WK. Wound-healing potential of human umbilical cord blood-derived mesenchymal stromal cells in vitro--a pilot study. Cytotherapy. 2015;17:1506–1513. doi: 10.1016/j.jcyt.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang DW, Cheng Y, Wang NL, Zhang JC, Yang MS, Yao XS. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine. 2008;15:55–61. doi: 10.1016/j.phymed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C, Lin Y. Osteogenic differentiation of adipose-derived stem cells promoted by quercetin. Cell Prolif. 2014;47:124–132. doi: 10.1111/cpr.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalgaard L. Comparison of minipig, dog, monkey and human drug metabolism and disposition. J Pharmacol Toxicol Methods. 2015;74:80–92. doi: 10.1016/j.vascn.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Oda Y, Tani K, Asari Y, Quintanilha LF, Haraguchi T, Momota Y, Katayama M, Itamoto K, Nakazawa H, Taura Y. Canine bone marrow stromal cells promote functional recovery in mice with spinal cord injury. J Vet Med Sci. 2014;76:905–908. doi: 10.1292/jvms.13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JB. The effects of dexamethasone, ascorbic acid, and beta-glycerophosphate on osteoblastic differentiation by regulating estrogen receptor and osteopontin expression. J Surg Res. 2012;173:99–104. doi: 10.1016/j.jss.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Nakai K, Kawato T, Morita T, Yamazaki Y, Tanaka H, Tonogi M, Oki H, Maeno M. Angiotensin II suppresses osteoblastic differentiation and mineralized nodule formation via AT1 receptor in ROS17/2.8 cells. Arch Med Sci. 2015;11:628–637. doi: 10.5114/aoms.2015.52369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millan JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71:567–577. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 30.Fei C, Guo J, Zhao Y, Gu S, Zhao S, Li X, Chang C. Notch-Hes pathway mediates the impaired osteogenic differentiation of bone marrow mesenchymal stromal cells from myelodysplastic syndromes patients through the down-regulation of Runx2. Am J Transl Res. 2015;7:1939–1951. [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar Y, Kapoor I, Khan K, Thacker G, Khan MP, Shukla N, Kanaujiya JK, Sanyal S, Chattopadhyay N, Trivedi AK. E3 ubiquitin ligase Fbw7 negatively regulates osteoblast differentiation by targeting Runx2 for degradation. J Biol Chem. 2015;290:30975–30987. doi: 10.1074/jbc.M115.669531. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Creff G, Safi S, Roques J, Michel H, Jeanson A, Solari PL, Basset C, Simoni E, Vidaud C, Den Auwer C. Actinide(IV) deposits on bone: potential role of the osteopontin-thorium complex. Inorg Chem. 2016;55:29–36. doi: 10.1021/acs.inorgchem.5b02349. [DOI] [PubMed] [Google Scholar]
- 33.Zappitelli T, Chen F, Aubin JE. Up-regulation of BMP2/4 signaling increases both osteoblast-specific marker expression and bone marrow adipogenesis in Gja1Jrt/+ stromal cell cultures. Mol Biol Cell. 2015;26:832–842. doi: 10.1091/mbc.E14-06-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renn J, Winkler C. Osterix/Sp7 regulates biomineralization of otoliths and bone in medaka (oryzias latipes) Matrix Biol. 2014;34:193–204. doi: 10.1016/j.matbio.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Vieira NM, Brandalise V, Zucconi E, Secco M, Strauss BE, Zatz M. Isolation, characterization, and differentiation potential of canine adipose-derived stem cells. Cell Transplant. 2010;19:279–289. doi: 10.3727/096368909X481764. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Song K, Rao N, Huang M, Huang Z, Cao Y. Roles of exogenously regulated bFGF expression in angiogenesis and bone regeneration in rat calvarial defects. Int J Mol Med. 2011;27:545–553. doi: 10.3892/ijmm.2011.619. [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Zheng Q, Kulbatski I, Yuan Q, Yang S, Shao Z, Wang H, Xiao B, Pan Z, Tang S. Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous beta-TCP ceramic scaffolds. Biomed Mater. 2006;1:93–99. doi: 10.1088/1748-6041/1/3/001. [DOI] [PubMed] [Google Scholar]
- 38.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Follo MY, Manzoli L, Poli A, McCubrey JA, Cocco L. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv Biol Regul. 2015;57:10–16. doi: 10.1016/j.jbior.2014.10.004. [DOI] [PubMed] [Google Scholar]


