Abstract
Recent studies have indicated that ATRA inhibits chondrogenesis and can lead to congenital clubfoot (CCF). The molecular mechanism of ATRA-induced chondrogenesis is not clear. As RhoA/ROCK and SDF-1/CXCR4 signaling play important molecular roles for a variety of cellular processes, we hypothesized that RhoA/ROCK2 and SDF-1/CXCR4 signaling are involved in ATRA-induced chondrogenesis in rat embryo hind limb bud mesenchymal cells (rEHBMCs). We found that ATRA dose-dependently inhibits proliferation and expression of chondrogenic transcription factors (SOX9 and COL2A1) in rEHBMCs. In contrast, ATRA increases the expression of ROCK2, SDF-1 and CXCR4. Pharmacological inhibition of ROCK signaling and SDF-1/CXCR4 signaling by Y27632 and AMD3100, respectively, resulted in elevated expression of SOX9 and COL2A1. In addition, we found that disturbing SDF-1/CXCR4 signaling by AMD3100 decreases ATRA-induced ROCK2 expression. In vivo studies we also confirm that SOX9 expression of early-stage cartilage progenitors in the proliferative zone and COL2A1 expression in prehypertrophic chondrocytes are decreased in ATRA-treated rat embryo hind limb. Together, these results show that ATRA activates SDF-1/CXCR4/ROCK2 signaling to inhibit chondrogenesis to lead to CCF by suppressing differentiation through down-regulation of SOX9 and COL2A1 expression in rat embryo hind limb bud mesenchymal cells.
Keywords: All-trans retinoic acid (ATRA), congenital clubfoot (CCF), rat embryo hind limb bud mesenchymal cells (rEHBMCs), ROCK2, SDF-1, CXCR4
Introduction
Congenital clubfoot (CCF) is typically an isolated defect and one of the most common serious congenital anomalies in the musculoskeletal system. The birth prevalence in the general population is approximately 1 in 1000 live births [1,2]. Clubfoot is characterized by the presence of forefoot and midfoot adductions, hindfoot varus and ankle equinus, and presents as a rigid inward turning of the foot towards the midline of the body resembling a persistently contracted foot [3]. We previously showed that inhibition of hind limb cartilage development is a crucial factor for ATRA-induced congenital clubfoot [4,5]. In vertebrates, chondrogenesis involves a complex network of transcription factor and signaling molecule expression leading to sequential aggregation, proliferation, differentiation, and hypertrophy of chondrocytes to provide the initial patterning of the vertebrate skeleton and lead to the formation of cartilage growth plates, which drive body growth and have primary roles in endochondral ossification [6-9]. Retinoic acid (RA), an oxidative metabolite of vitamin A, is present in the vertebrate embryonic limb and controls many important biological processes, including embryonic development [10]. Physiologically, either too high or too low levels of RA is detrimental for early embryogenesis and morphogenesis due to the narrow concentration window for proper RA function [11]. Thus, RA is indispensable for embryonic development and other biological processes, but aberrant RA metabolism and signaling lead to many congenital diseases. A single intragastric dose of retinoic acid (120 mg/kg body weight) on day 10 of pregnancy, in rats, has been shown to establish limb defects, and serves as a model for fetal clubfoot [12]. However, the cellular and molecular mechanisms whereby prenatal RA causes abnormalities in fetal hind limb chondrogenesis and induces limb skeletal malformation (clubfoot), are not clear.
ROCK2, also known as the kinase ROCKII, is an important effector of RhoA. The activation of RhoA and ROCK2, and the resultant cytoskeletal isometric tension that accompanies their activation, are important factors in mesenchymal stem cell fate [13]. Stromal cell-derived factor 1 (SDF-1), also known as CXCL12, belongs to the CXC chemokine subfamily and specifically activates C-X-C motif chemokine receptor 4 (CXCR4) [14]. SDF-1 is widely involved in the hematopoietic system, being essential for B-cell lymphopoiesis and bone marrow myelopoiesis [15]. The accumulating data support an emerging hypothesis that SDF-1/CXCR4 also plays pivotal roles in the biological and physiological functions of mesenchymal stem cells (MSCs) [16]. SDF-1a activates RhoA-ROCK-MYL2 through the CXCR4/G (i/o)-PI3K signaling pathway or the CXCR4-MEK pathway [17]. SDF-1 controls the actin organization of primary chondrocytes, and the absence of SDF-1 impairs the cytoskeleton of hypertrophic chondrocytes [18]. It has been shown previously that ATRA induces the expression of SDF-1 during acute APL U937 cell differentiation, and enhances expression of CXCR4 at the NB4 cell surface [19,20].
In this study, we investigated whether SDF-1/CXCR4 signaling and RhoA/ROCK signaling are related to the effects of ATRA on chondrogenesis in rEHBMCs. We first examined how ATRA affects cell proliferation, chondrocyte-specific gene expression, SDF-1/CXCR4 signaling and ROCK signaling. We then used pharmacological agents to inhibit CXCR4 and ROCK2 to investigate the mechanisms whereby RA influences chondrogenesis in vitro. Finally, the in vivo effects of ATRA were assessed using an ATRA-induced CCF model.
Materials and methods
Animals
Primiparous female and adult male Sprague-Dawley (SD) rats (the Experimental Animal Center of Shantou University Medical College) were from the same strain and suppliers. The day of mating was considered as gestation day 0 (E0). Pregnant female rats were individually housed in clear polycarbonate cages with stainless steel wire lids and corncob granules as bedding. Food pellets and filtered tap water were available ad libitum. Animals were maintained at 24°C, 50% humidity and a 12-hour light and dark cycle. All animal protocols were approved by the Institutional Animal Care and Use Committee of Shantou University Medical College, and the study was carried out in accordance with the established institutional and national guidelines on experimental use and care of animals.
Cell isolation and culture of rat hind limb bud mesenchymal stem cells
Pregnant rats, at E12.5, were euthanized with CO2, and embryos were removed and placed in phosphate-buffered saline (PBS). The limb buds were separated under a microscope and digested with 0.1% trypsin for 10 minutes at 37°C, with slight agitation with a plastic pipette. Serum was added to terminate the digestion, and cells were filtered through a pre-wetted 40-micron cell strainer, and centrifuged at 1000 rpm for 5 minutes. Cells were resuspended in DMEM/F12 (Hyclone) containing 10% fetal bovine serum (FBS) (GIBCO), 1% penicillin and 1% streptomycin (Sigma-Aldrich, St. Louis, MO), and cultured in 60 mm plates at 37°C in a humidified atmosphere containing 5% CO2. After 12 hours, medium was replaced with medium containing 1% fetal bovine serum for 24 h in order to ensure the consistency of cell stage, then cells were treated with 0.01-10 μM ATRA (Sigma) in dimethyl sulfoxide (DMSO).
Drug treatment
The ATRA was dissolved in dimethyl sulfoxide (DMSO) and prepared to be 0.01-10 μM. The following pharmacological agents were used: 25 μM AMD3100 octahydrochloride hydrate (Sigma-Aldrich, St. Louis, MO), a chemokine receptor CXCR4 antagonist; and 10 μM Y-27632 dihydrochloride (Tocris Bioscience, Bristol, UK), an inhibitor of p160 ROCKII activity. Cells were pretreated with inhibitors for 1 hour, then cultured with ATRA and inhibitors in DMEM/F12 (Hyclone) containing 10% fetal bovine serum (FBS), 1% penicillin and 1% streptomycin. The extent of proliferation, differentiation, expression of chondrogenic genes, and chondrogenic protein levels were analyzed at various time points.
Lactate dehydrogenase (LDH) assay and proliferation assay
The rEHBMCs were inoculated at 5,000 cells per well in 96-well plates and allowed to attach for 24 hours. Cells were then treated with 0, 0.01, 0.1, 1 and 10 μM ATRA for 24 hours. The LDH concentration in cultured medium was assayed utilizing a commercial kit (Cytotoxicity Detection Kit, Roche), which was instructed by the manufacturer. Cell proliferation was determined using the Cell Counting Kit-8 (CCK-8) (Beyotime, China) assay and absorbance at 490 nm was read immediately and at 6-48 h using a microplate reader (Thermo Scientific, Beijing, China). Cell proliferation was expressed as the optical density (OD) value.
Histology and immunohistochemistry
A single intragastric dose of retinoic acid (120 mg/kg body weight) was administered on E10 to pregnant female SD rats. At E16.5, rats were euthanized by CO2, then embryos were removed and placed in phosphate-buffered saline (PBS). Embryonic limbs were separated under a microscope and fixed in 4% paraformaldehyde/PBS at 4°C overnight. For histological sections, the hind limbs were processed and embedded in paraffin. Sections were cut to 5 μm thickness and subjected to H&E staining or immunostaining. Paraffin sections were first incubated with primary antibodies at 4°C overnight, and then incubated with the appropriate secondary antibody at room temperature, processed further with an ultrasensitive ABC IgG Staining Kit (Pierce), and visualized with diaminobenzidine (DAB) staining or immunofluorescence. Anti-SOX9 (Abcam, UK) and Anti-COL2A1 (Abcam, UK) antibody staining was performed as primary antibodies.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to analyze the expression of chondrogenesis genes. Total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Total RNA was reverse-transcribed into cDNA by using a High Capacity cDNA Reverse Transcription Kit (Invitrogen). Quantitative real-time PCR was performed using SuperReal PreMix (Tiangen, Beijing, China) on an ABI Prism 7300 Sequence Detection System (Applied Biosystems). The cDNA was amplified with SDF-1, CXCR4, SOX9, CO2A1, ROCK1, ROCK2, HOXD9, SHH, BSP, BMP4, MLC-2, HES-1 and GAPDH primers by using PCR. The primer sequences were listed in Table 1. PCR was performed at 95°C for 15 min followed by 40 cycles of 10 s at 95°C, and 31 s at 62°C. Melting curves were generated to confirm the specificity of the PCRs. The relative mRNA expression level was calculated using the 2-ΔΔCt method and normalized against the housekeeping gene glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). All quantitative real-time PCR experiments were performed in triplicate in independent experiments.
Table 1.
PCR primer sequences for quantitative real-time PCR
| Genes | Forward primer sequence | Reverse primer sequence |
|---|---|---|
| SDF-1 | 5’-CAGGTCCGAGGAATGCTGC-3’ | 5’-CCCTGACACTGAACTGGA-3’ |
| CXCR4 | 5’-ACAGGATCTTCCTGCCCACCAT-3’ | 5’-GGGGAGTGTGATGACAAAGAGG-3’ |
| SOX9 | 5’-AACAGACGTGCAAGCTGGGAA-3’ | 5’-CGGCAGGTATTGGTCAAACTCA-3’ |
| COL2A1 | 5’-GAACTGGTGGAGCAGCAAGAGC-3’ | 5’-CCCTCAGTGGACAGTAGACGGA-3’ |
| ROCK1 | 5’-TTCATGTCCGACCTGTAACC-3’ | 5’-TTGACAGCGTTCGAGGAGAG-3’ |
| ROCK2 | 5’-TTCACGTCCGACCTGTTACC-3’ | 5’-GTGGCACCTACGGCACTCTA-3’ |
| HOXD9 | 5’-CCGCTGAAGGAGGAGGAGAAG-3’ | 5’-GCGTCTGGTATTTGGTGTAGGG-3’ |
| SHH | 5’-GGACGGCACCATTCTCATCAAC-3’ | 5’-CTCGCTTCCGCTACAGATTGC-3’ |
| BSP | 5’-GGTCAGCAAGAGCGAGTGAA-3’ | 5’-TCGAGAAAGCACTCGCCATC-3’ |
| BMP4 | 5’-GAGCCATTCCGTAGTGCCAT-3’ | 5’-AACGACCATCAGCATTCGGT-3’ |
| MLC-2 | 5’-AGCCTCAGACACCATGTCAC-3 | 5’-TGAAGCCGTCTCTGTTCTGG-3’ |
| HES-1 | 5’-CAACACGACACCGGACAAAC-3’ | 5’-TTGGAATGCCGGGAGCTATC-3’ |
| GAPDH | 5’-CAAGTTCAACGGCACAGTCAAGG-3’ | 5’-ACATACTCAGCACCAGCATCACC-3’ |
Western blotting for protein expression
Total cellular lysates were prepared using RIPA lysis buffer (Boster, Wuhan, China) following the manufacturer’s instructions. Immunoblotting was carried out as previously described [21]. The following antibodies were used to assess protein levels: anti-SDF-1 antibody (Sigma-Aldrich, St. Louis, MO), anti-CXCR4 (Sigma-Aldrich, St. Louis, MO), anti-ROCK2 antibody (Sigma-Aldrich, St. Louis, MO), anti-SOX9 antibody (Abcam, UK) and anti-SOX9 antibody (Abcam, UK). Protein bands were visualized using a SuperSignal Western Blotting Kit (Pierce, Rockford, IL). Secondary antibodies used were horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG (Santa Cruz Biotechnology Inc.). Protein bands were visualized using a SuperSignal Western Blotting Kit (Pierce, Rockford, IL). Densitometric analysis was performed using Quantity One Software v4.62 (Bio-Rad, Hercules, CA). β-Actin was used as the loading control.
Statistical analyses
Data are presented as the mean ± standard deviation (SD) of three independent experiments, and analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL). Significant differences between the groups were determined using one-way or two-way analysis of variance, and multiple comparisons were conducted using the Scheffe method. Probability (P) values of less than 0.05 were considered to be statistically significant and highly significant, respectively.
Results
ATRA inhibits rat embryo hind limb bud mesenchymal cell proliferation in vitro
Hind limb bud mesenchymal cells were isolated from E12.5 embryos, according to a previous method (Figure 1A, 1B) [21]. We examined the optimal dose of ATRA that inhibited chondrogenesis in rEHBMCs. The cell toxicity data showed that 1 μM ATRA was the optimal dose for inhibiting chondrogenesis, and 10 μM ATRA enhanced significant cytotoxicity (Figure 1C). Therefore, we used 1 μM ATRA for our further experiments. To determine whether ATRA affects the proliferation on rEHBMCs, we performed a CCK-8 assay to measure cell proliferation. We found that ATRA caused increasing inhibition of cell proliferation with increasing dose. Doses of 0.01 and 0.1 μM ATRA caused no apparent effect on the growth of the mesenchymal cells at all time points examined, compared to controls (P>0.05). 1 μM ATRA caused a 30% reduction in the growth of the mesenchymal cells, when measured at 24 h and 48 h, compared to control (P<0.05). 10 μM ATRA caused a 70% reduction at 24 h and 48 h compared to controls (P<0.05) (Figure 1D). These findings indicate that ATRA causes a dose-dependent inhibition in the proliferation of rEHBMCs.
Figure 1.
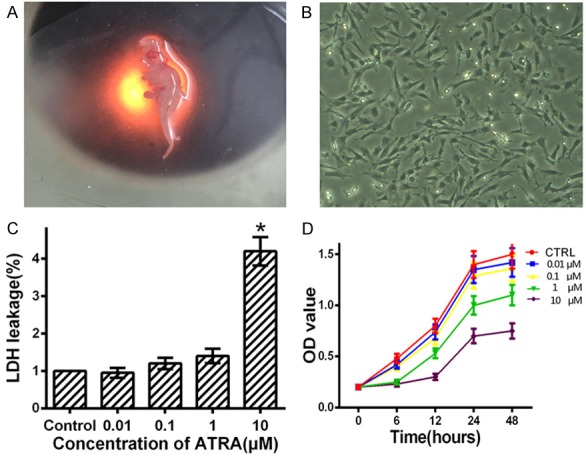
ATRA inhibits the proliferation of rat embryo hind limb bud mesenchymal cells. A: The hind limb buds were separated from E12.5 embryos under a microscope. B: The rat hind limb bud mesenchymal cells were cultured in 60 mm plates. C: Cells were cultured in medium with 0, 0.01, 0.1, 1 and 10 μM ATRA for 24 h and using LDH cytotoxicity assay to measure cytotoxicity. D: ATRA dose-dependently inhibits proliferation of rEHBMCs in vitro. CCK-8 was used to measure cell proliferation at 6-48 h after rEHBMCs were treated with ATRA at the indicated concentrations. Data are presented as the mean ± SD of at least three independent experiments done in triplicate. *P<0.05 compared to the control.
ATRA inhibits chondrogenesis of hind limb bud mesenchymal cells by downregulating SOX9 and COL2A1 expression
As a key transcription factor required for chondrogenesis, SOX9 is involved at many steps of the chondrocyte differentiation pathway, and COL2A1 is a downstream target gene of SOX9. To identify whether ATRA decreased the expression of SOX9 and COL2A1, rEHBMCs were treated with 0, 0.01, 0.1, 1 and 10 μM ATRA for 24 hours, then SOX9 and COL2A1 were measured by qRT-PCR and western blotting. Results showed that ATRA caused a dose-dependent reduction in the mRNA transcriptional levels of SOX9 and COL2A1 (P<0.05) (Figure 2A). Consistent with the qRT-PCR results, western blotting revealed that ATRA caused significant and dose-dependent reductions of the protein expressions of SOX9 and COL2A1 within 24 hours (P<0.05) (Figure 2B). Though the highest concentration of ATRA (10 μM) caused a clear decrease of SOX9 and COL2A1, 10 μM ATRA was strongly toxic and caused a large amount of cell death. Together, these results indicate that ATRA inhibits chondrogenesis by downregulating the expression of SOX9 and COL2A1 in rEHBMCs.
Figure 2.
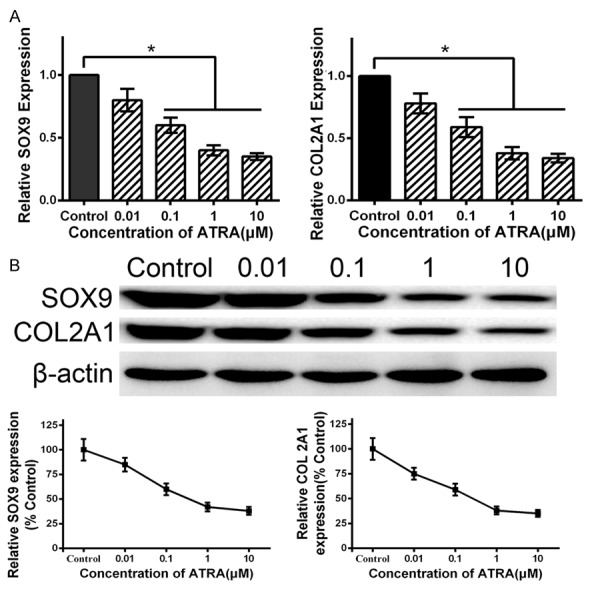
Expression of SOX9 and COL2A1 on ATRA-treated rat hind limb bud mesenchymal cells. A: Cells were cultured in medium with 0, 0.01, 0.1, 1 and 10 μM ATRA for 24 h, then detected mRNA transcript levels of SOX9 and COL2A1 by qRT-PCR. ATRA dose-dependently downregulates the mRNA transcript levels of SOX9 and COL2A1. B: Cells were cultured in medium with 0, 0.01, 0.1, 1 and 10 μM ATRA for 24 h, then detected protein levels of SOX9 and COL2A1 by western blotting. ATRA dose-dependently downregulates the protein levels of SOX9 and COL2A1. Data are presented as the mean ± SD for at least three independent experiments done in triplicate. *P<0.05 compared to the control.
ATRA up-regulates ROCK2 expression in hind limb bud mesenchymal cells
As previously reported, ATRA is widely involved in embryonic development, including the development of the limbs. ATRA might have an important role in chondrogenesis of rEHBMCs. We analyzed cytokines and factors, including HOXD9, SHH, BSP, BMP4, MLC-2, HES-1, ROCK1 and ROCK2, involved in chondrogenesis. Our qRT-PCR results demonstrated the effect of ATRA has a obvious correlation with the expression of ROCK2 in rEHBMCs (Figure 3A). Specifically, cells were incubated with 0, 0.01, 0.1, 1 or 10 μM ATRA for 24 h, and then measured by quantitative real-time PCR to visualize the expression of ROCK2. The qRT-PCR assays showed that ATRA caused a dose-dependent increase in mRNA transcript levels of ROCK2 (Figure 3B). When treated with 1 μM ATRA, the gene expression level of ROCK2 was increased to 325% compared to untreated control cells (P<0.05). The results suggest that ATRA up-regulates ROCK2 expression in rEHBMCs, and 1 μM ATRA caused the greatest increase among the concentrations tested, which is in line with previous results showing that 1 μM is the optimal concentration.
Figure 3.
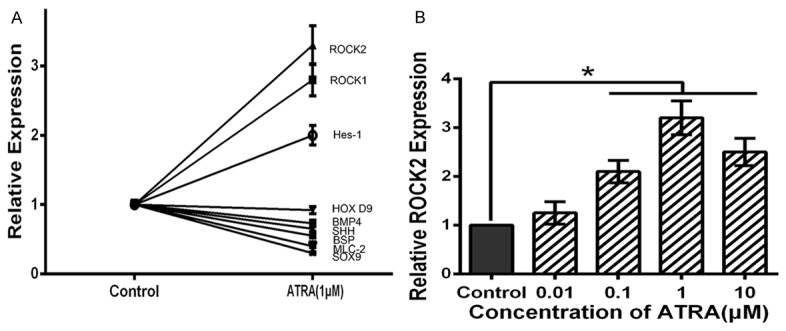
ATRA up-regulates ROCK2 expression in hind limb bud mesenchymal cells. A: The effects of 24 h ATRA (1 μM) treatment on HOXD9, SHH, BSP, BMP4, MLC-2, HES-1, ROCK1 and ROCK2 measured by qRT-PCR. The expression level of each factor is expressed as a percentage. The effect of ATRA has a obvious correlation with the expression of ROCK2. B: ATRA dose-dependently upregulates mRNA transcript levels for ROCK2, and 1 μM ATRA caused the greatest increase among the concentrations tested. Data are presented as the mean ± SD for at least three independent experiments done in triplicate. *P<0.05 compared to the control.
ATRA suppresses chondrogenesis by up-regulating ROCK2 expression
For the reason that SOX9 and COL2A1 are required transcription factors for chondrogenesis, we doubted whether SOX9 and COL2A1 expression are affected by RhoA/ROCK2 signaling. Quantitative RT-PCR assays showed that ATRA-mediated overexpression of ROCK2 in rat embryo hind limb bud mesenchymal cells correlated with a 50% reduction in SOX9 mRNA levels and 60% reduction in COL2A1 mRNA levels (P<0.05). This reduction was rescued by the addition of the ROCK2 inhibitor Y27632 (10 μM), suggesting that ROCK signaling controls SOX9 mRNA levels through transcriptional mechanisms (Figure 4A). Similarly, western blotting assays showed overexpression of ROCK2 correlated with markedly decreased SOX9 and COL2A1 protein levels (P<0.05). The effects were reversed by treatment with Y27632 (Figure 4B). Altogether, these results suggest that ATRA up-regulates the expression of ROCK2 to inhibit hind limb chondrogenesis through suppressing expression of SOX9 and COL2A1.
Figure 4.
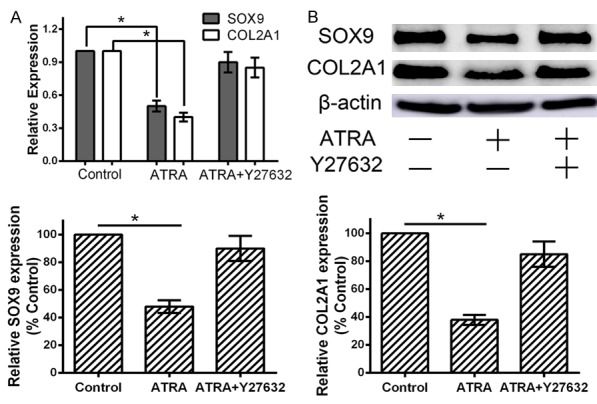
ATRA suppresses expression of SOX9 and COL2A1 by up-regulating ROCK2. A, B: Cells were pretreated with 10 μM Y27632 for 1 hour, then cultured with 1 μM ATRA for 24 h, then detected mRNA transcript and protein levels of SOX9 and COL2A1 by qRT-PCR and western blotting. ATRA-induced overexpression of ROCK2 contributes to decreased mRNA transcript and protein level of SOX9 and COL2A1. The effects were reversed by treatment with 10 μM Y27632. Data are presented as the mean ± SD for at least three independent experiments done in triplicate. *P<0.05 compared to the control.
ATRA suppresses chondrogenesis by up-regulating SDF-1 and CXCR4 expression
ATRA induces the expression of SDF-1 and CXCR4 in APL U937 cells and NB4 cells [19,20]. Whether ATRA modulates the SDF-1/CXCR4 signaling pathway is still not clear in rat hind limb bud mesenchymal cells. We treated hind limb bud mesenchymal cells with 1 μM ATRA for 24 h, and then measured the expression of SDF-1 and CXCR4 by qRT-PCR. Quantitative RT-PCR showed that the gene expression levels of SDF-1 and CXCR4 were increased to 275% and 245% respectively, significantly higher than untreated control cells (P<0.05) (Figure 5A).
Figure 5.
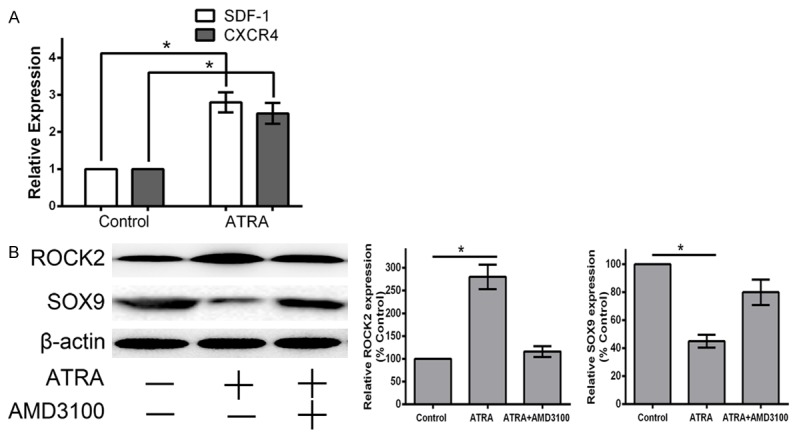
ATRA activates SDF-1/CXCR4/ROCK2 signaling to supress expression of SOX9 and further inhibit chondrogenesis. A: Cells were cultured in medium with 1 μM ATRA for 24 h, then detected mRNA transcript levels of SDF-1 and CXCR4 by qRT-PCR. ATRA upregulates the mRNA transcript levels of SDF-1 and CXCR4. B: Cells were pretreated with 25 μM AMD3100 for 1 hour, then cultured with 1 μM ATRA for 24 h, then detected expression levels of ROCK2 and SOX9 by qRT-PCR and western blotting. Overexpression of CXCR4 induced by ATRA contributes to increased expression of ROCK2 and decreased expression of SOX9. The effects were blocked by treatment with 25 μM AMD3100. Data are presented as the mean ± SD for at least three independent experiments done in triplicate. *P<0.05 compared to the control.
As RhoA/ROCK plays important roles for a variety of cellular processes that influence cell proliferation and cytoskeletal tension, we tested the hypothesis that SDF-1 binding to CXCR4 activates ROCK signaling to further inhibit chondrogenesis in rat embryo hind limb. We performed SDF-1 loss-of-function experiments using 25 μM AMD3100 in vitro. Western blotting showed a significant decrease in ROCK2 and increase in SOX9 expression in the ATRA+AMD3100-treated group compared with the ATRA-treated group (P<0.05) (Figure 5B). These results suggest that ROCK2 signaling was sufficiently blocked by AMD3100, and that ATRA enhanced the expression of ROCK2 through increasing the expression of SDF-1 and CXCR4. In total, the results demonstrate that increased CXCR4, induced by ATRA, inhibits chondrogenesis through a ROCK-dependent mechanism. ATRA suppresses chondrogenesis by activating an SDF-1/CXCR4/ROCK2 signaling pathway.
ATRA inhibits the expression of SOX9 and COL2A1 in vivo
We used a fetal rat clubfoot model, involving administration of a single intragastric dose of retinoic acid (120 mg/kg body weight) on day 10 of pregnancy, to confirm that abnormal expression of SOX9 and COL2A1 were involved in ATRA-induced chondrogenesis. Immunohistochemical analysis of E16.5 hind limb sections demonstrated decreased SOX9 expression in resting zone and proliferative zone in ATRA-treated embryos, compared with the control group (Figure 6A). In addition, immunohistochemical staining demonstrated decreased COL2A1 expression in proliferative zone and prehypertrophic zone in ATRA-treated embryos (Figure 6B). Together, these analyses indicate that the differentiation of chondrocyte progenitors and prehypertrophic chondrocytes are inhibited by ATRA treatment in vivo.
Figure 6.
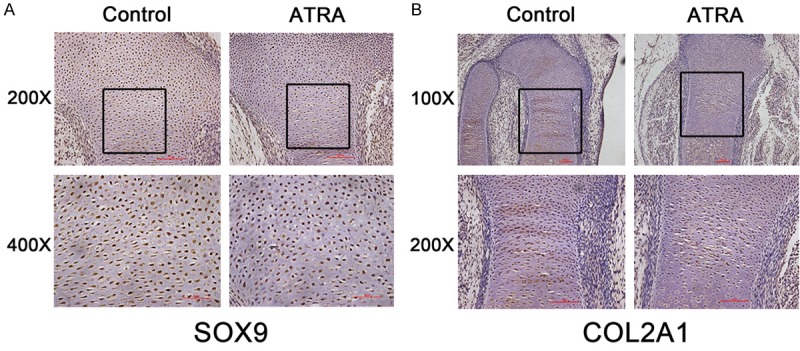
Expression pattern of SOX9 and COL2A1 proteins in E16.5 embryo limbs determined by immunohistochemistry. A: The expression of SOX9 in untreated and ATRA-treated E16.5 embryo limbs (200× and 400× objective). B: The expression of COL2A1 in untreated and ATRA-treated E16.5 embryo limbs (100× and 200× objective).
Discussion
RA is essential for embryonic development, but whose aberrant metabolism and signaling results in many congenital diseases. Defective chondrogenesis of primary hind limb bud mesenchymal cells may contribute to congenital clubfoot [4,5]. Our previous studies have demonstrated that a single intragastric dose of ATRA, to pregnant rats, results in hind limb congenital malformations, impaired chondrogenesis and increased cellular apoptosis, indicative of clubfoot. In this study, we demonstrate that ATRA dose-dependently inhibits the proliferation of rat embryo hind limb bud mesenchymal cells in vitro. ATRA also causes significant and dose-dependent reductions in the expression of SOX9 and COL2A1 after a 24-hour treatment. However, the underling cellular and molecular mechanisms are not well understood.
Using a qRT-PCR for genes that are involved in chondrogenesis, we found a positive correlation between ROCK2 and rat embryo hind limb chondrogenesis, and additionally demonstrated that ATRA significantly causes a dose-dependent increase of ROCK2. ATRA further decreased expression of chondrocyte-specific genes SOX9 and COL2A1. These results indicate that ATRA exerts its effects on early events in chondrogenesis.
Inhibition of the RhoA/ROCK2 pathway has been shown to promote chondrogenic differentiation via increased SOX9 expression, whereas overexpression of RhoA in the chondrogenic cell line ATDC5 has the opposite effect [22,23]. We similarly show that overexpression of ROCK2, induced by ATRA, leads to markedly decreased SOX9 mRNA transcript and protein levels, which is reversed by treatment with the ROCK inhibitor Y27632. This suggests that ATRA up-regulates the expression of ROCK2 to inhibit the hind limb chondrogenesis through suppressing expression of SOX9.
ATRA has been shown to induce the expression of SDF-1 during APL U937 cell differentiation and enhance the expression of CXCR4 on NB4 cells [19,20]. We extend these results to show that ATRA similarly increases the expression of SDF-1 and CXCR4 in rat hind limb bud mesenchymal cells.
CXCR4 has been demonstrated to have an inhibitory effect on cell proliferation on HSCs isolated from CXCR4-/- mice [24]. Interestingly, SDF-1 can induce matrix degradation and glycosaminoglycan release, a marker of early chondrogenic differentiation in cultures of human cartilage explants [25]. On the contrary, blocking the SDF-1/CXCR4 pathway has been shown to suppress BMP2-induced SOX9 and RUNX2 expression in ATDC5 and murine BMSCs, and consequently inhibit BMP2-induced chondrogenic differentiation [26]. To determine whether ATRA inhibits chondrogenesis through modulating SDF-1/CXCR4 expression, we performed SDF-1/CXCR4 signaling loss-of-function experiments using the CXCR4 inhibitor (AMD3100). In contrast to the above reports suggesting inhibition of the CXCR4 pathway inhibits chondrogenesis, our data demonstrate that CXCR4 inhibitor decrease the suppressing effect of SOX9 expression level by ATRA, which indicates that overexpression of SDF-1/CXCR4, stimulated by ATRA, suppresses chondrogenesis through downregulating SOX9 and COL2A1 in rat hind limb bud mesenchymal cells. In particular, SDF-1 and CXCR4 are highly expressed in ATRA-induced hind limb bud mesenchymal cells in our experiments.
Our contrasting results raise a question regarding whether SDF-1/CXCR4 increases or decreases SOX9, a key transcription factor required for chondrogenesis, and COL2A1, encoding α1 type II collagen. However, the previous studies and our current study were performed under different conditions that limit comparison. First, in our study the cells are isolated from the hind limb bud in embryos of 12.5 days pregnant female SD rats, whereas the cells were murine BMSCs and ATDC5 cells in the prior study, and were cultured under different conditions. Second, the dose and time of AMD3100 treatment are different. Third, different SDF-1/CXCR4 expression levels might lead to different effects on signaling pathways. Thus, the above factors could be responsible for the discrepancy. Furthermore, in OA and RA, the concentration of SDF-1 is sufficient to induce chondrocyte cell death in vitro through CXCR4 activation [27]. Indeed, infusion of SDF-1 in the proximal tibial physis of rabbits results in early growth plate closure in hypertrophic chondrocytes, which express the highest levels of CXCR4 in the cartilage [28,29]. We speculate that SDF-1/CXCR4 signaling functions within an expression window and both too high or too low levels of CXCR4 are detrimental for chondrogenesis during embryogenesis, similar to the requirement for ATRA. Moreover, our pharmacological studies show that SDF-1/CXCR4 overexpression causes enhanced expression of ROCK2. The reduction of the chondrogenic transcription factors SOX9 and COL2A1 induced by ATRA in this study are mediated by SDF-1/CXCR4/ROCK, because the ROCK inhibitor Y27632 reverses the effects of SOX9 and COL2A1 reduction in cells. However, further in vivo studies are needed to confirm and quantify the role of SDF-1 in chondrogenesis.
In conclusion, we have identified the SDF-1/CXCR4/ROCK2 pathway is an important negative regulator of early chondrogenic differentiation that controls cell morphology and chondrogenic gene expression. We find that ATRA suppresses chondrogenesis of rat hind limb bud mesenchymal cells by activating the SDF-1/CXCR4/ROCK2 signaling pathway. Inhibiting the ATRA-mediated overexpression of both CXCR4 and ROCK2 increase SOX9 and COL2A1 expression in rat hind limb bud mesenchymal cells. These results suggest the SDF-1/CXCR4/ROCK2 signaling pathway is important for chondrogenesis and the disruption of chondrogenesis program contributes to a significant proportion of ATRA-induced congenital clubfoot.
Acknowledgements
The study is supported by National Natural Science Foundation of China (81341103, 81271947), Administration of Traditional Chinese Medicine of Guangdong Province, China (20131248, 20142084), The Department of Education, Guangdong Government, under the Top-Tier University Development Scheme for Research and Control of Infectious Diseases (2015092, 2016011), and Natural Science Foundation of Guangdong Province, China (2014A030313467). All the research work was completed at the Laboratory of Molecular Cardiology, First Affiliated Hospital of Shantou University Medical College.
Disclosure of conflict of interest
None.
References
- 1.Dobbs MB, Gurnett CA. Update on clubfoot: etiology and treatment. Clin Orthop Relat Res. 2009;467:1146–1153. doi: 10.1007/s11999-009-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobbs MB, Gurnett CA. Genetics of clubfoot. J Pediatr Orthop B. 2012;21:7–9. doi: 10.1097/BPB.0b013e328349927c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miedzybrodzka Z. Congenital talipes equinovarus (clubfoot): a disorder of the foot but not the hand. J Anat. 2003;202:37–42. doi: 10.1046/j.1469-7580.2003.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong YS, Zheng C, Jia Y, Xu GT, Liu ZY, Chen B, Du SX. Quantitative evaluation in vivo of the degree of differentiation of hindlimb cartilage in a rat clubfoot model. Toxicol Mech Methods. 2009;19:292–297. doi: 10.1080/15376510802609737. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZY, Li XD, Chen B, Zheng CY, Zhong YS, Jia YL, Du SX. Retinoic acid retards fetal and hindlimb skeletal development asymmetrically in a retinoic acid-induced clubfoot model. Exp Toxicol Pathol. 2010;62:663–670. doi: 10.1016/j.etp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–348. doi: 10.1038/nature01659. [DOI] [PubMed] [Google Scholar]
- 7.Tickle C. Patterning systems--from one end of the limb to the other. Dev Cell. 2003;4:449–458. doi: 10.1016/s1534-5807(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 8.Fritsch H, Eggers R. Ossification of the calcaneus in the normal fetal foot and in clubfoot. J Pediatr Orthop. 1999;19:22–26. [PubMed] [Google Scholar]
- 9.Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- 10.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 11.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428. doi: 10.3390/nu3040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado-Baeza E, Santos-Alvarez I, Martos-Rodriguez A. Retinoic acid-induced clubfoot-like deformity: pathoanatomy in rat fetuses. J Pediatr Orthop B. 1999;8:12–18. [PubMed] [Google Scholar]
- 13.Sarasa-Renedo A, Tunc-Civelek V, Chiquet M. Role of RhoA/ROCK-dependent actin contractility in the induction of tenascin-C by cyclic tensile strain. Exp Cell Res. 2006;312:1361–1370. doi: 10.1016/j.yexcr.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 15.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 16.Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, Margalit R, Zsak M, Nagler A, Hardan I, Resnick I, Rot A, Lapidot T. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–1046. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 17.Choi DS, Stark DJ, Raphael RM, Wen J, Su J, Zhou X, Chang CC, Zu Y. SDF-1a stiffens myeloma bone marrow mesenchymal stromal cells through the activation of RhoA-ROCK-Myosin II. Int J Cancer. 2015;136:E219–229. doi: 10.1002/ijc.29145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murata K, Kitaori T, Oishi S, Watanabe N, Yoshitomi H, Tanida S, Ishikawa M, Kasahara T, Shibuya H, Fujii N, Nagasawa T, Nakamura T, Ito H. Stromal cell-derived factor 1 regulates the actin organization of chondrocytes and chondrocyte hypertrophy. PLoS One. 2012;7:e37163. doi: 10.1371/journal.pone.0037163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KJ, Kim HH, Kim JH, Choi YH, Kim YH, Cheong JH. Chemokine stromal cell-derived factor-1 induction by C/EBP activation is associated with all-trans-retinoic acid-induced leukemic cell differentiation. J Leukoc Biol. 2007;82:1332–1339. doi: 10.1189/jlb.1106697. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Hu L, Cui Z, Jiang X, Wang G, Krissansen GW, Sun X. Interaction of SDF-1a and CXCR4 plays an important role in pulmonary cellular infiltration in differentiation syndrome. Int J Hematol. 2010;91:293–302. doi: 10.1007/s12185-009-0488-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang TG, Li XD, Yu GY, Xie P, Wang YG, Liu ZY, Hong Q, Liu DZ, Du SX. All-trans-retinoic acid inhibits chondrogenesis of rat embryo hindlimb bud mesenchymal cells by downregulating p53 expression. Mol Med Rep. 2015;12:210–218. doi: 10.3892/mmr.2015.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281:13134–13140. doi: 10.1074/jbc.M509433200. [DOI] [PubMed] [Google Scholar]
- 23.Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–11634. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- 24.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei F, Moore DC, Wei L, Li Y, Zhang G, Wei X, Lee JK, Chen Q. Attenuation of osteoarthritis via blockade of the SDF-1/CXCR4 signaling pathway. Arthritis Res Ther. 2012;14:R177. doi: 10.1186/ar3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guang LG, Boskey AL, Zhu W. Regulatory role of stromal cell-derived factor-1 in bone morphogenetic protein-2-induced chondrogenic differentiation in vitro. Int J Biochem Cell Biol. 2012;44:1825–1833. doi: 10.1016/j.biocel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Wei L, Sun X, Kanbe K, Wang Z, Sun C, Terek R, Chen Q. Chondrocyte death induced by pathological concentration of chemokine stromal cell-derived factor-1. J Rheumatol. 2006;33:1818–1826. [PubMed] [Google Scholar]
- 28.Wei L, Kanbe K, Lee M, Wei X, Pei M, Sun X, Terek R, Chen Q. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol. 2010;341:236–245. doi: 10.1016/j.ydbio.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanbe K, Takemura T, Takeuchi K, Chen Q, Takagishi K, Inoue K. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br. 2004;86:296–300. doi: 10.1302/0301-620x.86b2.14474. [DOI] [PubMed] [Google Scholar]


