Abstract
Insulin resistance is associated with obesity and type 2 diabetes. The aim of this study was to explore the mechanism of how Astragalus Polysaccharides (APS) improves insulin resistance in 3T3-L1 adipocytes. A cell culture model of insulin resistance was established in mature 3T3-L1 adipocytes by treating them with TNF-α, high glucose and insulin. Glucose uptake levels were detected in each group. To determine the mechanism by which APS improves insulin resistance in 3T3-L1 adipocytes, qRT-PCR was used to detect the expression of miR-721, and Western blots were used to detect the expression or activity of PPAR-γ, PAKT, PI3K, AKT, and GLUT4. Immunostaining was used to detect the expression of GLUT4. We successfully madea model of insulin resistance in mature 3T3-L1 adipocytes. APS increased glucose uptake levels in insulin-resistant adipocytes in a dose- and time-dependent manner, and also increased insulin sensitivity. APS suppressed miR-721 with its target gene PPAR-γ in a dose-dependent manner. miR-721 or PPAR inhibitor T0070907 inhibited the expressions of PPAR-γ, pAKT, and GLUT4 and also reduced glucose accumulation. APS attenuated these miR-721- and PPAR-γ-induced changes. APS increased insulin sensitivity by attenuating the effects of miR-721. The PI3K inhibitor wortmannin reduced the APS-increased pAKT, glucose uptake, and GLUT4 levels, and also reduced those levels in the presence of insulin with or without APS. Taken together, our findings suggest that APS promotes glucose uptake and increases insulin sensitivity in 3T3-L1 adipocytes and may involve the miR-721-PPAR-γ-PI3K/AKT-GLUT4 signaling pathway. These might be new therapeutic targets for treating insulin resistance in obesity and diabetes.
Keywords: Astragalus polysaccharides, 3T3-L1 adipocyte, PPAR-γ, PI3K/AKT, GLUT4
Introduction
Insulin resistance is an important contributor to the pathogenesis of obesity and type 2 diabetes, which is in part induced by tumor necrosis factor-α (TNF-α) [1]. TNF-α is overexpressed in adipose tissues of obese animals and humans, and lacks either TNF-α or its receptor-induced protection against the development of insulin resistance [2]. Insulin resistance results in changes in the function of the glucose transporter, leading to high blood glucose levels due to a failure to suppress the release of glucose into blood. Insulin resistance is usually accompanied with high cholesterol and triglyceride levels, or high blood pressure [3]. Insulin resistance is also associated with various chronic diseases, including obesity, liver failure, atherosclerosis, hypertension, and certain cancers. Thus, an important strategy for the treatment of these glucose metabolism-related diseases is to maintain a normal bold glucose level by regulating glucose transport.
In traditional Chinese medicine, Astragulus has been used in the development of alternative therapeutics for type 2 diabetes [4,5]. Astragalus Polysaccharides (APS) are active components of the polysaccharide extract of Astragulus and are obtained by thin-layer chromatography (TLC) and Sephadex G-100 chromatography. Astragalus polysaccharides have an α-(1→4)-d-glucan with α-(1→6)-linked branches attached to the O-6 branch points [6]. APSh as a strong antioxidant, anti-hypertensive, and immunomodulatory effect and has been shown to activate, insulin-sensitizing and hypoglycemic activity in rats with type 2 diabetes [7-9]. APS has been shown to improve the systemic metabolic disorder and cardiac dysfunction in diabetic models [10]. In rats with type 2 diabetes, APS exerts insulin-sensitizing and hypoglycemic activities [11]. In diet-induced insulin-resistant C57BL/6J mice, APS alleviates insulin resistance [7]. However, the molecular mechanisms by which APS attenuates insulin resistance remain unknown.
APS has a strong anti-inflammatory effect, and enhances the gene expression of an inflammatory marker peroxisome proliferator-activated receptor gamma (PPAR-γ) in a time- and dose-dependent manner [12]. Besides its anti-inflammatory role, PPAR-γ promotes fatty acid catabolism and reduces circulating lipids [13]. Activated PPAR-γ increases peripheral insulin sensitivity and alleviates hepatic glucose production, reducing hyperglycemia [14]. PPAR-γ plays an important role in the differentiation and maturation of fat cells, such as 3T3-L1 preadipocytes [13,15]. APS dramaticallyimproves myocyte triglyceride accumulation and cardiac dysfunction in diet-induced insulin-resistant mice, and also has benefical effects on insulin resistance and hyperglycemia [7]. APS reversed the PPAR-mediated suppression of genesinvolved in glucose utilization [7]. PPAR-γ activity induced by APS alleviates high blood glucose levels, suggesting an important role of PPAR-γ in APS-attenuated insulin resistance. A recent study showed that APS suppressed insulin resistance and was accompanied by improved hepatic PPAR-γ coactivator intracellular signaling and reduced chronic inflammation [16]. The role of PPAR-γ in APS-attenuated insulin resistance needs to be further investigated.
The polysaccharide fraction has been shown to have the potential of reducing hyperglycemia by inducing glucose translocation enzymes and proteins [17]. APS promoted the expression and translocation of the glucose transporter protein-4 (GLUT4) in adipose tissues [18]. Insulin-mediated glucose uptake is dependent on the insulin-sensitive glucose transporter, GLUT4 in adipocytes [19]. Activation of the insulin-AKT signaling pathways have been shown to mediate insulin utilization [20].
MicroRNA (miRNA) is an endogenous noncoding RNA that serves as a post-transcriptional regulator of gene expression by binding to the 3’-untranslated region (UTR) of target mRNAs, and induces the degradation of those target mRNAs, thus, playing important roles in the regulation of development, and diabetes [21].
Here, we report that in the presence of APS, miR-721 regulates insulin resistance. Furthermore, we identify PPAR-γ as a target of miR-721 in 3T3-L1 adipocytes, and investigate the involvement of PI3K/AKT and GLUT4 in APS-attenuated insulin resistance.
Materials and methods
3T3-L1 cell culture
Mouse 3T3-L1 cell line was obtained from the American Type Culture Collection (ATCC, USA). The 3T3-L1 preadipocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 2 mmol/L glutamine and 20 mmol/L HEPES (pH 7.4) in a humidified atmosphere of 5% CO2 at 37°C. These cells were induced to differentiate into adipocytes, as previously described [22]. After reaching 100% confluence, 3T3-L1 preadipocytes were stimulated to differentiate using a differentiation mixture containing 0.5 mM 3-isobutyl-1-methylxanthine, 1 μM dexamethasone and 10 μg/ml insulin in DMEM with 10% FBS. After 48 h, the cells were cultured in DMEM supplemented with 10% FBS and 10 μg/ml insulin for 10-12 days. The mature adipocytes were confirmed by light microscopy and oil red O staining, and then used for further analysis. For Oil Red O staining, 3T3-L1 cells were fixed with ice-cold acetone for 30 min and then stained with 0.3% Oil Red O/60% isopropanol solution for 2 h. Lipid droplets were visualized by phase contrast microscopy.
Establishment of insulin resistance adipocyte model
Well-differentiated 3T3-L1 adipocytes were pretreated with DMEM (high glucose) and supplemented with 0.5% (w/v) FBS for 3 h, and the cells were then exposed to 10 ng/ml TNF-α in DMEM (high glucose) containing 10% (w/v) FBS for 24 h. Cell medium was replaced to DMEM (high glucose) containing 100 nM insulin and 10% FBS for 30 min incubation. Well-differentiated 3T3-L1 adipocytes without TNF-α and/or insulin treatment were used as controls. Glucose uptake tests using calorimetric assay kits (sigma, USA) were performed to confirm the establishment of a cell model that was insulin resistant.
2-Deoxy-D-[3H] glucose (3H-2-DG) uptake measurement
The glucose uptake tests were performed using a modified protocol, as previously described [22]. In brief, after treatment, cells were washed three times with Krebs-Ringer phosphate buffer (1.32 mM NaCl, 4.71 mM KCl2, 47 mM CaCl2, 1.24 mM MgSO4, 2.48 mM Na3PO4, and 10 mM HEPES (pH 7.4)). Then, cells were incubated with a fiHEP concentration of 1 μCi/ml 3H-2-DG (GE healthcare, USA) for 10 min, and then the reaction was terminated by washing with Krebs-Ringer phosphate buffer. After cells lysed with 0.1 N NaOH, the radioactivity (DPM) was determined using a scintillation counter (LS 6500, Beckman, USA). Finally, the DPM was corrected for protein content measured using the BCA protein assay in each well.
Transfection of miR-721 mimics
The insulin-resistant 3T3-L1 cells were seeded on 96-well plates. When the cells were 50% confluent, Lipofectamine™ 2000 was transfected with miR-721 mimics and miR-721 NC (GenePharma company, China) respectively. Cultures were incubated for 24 h and used for subsequent detection.
Treatment of APS
APS (20000-60000 mol/L) were purchased from the Gracia Chemical Technology Co, Chengdu, China. APS is composed of α-1,4 (1,6) glucan, arabinose-galactose polysaccharides, rhamnose-galacturonic acid polysaccharides, and arabinose-galactose protein polysaccharide. After inducing insulin resistance to 3T3-L1 cell, we examined the effect of APS on glucose uptake. Glucose uptake tests of the insulin resistance resistant 3T3-L1 cells were performed after treatment with APS (0.01, 0.05, 0.1, 0.5, 1, 5, and 10 μg/ml) for 60 min to obtain an optimum concentration of APS. And then, the glucose uptake tests of the insulin-resistant 3T3-L1 cells were performed after treatment with the optimum concentration of APS for 0, 15, 30, 60, 120, 240, and 480 min to further obtain the optimal incubation time. To investigate the effect of APS on insulin sensitivity, the insulin-resistant 3T3-L1 cells were treated with 0.1 or 1 μg/ml APS in the presence of 100 nM insulin for 60 min, and then followed by glucose uptake tests.
The expressions of miR-721 and PPAR-γ were detected in insulin-resistant 3T3-L1 cells treated with 0.1, 0.5, and 1 μg/ml APS for 60 min. To further determine the role of miR-721, PPAR-γ, and PI3K, the miR-721 mimics were transfected-, or pretreated with PPAR inhibitor T0070907 or PI3K inhibitor wortmannin- in insulin-resistant 3T3-L1 cells that were treated with 1 μg/ml APS with or without insulin for 60 min.
qRT-PCR
The miRNeasy Mini Kit (Qiagen, USA) was used to isolate total RNA including miRNAs. RNA purity was determined with a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, USA). TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, USA) was employed to reversely transcribe RNA into cDNA by using miRNA-specific primers. Real-time PCR was performed on an ABI PRISM 7300 instrument (ABI, USA). The PCR reaction mixture of 20 µL contained: 9 µL miRNA-specific cDNA, 10 µL TaqMan 2× Universal PCR Master Mix (No AmpErase UNG), 1 µL gene-specific primer. The PCR conditions were set as follows: initial activation of Taq polymerase at 95°C for 10 min, 40 cycles of PCR amplification at 95°C for 15 sec, annealing/elongation at 60°C for 1 min. The expression level of miR-721 in an individual sample was normalized to the U6 snRNA to compensate for sample variations. The relative expression of miR-721 was calculated by the 2-ΔΔCt formula. Samples were determined in duplicate. The miRNA sequences, specific stemloop primers and TaqMan probe were: for miR-721, CAGTGCAATTAAAAGGGGGAA (miR-721), CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTTCCCCC (RT); and ACACTCCAGCTGGGCAGTGCAATTAAAAG (probe). For U6, CTCGCTTCGGCAGCACA (U6), AACGCTTCACGAATTTGCGT (RT), and CTCAACTGGTGTCGTGGA (probe).
Western blot
Cells were harvested in protein extraction solution (Intron Biotechnology) and incubated for 30 min at 4°C. After cell debris was removed, supernatant containing proteins were collected and the concentrations determined using Bio-Rad protein assay reagent, according to the manufacturer’s instructions. Then, 30 μg proteins were separated by 10% SDS-PAGE, and transferred to PVDF membranes. Blots were incubated with 4% BSA blocking solution at 4°C, overnight with a primary antibody against PPAR-γ, PAKT, PI3K, AKT, GLUT4 and GAPDH, washed three times with TBST, and incubated with horseradish peroxidase-conjugated secondary antibody (1:1000) for 1 h at room temperature. After three washes with TBST, blots were developed using an enhanced chemiluminescence kit (Amersham Life Science, UK).
Luciferase reporter assay
The Psi-Check2-wild-type 3’-untranslated sequences (wt-3’UTR) of PPAR-γ, containing the miR-721 binding site ligated to pluc-Reporter luciferase vector were synthesized by Kangbio Company (TransGen Biotech, China). The corresponding Psi-Check2 mutated vectors (mut-3’UTR) were achieved by Fast Mutagenesis System (TransGen Biotech, China). All constructs were verified by DNA sequencing. HEK-293T cells were seeded in 24-well plates, co-transfected with 10-nmol pre-miR-721 or pre-miR-NC and 100-ng pluc-3’-UTR, and harvested 24 hours after transfection. Luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, USA) on a Glomax Luminometer (Promega, USA). Renilla luciferase activity was normalized to firefly luciferase.
Immunofluorescence staining and confocal microscopy examination
We performed immunofluorescence staining for GLUT4 to precisely evaluate GLUT4 expression in the insulin-resistant 3T3-L1 cells. Briefly, the cells were treated and washed with ice-cold PBS and then fixed in cold acetone for 15 min. After washing and blocking in 10% normal goat serum at 37°C for 1 h, the cells were washed and incubated at 37°C with a rabbit anti-GLUT4 antibody (1:200; Life Technologies, USA) for 1 h. After three washes, the FITC-conjugated goat anti-rabbit secondary antibodies were incubated for 1 h. The slides were mounted with DAPI (4’,6-diamidino-2-phenylindole) mounting medium [23,24]. Confocal images were collected under a confocal microscope (Olympus, Japan) using the excitation and emission wavelengths of 495/517 for FITC, and 358/463 for DAPI nuclear staining.
Statistical analysis
Results were expressed as means ± SD. The analysis was done using GraphPad Prism 6. For comparisons, Dunnett t test or Twoway ANOVA was used. The main factors of dosage and time are significant. P<0.05 was considered statistically significant.
Results
Confirmation of mature adipocytes
The 3T3-L1 preadipocytes were stimulated to differentiate, and these were then detected by light microscopy and Oil Red O staining (Figure 1A, 1B). The 3T3-L1 preadipocytes appeared as fibroblast-like spindle cells and there were no lipid droplets in the cytoplasm. After differentiation and maturation, the cells appeared rounder and larger, showing a typical ring shape with clear lipid droplets around the nucleus.
Figure 1.
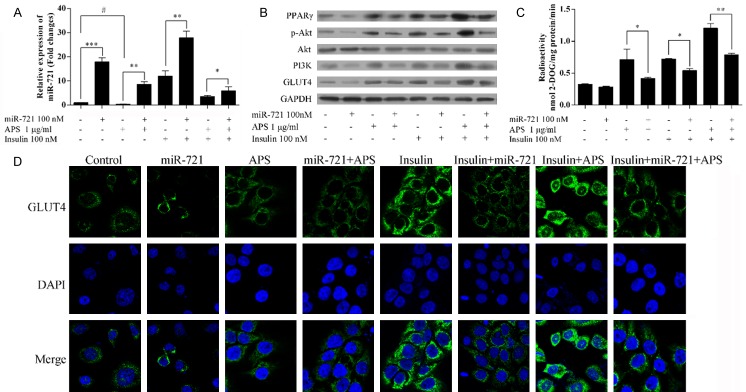
Establishment of a model of insulin resistance in adipocytes. Mature adipocytes were confirmed by light microscopy (A) and oil red O staining (B). (C) Glucose uptake tests were performed to confirm the establishment of insulin resistance adipocyte model. **P<0.01.
Establishment of insulin resistance adipocytemodel
Insulin increased the glucose uptake levels in well-differentiated 3T3-L1 adipocytes (Figure 1C). After pretreating with TNF-α, the glucose uptake levels in 3T3-L1 adipocytes did not change, suggesting the successful establishment of an insulin resistancemodel in adipocytes.
APS improved insulin sensitivity
The level of glucose uptake showed the greatest increase in the presence of 1 μg/ml of APS compared tono APS, in the insulin-resistant 3T3-L1 adipocytes (Figure 2A). After incubation with 1 μg/ml APS for 60 min, the level of glucose uptake showed the greatest increase in the insulin-resistant 3T3-L1 adipocytes (Figure 2B). Thus, the optimum concentration and incubation time were 1 μg/ml and 60 min, respectively.
Figure 2.
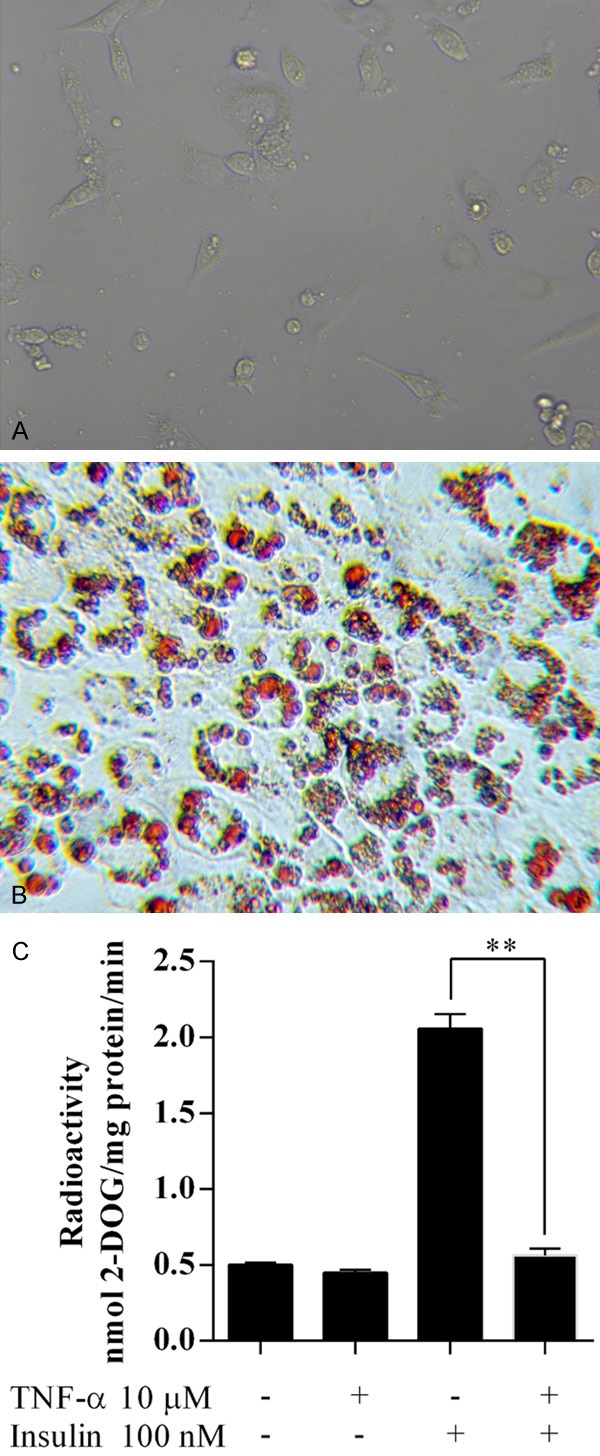
The glucose uptake tests were performed in insulin-resistant 3T3-L1 cells (A) treated with APS (0.01, 0.05, 0.1, 0.5, 1, 5, and 10 μg/ml) for 60 min; (B) Treated with 1 μg/ml APS for 0, 15, 30, 60, 120, 240, and 480 min; and (C) treated with 0.1 or 1 μg/ml APS in the presence of 100 nM insulin. For (A) and (B), *P<0.05, **P<0.01 vs 0 μg/ml; for (C), *,#P<0.05, **P<0.01.
The effect of APS on insulin sensitivity was further investigated (Figure 2C). APS with insulin increased the glucose uptake level, compared with insulin or APS alone. These results suggest that APS increases insulin sensitivity.
APS repressed expression of miR-721, and increased expression of PPAR-γ
The expression of miR-721 was suppressed by APS in a dose-dependent manner (Figure 3A). Also, the expression of PPAR-γ was increased in a dose-dependent manner (Figure 3B).
Figure 3.
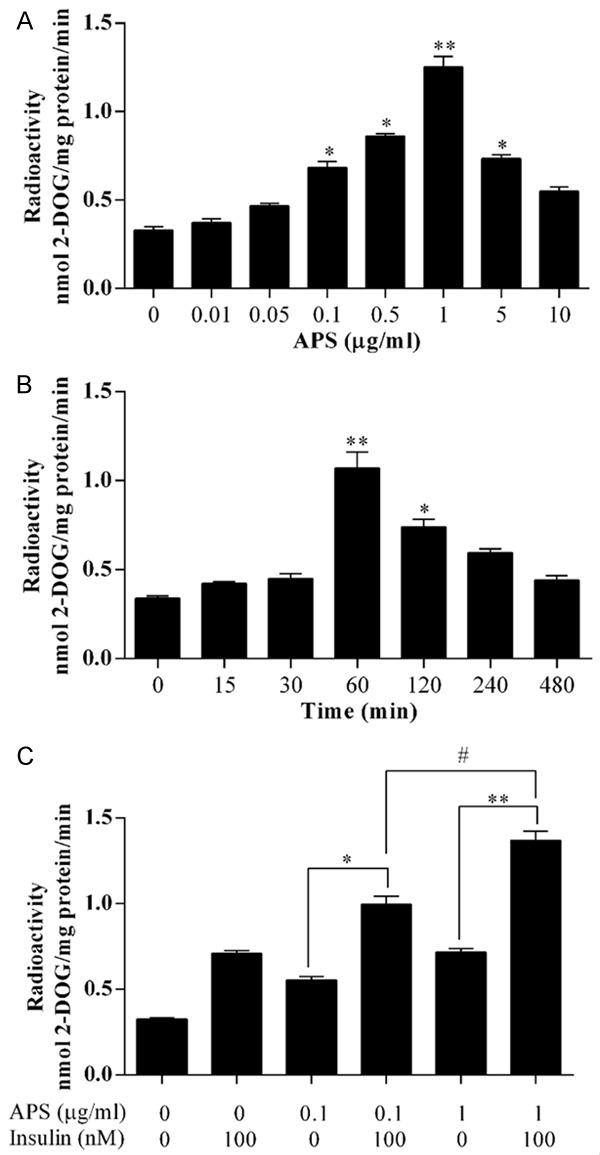
A. APS suppressed the expression of miR-721. *P<0.05, **P<0.01 vs 0 μg/ml. B. APS increased the expression of PPAR-γ. C. Comparison of the peak of the partial sequence of the wild-type and site-mutation plasmids. D. miR-721 mimics (mimics) suppressed the expression of PPAR-γ. E. Luciferase reporter assay. **P<0.01 vs NC.
PPAR-γ is a direct target of miR-721
The pluc-Reporter luciferase vector, wt-3’UTR of PPAR-γ containing the miR-721 binding site and the corresponding mutated vectors (mut-3’UTR) were obtained (Figure 3C) and transfected into cells. The expression of PPAR-γ was significantly reduced by miR-721 mimics, further confirming the interaction between miR-721 and PPAR-γ (Figure 3D). Furthermore, the miR-721 reduced the luciferase activity in wt-3’UTR of PPAR-γ, but did not change the luciferase activity in mut-3’UTR of PPAR-γ, suggesting that PPAR-γ is a direct target of miR-721 (Figure 3E).
APS increased increased the insulin sensitivity by attenuating the miR-721-downregulated PPAR-γ and -suppressing PI3/AKT activation in insulin-resistant 3T3-L1 adipocytes
After transfection with miR-721 mimics, miR-721 was overexpressed in insulin resistance 3T3-L1 adipocytes (Figure 4A). After miR-721 mimics were transfected, treatment with APS attenuated the expression of miR-721 in either the presence or absence of insulin (Figure 4A).
Figure 4.
APS increased insulin sensitivity byattenuating the miR-721-downregualted PPAR-γ in insulin-resistant 3T3-L1 adipocytes. A. Relative expression of miR-721. B. Expression of PPAR-γ, pAKT, PI3K, AKT, and GLUT4. C. Glucose uptake levels. D. Staining of GLUT4. *,#P<0.05, **P<0.01, ***P<0.001.
The miR-721 mimics inhibited the expressions of PPAR-γ, pAKT, and GLUT4, compared with their respective controls (Figure 4B). Treatment with APS attenuated the miR-721-inhibited expressions of PPAR-γ, pAKT, and GLUT4. In the presence of insulin, APS upregulated the expression of PPAR-γ, pAKT, PI3K, and GLUT4 in the miR-721 mimics. The expression levels of PPAR-γ, pAKT, PI3K, and GLUT4 in miR-721 + APS + insulin group were lower than that in the APS + insulin group.
Consistent with the above observations, the glucose uptake levels were reduced in miR-721 mimics (Figure 4C). APS increased the glucose uptake levels in miR-721 mimics. In the presence of insulin, APS increased the glucose uptake levels in miR-721 mimics, and the levels were lower than in insulin-resistant adipocytes without mimics (APS + insulin group), suggesting that there are other signaling pathways that are involved in the APS-increased insulin sensitivity, besides miR-721. The expression of GLUT4 was further confirmed by immunostaining (Figure 4D).
Thus, APS increased the miR-721-inhibited expressions of PPAR-γ, pAKT, and GLUT4 and also increased glucose accumulation. In addition, APS increased insulin sensitivity by attenuating the effects of miR-721 on the expressions of PPAR-γ, pAKT, PI3K and GLUT4, and glucose accumulation.
APS increased the insulin sensitivity via activation of PPAR-γ
The PPAR inhibitor T0070907 inhibited the expressions of PPAR-γ, pAKT, and PI3K (Figure 5A), and also reduced glucose uptake levels (Figure 5B), and GLUT4 levels (Figure 5A, 5C). APS abolished the inhibitory effect of T0070907.
Figure 5.
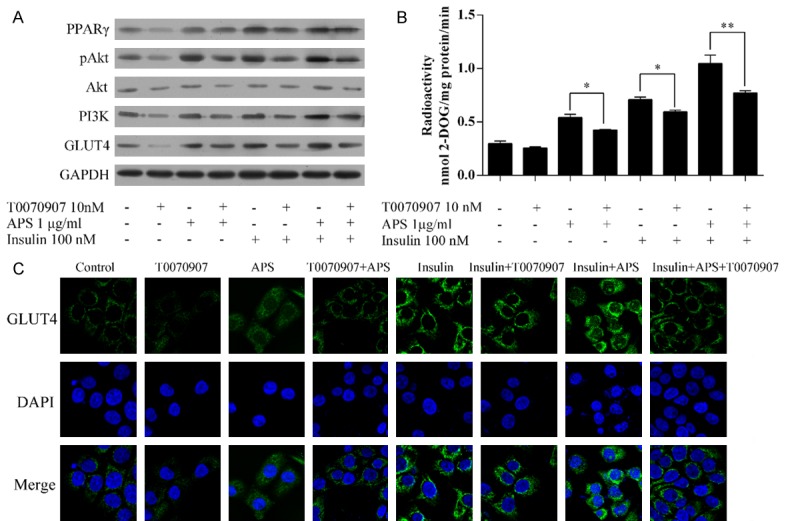
APS increased the insulin sensitivity via activation of PPAR-γ. A. Expression of PPAR-γ, pAKT, PI3K, AKT, and GLUT4. B. Glucose uptake levels. C. Staining of GLUT4. *P<0.05, **P<0.01.
In the presence of insulin, T0070907 also inhibited the expressions of PPAR-γ, pAKT, and PI3K (Figure 5A), glucose uptake levels (Figure 5B), and GLUT4 levels (Figure 5A, 5C). These effects were almost abolished by APS.
Thus, PPAR-γ plays a central role in APS-increased insulin sensitivity.
APS increased the insulin sensitivity via activation of PI3K
The PI3K inhibitor wortmannin reduced the APS-increased pAKT (Figure 6A), glucose uptake (Figure 6B), and GLUT4 levels (Figure 6A, 6C), and also reduced those levels in the presence of insulin with or without APS. These data suggest that APS increased the insulin sensitivity via the activation of PI3K.
Figure 6.
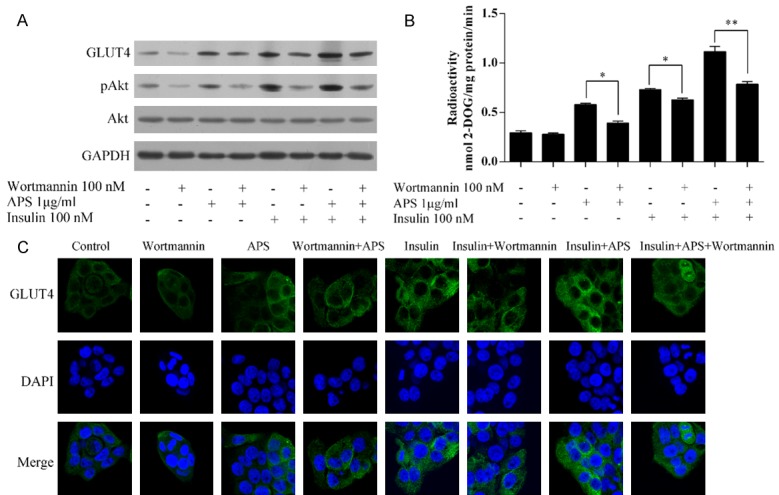
APS increased insulin sensitivity via activation of PI3K. A. Expression of pAKT, AKT, and GLUT4. B. Glucose uptake levels. C. Staining of GLUT4. *P<0.05, **P<0.01.
Discussion
Astragalus Polysaccharides (APS) are active components of the polysaccharides extract of Astragulus, which has important antioxidant, anti-hypertensive, and immunomodulatory roles [7-9]. In rats with type 2 diabetes, APS exerts insulin-sensitizing and hypoglycemic activities [11]. In diet-induced insulin-resistant C57BL/6J mice, APS alleviates insulin resistance [7]. The present study showed that APS attenuates TNF-α-induced insulin resistance by suppressing miR-721 and activating PPAR-γ and PI3K/AKT in 3T3-L1 adipocytes.
TNF-α is overexpressed in adipose tissues of obese animals and humans [2]. TNF-α-induced insulin resistance is an important contributor to the pathogenesis of obesity and type 2 diabetes [1]. High levels of TNF-α are associated with insulin resistance in many kinds of cells, such as human hepatocellular liver carcinoma cell line [25], endothelial cells [26], and adipocytes [27,28]. The white 3T3-L1 preadipocytes were well-differentiated to brown adipocytes and became rounder and, larger, showing a typical ring shape with clear lipid droplets around the nucleus. Treatment with TNF-α increased MCP-1 and decreased adiponectin secretion in a dose-dependent manner, in the 3T3-LA L1 adipocytes [29]. Here, we successfully created a model of insulin resistance in TNF-α-mediated mature 3T3-L1 cells mediated by TNF-α. After treatment with insulin, the glucose uptake levels increased significantly in mature 3T3-L1 adipocytes compared with insulin-resistant adipocytes (TNF-α).
APS increased the glucose uptake levels in insulin-resistant adipocytes in a dose- and time-dependent manner. Data from our study suggested that the optimum concentration and incubation time were 1 μg/ml and 60 min, respectively. APS also increased the insulin sensitivity. In Caenorhabditis elegans, miR-124 with its target gene atf-6 is involved in the lifespan-extending effects of APS [30]. miRNAs also plays important roles in the regulation of development, and diabetes [21]. We found that APS suppressed miR-721 and PPAR-γ in a dose-dependent manner. miRNA often induces the degradation of those target mRNAs via by binding to the 3’-untranslated region (UTR) of target mRNAs. After transfection with the binding site, mutated vectors for PPAR-γ, did not change luciferase activity, while however activity was reduced in WT vectors. Also, miR-721 mimics significantly reduced the expression of PPAR-γ. Therefore, we conclude that miR-721 induced the degradation of the target genes of PPAR-γ by binding to the 3’-UTR.
APS promotes expression and translocation of GLUT4 in adipose tissues [18]. Insulin-mediated glucose uptake is dependent on GLUT4 in adipocytes [19]. miR-721 inhibited expressions of PPAR-γ, pAKT, and GLUT4 and reduced glucose accumulation. APS attenuated these miR-721-induced changes. APS increased the insulin sensitivity by attenuating the effects of miR-721.
PPAR-γ is a member of the nuclear receptor superfamily of ligand-activated transcription factors that control energy homeostasis through the regulation of carbohydrate and lipid metabolism [31,32]. PPARγ is a master regulator of adipocyte differentiation [15]. PPAR-γ plays an important role in the differentiation and maturity of fatty cells, such as 3T3-L1 preadipocytes [13,15]. PPARγ also plays important roles in glucose accumulation and lipid homeostasis [31,32]. Activated PPAR-γ increased peripheral insulin sensitivity and alleviated hepatic glucose production, reducing hyperglycemia [14]. APS reversed the PPAR-mediated suppression of genes that might be involved in glucose utilization [7]. The activity of PPAR-γ induced by APS alleviates high blood glucose levels, suggesting an important role for PPAR-γ in APS-attenuated insulin resistance. A recent study showed that APS suppressed insulin resistance accompanied by improved hepatic PPAR-γ coactivator intracellular signaling and reduced chronic inflammation [16]. We showed that APSsuppressed insulin resistance via activation of PPAR-γ. Activation of the insulin-AKT signaling pathways mediate insulin utilization [20]. The PI3K inhibitor wortmannin reduced the APS-increased mediated pAKT, glucose uptake, and GLUT4 levels, and also reduced those levels in the presence of insulin with or without APS. Our data suggest that APS increased the insulin sensitivity via activation of PI3K.
Other PPAR receptors might also be involved in APS-mediated insulin sensitivity. In both db/db mice and myosin heavy chain (MHC)-peroxisome proliferator-activated receptor (PPAR) α mice, APS strikingly improved the myocyte triacyglyceride accumulation and cardiac dysfunction. APS repressed the activation of PPARα target genes involved in myocardial fatty acid uptake in db/db diabetic and MHC-PPARα hearts, and also reversed the PPARα-mediated suppression of genes involved in glucose utilization [7]. The activity of PPARα induced by APS alleviates high blood glucose levels, suggesting an important role for PPARα in APS-attenuated insulin resistance. A recent study has showed that APS suppressed insulin resistance and also improved hepatic PPARα intracellular signaling and reduced chronic inflammation [16].
In conclusion, our findings suggest that APS could promote glucose uptake and increase insulin sensitivity via the miR-721-PPAR-γ-PI3K/AKT-GLUT4 signaling pathway in adipocytes. These could potentially be new therapeutic targets for the treatment of insulin resistance in obesity and diabetes.
Acknowledgements
The study was supported by grants from National Natural Science Foundation of China (No. 81302877), Science and Technology Project of Guangdong Province of China (No. 2014A020212056), Natural Science Foundation of Guangdong Province of China (No. 2016A030313248) and Bureau of Traditional Chinese Medicine Foundation of Guangdong Province of China (No. 20141057, No. 20161056). Science and Technology project of Guangzhou city of China (201604020129), Science and Technology project of Shenzhen city of China (JCYJ20150402094341897).
Disclosure of conflict of interest
None.
Authors’ contribution
Conceived and designed the experiments: All authors; Performed the experiments: All authors; Analyzed the data and wrote the manuscript: All authors; All authors have read and approved the final submitted manuscript.
References
- 1.Lorenzo M, Fernandez-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci. 2008;86:E94–104. doi: 10.2527/jas.2007-0462. [DOI] [PubMed] [Google Scholar]
- 2.Huang CY, Yao WF, Wu WG, Lu YL, Wan H, Wang W. Endogenous CSE/H2 S system mediates TNF-alpha-induced insulin resistance in 3T3-L1 adipocytes. Cell Biochem Funct. 2013;31:468–475. doi: 10.1002/cbf.2920. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BB, Shulman GI, DeFronzo RA, Cushman SW, Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest. 1991;87:561–570. doi: 10.1172/JCI115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao CP, Xie ML, Gu ZL. Effects of konjac extract on insulin sensitivity in high fat diet rats. Acta Pharmacol Sin. 2002;23:855–859. [PubMed] [Google Scholar]
- 5.Wu F, Chen X. [A review of pharmacological study on astragalus membranaceus (Fisch. ) Bge] . Zhong Yao Cai. 2004;27:232–234. [PubMed] [Google Scholar]
- 6.Li R, Chen WC, Wang WP, Tian WY, Zhang XG. Extraction, characterization of astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohyd Polym. 2009;78:738–742. [Google Scholar]
- 7.Mao XQ, Yu F, Wang N, Wu Y, Zou F, Wu K, Liu M, Ouyang JP. Hypoglycemic effect of polysaccharide enriched extract of astragalus membranaceus in diet induced insulin resistant C57BL/6J mice and its potential mechanism. Phytomedicine. 2009;16:416–425. doi: 10.1016/j.phymed.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Sheikh Y, Maibam BC, Biswas D, Laisharm S, Deb L, Talukdar NC, Borah JC. Anti-diabetic potential of selected ethno-medicinal plants of north east India. J Ethnopharmacol. 2015;171:37–41. doi: 10.1016/j.jep.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Xia Y, Zhao X, Wang H, Chen W, Yu M, Li Y, Ye H, Zhang Y. The critical role of astragalus polysaccharides for the improvement of PPARalpha [correction of PPRAalpha] -mediated lipotoxicity in diabetic cardiomyopathy. PLoS One. 2012;7:e45541. doi: 10.1371/journal.pone.0045541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Ou-Yang JP, Wu K, Wang Y, Zhou YF, Wen CY. Hypoglycemic effect of astragalus polysaccharide and its effect on PTP1B. Acta Pharmacol Sin. 2005;26:345–352. doi: 10.1111/j.1745-7254.2005.00062.x. [DOI] [PubMed] [Google Scholar]
- 12.Li RJ, Qiu SD, Chen HX, Wang LR. [Immunomodulatory effects of astragalus polysaccharide in diabetic mice] . Zhong Xi Yi Jie He Xue Bao. 2008;6:166–170. doi: 10.3736/jcim20080212. [DOI] [PubMed] [Google Scholar]
- 13.Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003;52:2331–2337. doi: 10.2337/diabetes.52.9.2331. [DOI] [PubMed] [Google Scholar]
- 14.McGuire DK, Inzucchi SE. New drugs for the treatment of diabetes mellitus: part I: thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008;117:440–449. doi: 10.1161/CIRCULATIONAHA.107.704080. [DOI] [PubMed] [Google Scholar]
- 15.Kwak HJ, Choi HE, Jang J, Park SK, Cho BH, Kim SK, Lee S, Kang NS, Cheon HG. Suppression of adipocyte differentiation by foenumoside B from lysimachia foenum-graecum is mediated by PPARgamma antagonism. PLoS One. 2016;11:e0155432. doi: 10.1371/journal.pone.0155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu C, Zeng Y, Tang Z, Wang C, He Y, Feng X, Zhou L. Astragalus polysaccharides affect insulin resistance by regulating the hepatic SIRT1-PGC-1alpha/PPARalpha-FGF21 signaling pathway in male sprague dawley rats undergoing catch-up growth. Mol Med Rep. 2015;12:6451–6460. doi: 10.3892/mmr.2015.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agyemang K, Han L, Liu E, Zhang Y, Wang T, Gao X. Recent advances in astragalus membranaceus anti-diabetic research: pharmacological effects of its phytochemical constituents. Evid Based Complement Alternat Med. 2013;2013:654643. doi: 10.1155/2013/654643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu HF, Ren YH, ZX H. Effect of astragalus poly saccharides on insulin resistance and gene expression of GLUT4 in type 2 diabetes mellitus rats. Chinese Journal of Gerontology. 2011;31:3988–3989. [Google Scholar]
- 19.Cong LN, Chen H, Li Y, Zhou L, McGibbon MA, Taylor SI, Quon MJ. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Li H, Jeong JH, Noh M, Ryu JH. Kazinol B from broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. Fitoterapia. 2016;112:90–96. doi: 10.1016/j.fitote.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, Ramos KS, Young KH, Li Y. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene. 2011;30:843–853. doi: 10.1038/onc.2010.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu S, Sun F, Li W, Cao Y, Wang C, Wang Y, Liang D, Zhang R, Zhang S, Wang H, Cao F. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem. 2011;353:305–313. doi: 10.1007/s11010-011-0799-0. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y, Liu XH, Tarbell J, Fu B. Sphingosine 1-phosphate induced synthesis of glycocalyx on endothelial cells. Exp Cell Res. 2015;339:90–95. doi: 10.1016/j.yexcr.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363–372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Ke Y, Feng A, Yuan P, Zhou J, Yu Y, Wang X, Feng W. The mechanism by which amentoflavone improves insulin resistance in HepG2 cells. Molecules. 2016:21. doi: 10.3390/molecules21050624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batumalaie K, Amin MA, Murugan DD, Sattar MZ, Abdullah NA. Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci Rep. 2016;6:27236. doi: 10.1038/srep27236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao XY, Shao K. Roles of MicroRNA-21 in the pathogenesis of insulin resistance and diabetic mellitus-induced non-alcoholic fatty liver disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:144–149. doi: 10.3881/j.issn.1000-503X.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Huang Y, Bu D, Chen S, Tang C, Wang G, Du J, Jin H. Endogenous sulfur dioxide is a novel adipocyte-derived inflammatory inhibitor. Sci Rep. 2016;6:27026. doi: 10.1038/srep27026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagahara K, Dobashi K, Ishikawa T, Nakano Y, Abe Y, Tanaka D, Itabashi K. AICAR attenuates TNFalpha-induced inappropriate secretion of monocyte chemoattractant protein-1 and adiponectin in 3T3-L1 adipocytes. J Atheroscler Thromb. 2016;23:1345–1354. doi: 10.5551/jat.34835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang N, Liu J, Xie F, Gao X, Ye JH, Sun LY, Wei R, Ai J. miR-124/ATF-6, a novel lifespan extension pathway of astragalus polysaccharide in caenorhabditis elegans. J Cell Biochem. 2015;116:242–251. doi: 10.1002/jcb.24961. [DOI] [PubMed] [Google Scholar]
- 31.Evans RM. 2003 Keio medical science prize commemorative lecture. PPARs and the complex journey to obesity. Keio J Med. 2004;53:53–58. doi: 10.2302/kjm.53.53. [DOI] [PubMed] [Google Scholar]
- 32.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]