Abstract
Mesangial cells of glomerulus which could produce and degrade several ECMs, take part in the repair and update of mesangial matrix and GBM, regulate glomerular filtration rate, secret cytokines and phagocytose immune complexes contribute a lot to physiological functions and pathological reactions of glomerular. There inflammation response of abnormal proliferation induced by LPS could lead to renal damage. Herein, wedelolactone, an active chemical constituent extracted from leaves of Eclipta alba, was used to explore if it could be an effective inhibitor of the proliferative response of HRMCs. The effects of different concentration wedelolactone on the secretion of cytokines, cell viability and NF-κB pathway were all detected by qPCR, western blotting and ELISA. The results indicated that wedelolactone could inhibit the abnormal proliferation of HRMCs via regulating the activity of several key members of NF-κB signaling pathway.
Keywords: Wedelolactone, renal mesangial cells, cell proliferation, NF-κB signaling pathway
Introduction
Glomerular mesangial cell (GMC) is one of the four kinds of cells constituting a glomerulus. Mesangial cells of glomerulus contribute a lot to physiological functions and pathological reactions of glomerular [1]. GMCs could produce and degrade several extracellular matrixes (ECMs), take part in the repair and update of mesangial matrix and glomerular basement membrane (GBM), regulate glomerular filtration rate, secret cytokines and phagocytose immune complexes [2]. Thus, MC stimulated by immunological or inflammatory responses could always secrete chemokines and cytokines, such as interleukin-6 (IL-6), IL-1β, transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), and NO [2-4]. Meanwhile, Lipopolysaccharide (LPS), polymeric immunoglobulin A1 (pIgA1) and hyperglycemia have been identified to activate mesangial cells as well [4,5]. Renal damage could be induced by large amount of growth factors and cytokines secreted by MC during the progression of kidney diseases [6]. Current clinical approaches to treat inflammation focus not only on the inhibition of proinflammatory mediator production but also on the suppression of initiation of the inflammatory response (i.e., suppression of positive signaling pathways of proinflammatory cytokines).
Nuclear factor κB (NF-κB) is the key regulator of inflammation. In resting cells, NF-κB exists in the cytosolbond with inhibitor of κB (IκB), which prevents NF-κB from entering nucleus and functioning during transcription. Under the stimulus of external factors, such as cytokines, oxidant, virus, immunostimulant, UV and LPS, IκBs could be degraded and release free NF-κB dimers, which will be translated to nucleus and bind to the κB sequences of the target gene to affect the transcription of several adhesion factors, cytokines, immune receptors, acute phase proteins and stress response proteins [7]. Thus, NF-κB is an important immune-regulator. In addition, its signaling pathways also play a vital role in the viability, differentiation, proliferation and apoptosis of cells, and are key factors of genesis, development and prognosis of several diseases as well [7]. It was noticed that the activation of NF-κB signaling pathway plays an important role in the development, progression and prognosis of several kidney diseases caused by the activation of HRMCs, which could be induced by LPS stimulation [8]. NF-κB consists of the protein members from Rel family in the forms of homodimers or heterodimers [9]. Different constructions of different members of NF-κB family make their different functions. Specially, the dimers possessing p65 could activate target genes and the function of p65 could not be complemented by other proteins [10]. Moreover, it had been reported that the genesis and pathogenic processes of a series of diseases, including glomerulonephritis, all had close correlation with the excessive activation of NF-κB [11]. So the expression level of NF-κB p65 could be used to decide the activation intensity of NF-κB, and it was associated with the development of renal damage.
In the pathogenesis of renal damage, there is a complicated network involving cytokines, chemotactic factors, adhesion molecules and abnormal immune responses centering on NF-κB signaling pathway [12,13]. In consideration of the essential role of NF-κB in the development of renal damage, every part of the activation of NF-κB signaling pathway could be the target of therapies [7,14]. In addition, NF-κB could only be activated through activating IKK and ubiquitin-mediated degradation of IκB, which means the IKK/IκB/NF-κB signaling pathway could be regarded as a potential therapy target for the treatment of renal damage [15-18]. There are at least three types of enzymes taking part in the interaction of ubiquitin-proteasome system: ubiquitin-activating enzyme E1, ubiquitin conjugating enzyme E2 (UBC5) and ubiquitin-protein ligase E3 (E3RS IκB), which could recognize IκBα to help E2 bind and lead to its proteolysis [19,20].
Therefore, determining the changes of IκBα, IKKβ, and ubiquitin-proteasome system during the development and recovery of renal damage, as well as the effects of herbs on them, has great theoretical significance for investigating the mechanisms underlying herb treatment of renal damage.
Wedelolactone is an active chemical constituent extracted from leaves of Eclipta alba, which is a herbaceous plant used as traditional medicine in many countries in America, Asia, Africa [21]. Eclipta alba has been demonstrated to be an inflammation inhibitor [22]. Wedelolactone has been shown to possess a number of biological activities, including antibacterial, anti-venom, anti-hepatotoxic, and antitrypsin [23-25]. Although various cellular responses to wedelolactone treatment have been evaluated, whether wedelolactone is able to directly reduce the inflammation-causing effect of LPS on HRMCs and the mechanisms involved are not completely clear. In addition, there is little information about the relationship between the ubiquitin-proteasome system and the mechanisms of renal damage. Therefore, this study examined the cytoprotective effects of wedelolactone on mesangial cells in the conditions induced by LPS. The signaling mechanisms focusing on NF-κB during the process were also studied. And the inhibitory effect of wedelolactone on NF-κB activation was further investigated through determining its effects on expression levels of E1, UBC5 and E3RS IκB in HRMCs.
Materials and methods
Cell culture
Human renal mesangial cells (HRMCs) were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China). HRMCs were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 5.6 mmol/L glucose, glutamine, penicillin and streptomycin at 37°C under 5% CO2 in a 95% humidified atmosphere.
CCK-8 assay for cell proliferation
HRMCs (1 × 104 cells/well in 96-well plate) were treated with LPS (0, 0.5, 1, 2, 3, 5 and 10 μg/mL; Sigma Chemical Co., USA) or wedelolactone (0, 5, 10, 20, 40 and 80 μmol/L, Shanghai Shfeng Biological Technology Co., Ltd, Shanghai, China) for 24 h, and cell proliferation viability was evaluated through CCK-8 assay. HRMCs were seeded into 96-well plates at initial density of 3 × 103 cells/well. After different treatments described above for 24 h, cell proliferation was measured through detecting the absorbance at 595 nm using CCK-8 assay kit (Dojindo) according to manufacturer’s instructions. The experiment was performed in triplicate.
Determination of cytokine production and the components of ubiquitin proteasome system by ELISA assay
The inhibitory effect of wedelolactone on IL-1β, TNF-α, and TGF-β1 expressions in LPS-activated HEMCs was determined with the ELISA assay. HRMCs (3 × 105/well in 24-well plate) were first treated with wedelolactone (0, 1.25, 2.5, 5, 10, and 20 μmol/L) for 1 h and then incubated in 1 μg/mL LPS for 24 h or not. The expression levels of IL-1β, TNF-α, UBE1, UBC5 and E3RS IκB in the supernatants were then examined using ELISA kits (Human TNF-α ELISA Kit; Human IL-1β ELISA Kit; Human E1/ubiquitin-activating enzyme ELISA Kit; Human ubiquitin-conjugating enzyme UBC5 ELISA Kit; Human beta-transduction repeat containing ELISA kit, HuaMei Biotechnology, Wuhan, China) according to the manufacturer’s instructions (R&D Systems).
NO assay
HRMCs were first incubated with wedelolactone (0, 1.25, 2.5, 5, 10, and 20 μmol/L) for 1 h. After continuous incubation with 1 μg/mL LPS for 24 h, nitrite was determined using the Griess reagent (R&D Systems, USA) through detecting the absorbance of the product dye at 540 nm using a spectrophotometer. The sensitivity of the NO assay was less than 0.78 μmol/L.
Determination of DNA binding activity of NF-κB p65 in HRMCs
Divide HRMCs into groups undergoing different pretreatments: no treatment or treatments with wedelolactone at different doses (0, 1.25, 2.5, 5, 10 and 20 μmol/L) for 1 h. Then all groups were stimulated with LPS to build the inflammatory model except the blank group (totally no treatment) for 24 h. Subsequently, all groups of HRMCs were collected and their nucleoprotein was extracted using a nuclear extract kit (Active Motif, USA) respectively. The Trans AMTM NF-κB p65 kit (Active Motif, USA) was then used to determine the DNA binding activity of the NF-κB p65 in the extracted nucleoprotein.
Quantitative real-time PCR
HRMCs were planted on the 6-well plate at a density of 5 × 104 per well. After being cultured with different doses of wedelolactone (0, 1.25, 2.5, 5, 10 and 20 μmol/L) for 1 h and then LPS (1 μg/mL) for 24 h in non-serum medium, the cells were collected and 1 mg total RNA was extracted from each sample to be reverse transcribed into cDNA using a PrimeScript RT Reagent Kit (Takara, Shiga, Japan), following the manufacturer’s instructions. Oligonucleotide primers and probes were designed according to GeneBank by Shanghai Biological Engineering Co. The primer sequences are listed below: IκBα, forward: 5’-CTGTACGCCCCAGCATCT-3’, reverse: 5’-GCACCCAAAGTCACCAAG-3’; IKKβ, forward: 5’-AAATGAAAGAGCGCCTTGG-3’, reverse: 5’-CACTGCTTGATGGCAATCTG-3’; E1, forward: 5’-AGTCCGTGTTGTCCGAAGTG-3’, reverse: 5’-GCAGTGCCCTGGTCATGTAG-3’; UBC5, forward: 5’-GGCAGCATTTGTCTCGATA-3’, reverse: 5’-AGCATCACATGGCATACTTC-3’; E3RS IκB, forward: 5’-ACCTGGATGCCAAATCAC-3’, reverse: 5’-TCTCGAAGGCCGCTA-3’; GAPDH, forward: 5’-GAAGGTCGGAGTCAACGGAT-3’, reverse: 5’-CTGGAAGATGGTGATGGGATT-3’. Real-time PCR cycles consisted of: 2 minutes at 50°C, 4 minutes at 95°C for polymerase activation, 45 cycles of 10 seconds at 95°C (denaturation), 5 seconds at 54°C, 5 seconds at 72°C and 15 seconds at 83°C (annealing and extension). In addition, melting was performed at 72-95°C (0.5°C increments) for 5 seconds for each step. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as intrinsic positive control. The threshold cycle (CT) of each sample was normalized to GAPDH. Each sample was run in triplicate.
Western blotting
HRMCs (5 × 106/10 cm dish) were pretreated with wedelolactone (0, 1.25, 2.5, 5, 10 and 20 μmol/L) for 1 h and further incubated in the presence or absence of LPS (1 μg/mL) for 24 h. Cells were collected and lysed with a lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 1% NP-40, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM leupeptin, 1 mM PMSF) for 30 min at 4°C. After determining the concentration of protein in each cell lysate using a BCA protein assay kit (Pierce, IL, USA), 15 micrograms of cell protein lysates per sample were mixed with 2 × SDS loading buffer containing DTT and heated at 100°C for 10 min, and subsequently resolved by SDS-PAGE. Proteins were transferred into a PVDF membrane and blocked with 1% bovine serum albumin in TBST at room temperature for 1 h. The membrane was incubated with primary antibodies overnight at 4°C. The membrane was washed with TBST and incubated for 2 h at room temperature with secondary antibodies. After further washing, the membrane was detected with ECL chemiluminescence. β-actin was used as intrinsic l control. The primary antibodies used were as follows: mouse anti-pIκBα (Santa Cruz, USA), rabbit anti-IKKβ (Cell Signaling, USA), mouse anti-IκBα (Cell Signaling, USA), mouse anti-β-actin (Santa Cruz, USA); the secondary antibodies used were goat anti-rabbit HRP-conjugated secondary antibody (Bioworld Technology, USA) and goat anti-mouse HRP-conjugated secondary antibody (Bioworld Technology, USA).
Statistical analysis
Data were analyzed by Student’s t test and one-way ANOVA using SPSS 16.0 software. P values less than 0.05 were considered as statistical significance. Data were presented as mean ± standard deviation.
Results
Effects of LPS on HRMCs viability and expression of TNF-α and IL-1β
A pre-experiment was performed to examine the effects LPS has on HRMCs and determine the optimum concentration of LPS which was used in this study. The viability and the levels of TNF-α and IL-1β in the supernate of HRMCs incubated with different concentrations of LPS (0, 0.5, 1, 2, 3, 5 and 10 μg/mL) for 24 h were examined by using CCK-8 and ELISA assays. The results indicated that under the concentrations between 1 and 2 μg/mL, LPS could promote cell viability, while LPS concentration over 2 μg/mL could inhibit HRMCs viability in reverse (Figure 1A). In addition, 1-2 μg/mL LPS could significantly increase the expression levels of TNF-α and IL-1β in HRMCs comparing with other concentrations of LPS (Figure 1B, 1C). According to the aim of the study to explore the effects of wedelolactone on LPS-enhanced proliferation of HRMCs, LPS with a concentration of 1 μg/mL was selected to construct the inflammatory HRMC model.
Figure 1.
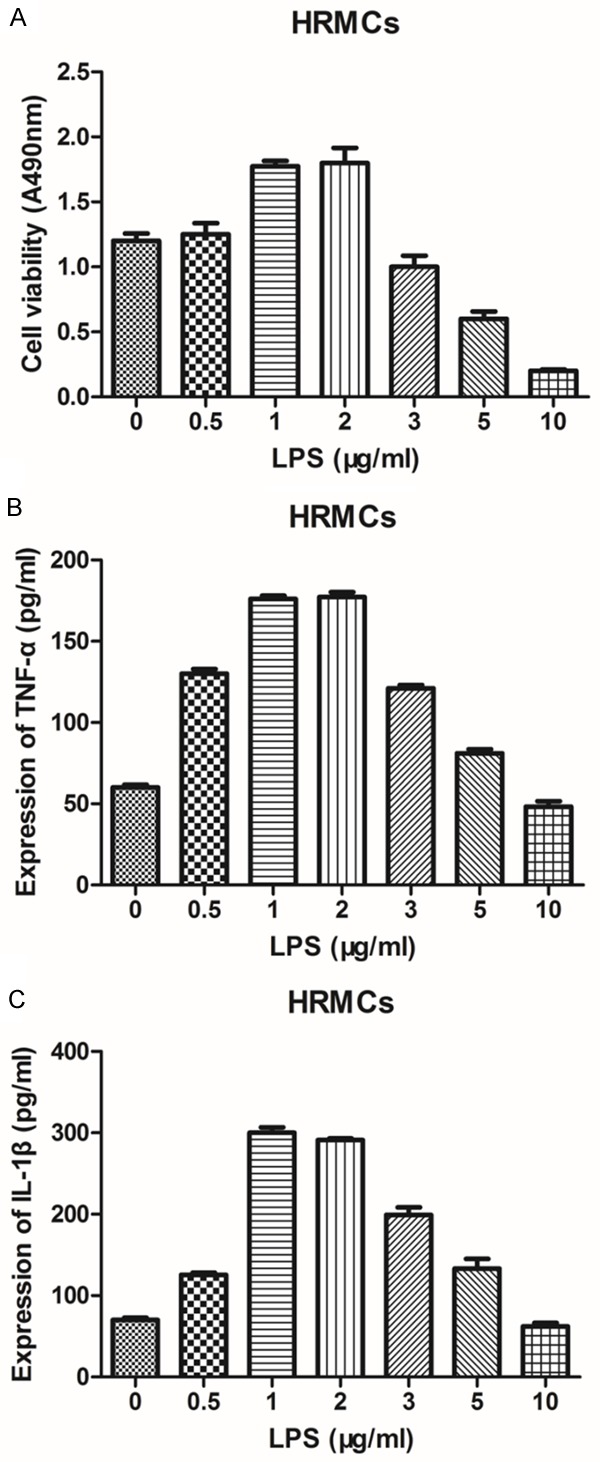
Effects of LPS on HRMCs viability and the expression of TNF-α and IL-1β. A: The viability of HRMCs incubated with different concentration of LPS for 24 h was determined by CCK-8 assay. B: The supernatant level of TNF-α in HRMCs incubated with different concentration of LPS for 24 h was determined by ELISA assay. C: The supernatant level of IL-1β in HRMCs incubated with different concentration of LPS for 24 h was determined by ELISA assay. Data represent mean ± S.E.M. (n=3).
Effects of wedelolactone on normal HRMCs viability
Effect of wedelolactone on normal HRMCs was investigated before studying its effects on inflammatory HRMCs to exclude its own cytotoxicity. The viabilities of HRMCs treated with different concentrations of wedelolactone (0, 5, 10, 20, 40, 80 μmol/L) for 24 h were determined by CCK-8 assay (Figure 2). It was demonstrated that HRMCs viability was not obviously changed after 24 h treatment of 0-20 μmol/L wedelolactone which means under the concentration of 20 μmol/L, wedelolactone has little cytotoxicity on HRMCs. However, wedelolactone with concentration higher than 40 μmol/l could significantly inhibit cell viability of HRMCs, which means wedelolactone had serious cytotoxicity in this condition. Therefore, low concentrations under 20 μmol/L of wedelolactone could be used to explore its pharmacological action without any damage to normal cell function.
Figure 2.

Effect of wedelolactone on the cell viability of normal HRMCs. The viability of healthy HRMCs treated with different concentrations of wedelolactone was detected via CCK-8 assay. Data represent mean ± S.E.M. (n=3).
Inhibitory effect of wedelolactone on the LPS-induced faster proliferation of HRMCs
To examine the effects of wedelolactone on the LPS-induced more vibrant proliferation of HRMCs, CCK-8 assay was carried out to detect the viability of HRMCs before and after 1 h treatment of wedelolactone at low concentrations (0, 1.25, 2.5, 5, 10, and 20 μmol/L) following stimulus with 1 μg/ml LPS for 24 h. As is shown in Figure 3, wedelolactone with low concentrations higher than 2.5 μmol/L could significantly slow down the LPS-enhanced proliferation of HRMCs.
Figure 3.

Effect of wedelolactone on the enhanced proliferation of LPS-induced HRMCs. Cell viability of LPS-induced HRMCs treated with different concentrations (no more than 20 μmol/l) of wedelolactone for 1 h was determined by CCK-8 assay. Data represent mean ± S.E.M. (n=3).
Inhibitory effect of wedelolactone on the LPS-induced upregulation of inflammatory factor production in HRMCs
To evaluate the inhibitory effect of wedelolactone on LPS-mediated production of inflammatory cytokines, the levels of IL-1β, TNF-α and NO in the supernatant of HRMCs were determined by ELISA and Griess assays. Cells were pretreated with various concentrations of wedelolactone (0, 1.25, 2.5, 5, 10, and 20 μmol/L) following the culture with or without LPS (1 μg/mL) for 24 h. Wedelolactone at the concentration between 10 and 20 μmol/l significantly obliterated the LPS-induced upregulation of all examined cytokine production (Figure 4), indicating that wedelolactone may inhibit the LPS-mediated inflammatory responses of HRMCs. Furthermore, the inhibitory effect was in a dose-dependent manner. Indeed, wedelolactone almost completely reversed the expression levels of IL-1β, TNF-α AND NO at the concentration of 10 and 20 μmol/L (Figure 4).
Figure 4.
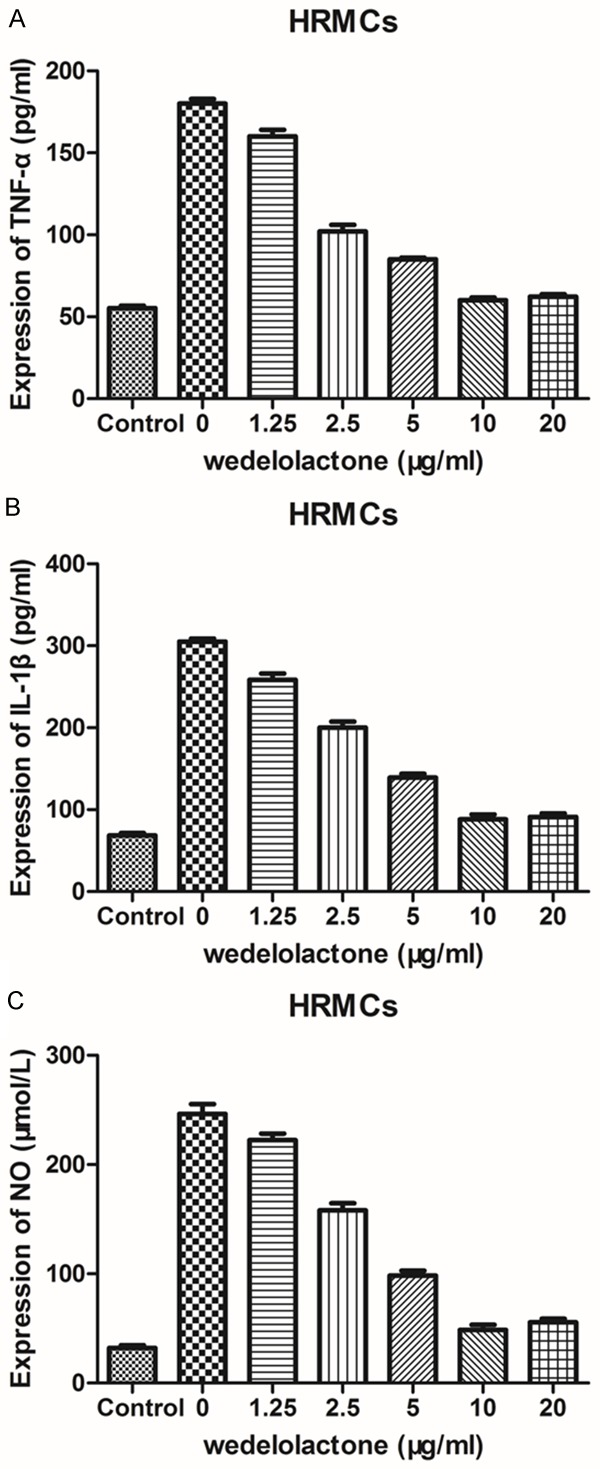
Effects of low concentration wedelolactone on LPS-mediated production of inflammatory cytokines in HRMCs. A: The supernatant level of TNF-α in LPS-induced HRMCs incubated with low concentrations of wedelolactone for 1 h was determined by ELISA assay. B: The supernatant level of IL-1β in LPS-induced HRMCs treated with low concentrations of wedelolactone for 1 h was determined by ELISA assay. C: The supernatant level of NO in LPS-induced HRMCs treated with low concentrations of wedelolactone for 1 h was determined by Griess assay. Data represent mean ± S.E.M. (n=3).
Wedelolactone could inhibit LPS-induced high binding activity of NF-κB p65 in HRMCs
To investigate the relationship between the molecular mechanisms by which wedelolactone blocks various inflammatory processes and NF-κB signaling pathways, the effect of wedelolactone on LPS-enhanced DNA binding activity of NF-κB p65 was investigated. As is shown in Figure 5, the DNA binding activity of NF-κB p65 in LPS-induced HRMCs (inflammation model group) was significantly higher than the blank group (***P<0.001). However, NF-κB p65 in HRMCs pretreated with 10 μmol/L and 20 μmol/L held the DNA binding activities which were significantly weaker than the inflammation model group (***P<0.001).
Figure 5.
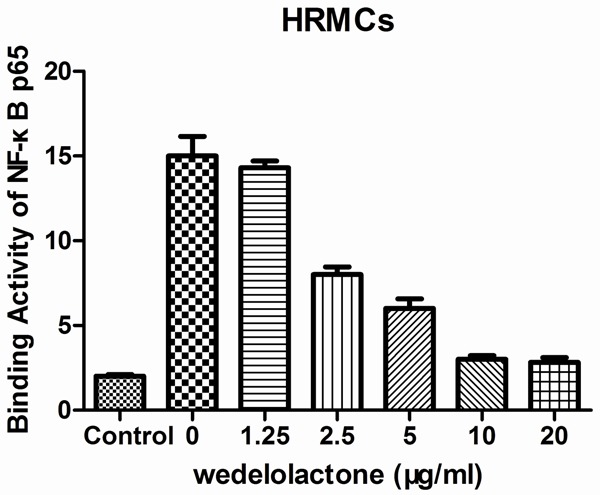
Effect of wedelolactone on LPS-enhanced DNA binding activity of NF-κB p65. The DNA binding activity of NF-κB p65 in LPS-induced HRMCs treated with different concentrations of wedelolactone was detected using the Trans AMTM NF-κB p65 kit. Data represent mean ± S.E.M. (n=3). ***P<0.001.
Effects of wedelolactone on the mRNA and protein expression levels of IκBα, p-IκBα and IKKβ in LPS-induced HRMCs
In order to explore the mechanism underlying the inhibitory action of wedelolactone in LPS-enhanced inflammatory proliferation of HRMCs, the changes of mRNA and protein expression levels of IκBα, p-IκBα, and IKKβ were determined (Figure 6). As is shown in the results, after treatments of different concentrations of wedelolactone for 1 h and 24 h incubation with or without LPS, both mRNA and protein expression levels of IκBα in LPS-induced HRMCs without treatment of wedelolactone was obviously lower than that in the blank group (P<0.05) (Figure 6A). The mRNA expression of IκBα in HRMCs treated with 20 μmol/L wedelolactone was significantly higher than the inflammation model group (P<0.05) (Figure 6A), while the protein expressions of IκBα in HRMCs treated with both 10 and 20 μmol/l wedelolactone were also significantly higher than the inflammation model group (P<0.05) (Figure 6C). As for IKKβ, there was an opposite direction. Compared with blank group, the inflammation model group held much more IKKβ mRNA and protein (P<0.05), the mRNA and protein expressions of IKKβ in HRMCs treated with 10 and 20 μmol/L wedelolactone were all significantly lower than the inflammation model group (P<0.05) (Figure 6B, 6D). The protein expression of pIκBα had a similar change with IKKβ, which was increased in inflammation model group (P<0.05) and almost recovered by 10 and 20 μmol/L wedelolactone (P<0.05) (Figure 6E).
Figure 6.
Effects of wedelolactone on the mRNA and protein expression levels of IκBα, p-IκBα and IKKβ in LPS-induced HRMCs. A: Relative mRNA expression of IκBα in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by qPCR. B: Relative mRNA expression of IKKβ in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by qPCR. C: Relative protein expression of IκBα in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by western blotting. D: Relative protein expression of IKKβ in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by western blotting. E: Relative protein expression of p-IκBα in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by western blotting. Data represent mean ± S.E.M. (n=3) *P<0.05.
Effects of wedelolactone on the mRNA and protein expression levels of UBE1, UBC5 and E3RS IκB in LPS-induced HRMCs
According to the classic activation pathway of NF-κB, ubiquitination and degradation of IκB should be an indispensable step. Herein, effects of wedelolactone on the mRNA and protein expression levels of UBE1, UBC5 and E3RS IκB in LPS-induced HRMCs were analyzed to further investigate the mechanisms wedelolactone restores the inflammatory responses of HRMCs induced by LPS. As is shown in Figure 7, there was no significant difference of E1 mRNA and protein expressions between HRMCs induced by LPS and those treated with a series of doses of wedelolactone. The results of UBC5 were similar to E1 that only protein expression of the group treated with 20 μmol/L wedelolactone was significantly decreased (P<0.05). However, when it comes to E3RS IκB, there were some different results that the mRNA and protein expressions of E3RS IκB in HRMCs treated with 20 μmol/l wedelolactone were both significantly lower than the inflammation model group (P<0.05), and the protein level of E3RS IκB in HRMCs treated with 10 μmol/l wedelolactone was also significantly lower than those only induced by LPS (inflammation model group) (P<0.05).
Figure 7.
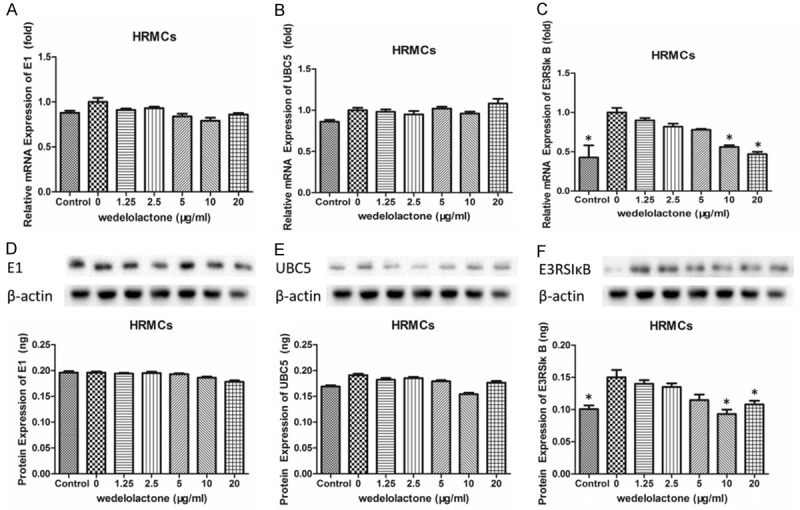
Effects of wedelolactone on the mRNA and protein expression levels of UBE1, UBC5 and E3RS IκB in LPS-induced HRMCs. A: Relative mRNA expression of UBE1 in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by qPCR. B: Relative mRNA expression of UBC5 in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by qPCR. C: Relative mRNA expression of E3RS IκB in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by qPCR. D: Protein expression of UBE1 in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by ELISA assay. E: Protein expression of UBC5 in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by ELISA assay. F: Protein expression of E3RS IκB in LPS-induced HRMCs treated with different concentrations of wedelolactone was measured by ELISA assay. Data represent mean ± S.E.M. (n=3) *P<0.05.
Discussion
Glomerular mesangial cell (GMC) is a unique kind of vascular smooth muscle cells, which possesses a variety of physiological functions and pathological significances including regulating the glomerular filtration rate, synthetizing mesangial matrix, supporting and protecting glomerulus capillary loops, secreting cytokines, phagocytosis and clearance [26]. Above all, GMCs are the most active intrinsic cells in glomerulus that they are both the injured and participants in glomerular lesion. Proliferative reaction is their most common way in response to several stimulations, such as immune complexes, macromolecular substances and hypoxia. The activated and proliferative GMCs could produce kinds of vasoactive substances and cytokines, which further stimulates their own to proliferate faster and affects the cells around [2]. During the activation process of GMCs, NF-κB signaling pathways paly an essential role. There are two forms of NF-κB existing in cells, p65/p50 which is activated in nucleus and p65/p50/IκB which is inactivated in cytoplasm. IκB could bind to the p65 subunit of NF-κB to protect it from transferring into nucleus to regulate transcription, so that IκB is the inhibitor of NF-κB [27]. Some immune molecules or inflammation cytokines, such as LPS, IL-1β and TNF-α, could bind to their receptors to activate IKK, which could catalytic the phosphorylation of IκB [28,29]. Once phosphorylated, IκB will be recognized by ubiquitin-proteasome system and polyubiquitinated to be degraded, so that NF-κB is released and the signaling pathway is activated. Thus, the ubiquitin-mediated proteolysis of IκB is the key stage of activating NF-κB [15,16,18]. Subsequently, the proliferative response of GMCs could be induced to result in nephritis [30,31].
Most of the traditional drugs for kidney diseases are immunosuppresses and cytotoxic drugs, some of which possess the functions of inhibiting the proliferation of GMC, synthesis of ECM (extracellular matrix)and production of inflammatory mediators [32-34]. However, some patients are not sensitive to such drugs and there are some serious side effects when taking the drugs for a long time. As a result, more and more studies about treating kidney diseases with herbs had been carried out, especially the investigation into the effective constituents in herbs.
Wedelolactone has been reported to suppress the LPS-induced caspase-11 expression in mouse embryo fibroblasts through inhibiting NF-κB-mediated transcription, indicating that wedelolactone is an inhibitor of IKK, whose activation is a critical step to activate NF-κB [35]. However, there is little information about its role in LPS-induced mesangial cell injury leading to renal damage. In this study, the anti-inflammatory effect of wedelolactone and its regulating mechanisms in HRMCs induced by LPS were investigated.
The cytotoxic effect of LPS or wedelolactone was evaluated by the CCK-8 assay 24 h after treatment of HRMCs with variable LPS or wedelolactone concentrations before deeper research through detecting the expression of TNF-α and IL-1β in the supernatant caused by gradient LPS concentrations by ELISA assay. Figure 1 shows that at the concentration of 1 μg/mL, LPS didn’t inhibit the cell viability of HRMCs, meanwhile it obviously increased the expression levels of TNF-α and IL-1β. Therefore, the concentration of 1 μg/ml was selected for our following experiments. Although no cytotoxicity of wedelolactone was detected at concentrations less than 20 μmol/l, there seemed to have a dose-dependent manner, so several concentrations no higher than 20 μmol/L of wedelolactone were used then (Figure 2). According to Figure 3, wedelolactone at low concentrations could significantly decrease the increased cell viability of HRMCs induced by LPS which means wedelolactone has an inhibitory effect on HRMCs against LPS, and this was confirmed through detecting the changes of TNF-α and IL-1β expression in LPS-induced HRMCs caused by different concentrations of wedelolactone (Figure 4). In order to explore the mechanisms of the inhibitory effect of wedelolactone displayed in Figures 3 and 4, the DNA binding ability of NF-κB p65 in HRMCs that went through different treatments was determined and the results indicated that wedelolactone may inhibit the activation of NF-κB through the ubiquitin proteasome pathway, which could be the mechanism of wedelolactone to treat kidney diseases led by abnormal proliferation of HRMCs (Figure 5). Further investigations about the effects of wedelolactone on NF-κB pathways were carried out and the result that wedelolactone could upregulates the expression of IκBα and downregulates the expression of IKKβ and pIκBα demonstrated that wedelolactone could inhibit the activation of IKKβ to inhibit the phosphorylation of IκBα to protect IκB from proteolysis, so that the activation of wedelolactone could be suppressed in this way (Figure 6). Subsequently, the mechanism of wedelolactone inhibiting the activity of IKKβ and degradation of IκBα was explored through examines the expression of related enzyme in LPS-induced HRMCs before and after treatments with wedelolactone. The results suggested that wedelolactone may affect the activity of the key enzyme E3RS IκB to prevent the ubiquitin-mediated proteolysis of IκBα to inhibit the activation of NF-κB to control the inflammation response of HRMCs (Figure 7). In conclusion, wedelolactone from Eclipta alba could be a novel medicine for the therapy of renal damage caused by the inflammation response of HRMCs through affecting the NF-κB signaling pathway.
Acknowledgements
This study was supported by National Natural Science Foundation of China Grant (81173219), Shanghai Science & Technology Commission Grant (14401972203 and15401930100), the project of Shanghai Municipal Commission of Health and Family Planning (201440488), three years of development project for Traditional Chinese Medicine (ZY3-JSFC-2-1029 and ZY3-LCPT-1-1006) and senior Chinese Integrative Medicine talent cultivation project (ZYSNXD012-RC-ZXY003) of Shanghai Municipal Commission of Health and Family Planning, and Innovative Research Team in Universities, Shanghai Municipal Education.
Disclosure of conflict of interest
None.
References
- 1.Rodriguez-Barbero A, L’Azou B, Cambar J, Lopez-Novoa JM. Potential use of isolated glomeruli and cultured mesangial cells as in vitro models to assess nephrotoxicity. Cell Biol Toxicol. 2000;16:145–153. doi: 10.1023/a:1007683320660. [DOI] [PubMed] [Google Scholar]
- 2.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung JC, Tang SC, Chan LY, Chan WL, Lai KN. Synthesis of TNF-alpha by mesangial cells cultured with polymeric anionic IgA-role of MAPK and NF-kappaB. Nephrol Dial Transplant. 2008;23:72–81. doi: 10.1093/ndt/gfm581. [DOI] [PubMed] [Google Scholar]
- 4.Wu F, Yao H, Bader A, Dong F, Zhu F, Wu N, Wang B, Li H, Brockmeyer NH, Altmeyer P. Decorin gene transfer inhibited the expression of TGFbeta1 and ECM in rat mesangial cells. Eur J Med Res. 2007;12:360–368. [PubMed] [Google Scholar]
- 5.Lee SY, Cho JY. Inhibitory effects of honokiol on LPS and PMA-induced cellular responses of macrophages and monocytes. BMB Rep. 2009;42:574–579. doi: 10.5483/bmbrep.2009.42.9.574. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Lopez E, Rodriguez-Vita J, Cartier C, Ruperez M, Esteban V, Carvajal G, Rodrigues-Diez R, Plaza JJ, Egido J, Ruiz-Ortega M. Inhibitory effect of interleukin-1beta on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesangial cells. Am J Physiol Renal Physiol. 2008;294:F149–160. doi: 10.1152/ajprenal.00129.2007. [DOI] [PubMed] [Google Scholar]
- 7.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo G, Zhu X, Gao Z, Ge H, Yu Y, Guo Y, Zheng JP, Liu L. Effects of Yishen Pinggan Recipe on renal protection and NF-kappaB signaling pathway in spontaneously hypertensive rats. Evid Based Complement Alternat Med. 2016;2016:6435040. doi: 10.1155/2016/6435040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng C, Yin Q, Wu H. Structural studies of NF-kappaB signaling. Cell Res. 2011;21:183–195. doi: 10.1038/cr.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 11.Kwak JH, Jung JK, Lee H. Nuclear factor-kappa B inhibitors; a patent review (2006-2010) Expert Opin Ther Pat. 2011;21:1897–1910. doi: 10.1517/13543776.2011.638285. [DOI] [PubMed] [Google Scholar]
- 12.Guo W, Guan X, Pan X, Sun X, Wang F, Ji Y, Huang P, Deng Y, Zhang Q, Han Q, Yi P, Namaka M, Liu Y, Deng Y, Li X. Post-natal inhibition of NF-kappaB activation prevents renal damage caused by prenatal LPS exposure. PLoS One. 2016;11:e0153434. doi: 10.1371/journal.pone.0153434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin Y, Li G, Liu H, Ai D. AS-IV protects against kidney IRI through inhibition of NF-kappaB activity and PUMA upregulation. Int J Clin Exp Med. 2015;8:18293–18301. [PMC free article] [PubMed] [Google Scholar]
- 14.Chakraborty JB, Mann DA. NF-kappaB signalling: embracing complexity to achieve translation. J Hepatol. 2010;52:285–291. doi: 10.1016/j.jhep.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa] B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 16.Yaron A, Hatzubai A, Davis M, Lavon I, Amit S, Manning AM, Andersen JS, Mann M, Mercurio F, Ben-Neriah Y. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 1998;396:590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 18.Baldi L, Brown K, Franzoso G, Siebenlist U. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of I kappa B-alpha. J Biol Chem. 1996;271:376–379. doi: 10.1074/jbc.271.1.376. [DOI] [PubMed] [Google Scholar]
- 19.Ciechanover A. Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Bioorg Med Chem. 2013;21:3400–3410. doi: 10.1016/j.bmc.2013.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Clague MJ, Urbe S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–685. doi: 10.1016/j.cell.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Sidra S, Hussain S, Malik F. Accentuating the prodigious significance of Eclipta alba-an inestimable medicinal plant. Pak J Pharm Sci. 2013;26:1259–1266. [PubMed] [Google Scholar]
- 22.Singh A, Malhotra S, Subban R. Anti-inflammatory and analgesic agents from indian medicanal plants. International Journal of Integrative Biology. 2007:3. [Google Scholar]
- 23.Kalirajan J. Screening of antibacterial and antioxidant activities of leaves of eclipta prostrata (L) J Heat Transfer. 2007;134:723–732. [Google Scholar]
- 24.Uddin MN, Rahman MA, Ahmed NU, Rana MS, Akter R, Chowdhury AMMA. Antioxidant, cytotoxic and antimicrobial properties of eclipta alba ethanol extract. Biomedscidirect Publications. 2010 [Google Scholar]
- 25.Martinez-Rossi NM. Frontiers | antimicrobial activities of ethanol extract and coumestans from eclipta alba (L. ) Hassk (asteraceae) Latin American Journal of Pharmacy. 2009;26:863–868. [Google Scholar]
- 26.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 27.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 30.Shui HA, Ka SM, Wu WM, Lin YF, Hou YC, Su LC, Chen A. LPS-evoked IL-18 expression in mesangial cells plays a role in accelerating lupus nephritis. Rheumatology (Oxford) 2007;46:1277–1284. doi: 10.1093/rheumatology/kem136. [DOI] [PubMed] [Google Scholar]
- 31.Doi T. The contribution of mesangial cell proliferation to progressive glomerular injury. J Med Invest. 2001;48:1–4. [PubMed] [Google Scholar]
- 32.Hauser IA, Renders L, Radeke HH, Sterzel RB, Goppelt-Struebe M. Mycophenolate mofetil inhibits rat and human mesangial cell proliferation by guanosine depletion. Nephrol Dial Transplant. 1999;14:58–63. doi: 10.1093/ndt/14.1.58. [DOI] [PubMed] [Google Scholar]
- 33.Xiao W, Liu Y, Templeton DM. Ca(2+)/calmodulin-dependent protein kinase II inhibition by heparin in mesangial cells. Am J Physiol Renal Physiol. 2005;288:F142–149. doi: 10.1152/ajprenal.00145.2004. [DOI] [PubMed] [Google Scholar]
- 34.Kurogi Y. Mesangial cell proliferation inhibitors for the treatment of proliferative glomerular disease. Med Res Rev. 2003;23:15–31. doi: 10.1002/med.10028. [DOI] [PubMed] [Google Scholar]
- 35.Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung YK, Gakidis MA, Rao A, Sekine T, Ikegami F, Yuan C, Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK complex. Cell Death Differ. 2004;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]



