Abstract
Previously, we found that cancerous inhibitor of protein phosphatase 2A (CIP2A) plays a key role in the malignant transformation of cervical cancer. Here, we further explore whether and how CIP2A is regulated by human papillomavirus E7 (HPV E7) and the prognostic value of CIP2A in cervical cancer. We demonstrated a positive feedback loop between the E2F transcription factor 1 (E2F1) and CIP2A at the transcription level in HeLa and SiHa cells by real-time PCR and western blot analysis. The feedback, regulated by HPV E7, was further confirmed by their sub-cellular co-expression seen on immunofluorescence and immunohistochemistry staining in vitro and in vivo. Moreover, CIP2A and E2F1 expression was greatly elevated in human cervical cancer tissue. CIP2A expression was tightly associated with tumor size, depth of invasion and lymph node metastasis in 184 cases of cervical cancer. Kaplan-Meier and Cox proportional-hazards regression analyses revealed poor overall and disease-free survival of patients with CIP2A-E2F1 co-expression, and high CIP2A-E2F1 co-expression was an independent risk factor for overall survival of patients. Therefore, CIP2A-E2F1 expression might be a valuable indicator to predict outcome and guide personal treatment in cervical cancer.
Keywords: CIP2A, E2F1, HPV E7, cervical cancer, prognosis
Introduction
Cervical cancer is one of the most common cancers in women worldwide. Approximately 500,000 new cases are diagnosed each year, and more than one-third of these cases result in death [1]. From the observation of large cohorts of patients, a series of factors have been found associated with the prognosis of cervical cancer, such as FIGO staging (the International Federation of Gynecology and Obstetrics Staging), histological type, depth of tumor invasion, and lymph node metastasis [2-5]. However, the results are not consistent and even controversial. Hence, current predictive factors are not sufficient to predict and stratify the risk for patients with cervical cancer. Precise indicators are still needed to facilitate the assessment of prognosis and treatment.
Human papillomavirus (HPV) infection is well known as the causative agent of cervical cancer [6]. HPV oncoproteins (E6 and E7) are crucial for the cancer development and progression. HPV E6 can deregulate host genes by inactivating the tumor suppressor p53. HPV E7 can destabilize the tumor suppressor RB1, to release the E2F family, leading to the deregulation of cell cycle progression [6]. Although researchers worldwide have invested much effort to untangle the role of E6 and E7 in the last 2 decades, the molecular alteration of host cells after HPV infection and the cellular targets of HPV E6/E7 are not fully understood. We need to address the detailed molecular mechanism of HPV oncoproteins in the carcinogenesis of cervical cancer and discover novel molecular surrogate markers for diagnosis and prognosis prediction of cervical cancer [6].
Cancerous inhibitor of PP2A (CIP2A) is a newly identified oncoprotein with many roles [7]. It promotes c-Myc protein stability by its ability to inhibit PP2A activity directed toward c-Myc at Ser 62 [7] and thereby promotes the carcinogenesis of many solid cancers. It participates in the malignant transformation of liver cancer [8], breast cancer [9], gastric cancer [10], leukemia [11], and ovarian cancer [12]. In 2007 and 2011, we found that CIP2A plays a key role in the malignant transformation of gastric cancer [10] and cervical cancer in vitro and in vivo [13]. However, the role of CIP2A expression in the prognosis of cervical cancer remains unclear.
In the present study, we explored whether CIP2A is regulated by HPV E7 and whether it has prognostic value in cervical cancer. We discovered that high CIP2A-E2F1 expression is an independent risk factor for the overall survival of patients. The synergistic effect of E2F1 and CIP2A on the prognosis prediction might be due to a positive feedback loop regulated by HPV E7 at the transcription level.
Materials and methods
Ethics statement
The study was approved by the ethics committee of Medical School of Shandong University (ethic vote 201301050). Written informed consent was obtained from all patients for use of materials.
Patient population
We collected cervical cancer tissue from patients who underwent radical surgery and systematic pelvic lymphadenectomy because of cervical cancer and normal cervical tissue from patients who underwent total hysterectomy because of uterine fibroids at Qilu Hospital of Shandong University between July 2005 and July 2012. Patient information was retrieved from patient medical records at Qilu Hospital. Hematoxylin and eosin (HE)-stained sections and paraffin-embedded tissue sections were obtained from the Department of Pathology, Qilu Hospital. HE sections were reviewed by a gynecologic pathologist. Diagnoses were according to the World Health Organization Classification of Tumors.
Follow-up
Patients were followed up until death or December 2015. Survival data were recorded including survival time, disease-free interval and development of metastasis. The overall survival (OS) time was defined as the interval between the date of operation and death from any cause. The disease-free survival (DFS) time was calculated from the date of the surgery to the date of the recurrence or death resulting from any cause. Patients alive were censored.
Cell lines and culture
The HeLa cell line (from cervical adenocarcinoma) and SiHa cell line (from cervical squamous cell carcinoma) were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured as routine in DMEM or 1640 medium with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C in a humidified atmosphere with 5% CO2.
Small-interfering RNA (siRNA) and plasmid transfection
HeLa and SiHa cells were seeded in 6-well culture plates at 2×105 cells per well and cultured in medium without antibiotics for 24 h before transfection. The sequences of double-stranded siRNA oligonucleotide were as follows: CIP2A: 5’-CUGUGGUUGUGUUUGCACUTT-3’; E2F1: 5’-GUCACGCUAUGAGACCUCATT-3’; HPV16 E7: 5’-AGGAGGAUGAAAUAGAUGGTT-3’; HPV18 E7: 5’-CUAGCACGAGCAAUUAAGCGA-3’; and negative control, 5’-UUCUCCGAAC-GUGUCACGUTT-3’ (GenePharma, Shanghai, China). The CIP2A expression plasmid (pBabe-CIP2A) and pBabe vector were a gift from Professor Jukka Westermarck (Institute of Medical Technology, University of Tampere). The E2F1 expression plasmid (pCMV-E2F1) and pCMV vector were bought from Addgene Company (Cambridge, MA, USA). Lipofectamine 2000 (Invitrogen, Calsbad, USA) was used to transfect the siRNA or plasmids into cells. After 72 h, cells were collected for mRNA and protein analysis.
RNA extraction and real time PCR
Total RNA was extracted from cell lines by using Trizol (Ambion, Calsbad, USA) and synthesized into cDNA with the RevertAid First Strand cDNA Synthesis kit (Toyobo, Osaka, Japan). The primer sequences were in Table 1. GAPDH was an internal control. Real-time PCR involved the ABI Prism 7000 Sequence Detection System with SYBR Premix Ex Taq (Toyobo, Osaka, Japan).
Table 1.
Primer sequences for real-time PCR in cervical cancer cells
| Gene name | The primer sequences |
|---|---|
| CIP2A | Forward: 5’-CCATATGCTCACTCAGATGATGT-3’ |
| Reverse: 5’-GTGTATCATCTCCACAGAGAGTT-3’ | |
| E2F1 | Forward: 5’-CATCCAGCTCATTGCCAAGAAG-3’ |
| Reverse: 5’-GATCCCACCTACGGTCTCCTCA-3’ | |
| HPV18 E7 | Forward: 5’-TGTATTGCATTTAGAGCCCCAAA-3’ |
| Reverse: 5’-CTTCCTCTGAGTCGCTTAATTGC-3’ | |
| HPV 16 E7 | Forward: 5’-AGTGTGACTCTACGCTTCGGTTG-3’ |
| Reverse: 5’-CTGAGAACAGATGGGGCACAC-3’ | |
| GAPDH | Forward: 5’-AGAAGGCTGGGGCTCATTTG-3’ |
| Reverse: 5’-AGGGGCCATCCACAGTCTTC-3’ |
Western blot analysis
Total protein was extracted from cells by using Cel LyticTM MT Cell Lysis Reagent (Sigma, St. Louis, USA) and qualified by using the BCA reagent kit (Beyotime, Shanghai, China). Western blot analysis was performed as described [13]. Briefly, membranes were incubated with monoclonal antibodies for CIP2A (1:2000, Novus, Saint Charles, USA), E2F1 (1:1000, Santa Cruz, CA, USA) or GAPDH (1:1000, Santa Cruz, CA, USA) separately. GAPDH was used as the loading control. The protein bands were visualized by use of an ECL kit (Beyotime, Shanghai, China) and data were analyzed by using Quantity One v4.62 software.
Immunofluorescence staining
Cells were fixed and permeabilized with 85% ethanol, blocked with 5% skim-milk phosphate buffered saline (PBS), and then probed with a monoclonal antibody for CIP2A (1:40, Novus, Saint Charles, USA) and FITC-conjugated goat anti-mouse secondary antibody (Zhongshan, Beijing, China). The labelled cells were further blocked with 5% skim-milk PBS, and then probed with a polyclonal antibody for E2F1 (1:200, Abcam, Cambridge, USA) and rhodamine123-conjugated goat anti-rabbit secondary antibody (Zhongshan, Beijing, China). Slides were counterstained with 4’,6’-diamidino-2-phenylindole (DAPI) and observed by confocal laser immunofluorescence microscopy (LSM780, ZEISS, Germany).
Immunohistochemistry staining
Immunohistochemistry staining was performed as described [13]. Sections of paraffin-embedded specimens were baked at 60°C for 60 min, deparaffinized with xylene and rehydrated with gradient ethanol. Then slides were incubated and heated in 0.01 M citric buffer (pH 6.0) for antigenic retrieval and cooled to room temperature. After quenching endogenous peroxidase activity with 3% H2O2, the slides were incubated with antibodies for CIP2A (1:150, Novus, Saint Charles, USA) or E2F1 (1:100, Santa Cruz, CA, USA) overnight at 4°C. On the second day, slides were incubated with Reagent 1 (containing anti-mouse secondary antibody) and Reagent 2 (containing streptavidin-horseradish peroxidase complex) (PV-9000 kit, Zhongshan, Beijing, China). Finally, slides were stained with diaminobenzidine and counterstained with hematoxylin. PBS was used as the negative control.
All stained slides were scored by 2 independent blinded investigators. Tumor cell proportion was scored as 0, no positive tumor cells; 1, ≤25% positivity; 2, 25-50% positivity; 3, 51-75% positivity; and 4, >75% positivity. Staining intensity was graded as 0, none; 1, weak; 2, moderate; and 3, strong. The staining index (SI) score was used to define the result by the product of staining intensity score and the percentage of positive tumor cells. The staining results were finally recorded as 0, negative (-); ≤4, low expression (+); 5-8, moderate expression (++); and ≥9, high expression (+++). Tumor samples scored (+) to (+++) were considered positive. If the staining interpretation differed between the 2 investigators, the data for the slide were discarded.
Statistical analysis
Statistical analysis involved use of SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism5 (GraphPad Software Inc., San Diego, CA, USA). Data were presented as mean ± SEM. E2F1 and CIP2A protein expression in cervical tissue was analyzed by Fisher’s exact test or chi-square test. The correlation between CIP2A and E2F1 protein levels was analyzed by Spearman correlation analysis. DFS and OS were analyzed by the Kaplan-Meier method and compared by log-rank test. Cox proportional-hazards regression was used for univariate and multivariate analyses with stepwise variable selection, estimating hazard ratios (HRs) and 95% confidence intervals (95% CIs). P<0.05 was considered statistically significant.
Results
HPV E7 upregulated CIP2A transcription and translation in cancer cells
E7 is the key oncoprotein of HPV virus responsible for the transformation of the host cells. Previously, we reported the tight association of HPV E7 and CIP2A protein in cervical cancer tissue [13]. Therefore, we hypothesized that HPV E7 might regulate the expression of CIP2A in cervical cancer cells. In agreement, when we transfected HeLa and SiHa cells with siRNA targeting the E7 gene, the E7 mRNA level was decreased greatly (Figure 1A, 1B), and the mRNA and protein levels of CIP2A were reduced significantly (P<0.05) (Figure 1A, 1B). This result was consistent with our previous observation that the overexpression of HPV 16E7 and 58E7 could upregulate CIP2A expression at the transcription level [14]. Thus, HPV E7 could upregulate CIP2A gene transcription, and the regulatory role of E7 on CIP2A was not limited to one type of HPV E7.
Figure 1.
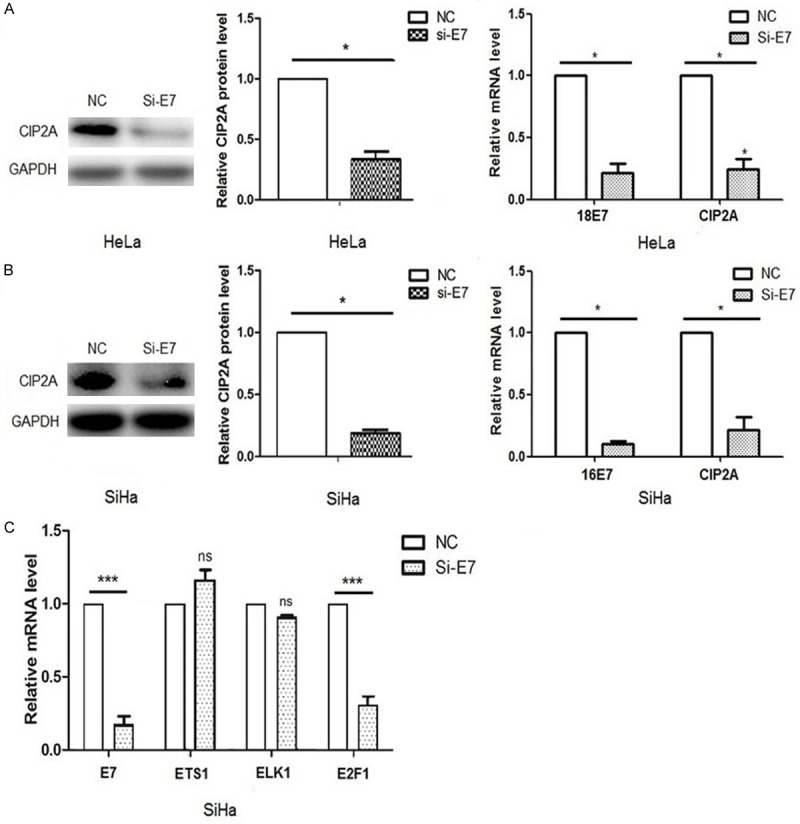
Effect of HPV E7 on the gene expression of CIP2A. A, B. Quantified real-time PCR and western blot analysis of CIP2A mRNA and protein levels after siRNA silencing of HPV E7 in HeLa and SiHa cells. C. Quantified real-time PCR analysis of mRNA levels of ETS1, ELK1 and E2F1 with siRNA silencing of HPV E7 in SiHa cells. Cells were transfected with siRNA targeting E7 (siE7) or negative control siRNA (NC). Data are mean SEM of triplicate experiments (*P<0.05, ***P<0.001).
HPV E7 upregulated CIP2A expression via the transcription factor E2F1 in cancer cells
Previous researchers have screened several transcription factors, such as ETS1 [15,16], ELK1 [15] and E2F1 [17], that bind to the promoter region of CIP2A gene. To determine which transcription factor was responsible for the regulatory role of E7 on CIP2A gene expression, we transfected HPV 16E7 siRNA into SiHa cells, and examined the mRNA levels of ETS1, ELK1 and E2F1. ETS1 and ELK1 mRNA levels showed no changes with siRNA transfection (P>0.05), but E2F1 mRNA level was decreased significantly (P<0.001) (Figure 1C). The result indicated that HPV E7 might regulate CIP2A expression via E2F1.
To prove this possibility, we first transfected HPV 18E7 siRNA into HeLa cells and examined the mRNA and protein expression of E2F1 and CIP2A. As shown in Figure 2A, the mRNA and protein levels of E2F1 and CIP2A were reduced simultaneously, along with decreased E7 mRNA level (P<0.05). Moreover, with siRNA knockdown of E2F1 mRNA expression, the mRNA level of CIP2A was decreased (P<0.05) and with overexpression of E2F1, the transcription of CIP2A was increased (P<0.05) (Figure 2B). The result was consistent with the changes in protein levels in our previous report [18]. Similar results were observed in SiHa cells when transfected with HPV 16E7 siRNA, E2F1 siRNA and pCMV-E2F1 plasmid separately (Figure 2C, 2D). Therefore, E2F1 is an essential transcription factor that promotes the transcription of CIP2A, and HPV E7 can upregulate CIP2A mRNA expression via E2F1.
Figure 2.
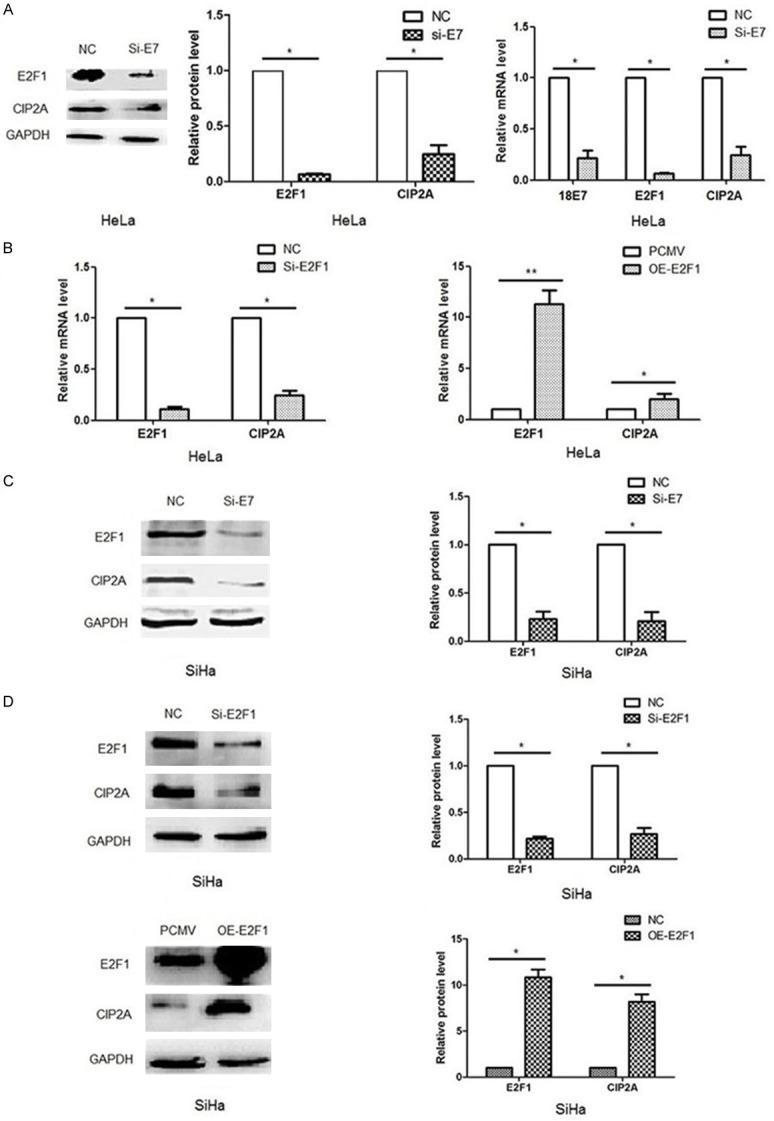
HPV E7 upregulated CIP2A expression via the transcription factor E2F1. A, C. Quantified real-time PCR and western blot analysis of E2F1 and CIP2A mRNA and protein levels after siRNA silencing of HPV E7 in HeLa and SiHa cells. B, D. Quantified real-time PCR and western blot analysis of CIP2A mRNA or/and protein levels after siRNA silencing or plasmid overexpression of E2F1 in HeLa and SiHa cells. For gene silencing, cells were transfected with siRNA targeting E7 (siE7), siRNA targeting E2F1 (siE2F1) or negative control siRNA (NC). For gene overexpression, cells were transfected with pCMV-E2F1 plasmid (OE-E2F1) or pCMV vector (PCMV). Data are mean SEM of triplicate experiments (*P<0.05, **P<0.01).
CIP2A upregulated E2F1 gene expression at the transcription level in cancer cells
To clarify whether a feedback exists between E2F1 and CIP2A, we knocked down CIP2A expression in HeLa cells and examined changes in E2F1 mRNA and protein expression. E2F1 mRNA and protein levels were significantly decreased with CIP2A knockdown and greatly increased with its overexpression (P<0.05; Figure 3A). Similar results were observed in SiHa cells with transfection of CIP2A siRNA and pBabe-CIP2A plasmid (Figure 3B). So we confirmed that CIP2A upregulated E2F1 mRNA expression at the transcription level. Considering the regulatory role of E2F1 on CIP2A gene expression, we demonstrated a positive feedback loop between E2F1 and CIP2A in cervical cancer cells, and the crosstalk between the two genes was at the transcription level.
Figure 3.
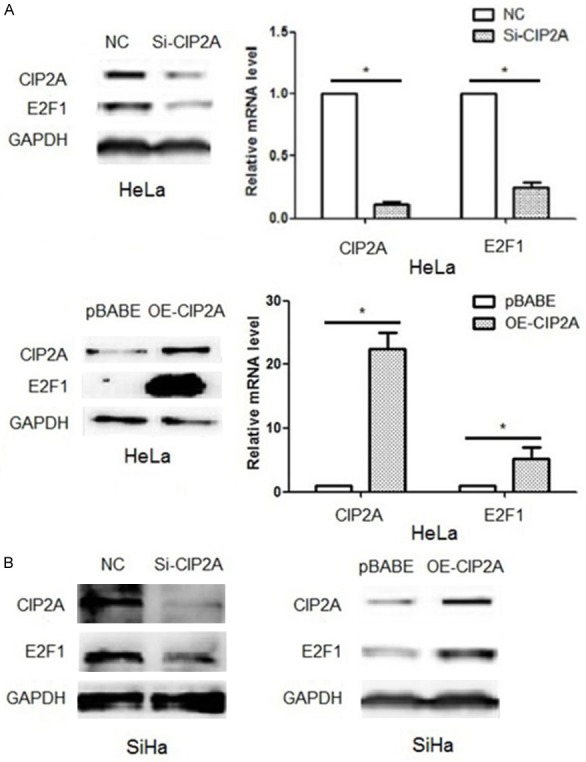
CIP2A upregulated E2F1 gene expression at the transcription level. A. Silencing or over-expression of CIP2A could decrease or increase transcription and thus translation of E2F1 in HeLa cells. B. Silencing or over-expression of CIP2A could decrease or increase the translation of E2F1 in SiHa cells. For gene silencing, cells were transfected with siRNA targeting CIP2A (siCIP2A) or negative control siRNA (NC). For gene overexpression, cells were transfected with pBabe-CIP2A plasmid (OE-CIP2A) or pBabe vector (pBabe). Data are mean SEM of triplicate experiments (*P<0.05).
Sub-cellular co-expression of E2F1 and CIP2A in cancer cells
Considering the tight association between E2F1 and CIP2A, we deduced that the two proteins might have sub-cellular co-expression in cervical cancer cells. We examined the expression and location of E2F1 and CIP2A in HeLa and SiHa cells by immunofluorescence staining. E2F1 protein mainly located in the nucleus and CIP2A protein mainly located in the cytoplasm (Figure 4A). E2F1 and CIP2A showed significant positive co-immunoreactivity. Hence, we confirmed the sub-cellular co-expression of E2F1 and CIP2A in cervical cancer cells and furthermore, the crosstalk between the two proteins.
Figure 4.
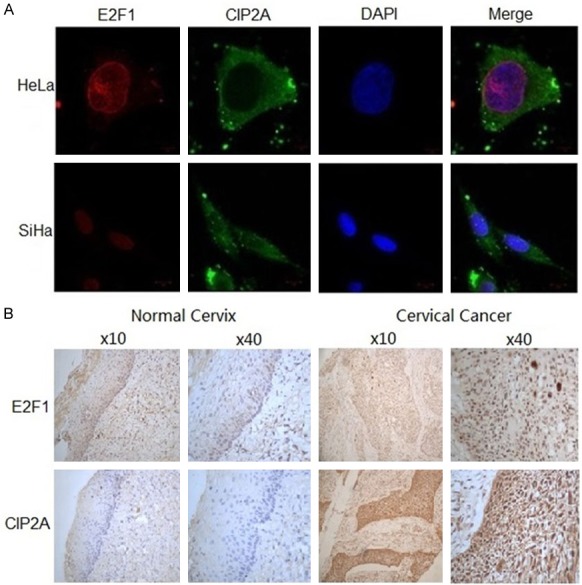
Sub-cellular co-expression of E2F1 and CIP2A in vivo. A. Representative immunofluorescence staining of CIP2A (green) and E2F1 (red) protein in HeLa and SiHa cells by confocal laser scanning microscopy. The nucleus was stained blue by DAPI. B. Immunohistochemistry staining of E2F1 and CIP2A protein in paraffin-embedded cervical tissue. Positive staining for E2F1 was defined as brown stain in the nucleus and for CIP2A in the cytoplasm.
Expression of E2F1 and CIP2A in cervical cancer tissue
To confirm our in vitro observations, we further examined the protein signals of E2F1 and CIP2A in normal cervix (n=56 samples) and cervical cancer tissue (n=184 samples). We found no signal in the 56 normal cervix tissue samples, which indicates low expression of E2F1 and CIP2A in the normal cervical epithelium (Figure 4B). In contrast, we detected cytoplasmic CIP2A protein expression in 38.04% (70/184) of cervical cancer tissue and nuclear E2F1 expression in 37.50% (69/184). So both E2F1 and CIP2A expression was significantly increased in cervical cancer tissue, which suggests their possible role in the carcinogenesis and transformation of cervical cancer. Moreover, E2F1 and CIP2A protein exhibited a uniform location and immunointensity in the same specimens. E2F1 and CIP2A protein levels were positively correlated in cervical cancer tissue (r=0.239, P<0.01).
Association of CIP2A and E2F1 expression with clinicopathological features of cervical cancer patients
Tumor size, lymph node metastasis and invasion depth of cervical cancer patients were significantly associated with CIP2A expression (P<0.05; Table 2), but no factor was associated with E2F1 protein level (P>0.05). The detailed information for patients and disease features were in Table 2.
Table 2.
Association of clinicopathological parameters with CIP2A and E2F1 expression
| CIP2A | E2F1 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| (-) | (+) | (-) | (+) | |||
| Age (years) | P=0.188 | P=0.239 | ||||
| ≤40 | 52 | 10 | 53 | 9 | ||
| >40 | 94 | 28 | 110 | 12 | ||
| Tumor size, cm | P<0.001* | P=0.388 | ||||
| <4 | 107 | 16 | 110 | 13 | ||
| ≥4 | 39 | 22 | 53 | 8 | ||
| LN metastasis | P=0.003* | P=0.573 | ||||
| No | 119 | 22 | 125 | 16 | ||
| Yes | 27 | 16 | 38 | 5 | ||
| Differentiation grade | P=0.267 | P=0.303 | ||||
| Well to moderate | 70 | 21 | 79 | 12 | ||
| Poor | 76 | 17 | 84 | 9 | ||
| FIGO staging (range) | P=0.270 | P=0.342 | ||||
| I | 127 | 31 | 141 | 17 | ||
| II to IV | 19 | 7 | 22 | 4 | ||
| Distant metastasis | P=0.571 | P=0.570 | ||||
| No | 133 | 35 | 149 | 19 | ||
| Yes | 13 | 3 | 14 | 2 | ||
| Depth of invasion | P=0.005* | P=0.402 | ||||
| <1/2 | 41 | 3 | 40 | 4 | ||
| ≥ 1/2 | 105 | 35 | 123 | 17 | ||
| Histotype | P=0.583 | P=0.162 | ||||
| SCC | 135 | 35 | 149 | 21 | ||
| AC | 11 | 3 | 14 | 0 | ||
Note: LN, lymph node; FIGO staging, the International Federation of Gynecology and Obstetrics Staging; SCC, squamous cell carcinoma; AC, adenocarcinoma.
Indicates statistical significance.
Prognostic role of CIP2A and E2F1 in OS and DFS of patients with cervical cancer
To investigate the role of CIP2A and E2F1 in the prognosis of cervical cancer, we followed up 184 patients; 127 were followed up successfully until December 2015. OS was lower for CIP2A-positive than CIP2A-negative patients (57.1% vs 92.5%), and the mean OS time was shorter [50.4 months (95% CI 39.2-61.5) vs 83.4 months (79.1-87.8)] (P<0.001) (Figure 5A). OS was lower for E2F1-positive than E2F1-negative patients (75.0% vs 88.3%), and the mean OS time was shorter [56.3 months (43.9-68.8) vs 80.0 months (75.0-85.0)] but not significantly (P>0.05) (Figure 5A).
Figure 5.
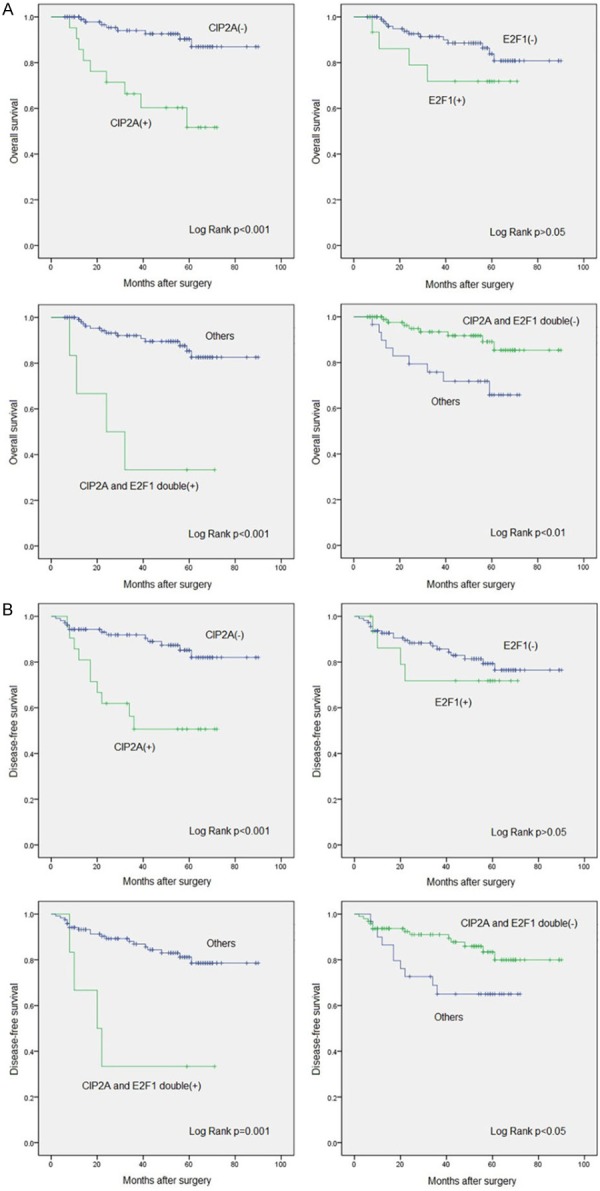
Kaplan-Meier curves for survival of 127 patients with cervical carcinoma by CIP2A and E2F1 positivity and negativity. Culminative overall survival (A) and disease-free survival (B).
Co-expression of CIP2A and E2F1 conferred strong prognostic value. DFS was lower for patients with than without CIP2A-E2F1 double-positive co-expression (33.3% vs 89.3%), and the mean OS time was shorter [36.2 months (95% CI 15.5-56.9) vs 80.9 months (76.3-85.5)] (P<0.001). Consistently, the mean OS time was longer for patients with than without CIP2A-E2F1 double-negative co-expression [82.7 months (77.9-87.5) vs 56.6 months (47.9-65.3)] (P=0.009) (Figure 5A).
Similar results were obtained when we analyzed the role of CIP2A and E2F1 in DFS. DFS was lower for CIP2A-positive than CIP2A-negative patients (52.4% vs 87.7%), and the mean DFS time was shorter [45.8 months (95% CI 33.9-57.7) vs 79.8 months (74.7-85.0)] (P<0.001) (Figure 5B). Moreover, DFS was lower for E2F1-positive than E2F1-negative patients (75.0% vs 82.9%), and the mean DFS time was shorter [55.2 months (42.1-68.4) vs 75.9 months (70.2-81.6)] but not significantly (P>0.05) (Figure 5B).
When analyzing the co-expression of CIP2A and E2F1, DFS was lower for patients with than without CIP2A-E2F1 double-positive co-expression (33.3% vs 84.3%), and the mean DFS time was shorter [33.7 months (95% CI 12.2-55.2) vs 77.1 months (71.9-82.4)] (P=0.001) (Figure 5B). The mean DFS time was longer with than without CIP2A-E2F1 double-negative co-expression [78.7 months (73.1-84.4) vs 53.4 months (43.9-62.9)] (P=0.032) (Figure 5B).
Thus, CIP2A-positive expression or CIP2A-E2F1 double-positive co-expression in cervical cancer tissue might indicate poor prognosis, and CIP2A-E2F1 double-negative co-expression might indicate better outcome.
Factors associated with survival of patients with cervical cancer
To investigate whether CIP2A and/or E2F1 could be an independent prognostic factor with cervical cancer, we used Cox univariate and multivariate models. On univariate analysis, CIP2A-positive expression, lymph node metastasis, tumor size, CIP2A-E2F1 double-positive and double-negative co-expression were associated with survival (Table 3). On multivariate analysis, CIP2A-E2F1 double-positive co-expression was an independent factor associated with poor overall survival of patients with cervical cancer (HR: 5.28, 95% CI 1.25-22.35, P=0.024), together with tumor size (HR: 4.37, 1.39-13.73, P=0.012) and lymph node metastasis (HR: 3.87, 1.36-11.03, P=0.011) (Table 4).
Table 3.
Cox univariate analysis of survival in 127 patients with cervical cancer
| Overall survival | Disease-free survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| CIP2A | ||||||
| (-) vs (+) | 5.75 | 2.22-14.91 | <0.001 | 4.24 | 1.85-9.68 | 0.001 |
| E2F1 | ||||||
| (-) vs (+) | 2.11 | 0.69-6.50 | 0.192 | 1.44 | 0.49-4.23 | 0.509 |
| Tumor size | ||||||
| <4 vs ≥4 cm | 6.94 | 2.26-21.31 | <0.001 | 4.94 | 2.03-12.02 | <0.001 |
| LN metastasis | ||||||
| No vs Yes | 5.09 | 1.93-13.38 | 0.001 | 4.06 | 1.79-9.21 | 0.001 |
| Differentiation grade | ||||||
| Well-moderate vs poor | 2.14 | 0.79-5.80 | 0.134 | 1.77 | 0.77-4.10 | 0.181 |
| Age, years | ||||||
| ≤ 40 vs >40 | 1.18 | 0.42-3.36 | 0.752 | 1.78 | 0.66-4.80 | 0.253 |
| FIGO stage | ||||||
| I vs (II to IV) | 1.45 | 0.41-5.10 | 0.560 | 1.70 | 0.63-4.61 | 0.294 |
| Distant metastasis | ||||||
| No vs Yes | 2.24 | 0.64-7.84 | 0.208 | 2.08 | 0.70-6.13 | 0.185 |
| Depth of invasion | ||||||
| <1/2 vs ≥1/2 | 2.55 | 0.58-11.16 | 0.214 | 1.62 | 0.55-4.77 | 0.380 |
| Histotype | ||||||
| SCC vs AC | 0.80 | 0.11-6.07 | 0.831 | 1.23 | 0.29-5.27 | 0.778 |
| CIP2A and E2F1 | ||||||
| Co-expression(+) vs others | 7.76 | 2.52-23.87 | 0.003 | 5.24 | 1.77-15.48 | 0.003 |
| CIP2A and E2F1 | ||||||
| Co-expression(-) vs others | 0.30 | 0.12-0.78 | 0.013 | 0.42 | 0.18-0.95 | 0.038 |
HR, hazard ratio; 95% CI, 95% confidence interval.
Table 4.
Cox multivariate analysis of survival in 127 patients with cervical cancer
| Overall survival | Disease-free survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | (95% CI) | P value | HR | (95% CI) | P value | |
| Tumor size ≥4 cm | 4.37 | 1.39-13.73 | 0.012 | 3.02 | 1.22-7.48 | 0.017 |
| LN metastasis | 3.87 | 1.36-11.03 | 0.011 | 2.92 | 1.23-6.91 | 0.015 |
| CIP2A and E2F1 co-expression (+) | 5.28 | 1.25-22.35 | 0.024 | 3.06 | 0.81-11.51 | 0.099 |
Discussion
CIP2A is a key oncoprotein discovered in 2007 [7]. However, previous research has mainly focused on the downstream pathways of CIP2A when exploring its carcinogenic capability [19], with little known about the upstream regulatory pathways [19]. Previously, we found that the CagA oncoprotein of Helicobacter pylori could increase CIP2A expression in gastric cancer cell lines [20] suggesting that CIP2A can be regulated by an oncoprotein synthesized by certain kinds of microbes. In agreement, we demonstrated that HPV oncoprotein E7 could upregulate CIP2A mRNA expression via the transcription factor E2F1 in cervical cancer cells. The result sheds new light on the molecular mechanism of HPV E7 and CIP2A in the carcinogenesis of cervical cancer.
Previous reports have showed that HPV E7 can degrade retinoblastoma protein (pRb), destroy the pRb-E2F complex, and release abundant E2Fs, which promote malignant transformation of cervical epithelium [21-24]. However, theoretically, E7 can only rescue E2F1 protein from the pRb-E2F1 complex and will not change the transcription level of E2F1 [21-24]. Our data showed that E7 could activate the transcription of E2F1, increase its protein level and initiate the expression of CIP2A. Considering that E7 is not a transcription factor, E7 might regulate E2F1 transcription via other unknown “bridge” transcription factors. However, the underlying mechanism is still needs to be clarified.
Here we demonstrate a crosstalk between E2F1 and CIP2A in cervical cancer cells. During our research, similar results were reported [17]. The authors found a feedback mechanism between E2F1 and CIP2A in breast cancer cells that affected cell senescence [17]. However, we found that CIP2A had no effect on cervical cancer cell aging (data not shown) but played a key role in cell proliferation [13]. Therefore, the cross-talk between E2F1 and CIP2A is not limited to one type of cancer, and the feedback might have variable functions in various tumors. Moreover, it was reported that increased CIP2A protein level could improve the phosphorylation of E2F1 at Ser 364 but not E2F1 transcription in breast cancer [17]. In contrast, we found that CIP2A could significantly increase E2F1 mRNA expression in cervical cancer cells. However, whether CIP2A regulates E2F1 transcription directly as a transcription factor or via other factors is unknown.
Previously, a series of factors found associated with the prognosis of cervical cancer have included FIGO staging [25], tumor size [26], lymph node metastasis [27,28], close surgical margins [29] and histological type [30]. However, obtaining consistent conclusions is difficult for guiding the precise prognosis of the patients. Recently, the mRNA level of HPV E6/E7 was found to be a novel prognostic indicator for cervical cancer [31]. However, the operation complexity, low specificity and high false-positive rate has hampered the use of this factor for prognosis prediction. As well, E7 protein is difficult to detect by western blot analysis or immunohistochemistry staining because of low molecular weight and easy degradation.
As a key oncoprotein with many roles, CIP2A has been found to be associated with the prognosis of several tumors, such as colon cancer [32], melanoma [33] and ovarian cancer [12]. In cervical cancer, Wu et al. found that CIP2A cooperated with H-Ras to initiate the progression of the epithelial-mesenchymal transition and enhanced the invasion and migration of cancer cells [34]. However, whether CIP2A can be an independent prognostic indicator for cervical cancer is unknown. In view of the tight regulation among HPV E7, E2F1 and CIP2A, we hypothesized that E2F1 and/or CIP2A, as the replacement for E7, might have prognostic value in cervical cancer. As expected, patients with CIP2A-positive expression alone or CIP2A-E2F1 double-positive co-expression showed markedly shortened OS and DFS. Hence, the detection of CIP2A and E2F1 can help to guide the outcome of cervical cancer patients. This observation was further confirmed by the finding that CIP2A-E2F1 double-positive co-expression is a strong predictor, together with large tumor size and lymph node metastasis, of poor prognosis with cervical cancer.
In summary, here we found a positive feedback between E2F1 and CIP2A that was regulated by HPV E7 in cervical cancer in vitro and in vivo. The crosstalk has prognostic value on the outcome of patients with cervical cancer. The findings have implications for a prognostic indicator and guidance for therapy in cervical cancer.
Acknowledgements
The research was supported by the Taishan Scholars Program of Shandong Province (no. ts201511096), the National Natural Science Foundation of China (no. 81000733 and 81472552), the Shandong Natural Science Project (no. ZR2016HM18), the Science and Technology Project of Shandong Province (no. 2012GGE27122) and the Doctoral Fund of Ministry of Education of China (no. 2014M560560).
Disclosure of conflict of interest
None.
Authors’ contribution
Xiao Wang, Peng Gao, Chenghao Guo and Qingwei Zhang designed the experiments. Meng Wang, Jing Liu and Jiaxiang Lin performed the experiments and follow-up. Shule Zhang, Yiwei Zhao and Jingwen Zhang collected the specimens. Wei Pan and Zeyu Sun and Feifei Sun analyzed the data. Chenghao Guo and Peng Gao reviewed the slides. Weiming Zhao optimized the design of the experiment. Xiao Wang drafted the manuscript. All authors approved the final manuscript for publication.
References
- 1.Yugawa T, Kiyono T. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev Med Virol. 2009;19:97–113. doi: 10.1002/rmv.605. [DOI] [PubMed] [Google Scholar]
- 2.Shim SH, Lee SW, Park JY, Kim YS, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Risk assessment model for overall survival in patients with locally advanced cervical cancer treated with definitive concurrent chemoradiotherapy. Gynecol Oncol. 2013;128:54–59. doi: 10.1016/j.ygyno.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, Burger MP, Buist MR. Prognostic model for survival in patients with early stage cervical cancer. Cancer. 2011;117:768–776. doi: 10.1002/cncr.25658. [DOI] [PubMed] [Google Scholar]
- 4.Horn LC, Fischer U, Raptis G, Bilek K, Hentschel B. Tumor size is of prognostic value in surgically treated FIGO stage II cervical cancer. Gynecol Oncol. 2007;107:310–315. doi: 10.1016/j.ygyno.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald OK, Chen J, Dodson M, Lee CM, Gaffney DK. Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol. 2009;32:411–416. doi: 10.1097/COC.0b013e31819142dc. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 7.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, Lu SL, Lin S, Chan EK, Wang XJ, Grenman R, Kast J, Kallunki T, Sears R, Kahari VM, Westermarck J. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Chen KF, Liu CY, Lin YC, Yu HC, Liu TH, Hou DR, Chen PJ, Cheng AL. CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene. 2010;29:6257–6266. doi: 10.1038/onc.2010.357. [DOI] [PubMed] [Google Scholar]
- 9.Niemela M, Kauko O, Sihto H, Mpindi JP, Nicorici D, Pernila P, Kallioniemi OP, Joensuu H, Hautaniemi S, Westermarck J. CIP2A signature reveals the MYC dependency of CIP2A-regulated phenotypes and its clinical association with breast cancer subtypes. Oncogene. 2012;31:4266–4278. doi: 10.1038/onc.2011.599. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Ge Z, Liu C, Liu Z, Bjorkholm M, Jia J, Xu D. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722–3728. [Google Scholar]
- 11.Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117:6660–6668. doi: 10.1182/blood-2010-08-304477. [DOI] [PubMed] [Google Scholar]
- 12.Bockelman C, Lassus H, Hemmes A, Leminen A, Westermarck J, Haglund C, Butzow R, Ristimaki A. Prognostic role of CIP2A expression in serous ovarian cancer. Br J Cancer. 2011;105:989–995. doi: 10.1038/bjc.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Wang X, Zhou G, Wang H, Xiang L, Cheng Y, Liu W, Wang Y, Jia J, Zhao W. Cancerous inhibitor of protein phosphatase 2A is overexpressed in cervical cancer and upregulated by human papillomavirus 16 E7 oncoprotein. Gynecol Oncol. 2011;122:430–436. doi: 10.1016/j.ygyno.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Chen H, Chen Y, Liu J, Wang X, Yu X, Chen JJ, Zhao W. Cancerous inhibitor of protein phosphatase 2A contributes to human papillomavirus oncoprotein E7-induced cell proliferation via E2F1. Oncotarget. 2015;6:5253–5262. doi: 10.18632/oncotarget.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallai R, Bhaskar A, Sodi V, Rice LM. Ets1 and Elk1 transcription factors regulate cancerous inhibitor of protein phosphatase 2A expression in cervical and endometrial carcinoma cells. Transcription. 2012;3:323–335. doi: 10.4161/trns.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna A, Okkeri J, Bilgen T, Tiirikka T, Vihinen M, Visakorpi T, Westermarck J. ETS1 mediates MEK1/2-dependent overexpression of cancerous inhibitor of protein phosphatase 2A (CIP2A) in human cancer cells. PLoS One. 2011;6:e17979. doi: 10.1371/journal.pone.0017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laine A, Sihto H, Come C, Rosenfeldt MT, Zwolinska A, Niemela M, Khanna A, Chan EK, Kahari VM, Kellokumpu-Lehtinen PL, Sansom OJ, Evan GI, Junttila MR, Ryan KM, Marine JC, Joensuu H, Westermarck J. Senescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1. Cancer Discov. 2013;3:182–197. doi: 10.1158/2159-8290.CD-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wang M, Zhang X, Wang Q, Qi M, Hu J, Zhou Z, Zhang C, Zhang W, Zhao W, Wang X. CIP2A is associated with multidrug resistance in cervical adenocarcinoma by a P-glycoprotein pathway. Tumour Biol. 2016;37:2673–2682. doi: 10.1007/s13277-015-4032-8. [DOI] [PubMed] [Google Scholar]
- 19.Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, Kallioniemi O, Thezenas S, Westermarck J. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15:5092–5100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 20.Zhao D, Liu Z, Ding J, Li W, Sun Y, Yu H, Zhou Y, Zeng J, Chen C, Jia J. Helicobacter pylori CagA upregulation of CIP2A is dependent on the Src and MEK/ERK pathways. J Med Microbiol. 2010;59:259–265. doi: 10.1099/jmm.0.014704-0. [DOI] [PubMed] [Google Scholar]
- 21.Munger K, Basile JR, Duensing S, Eichten A, Gonzalez SL, Grace M, Zacny VL. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20:7888–7898. doi: 10.1038/sj.onc.1204860. [DOI] [PubMed] [Google Scholar]
- 22.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 23.Yim EK, Park JS. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer Res Treat. 2005;37:319–324. doi: 10.4143/crt.2005.37.6.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S43–103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi T, Ishikawa H, Nawa A, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. The significance of tumor size in clinical stage IB cervical cancer: can a cut-off figure be determined? Int J Gynecol Cancer. 2000;10:397–401. doi: 10.1046/j.1525-1438.2000.010005397.x. [DOI] [PubMed] [Google Scholar]
- 27.Atasii T, Aksu MF, Cetinkaya MB. Prognostic factors that affect survival after the treatment of early stage cervical carcinoma. Int Surg. 2000;85:147–151. [PubMed] [Google Scholar]
- 28.Morice P, Piovesan P, Rey A, Atallah D, Haie-Meder C, Pautier P, Sideris L, Pomel C, Duvillard P, Castaigne D. Prognostic value of lymphovascular space invasion determined with hematoxylin-eosin staining in early stage cervical carcinoma: results of a multivariate analysis. Ann Oncol. 2003;14:1511–1517. doi: 10.1093/annonc/mdg412. [DOI] [PubMed] [Google Scholar]
- 29.McCann GA, Taege SK, Boutsicaris CE, Phillips GS, Eisenhauer EL, Fowler JM, O’Malley DM, Copeland LJ, Cohn DE, Salani R. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol. 2013;128:44–48. doi: 10.1016/j.ygyno.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang HJ, Chang TC, Hong JH, Tseng CJ, Chou HH, Huang KG, Lai CH. Prognostic value of age and histologic type in neoadjuvant chemotherapy plus radical surgery for bulky (>/=4 cm) stage IB and IIA cervical carcinoma. Int J Gynecol Cancer. 2003;13:204–211. doi: 10.1046/j.1525-1438.2003.13004.x. [DOI] [PubMed] [Google Scholar]
- 31.Hovland S, Muller S, Skomedal H, Mints M, Bergstrom J, Wallin KL, Karlsen F, Johansson B, Andersson S. E6/E7 mRNA expression analysis: a test for the objective assessment of cervical adenocarcinoma in clinical prognostic procedure. Int J Oncol. 2010;36:1533–1539. doi: 10.3892/ijo_00000640. [DOI] [PubMed] [Google Scholar]
- 32.Chen KF, Yen CC, Lin JK, Chen WS, Yang SH, Jiang JK, Lan YT, Lin CC, Yu HC, Hsu HM, Lin WL, Teng HW. Cancerous inhibitor of protein phosphatase 2A (CIP2A) is an independent prognostic marker in wild-type KRAS metastatic colorectal cancer after colorectal liver metastasectomy. BMC Cancer. 2015;15:301. doi: 10.1186/s12885-015-1300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi F, Ding Y, Ju S, Wu X, Cao S. Expression and prognostic significance of CIP2A in cutaneous malignant melanoma. Biomarkers. 2014;19:70–76. doi: 10.3109/1354750X.2013.871752. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Gu TT, Zheng PS. CIP2A cooperates with H-Ras to promote epithelial-mesenchymal transition in cervical-cancer progression. Cancer Lett. 2015;356:646–655. doi: 10.1016/j.canlet.2014.10.013. [DOI] [PubMed] [Google Scholar]


