Abstract
Development of the novel targeted therapies for glioblastoma multiforme is very important. Brachytherapy has been proven to provide a good alternative to surgical removal of the prostate, breast and cervix with reduced risk of certain long-term side effects. Thus, 125I brachytherapy was used to effect on the growth and invasion of glioma cells in vitro and in vivo. The inhibitory effect of 125I seeds on C6 cells proliferation was determined by MTT assay. A rat intracranial glioma model was established and the 125I seeds were implanted into the glioma area. CD31 expression was determined by immunohistochemical method to evaluate the angiogenesis. The ΔΨm detection and cell invasion assays were performed to detect the mitochondrial-induced apoptosis and invasion signaling in tumor cells. 125I brachytherapy could significantly inhibit C6 rat glioma cells growth and reduce cell viability in vitro. The seeds implantation also inhibited tumor growth in the rat glioma model and improved survival rate. Analysis revealed that ROS production and the intrinsic mitochondrial pathway of apoptosis was activated by 125I brachytherapy. The HE staining results revealed that the rat glioma model treated with 125I seeds exhibited better defined tumor margins and fewer invasive cells to the lateral striatum compared with the untreated group. The comparison of expression of CD31 in treated or untreated groups was performed to show that the 125I brachytherapy has potential antiangiogenic activity. Meanwhile, 125I brachytherapy can inhibit the growth and invasion of glioma cells via decreasing the expression of MMP-2 and MMP-9.
Keywords: 125I brachytherapy, apoptosis, glioblastoma, invasion, mitochondrion
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive type of primary human brain tumor. Glioma cells highly grow and diffusely infiltrate adjacent brain tissues [1,2]. Invasive glioblastoma cells rapidly infiltrate and disrupt normal tissue architecture, making complete surgical resection badly difficult. Despite many advances in surgical resection, chemotherapy and radiotherapy combination therapies, the mean survival time of glioblastoma diagnosed patients is up to 1 year [3-5]. Therefore, exploiting the novel targeted therapies for glioblastoma multiforme is significantly important.
Radioactive seed implantation therapy is a form of interstitial brachytherapy [6]. Implantation with 125I seeds is a useful and well tolerated way of treatment for many kinds of carcinoma, such as prostate carcinoma, choroidal melanoma, parotid gland carcinoma [7-9]. Compared with external radiotherapy, it is easy and involves low photon energy use (27-35 keV). The seeds can be screened, and the dose reduced sharply with distance, thereby minimizing the risk of damage to the adjacent vital structures. The quality of an implant can be assessed via performing post-implant dosimetry which is a valuable learning tool that provides dosimetric indices which can be correlated to clinical outcomes.
Here we used a rat intracranial glioma model to study the effect of 125I brachytherapy for glioma. The data suggested that 125I brachytherapy could inhibit the growth of C6 glioma cells and prolong the survival of animal significantly. Further study demonstrated that it may be concerned with triggering of ROS production and the intrinsic mitochondrial pathway of apoptosis. The HE staining results revealed that 125I brachytherapy could also inhibit invasion of glioma cells, for the rat glioma model treated with 125I seeds exhibited better defined tumor margins and fewer invasive cells to the meninges compared with the untreated group, in which the inhibitory effect of angiogenesis and the decreased expression of MMP-2 and MMP-9 were involved. Therefore, 125I interstitial brachytherapy may be a promising alternative method of external radiotherapy for glioma.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the China Pharmaceutical University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Materials
125I seeds strengths of 0.3 mCi per seed were purchased from Shanghai Syncor Pharmacutical Co. Ltd. with a matched peripheral dose (MPD) of 60 Gy and a half life of 60.1 days. Stereotaxic apparatus was obtained from Tai Meng Science and Technology Co. Ltd. (Chengdu, Sichuan). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Fluka, U.S.A. and was dissolved in 0.01 M PBS. Primary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), and IRDyeTM800 conjugated anti-goat and anti-rabbit second antibodies were obtained from Rockland Inc. (Philadelphia, PA, USA).
Cell culture
Rat C6 glioma cell line was purchased from Cell Bank of Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The cells were cultivated in DMEM, supplemented with 10% (v/v) heat inactivated fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin in a 5% CO2 atmosphere.
Cell morphological assessment
Rat C6 glioma cells were cultivated in DMEM till mid-log phase in 96-well plates. 125I seeds were added to the culture media with three seeds per well and incubated for 24 h. At the end of the incubation, the morphology of cells was observed under an inverted light microscope.
MTT assay
Rat C6 glioma cells were cultured in DMEM media till mid-log phase in 96-well plates. 125I seeds were added with three seeds per well for 24 h. At the end of incubation, the culture medium was removed and 20 μL MTT (5 mg/mL in phosphate buffered saline, PBS) was added and cells were further incubated for 4 h. Four hours later, the supernatant was discarded and formazan crystals formed were dissolved in 100 μL of DMSO (20% sodium dodecyl sulfate, 50% dimethylformamide) [10]. Then the mixture was shaken and the absorbance of solubilized formazan was measured at 570 nm using a Universal Microplate Reader (EL800, Bio-Tek Instruments, Inc.).
Colony formation assay
Rat C6 glioma cells were trypsinized and plated at a density of 500 perplate. Fourteen days later, 125I seeds in cell culture medium were added to the plates, and cells were incubated for a further 7 days. Cells were then fixed with 3% paraformaldehyde, stained with crystal violet and imaged with a light microscope. The experiment was performed in triplicate. The number of colonies, defined as containing > 50 cells, was counted.
Animals
Spraque-Dawley rats (Charles River Laboratories, Sulzfeld, Germany) weighting 200-250 g were used, which were divided in groups of two under standard conditions at a temperature of 22°C ± 1 and a 12-h light-dark cycle with free access to food and water. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85-23, revised 1985) and approved by IACUC (Institutional Animal Care and Use Committee of China Pharmaceutical University).
Orthotopic glioma model
Before the implantation of 125I, almost all the confluent C6 cells were trypsinized, rinsed by DMEM with 10% fetal calf serum and centrifuged at 1000 rpm for 5 min. The cell pellet resuspended in DMEM was placed on ice. Concentration of viable cells was adjusted to 1 × 105 cells/1 μL of DMEM. Each rat was anesthetized and placed in a stereotactic frame, and a hole was drilled at AP 0.0, R-3.0 relative to bregma according to the stereotaxic atlas. Tumor cells were injected into a rat of 0.5 μL/s, using a 2 μL Hamilton (#2701) syringe (Reno, NV, USA) with a 26s-gauge needle mounted on a stereotactic holder at a depth of 5 mm. The needle was left in place for a further 10 min to prevent reflux along the needle tract [11,12]. Seeds based on 125I most often contain a loose silver wire impregnated with iodine. During implantation, a metallic frame is used for precise parallel alignment and the implantation needles with respect to each other. The 125I seed activity was 0.3 mci. The MPD was 60 Gy, D90 was > 80 Gy, and V150 was < 50%.
Immunohistochemistry study
Two groups of micrometer-thick sections from formalin-fixed and paraffin-embedded tissues were placed on poly-L-lysine-coated slides [13]. The tissue sections were dewaxed and rehydrated. In order to reduce non-specific background staining attributable to endogenous peroxidase activity, slides were incubated in 0.3% hydrogen peroxide for 15 min. The tissue sections were boiled in 10 mM citrate buffer (pH 6.0) for 10 min followed by cooling at 25°C for 20 min. To reduce the non-specific binding while using mouse monoclonal antibody to mouse tissue, blocking solution was used for 65 min (Labvision, USA, cat. no. TQ-015-HD; Ultra V block for 5 min, Rodent block for 60 min). Sections were incubated with mouse monoclonal antibody against CD31 as the primary antibody at 4°C overnight. After being washed for 10 min in PBS, the CD31 specimens were then incubated with biotinylated goat anti-mouse immunoglobulin secondary antibodies for 10 min, followed by a 10 min PBS wash and 10 min incubation in streptavidin-peroxidase at room temperature. Finally, the binding of primary antibody to the sections was visualized by using a diaminobenzidine tetrahydrochloride (DAB) reagent kit (Zymed, USA). Then, specimens were mounted using aqueous mounting medium (Beckman Coulter, Japan). Statistical analysis of these measurements consisted of an independent t-test.
Apoptosis assessment
The apoptosis induced by 125I brachytherapy was detected by Annexin V-FITC apoptosis detection kit according to the manufacturer’s protocol. Briefly, Rat C6 glioma cells were treated with 125I seeds for 24 h at 37°C. Then the cells were collected, resuspended in binding buffer (pH 7.5, 10 mM HEPES, 2.5 mM CaCl2 and 140 mM NaCl), and incubated with Annexin V-FITC and then PI for 10 min in the dark at room temperature, cells were analyzed by flow cytometry (FACSCalibur, Becton Dickinson, USA) and a computer station running Cell-Quest software (BD Biosciences, Franklin Lakes, NJ, USA).
Mitochondrial transmembrane potential (ΔΨm) assessment
The electrical potential difference across inner mitochondrial membrane (ΔΨm) was monitored using the Mito-ID® Membrane Potential Kit according to the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY). Briefly, the C6 and U87 glioma cells (8 × 103 per well) in 96-well plates. After 24 h, the cells were washed with PBS and loaded with Mito-ID detection reagent for 30 min at 37°C in the dark. Afterwards, cells were washed with PBS and incubated with 125I seeds. The fluorescence was estimated using a plate reader (Fluoroskan Ascent FL, Labsystem, Finland) at 490 nm (excitation) and 590 nm (emission).
ROS measurement
The generation of ROS induced by 125I brachytherapy was determined with the cell permeant fluorogenic probe 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes, Invitrogen, Darmstadt, Germany) as described [14]. H2DCFDA diffuses into the cell, where it is enzymatically converted by intracellular esterases and oxidized into the high fluorescence compound DCF, which allows the determination of H2O2, peroxynitrite anions and peroxyl radicals. Approximately 4 × 103 C6 and U87 glioma cells per well were plated on white bottom 96-well plates in extra cellular fluid (140 nM NaCl, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 25 mM glucose; pH 7.4). After 24 h incubation, cells were loaded with 10 mM H2DCFDA for 30 min at 37°C and 5% CO2. Then, cells were treated with 125I seeds for 24 h at 37°C. DCF generation was measured over time using a fluorometer (Fluostar BMG Labtech, Offenburg, Germany) at 492 nm excitation and 520 nm emission.
H2O2 measurement
The production of H2O2 was determined with the Amplex® Red Reagent (10-acetyl-3,7-dihydroxyphenoxazine; Molecular Probes). In the presence of horseradish peroxidase (HPR) the Amplex® Red Reagent reacts in a 1:1 stoichiometry to produce the red-fluorescent oxidation product, resorufin. Consequently, resorufin generation allows the detection of the H2O2 released from biological samples. Approximately 4 × 103 C6 and U87 glioma cells were plated on black bottom 96-well plates in 120 mL of working solution containing 40 mM Amplex® Red Reagent and 0.1 U/mL HPR, and incubated in the presence of 125I seeds for 24 h at 37°C. Resorufin generation was measured over time using a fluorometer (Fluostar BMG Labtech) at 544 nm excitation and 590 nm emission.
Cell invasion and migration assay
Cell invasion and migration analysises were performed using a transwell system (Corning, NY, USA) according to the manufacturer’s guidelines. Briefly, cells treated with the indicated conditions were trypsinized and resuspended in serum-free medium. Then, cells were added into a transwell chamber (8 µm pore size) and seeded into the upper chamber. About 500 µL of culture medium containing 10% FBS was added into the lower chamber as a chemoattractant. For invasion assay, the upper surface of the transwell chamber membrane was coated with 0.5-mm thickness of matrigel (50 µg/chamber, BD Biosciences; Franklin Lakes, NJ, USA). The C6 and U87 glioma cells treated with 125I seeds for 24 h later, the residual cells on the upper membrane surface were gently wiped out with a cotton swab. Following fixation with 4% paraformaldehyde, cells that had migrated to the lower surface of membrane were stained with crystal violet. The average number of invading and migrating cells was counted on each membrane in five random microscopic fields.
Western-blot
At indicated time points, fresh tumor tissue was collected, cut into pieces, washed twice with cold PBS and lysed in lysis buffer (20 mM Tris-HCl, pH 7.4, 0.25 M NaCl, 2 mM EDTA, pH 8.0, 0.1% Triton-X-100, 1 mM DTT, 1 mM PMSF, 5 mg/mL leupeptin, 5 mg/mL aprotinin and 1.25 mg/mL bezamidine). Whole cell lysates were prepared for western blot analysis as described previously. Protein extracts were resolved on 10-12% SDS-PAGE. After electrophoresis, proteins were electrotransferred to nitrocellulose membrane and immune complexes were formed by incubation of the proteins probed with primary antibodies, mouse anti-Bax, rabbit anti-Bcl-2, rabbit anti-Caspase-3, rabbit anti-Caspase-8 and rabbit anti-p53, rabbit anti-PARP, mouse anti-MMP-2, mouse anti-MMP-9 and mouse anti-Actin (Boster, Inc. China) for overnight at 4°C. Blots were washed and incubated for 1 h with IRDyeTM800 conjugated anti-mouse and anti-rabbit second antibodies. All antibodies were obtained from Santa Cruz Biotechnology, CA, USA. Immunoreactive protein bands were visualized with an Odyssey Scanning System (LI-COR Inc., U.S. A.).
Statiatical evaluation
All results mentioned above represent the Mean ± S.E.M from triplicate experiments performed in a parallel manner unless otherwise indicated. Statistical analyses were performed using an unpaired, two-tailed Student’s t-test. All comparisons are made relative to untreated controls. A value of P < 0.05 was considered significant.
Results
125I seeds inhibited the cell growth and reduced the cellular viability in vitro
After treated with 125I seeds for 24 h, C6 glioma cells were seriously damaged and distorted, grew slowly and some cells turned round in shape. The control cells exhibited normal, healthy shapes verified by the clear skeletons in Figure 1A. Viability of rat C6 glioma cells was assessed by MTT assay. The results indicated that 125I brachytherapy could significantly reduce the cellular viability of C6 rat glioma cells in Figure 1B. Then, the colony forming assay (CFA) was used to detect the ability of 125I seeds to prevent the proliferation of C6 glioma cells. As we known, the CFA assay is an extensively used and well-established method for testing chemotherapeutic agents in vitro [15], reflecting the ability of agents to prevent the cell from dividing. Cells were treated with 125I seeds for 7 days. As shown in Figure 1C, apparently fewer colonies survived on the plates exposed to 125I seeds than those on the plates not treated with 125I seeds. Thus, 125I seeds were effective in inhibiting the proliferation of C6 glioma cells.
Figure 1.
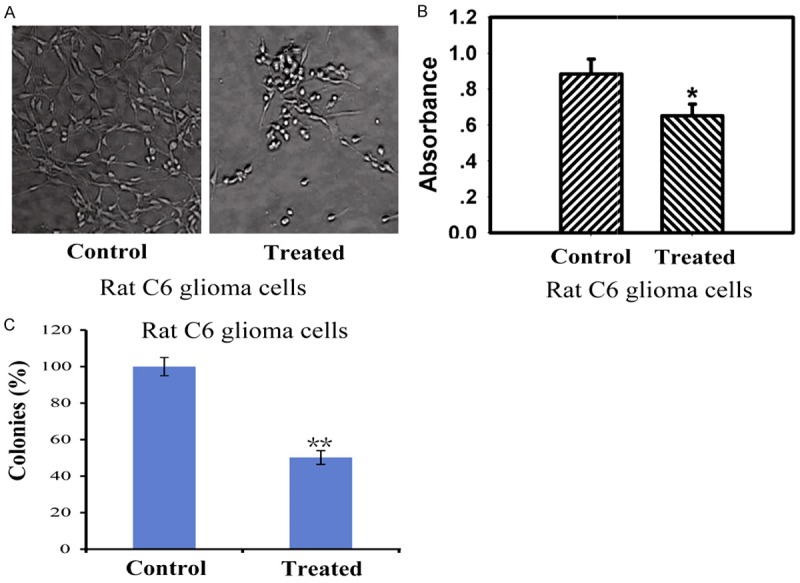
The implantation of 125I seeds induced inhibition of cell growth and reduction of cellular viability in vitro. A: Morphologic change of the cells observed under an inverted light microscope (× 400). B: The treatment of 125I seeds reduced cellular viability of rat C6 glioma cells in vitro. Viability of cells was assessed using the MTT assay. C: Long-term colony formation assays of C6 glioma cells. Cells were grown in the presence of drugs 125I seeds for 7 days. For each cell line, all dishes were fixed at the same time, stained, and photographed. Data are presented as the mean ± S.E.M., determined from three separate experiments. Error bars indicate SD. *P < 0.05; **P < 0.01.
125I seeds implantation inhibited the growth of orthotopic glioma model in rat
The results obtained by CT scans showed the reduced regions of the tumor after seeds implantation in Figure 2A. A magnification of the whole cross-section of the brain by HE staining displayed that the animals treated with 125I showed significantly smaller tumor regions compared with untreated animals in Figure 2B. The 125I-treated animals showed a significant reduction of tumor volumes compared with untreated animals according to the data from the CT scans. Untreated animals exhibited a median tumor volume of 369.0 mm3 ± 30.4 whereas 125I-treated animals revealed tumor volumes of 119.7 mm3 ± 25.6, reflecting 68% reduction in Figure 2C. Rats in 125I-treated group exhibited higher survival rate compared with untreated group. Asterisks indicate significant level in Student t-test: (*) P < 0.05 (n = 5) in Figure 2D.
Figure 2.
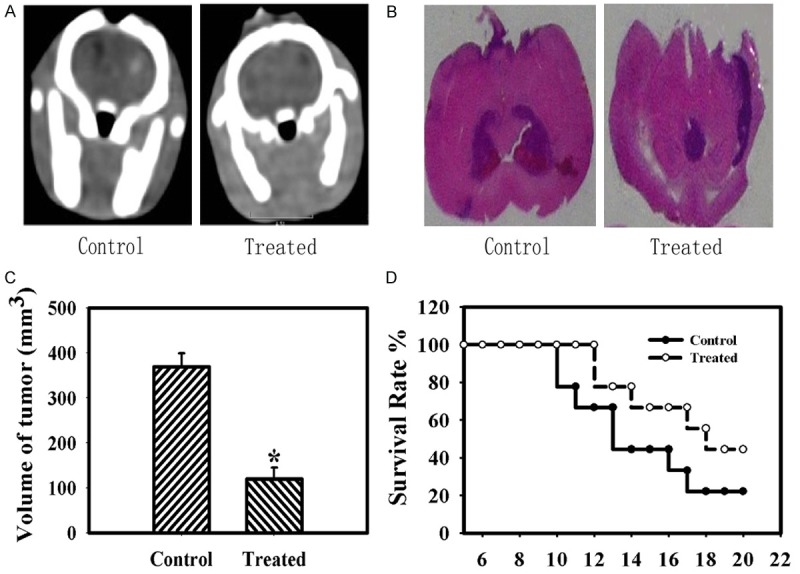
125I implantation effect on the tumor in the rat glioma model. A: CT verification of the positions of 125I seeds and the area of the tumor. B: A magnification of the whole cross-section of the brain by HE staining. 125I seeds-treated animals showed significantly smaller tumor area compared to untreated animals. C: Tumor volumes in mm3 of treated and untreated group. D: The survival rate of the animals treated with 125I seeds compared with untreated animals. Data are presented as mean ± S.E.M. of five animals per group. Data are presented as the mean ± S.E.M., determined from three separate experiments. Error bars indicate SD. *P < 0.05; **P < 0.01.
125I seeds triggered the mitochondria-dependent apoptotic pathway
To study the pathway involved in apoptotic cell death induced by 125I brachytherapy, the expressions of the proapoptotic proteins Bax, antiapoptotic protein Bcl-2, and pro-Caspase-3, pro-Caspase-8, cleaved-Caspase-3, cleaved-Caspase-8, PARP, P53 were detected in whole-hemisphere lysates of C6 glioma animals. Densitometric analysis at the endpoint of treatment revealed that protein levels of Bax, cleaved-Caspase-3, cleaved-Caspase-8 and P53 were upregulated and Bcl-2, pro-Caspase-3, pro-Caspase-8 and PARP was decreased in 125I-treated groups in Figure 3A and 3B. The apoptotic rates of 125I seeds were evaluated via Annexin V/PI stain assays. The results in Figure 3C showed that the apoptotic percentage (including the early and late apoptosis) of 125I seeds was 57.49% and 46.86%, indicating that 125I seeds showed much higher toxicity in Rat C6 glioma cells.
Figure 3.
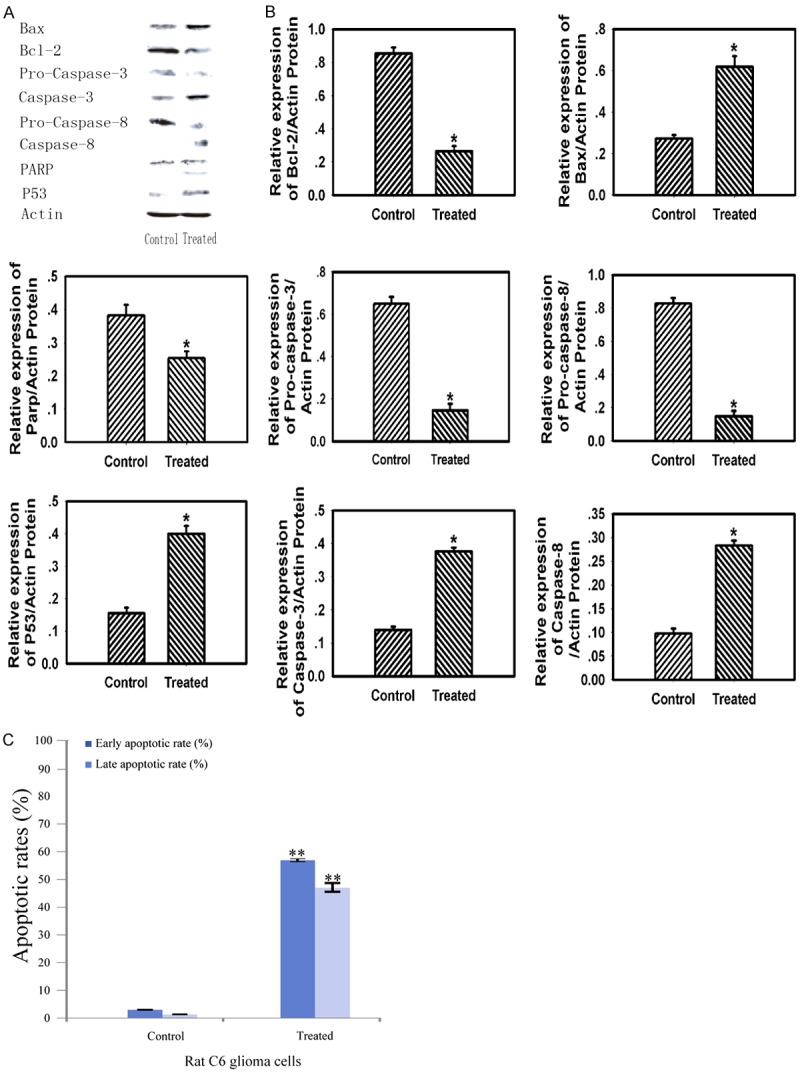
The implantation of 125I seeds induced the mitochondria-dependent apoptotic pathway compared with the control. A: Western Blotting analysis of Bax, Bcl-2, pro-Caspase-3, Caspase-3, pro-Caspase-8, cleaved-Caspase-8, PARP, P53, β-Actin expression. B: The relative expression of Bax, Bcl-2, pro-Caspase-3, Caspase-3, pro-Caspase-8, cleaved-Caspase-8, PARP and P53. C: Apoptosis inducing property of the measured samples by Annexin V-FITC/PI staining of cancer cells. The Y-axis shows the PI-labeled population and the X-axis shows FITC-labeled Annexin V-positive cells. Data are presented as the mean ± S.E.M., determined from three separate experiments. Error bars indicate SD. *P < 0.05; **P < 0.01.
To further study the detailed process of apoptosis induced by 125I brachytherapy in glioma cells, we examined the mitochondrial membrane potential (ΔΨm), which is an important proapoptotic index for early apoptosis. Consistently, a significant increase of cells with depolarized mitochondria was observed after treatment with 125I seeds (Figure 4A). These data suggested that 125I brachytherapy may activate the mitochondria-dependent apoptotic pathway.
Figure 4.
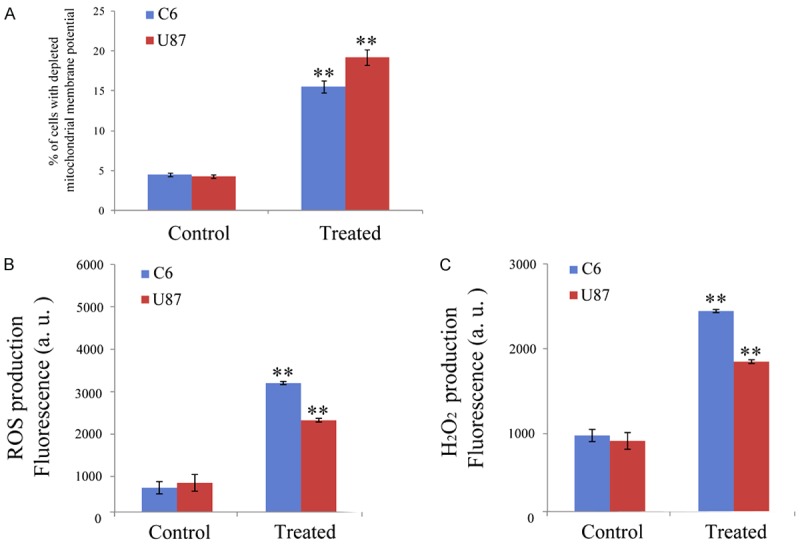
Effects of 125I brachytherapy on the mitochondria, ROS production and H2O2 level. A: Effects of 125I brachytherapy on the mitochondrial membrane potential. C6 and U87 glioma cells were treated for 24 with 125I seeds and stained with TMRM (10 nM). The fluorescence was estimated at 490 nm (excitation) and 590 nm (emission). B: ROS production in C6 and U87 glioma cells. Cells were pre-incubated in PBS/10 mM glucose medium for 20 min at 37°C in the presence of 10 mM CM-H2DCFDA and then treated with 125I seeds. The fluorescence of DCF was measured at 485 nm (excitation) and 527 nm (emission). C: Cells were exposed to 125I seeds for 24 h, then H2O2 level was measured. Data are presented as the mean ± S.E.M., determined from three separate experiments. Error bars indicate SD. *P < 0.05; **P < 0.01.
125I seeds induced ROS and H2O2 generation of cancer cells
Reactive oxygen species (ROS) play significant roles in a variety of physiological and pathophysiological processes [16]. Previous explorations have reported that excess accumulation of ROS is associated with disruption of mitochondrial membrane potential (MMP), therefore triggering a series of mitochondria-associated events including apoptosis [17]. Thus, the generation of ROS was investigated to further analyze the anticancer mechanism of 125I seeds (Figure 4B and 4C). C6 and U87 cancer cells were treated with 125I seeds, stained with H2DCFDA and then analyzed by flow cytometry. After 24 h incubation, the level of ROS cells increased after the treatment with 125I seeds as expected (Figure 4B), which was definitely higher than control groups.
Taking into account that among all ROS and other oxygen-derived free radicals, H2O2 has been recently suggested to act as a central player in signal transduction pathways [18], we evaluated H2O2 generation by a fluorometric assay in cancer cells incubated in the presence of 125I seeds. H2O2 measurement was carried out after 24 h incubations with 125I seeds. The production of H2O2 was significantly higher in cancer cells incubated with 125I seeds than that in the control group (Figure 4C). The results strongly suggested that 125I seeds could induce increased intracellular oxidative stress, thus triggering cell apoptotic pathways.
125I seeds implantation inhibited the tumor invasion
Traditional extra radiotherapy could inhibit glioma cell growth and migration, but the invasiveness of the remaining tumor cells seems to be unaffected [18]. Here we studied the effect of 125I brachytherapy on invasion of C6 and U87 glioma cells. Notably, 125I seeds suppressed the number of invading cells compared with control groups (Figure 5A). Moreover, 125I seeds also reduced cell migration (Figure 5B). Mechanisms involved in it may be for the decreasing expression of MMP-2 and MMP-9 in Figure 5C. The HE staining results revealed that 125I brachytherapy could also inhibit invasion of glioma cells, the rat glioma model treated with 125I seeds exhibited better defined tumor margins and fewer invasive cells to the meninges compared with the untreated group in Figure 5D. In addition, the inhibitory effect of angiogenesis in glioma may also contribute to this.
Figure 5.
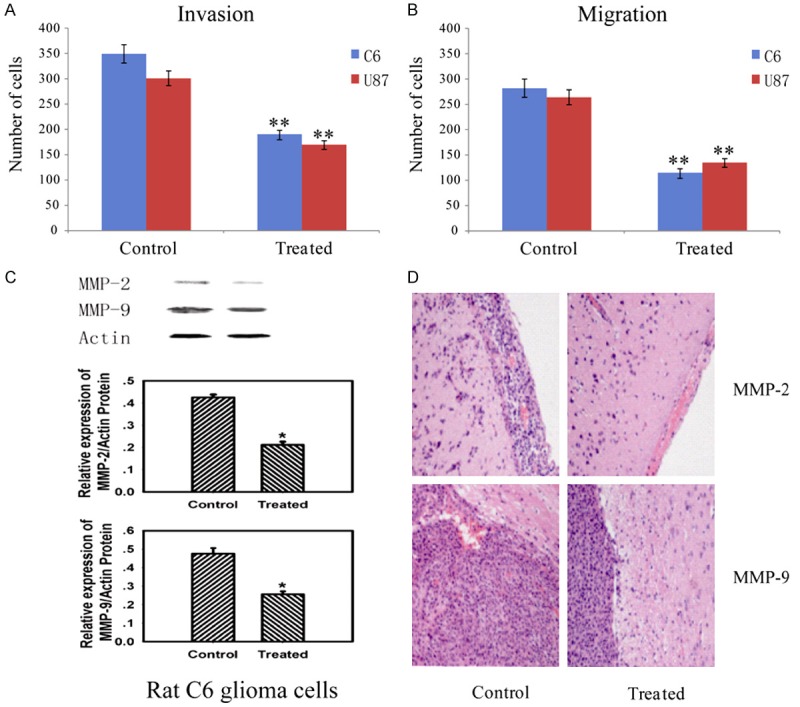
125I brachytherapy induced inhibition of tumor invasion. A: Effect of 125I brachytherapy on invasion of C6 and U87 glioma cells. B: Effect of 125I brachytherapy on cell migration. C: The implantation of 125I seeds reduced MMP-2, MMP-9 expression in treated groups. D: A magnification of the striatum with a representative part of the tumor by HE staining. The group treated with 125I seeds exhibited better defined tumor margins and fewer invasive cells to the meninges compared with the untreated. Data are presented as the mean ± S.E.M., determined from three separate experiments. Error bars indicate SD. *P < 0.05; **P < 0.01.
125I seeds implantation inhibited tumor angiogenesis
Histological examination of the sections from 125I-treated group showed a lower distribution of capillaries than in untreated group. The presence of CD31 positive cells indicated that endothelial monolayers were lining the walls of newly formed blood vessels. The groups embedded with 125I seeds showed lower levels of CD31 positive microvessels in Figure 6A. Quantification of capillary density demonstrated the extent of the vascularization achieved lower density in the 125I-treated group than that in the untreated group in Figure 6B.
Figure 6.
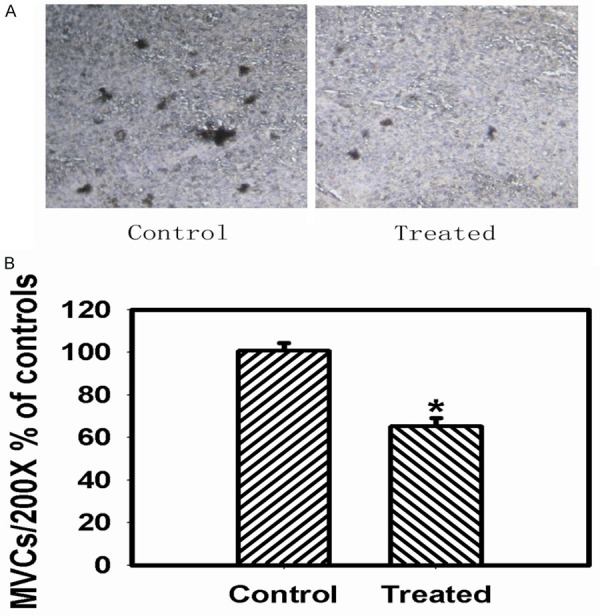
125I brachytherapy suppressed the tumor angiogenesis. A: The expression of CD31 in control and treated groups was shown by immunohistochemical method (× 200). B: Microvessel counts (MVCs) were assessed to demonstrate the extent of the vascularization in the control and treated groups. Data are presented as the mean ± S.E.M., determined from three separate experiments. Error bars indicate SD. *P < 0.05; **P < 0.01.
Discussion
Glioblastoma multiforme (GBM) which is considered as the most malignant glioma tumor of astrocytomas can occur at 50 years of age with a rate of one out of four surviving for two years. The treatment of patients with surgical resection is conventionally considered to be a palliative venture for diffusely infiltration of the tumor. The role of chemotherapy, specifically focusing on chloroethylating agents, has been controversial with arguing towards or against the therapy [19]. Traditionally, radiotherapy increases the duration of survival, but again is not a curative intervention.
Modern brachytherapy has led to effective treatments for maximizing survival and minimizing morbidity. 125I seed implantation therapy for rat C6 glioma cells can enhance the exposure dose of target area and reduce the irradiated scope of normal tissues. C6 cells were injected into rat striatum and treated with 125I for a week. The implantation of 125I seeds could reduce volume and weight of tumor and exhibit higher survival rate by inhibition of cell apoptosis, invasion and suppression of tumor angiogenesis. At the endpoint of 125I implantation, an increase in caspase-3, cleaved caspase-8, Bax, p53 and decrease of pro-Caspase-3, pro-Caspase-8, Bcl-2, PARP, MMP-2 and MMP-9 proteins were observed. Bcl-2 and Bax are two kinds of apoptosis related proteins [20], and caspase-3 is in the downstream of Bcl-2 and Bax, which play important roles in the control of apoptosis initiation and execution. Recent researches showed that Bax could form heterodimers with Bcl-2 to inhibit its anti-apoptotic function. Hence Bcl-2/Bax ratio is important to apoptosis induced by several agents and the decline of this ratio contributes to apoptosis induction. Additionally, Bax dimers or oligomers directly form channel in mitochondrial outer membrane in apoptosis. So mitochondria might be one of the targets of 125I brachytherapy. The increase of caspase-3 expression may induce the mitochondria-dependent apoptotic pathway with the possible mitochondria-independent pathway for the caspase-8 activation for that caspase-8-mediated apoptosis induced by oxidative stress is independent of the intrinsic pathway and dependent on cathepsins [21]. Moreover, p53 mediates apoptosis through a linear pathway involving Bax transactivation [22]. We suggested that perhaps the extrinsic and intrinsic apoptotic pathways were both involved in 125I brachytherapy. HE-staining revealed that the glioma model rat treated with 125I exhibited better defined tumor margins and fewer invasive cells to the contralateral striatum compared with the untreated rats, which led us to further investigate the effect of 125I brachytherapy on cell invasion. Among the MMP family, MMP-2 and MMP-9 expression play an important role on the invasion of rat C6 glioma cells. The higher expressions of two proteins might promote the invasion of rat C6 glioma cells. MMP-9 mediates fundamental processes underlying discreet steps in invasion and liberation of cancer cells from the surrounding tissues.
Antiangiogenic therapies are considered promising [23-25] for glioblastomas not only grow as solid tumor foci but also spread diffusely throughout the brain by high formation of new blood vessels. Tumor microvessel density can be measured using immunohistochemistry for endothelial-specific antigens such as CD31. Previous studies showed that tumor microvessel density to be related to disease progression and may function as a tumor prognostic factor [26]. Microvessel density was also increased in higher grade tumors [27]. Our study also provided evidences that 125I had a potent antiangiogenic activity in vitro. Although the exact mechanism of antiangiogenesis for 125I brachytherapy remains unclear.
The 125I brachytherapy could significantly inhibit glioma cells growth and reduce cell viability in vitro. The 125I seeds implantation also inhibited tumor growth in the rat glioma model and improved survival rate of the animals. Analysis revealed that ROS production, H2O2 level and the intrinsic mitochondrial pathway of apoptosis was activated by 125I brachytherapy. The results revealed that the rat glioma model treated with 125I seeds exhibited better defined tumor margins and fewer invasive cells to the lateral striatum compared with the untreated group. The comparison of expression of CD31 in treated or untreated groups was performed and the data suggested that the 125I brachytherapy has potential antiangiogenic activity.
In conclusion, the current data showed that 125I brachytherapy may be of potential use in treatment of glioblastoma by inducing of cell apoptosis and inhibiting of tumor angiogenesis and invasion.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (Grant No. 21571033) and the New Drug Creation Project of the National Science and Technology Major Foundation of China (Grant No. 2015ZX09101032) for financial aids to this work. The research was also supported by the National Natural Science Foundation of China (Grant No. 81503099) and Jiangsu Province Natural Science Foundation (Grant No. BK20150643). The authors would also like to thank the Fundamental Research Funds for the Central Universities (Project 2242016K30020) for supplying basic facilities to our key laboratory. China Pharmaceutical University was appreciated for completing the in vivo tests.
Disclosure of conflict of interest
None.
References
- 1.Kleihues P, Louis D, Scheithauer B. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 2.Qiang L, Yang Y, You QD, Ma YJ, Yang L, Nie FF, Gu HY, Zhao L, Lu N, Qi Q, Liu W. Inhibition of glioblastoma growth and angiogenesis by gambogic acid: an in vitro and in vivo study. Biochem Pharmacol. 2008;75:1083–1092. doi: 10.1016/j.bcp.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C, Grosu A, Astner S, Molls M. Treatment of unresectable glioblastoma multiforme. Anticancer Res. 2002;25:4605–4610. [PubMed] [Google Scholar]
- 4.Stupp R, Mason W, van den Bent M. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Astner S, Pihusch R, Nieder C, Rachinger W. Extensive local and systemic therapy in extraneural metastasized glioblastoma multiforme. Anticancer Res. 2006;26:4917–4920. [PubMed] [Google Scholar]
- 6.Jin L. Dosimetry for 125I radioactive seed implantation therapy for hepatocellular carcinoma. J Nanjing Med Univ. 2008;22:269–272. [Google Scholar]
- 7.Diener-West M, Earle J, Fine S, Hawkins B. The COMS randomized trial of iodine 125I brachytherapy for choroidal melanoma, III: initial mortality findings, COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982. doi: 10.1001/archopht.119.7.969. [DOI] [PubMed] [Google Scholar]
- 8.Hellawell G, Ho K, Halliwell M, Appleby H. Long-term outcomes and morbidity after 125I brachytherapy for localised prostate cancer: an early UK series. Clin Oncol (R Coll Radiol) 2005;17:68–69. doi: 10.1016/j.clon.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Song T, Zhen L, Zhang Y. 125I seed implant brachytherapy-assisted surgery with preservation of the facial nerve for treatment of malignant parotid gland tumors. Int J Oral Maxillofac Surg. 2008;37:515–520. doi: 10.1016/j.ijom.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Mei-Chin L, Shiuh-Lin H, Fang-Rong C, Yung-Husan C. Immunostimulatory effect of Antrodia camphorata extract on functional maturation of dendritic cells. Food Chem. 2009;113:1049–1057. [Google Scholar]
- 11.Stander M, Naumann U, Dumitrescu L, Heneka M. Decorin gene transfer-mediated suppression of TGF-beta synthesis abrogates experimental malignant glioma growth in vivo . Gene Ther. 1998;5:1187–1194. doi: 10.1038/sj.gt.3300709. [DOI] [PubMed] [Google Scholar]
- 12.Grommes C, Landreth G, Sastre M, Beck M. Inhibition of in vivo glioma growth and invasion by peroxisome proliferator-activated receptor gamma agonist treatment. Mol Pharmacol. 2006;70:1524–1533. doi: 10.1124/mol.106.022194. [DOI] [PubMed] [Google Scholar]
- 13.D’Amico F, Skarmoutsou E, Stivala F. State of the art in antigen retrieval for immunohistochemistry. J Immunol Methods. 2009;341:1–18. doi: 10.1016/j.jim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Zhang L, Qiang L, Yang Z, Wu T, Zou M, Tao L, You Q, Li Z, Yang Y, Guo Q. Reactive oxygen species-mitochondria pathway involved in LYG-202-induced apoptosis in human hepatocellular carcinoma HepG2 cells. Cancer Lett. 2010;296:96–105. doi: 10.1016/j.canlet.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Chen F, Pan Y, Li Z, Xue X. Nanodrug formed by coassembly of dual anticancer drugs to inhibit cancer cell drug resistance. Acs Appl Mater Inter. 2015;7:56–74. doi: 10.1021/acsami.5b05347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkel T, Holbrook N. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 17.Park M, Kim M, Bae S. Phytosphingosine in combination with ionizing radiation enhances apoptotic cell death in radiation-resistant cancer cells through ROS-dependent and -independent AIF release. Blood. 2005;105:1724–1733. doi: 10.1182/blood-2004-07-2938. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B, Cross C. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102:5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman HS. State-of-the-art therapy for glioblastoma multiforme. US Oncological Dis. 2007:16–7. [Google Scholar]
- 20.Gliemroth J, Feyerabend T, Gerlach C, Arnold H, Terzis A. Proliferation, migration and invasion of human glioma cells exposed to fractionated radiotherapy in vitro . Neurosurg Rev. 2003;26:198–205. doi: 10.1007/s10143-003-0253-1. [DOI] [PubMed] [Google Scholar]
- 21.Yin C, Knudson C, Korsmeyer S, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo . Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 22.Ning S, Pei-Hua Z, Chang-Qin M, Jian-Zhuang J. The synthesis, spectroscopy, electrochemistry and photophysical properties of novel, sandwich europium(III) complexes with a porphyrin ligand bearing four pyrenyl groups in meso-positions. Dyes Pigments. 2009;81:91–96. [Google Scholar]
- 23.Laurie N, Donovan S, Shih C, Zhang J, Mills N. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 24.Kargiotis O, Rao J. A. Kyritsis, Mechanisms of angiogenesis in gliomas. J Neuro Oncol. 2006;78:281–293. doi: 10.1007/s11060-005-9097-6. [DOI] [PubMed] [Google Scholar]
- 25.Naumov G, Bender E, Zurakowski D, Kang S, Sampson D. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98:316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 26.Jiménez JA, Kao C, Raikwar S, Gardner TA. Current status of anti-angiogenesis therapy for prostate cancer. Urol Oncol. 2006;24:260–268. doi: 10.1016/j.urolonc.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]


