Abstract
Cutaneous wound healing following trauma is a complex and dynamic process involving multiple overlapping events following trauma. Two critical elements affecting skin wound healing are neovascularization and inflammation. A nascent vessel can provide nutrition and oxygen to a healing wound. Therefore, treatments strategies that enhance angiogenesis and inhibit inflammation can promote skin wound healing. Previous studies have shown that the SIKVAV peptide (Ser-Ile-Lys-Val-Ala-Val) from laminin can promote angiogenesis in vitro. This study evaluated the effects of peptide SIKVAV-modified chitosan hydrogels on skin wound healing. We established skin wounds established in mice and treated them with SIKVAV-modified chitosan hydrogels. H&E staining showed that peptide-modified chitosan hydrogels accelerated the reepithelialization of wounds compared with the negative and positive controls. Immunohistochemistry analysis demonstrated that more myofibroblasts were deposited at wounds treated with peptide-modified chitosan hydrogels that at those treated with negative and positive controls. In addition, peptide-modified chitosan hydrogels promoted angiogenesis as well as keratinocyte proliferation and differentiation, but inhibited inflammation in skin wounds. Taken together, these results suggest that SIKVAV-modified chitosan hydrogels are a promising treatment component for healing-impaired wounds.
Keywords: SIKVAV, chitosan hydrogel, wound healing, angiogenesis, inflammation, re-epithelialization
Introduction
As the largest organ of the human body, the skin is a critical protective barrier against the environment. It helps prevent infection, water loss, and electrolyte imbalance and regulates body temperature [1]. Large areas of full-thickness skin defects result in clinical problems including severe infection and shock because of the large water and electrolytes losses. Cutaneous wound healing following trauma is a complex and dynamic process involving multiple overlapping events, including hemostasis, inflammation, neovascularization, epithelialization, granulation tissue formation, extracellular matrix (ECM) deposition, and tissue remodeling [2]. Some severe wounds result in high mortality, decreased quality of life, and high health care costs [3]. A better understanding of the mechanisms involved in wound healing is needed to develop novel and effective therapies for patients with skin wounds. Current treatment strategies, including autografts, allografts, xenografts, and bioengineered skin substitutes [4] have limitations, such as antigenicity, potential disease transmission risk, and insufficient availability. Biomaterial-based wound dressings are a research interest area because they can provide a straightforward treatment option.
Angiogenesis, the formation of new blood vessels, is important in physiological processes such as wound healing and tissue growth, as well as cell migration, proliferation, and differentiation [5]. Angiogenesis supplies nutrients for skin wound healing and is regulated by various vascular growth factors and cytokines, such as vascular endothelial growth factor (VEGF), base fibroblast growth factor (bFGF), platelet derived growth factor, and transforming growth factor beta (TGF-β1) [6]. Laminin, the major glycoprotein in the basement membranes of blood vessels, possesses numerous biological activities including enhancing cell adhesion, migration, growth, and proliferation [7], all of which are important during angiogenesis. Several of these activities have been localized to specific active sites on the laminin molecule. For example, the amino acid sequence serine-isoleucine-lysine-valine-alanine-valine (SIKVAV) from the long arm of the laminin A chain is involved in cell attachment, migration, and proliferation, neurite outgrowth, and tumor cell metastasis and growth [8]. SIKVAV directly interacts with endothelial cells in vitro and has biological activities including attachment, migration, and invasion [9,10], which are critical during vessel formation in vivo. In addition, Kibbey et al. [11] demonstrated that the SIKVAV peptide enhances tumor cell growth and angiogenesis. In vitro, the SIKVAV peptide promotes the formation of endothelial cell tubular structures [12] and facilitates angiogenesis. Therefore, the SIKVAV peptide may be a useful therapeutic agent in skin wound healing.
The process of skin wound healing is affected by inflammation [2], and thus a treatment strategy preventing inflammation can promote skin wound healing. Chitosan is a natural, cationic polymer that is biologically renewable, biodegradable, biocompatible, nonantigenic, nontoxic, and biofunctional [13,14]. Chitosan has high concentrations of hydroxyl and primary amino functional groups [15] and excellent adsorption, carrier, and antibacterial capabilities. These beneficial biological properties have enabled its use in numerous biomedical and industrial applications involving hemostasis, antimicrobial activity, wound healing, tissue-engineering scaffolds, biotechnology, agriculture, and environmental protection [13,16-19]. Chitosan can accelerate wound healing by enhancing the function of inflammatory cells, macrophages, and fibroblasts [14]. A recent study by Lefler et al. [20] demonstrated that bFGF/chitosan matrices promote re-epithelialization and inhibit bacterial growth.
In this study, we prepared a composite hydrogel sheet composed of chitosan and the SIKVAV peptides from laminin. We evaluated the in vivo effectiveness of the composite hydrogel for accelerating wound healing in full-thickness wounds in a mouse skin defect model. We hypothesized that the peptide-modified hydrogel would provide advantages as a wound dressing for the repair of healing-impaired wounds.
Materials and methods
Materials
Chitosan (85% deacetylation degree, molecular weight 100,000 Da) was purchased from Golden-Shell Pharmaceutical Co., Ltd. (Yuhuan, China). Methacrylic anhydride was purchased from APC Chemicals Company (Montreal, Canada). 3-(Maleimid) opropionic acid n-hydroxysuccinimide ester (SMP; 97%) was purchased from Polysciences Corporation (Tamil Nadu, India). N,N,N,N-tetramethylethylenediamine (TEMED), ammonium persulfate (APS), and dimethylformamide (DMF) were purchased from Sigma-Aldrich (Guangzhou, China). The SIKVAV peptide was purchased from Peptide Biotech Co., Ltd. (Shanghai, China). Sodium pentobarbital was purchased from Aladdin (Guangzhou, China). CD86 and CD163 polyclonal antibodies were purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). K1 monoclonal antibody was purchased from Abcam Ltd. (Hong Kong, China). K6 polyclonal antibody was purchased from Covance (New York, USA). CD31 monoclonal antibody was purchased from Dako (Guangzhou, China). Alpha-smooth muscle actin (α-SMA) polyclonal antibody, biotinylated secondary antibody, and streptavidin-biotin complex (SABC) detection kits were purchased from Wuhan Boster Biological Engineering Co., Ltd. (Wuhan, China).
Synthesis of peptide-modified chitosan hydrogels
Peptide-modified chitosan hydrogels were prepared as described in our previous report [21]. Briefly, methacrylic anhydride was reacted with chitosan in 3% acetic acid solution, and the resulting mixture was dialyzed against water. Chitosan macromers, which carried polymerizabledouble bonds due to the methacrylamide groups, were obtained as a dry powder after lyophilization. The chitosan macromers were combined with SMP via an amide coupling reaction. The resulting mixture was dialyzed against water and lyophilized to obtain maleimidopropionic-conjugated chitosan macromers. The maleimidopropionic-conjugated chitosan macromers were further reacted with SIKVAV peptides under nitrogen at room temperature. SIKVAV peptide-modified chitosan macromers were obtained after dialyzing the reaction mixture against water and lyophilizing. Peptide-modified chitosan hydrogels were synthesized from the peptide-modified chitosan macromers via free radical polymerization using APS and TEMED as the initiator and catalyst, respectively. For comparison, chitosan hydrogels without SIKVAV peptide were synthesized from the initial chitosan macromers, which carried polymerizable double bonds, using the same protocol for synthesizing peptide-modified chitosan hydrogels.
Peptide-modified chitosan hydrogel in vivo studies on skin wound healing in mice
The animal studies in this work were approved by the Institutional Animal Care and Use Committee. All experiments were approved by the Southern Medical University Ethics Committee and strictly complied with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Forty-eight female C57BL/6 mice, aged 8 to 12 weeks, were purchased from the Experimental Animal Center of Southern Medical University. The mice were anesthetized with an intraperitoneal injection of 1% pentobarbital sodium with an injection volume of 0.01 ml/g body weight. Following anesthetization, the hair was removed on a unilateral site of the back of each mouse. Next, a 6-mm full-thickness round wound was created. After the wound model was established, the mice were randomly divided into four groups. The negative control group received no treatment. The two positive control groups were treated with either the injection of the SIKVAV peptide solution (peptide group) or chitosan hydrogel dressing (chitosan group) at the wound site. The experimental group was treated with a peptide-modified chitosan hydrogel dressing (chitosan + peptide group) at the wound site. After treatment, each mouse was covered with Tegaderm tape to protect the wound, individually housed, and fed ad libitum. The wounds were photographed on days 3, 5, and 7 post-surgery using a digital camera before the animals were sacrificed. The wound area was determined using image analysis software (NIH Image). The residual wound percentage was calculated using Equation (1) as follows:
Residual wound percentage (%) = St/S0 × 100% (1)
where S0 was the initial wound area and St was the residual wound area at the indicated time for that mouse.
Histological observations
The wounds and surrounding tissues were collected from the mice on days 3, 5, 7 and 14 d post-treatment. The specimens were rinsed with phosphate buffered saline (PBS), fixed using 4% paraformaldehyde solution, dehydrated at room temperature in a series of increasing ethanol concentrations, and embedded in paraffin wax. From each embedded tissue sample, 5-mm thick sections were prepared from the middle of the specimen. The sections were then stained with hematoxylin and eosin (H&E) according to standard procedures [22].
Immunohistochemistry assays
The tissue sections were deparaffinized and rehydrated. Next, antigen retrieval was performed with 0.1 M citrate buffer. The tissue sections were incubated with 5% bovine serum albumin for 2 h and then incubated with monoclonal goat anti-mouse CD31 antibody at 4°C overnight. The sections were rinsed with PBS and incubated with biotinylated goat anti-rabbit secondary antibody for 2 h. Following this step, the sections were reacted with SABC for 20 min, colored with diaminobenzine (DAB), and stained with hematoxylin. The sections were then dehydrated with gradient ethanol, soaked with xylene, and sealed with resin. Each tissuesection was observed with a microscope (400 ×) at five randomly selected fields from one section (three sections from three mice in each group) to count the new capillaries with CD31-positive endothelial cells. Immunohistochemistry assays to detect CD86, CD163, K1, K6, and α-SMA in the tissue samples were performed using the above methods. Image-Pro Plus was used to analyze the average optical density values for K1, K6, and α-SMA expression. Five randomly selected fields of view were examined for each group at each time point and used to assess the average optical density value per unit area.
Statistical analysis
Numerical data were expressed as the means ± standard deviations. Statistical differences between the groups were assessed using one-way analysis of variance (ANOVA) in SPSS20.0 software. A p-value of less than 0.05 (*P < 0.05) indicates a statistical difference between the groups. A p-value less than 0.01 (**P < 0.01) indicates a statistically significant difference between the groups. All experiments were performed at least three times.
Results
Peptide-modified chitosan hydrogels promote skin wound contraction
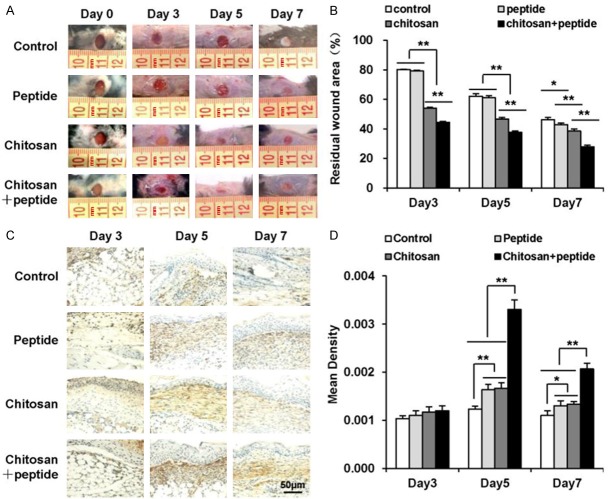
Peptide-modified chitosan hydrogels were synthesized and applied as wound dressings in a mouse model. Their effectiveness in skin wound healing was measured by calculating the residual wound area following treatment (Figure 1A, 1B). Treatment with the SIKVAV did not accelerate wound contraction compared with the negative control group until 7 days after treatment (Figure 1B). The two groups treated with either chitosan or peptide-modified chitosan hydrogels showed significantly greater wound contraction on days 3, 5, and 7 after the operation than the negative control group (Figure 1B). The peptide-modified chitosan hydrogels significantly promoted wound contraction compared with the chitosan hydrogels at all tested time points (Figure 1B).
Figure 1.
Peptide-modified chitosan hydrogels significantly promote skin wound healing in mice. A: Representative photographs of wounds in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups taken 3, 5, and 7 days after the initial treatment in mice. B: The residual wound percentages in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups (n = 3). C: α-SMA expression in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups detected on days 3, 5, and 7 after the initial treatments as detected by immunohistochemical staining (scale bar: 50 μm). D: Mean α-SMA expression in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups measured 3, 5, and 7 days after the initial treatments (n = 3).
Wound contraction is dependent upon myofibroblasts, which produce pulling effects and express large amounts of α-SMA. Therefore, we assessed α-SMA expression at the wound sites (Figure 1C). Quantitative analyses of α-SMA expression in the four groups showed that the chitosan + peptide group exhibited higher α-SMA expression than the other three groups on days 5 and 7 post-surgery (P < 0.01). No significant differences were found between the peptide and hydrogel groups on post-surgery days 5 and 7 or between the four groups on post-surgery day 3 (Figure 1D).
Peptide-modified chitosan hydrogels promote re-epithelialization of skin wounds
The H&E staining results showed that peptide-modified chitosan hydrogels promoted the skin wound re-epithelialization process (Figure 2). Keratinocytes migrated only slightly from the wound edge in the negative control, peptide, and chitosan groups 3 days after treatment. In contrast, keratinocytes migrated farther in the chitosan + peptide group. Five days after the treatment, the chitosan + peptide group completed re-epithelialization; however, in the other three groups, keratinocytes had not covered the wounds. Seven days after treatment, the keratinocytes still did not cover the wounds in the peptide and negative control groups.
Figure 2.
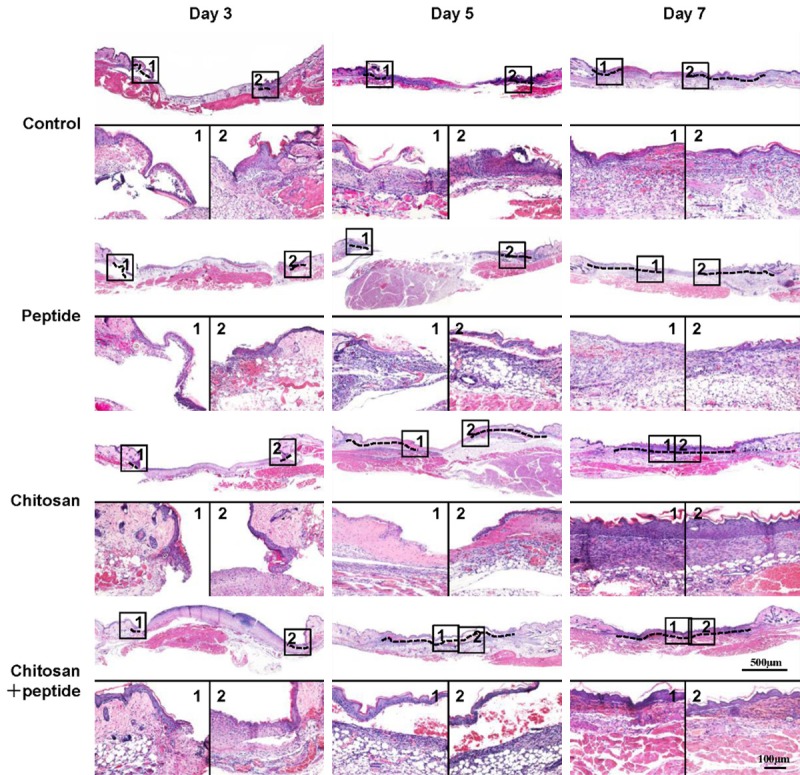
Peptide-modified chitosan hydrogels promote skin re-epithelialization in mice. H&E staining showed the re-epithelialization process in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on days 3, 5, and 7 after the initial treatments (scale bar: 500 μm).
Peptide-modified chitosan hydrogels promote angiogenesis in skin wounds
The immunohistochemistry assay results showed that the peptide-modified chitosan hydrogels promoted angiogenesis in the skin wounds (Figure 3). Capillary endothelial cells express CD31. Therefore, we assessed CD31 expression at the wound sites with an immunohistochemistry assay (Figure 3A). Significant angiogenesis was observed in both the peptide and chitosan hydrogel groups compared with the negative control group 5 and 7 days after the treatments. Moreover, there was significantly more angiogenesis for the wounds in the chitosan + peptide group than for the peptide or chitosan groups, and there was no difference between the peptide and chitosan groups at the time points tested (Figure 3B).
Figure 3.
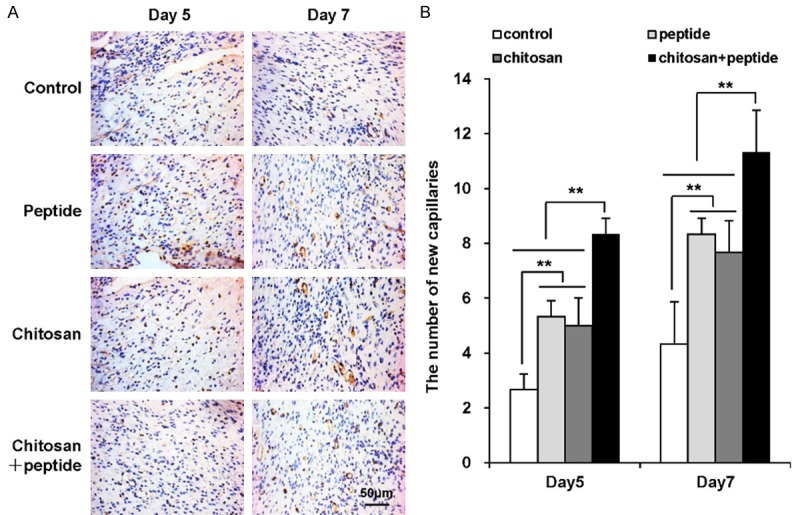
Peptide-modified chitosan hydrogels significantly promote angiogenesis in mouse skin wounds. A: Immunohistochemical staining for CD31 expression in vascular endothelial cells of wounded mouse skin 5 and 7 days after the initial treatments (scale bar: 50 μm). B: Mean numbers of new blood capillaries per field of view in mouse skin wounds in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups (n = 3).
Peptide-modified chitosan hydrogels promote keratinocyte proliferation and differentiation in skin wounds
The proliferation of keratinocytes plays a critical role in the re-epithelialization process for skin wounds. After trauma, many keratinocytes around the wound edges migrate towards the center of the wound and proliferate. Keratinocytes express large amounts of K6, which is an indicator of keratinocyte proliferation in skin wounds. The immunohistochemistry assays showed that the peptide-modified chitosan hydrogels promoted keratinocyte proliferation in skin wounds (Figure 4A). K6 expression in the chitosan + peptide group was significantly higher than in the other three groups on days 5 and 7 after trauma. K6 expression in the peptide group was not statistically different than in the chitosan group, and K6 expression was significantly higher in both the peptide and chitosan groups than in the control group. In contrast, 3 days after trauma, K6 expression was not statistically different among the four groups (Figure 4B).
Figure 4.
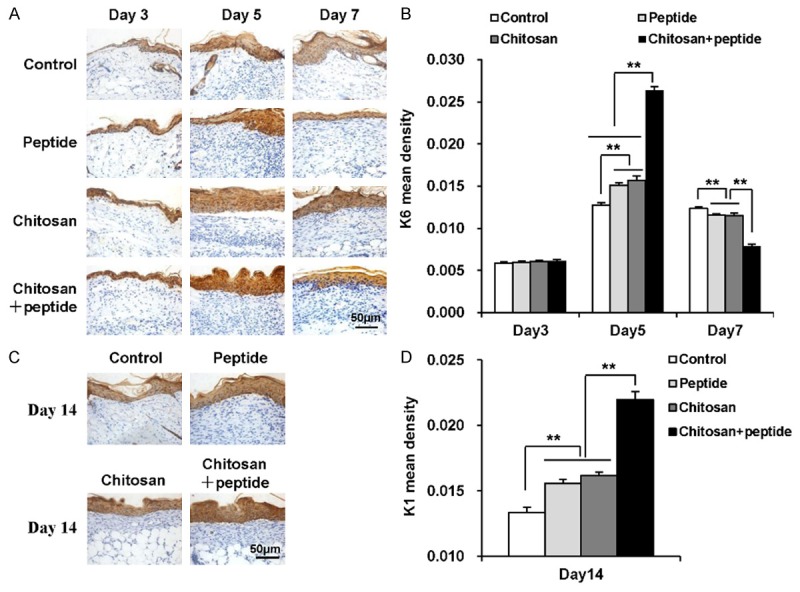
Peptide-modified chitosan hydrogels promote the proliferation and differentiation of keratinocytes in skin wounds. A: Immunohistochemical staining to show K6 expression in keratinocytes in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups 3, 5, and 7 days after the initial treatments (scale bar: 50 μm). B: K6 expression in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on days 3, 5, and 7 after the initial treatments (n = 3). C: Immunohistochemical staining of K1 expression in keratinocytes in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on day 14 after the initial treatments (scale bar: 50 μm). D: K1 expression in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on day 14 after the initial treatments (n = 3).
After trauma, keratinocytes around the wound edges begin proliferating and differentiating. K1 is one of the markers for keratinocyte terminal differentiation. We detected K1 expression at the re-epithelialized wound sites in the four groups 14 days after treatment. As shown in Figure 4C and 4D, K1 expression was significantly higher in the chitosan + peptide group than in the other three groups. K1 expression in the peptide group was not statistically different than in the chitosan group, and it was significantly higher in both the peptide and chitosan groups than in the control group on day 14 after treatment.
Peptide-modified chitosan hydrogels inhibitskin wound inflammation
The inflammatory response is a necessary pathway for wound healing, which often occurs after skin injury. Excessive inflammation prolongs skin wound healing. Macrophages play a critical role in the inflammatory response as the major inflammatory cells present in wounds. Macrophages have two subsets: M1 and M2. M2 macrophages are necessary for angiogenesis and wound healing, in contrast to proinflammatory M1 macrophages. In this study, we used CD86 to label M1 macrophages and CD163 to label M2 macrophages. As shown in Figure 5A and 5B, there were significantly more CD86-positive cells (M1 macrophages) in the wounds of the control and peptide groups than in the chitosan and chitosan + peptide groups on day 3, 5, and 7 after the operation. There was no statistical difference in the number of CD86-positive cells between the chitosan and chitosan + peptide groups. Additionally, as shown in Figure 5C and 5D, there were significantly more CD163-positive cells (M2 macrophages) in the skin wounds of the chitosan and chitosan + peptide groups than in the control and peptide groups 3 and 5 days after trauma, whereas there was no significant difference between the chitosan and chitosan + peptide groups. On day 7, there was no statistical difference in CD163 expression among the four groups.
Figure 5.
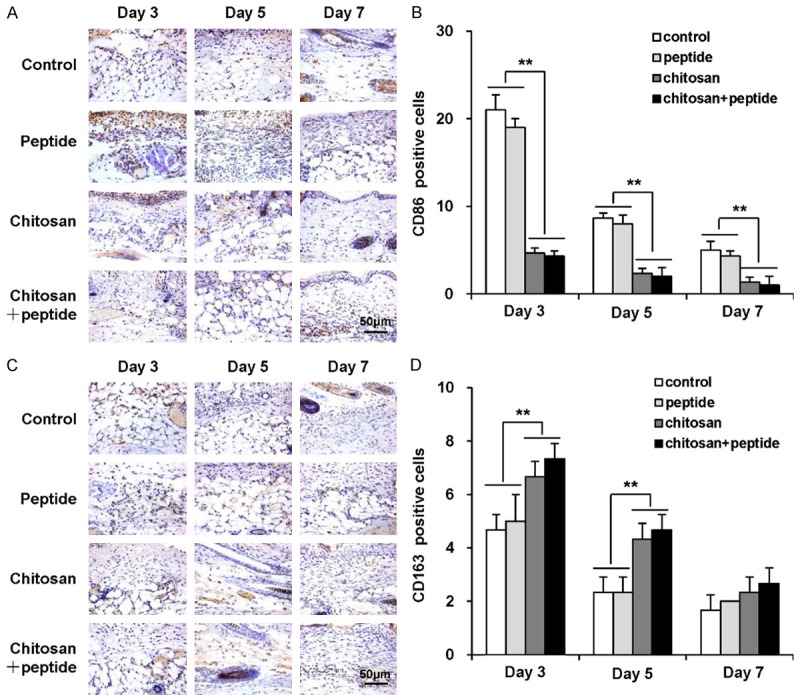
Peptide-modified chitosan hydrogels inhibit inflammation in skin wounds. A: Immunohistochemical staining of CD86-positive cells in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on days 3, 5, and 7 after the initial treatments (scale bar: 50 μm). B: Mean numbers of CD86-positive cells per field of view in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on days 3, 5, and 7 after the initial treatments (n = 3). C: Immunohistochemical staining of CD163-positive cells in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on days 3, 5, and 7 after the initial treatments (scale bar: 50 μm). D: Mean numbers of CD163-positive cells in the control, peptide, chitosan hydrogel, and peptide + chitosan hydrogel groups on days 3, 5, and 7 after the initial treatments (n = 3).
Discussion
Vascularization provides nutrition and oxygen to support cell viability and promote regeneration in a skin wound. In a nonhealing wound, angiogenesis can be a challenge. Therefore, tissue-engineered substrates enhance angiogenesis [23]. Myofibroblasts play critical roles in the process of skin wound contraction [24], and wound contraction is primarily determined by myofibroblasts produced pulling effects. The results of this study indicate that the peptide-modified chitosan hydrogel accelerates skin wound healing (Figure 1) and promotes angiogenesis in comparison to the positive and negative control groups (Figure 3).
Effective skin wound healing requires full re-epithelialization in the wound, meaning that keratinocytes must fully cover the skin wound. After trauma, keratinocytes migrate from the edge of the wound to its center where they differentiate. Angiogenesis provides nutrients and oxygen to the wound, supports keratinocyte migration, and supports skin regeneration for the wound [25]. Tissue-engineered substrates enhance angiogenesis, and scaffolds that exhibit good biocompatible structures support cell survival and growth [26]. The results of this study indicate that the peptide-modified chitosan hydrogel could accelerate skin wound healing (Figure 1) and re-epithelialization (Figure 2). During skin wound healing, the peptide-modified chitosan hydrogel significantly accelerated angiogenesis (Figure 3), allowing new blood vessels to supply enough oxygen and nutrition for keratinocytes to migrate and proliferate (Figure 4). Our results demonstrate that the process of re-epithelialization is faster in the peptide-modified chitosan hydrogel group than the other groups (Figure 2).
Skin injury often leads to an inflammatory response, which is a necessary stage in wound healing [27,28]. However, severe injury may result in an early stress response and a whole-body inflammatory response. This type of excessive inflammation can cause difficulties in wound healing. Because scaffolds are composed of foreign materials, they may affect the host immune response and promote inflammatory cell infiltration and a foreign body reaction. Previous studies demonstrate that chitosan hydrogel scaffolds inhibit inflammation after implantation for treatment of mouse skin defects [29], which is consistent with our results (Figure 5).
Macrophages modulate the wound healing process by secreting various types of cytokines [30], including chemokines and growth factors. Additionally, macrophages enhance the proliferation and migration of various types of repair cells and stimulate their repair functions. Therefore, an appropriate inflammatory response is beneficial to wound healing [31]. In the present study, the synthesized chitosan hydrogel could effectively reduce the loss of water and electrolytes from skin wounds. Due to its properties, the chitosan hydrogels could closely adhere to various types of unevenly surfaced wounds to insulate them from the external environment, protect them from bacterial invasion, and provide a relatively stable aseptic environment. Thus, the chitosan hydrogel could prevent the occurrence of an excessive inflammatory response and promote wound healing [32], consistent with our results (Figure 5). Chitosan hydrogels exhibit water absorption capacity and help prevent fluid accumulation in wounds. Due to their biocompatibility, chitosan hydrogels will not aggravate inflammatory responses in wounds.
Conclusion
In vivo studies on skin wounds in mice showed that peptide-modified chitosan hydrogels promoted skin wound healing and re-epithelialization, as well as proliferation and differentiation of keratinocytes. The peptide-modified chitosan hydrogels accelerated wound healing in a synergistic manner. These hydrogels inhibited the inflammation reaction in skin wounds in vivo. Therefore, these findings suggest that peptide-modified chitosan hydrogels are promising materials for the treatment of healing-impaired wounds, such as pressure sores.
Acknowledgements
This work was supported by the Natural Science Foundation of China (81371719, 81472166, and 81571860), the Colleges Pearl River Scholar Funded Scheme (GDUPS2013 and GDUPS2015), Science and Technology Program of Guangzhou (201604020002), Guangdong Natural Science Foundation (2014A030312013), and Base and talent plan/excellent young talent funding plan of Jiujiang Science and Technology Bureau (Jiu Cai Jiao Zhi [2016] 43-74).
Disclosure of conflict of interest
None.
References
- 1.Zoller N, Valesky E, Butting M, Hofmann M, Kippenberger S, Bereiter-Hahn J, Bernd A, Kaufmann R. Clinical application of a tissue-cultured skin autograft: an alternative for the treatment of non-healing or slowly healing wounds? Dermatology. 2014;229:190–198. doi: 10.1159/000362927. [DOI] [PubMed] [Google Scholar]
- 2.Takayama Y, Aoki R. Roles of lactoferrin on skin wound healing. Biochem Cell Biol. 2012;90:497–503. doi: 10.1139/o11-054. [DOI] [PubMed] [Google Scholar]
- 3.Curtis K, Lam M, Mitchell R, Black D, Taylor C, Dickson C, Jan S, Palmer CS, Langcake M, Myburgh J. Acute costs and predictors of higher treatment costs of trauma in New South Wales, Australia. Injury. 2014;45:279–284. doi: 10.1016/j.injury.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255. doi: 10.1002/14651858.CD011255.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadokoro S, Ide S, Tokuyama R, Umeki H, Tatehara S, Kataoka S, Satomura K. Leptin promotes wound healing in the skin. PLoS One. 2015;10:e0121242. doi: 10.1371/journal.pone.0121242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 7.Holmberg J, Durbeej M. Laminin-211 in skeletal muscle function. Cell Adh Migr. 2013;7:111–121. doi: 10.4161/cam.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesapane A, Di Giovanni C, Rossi FW, Alfano D, Formisano L, Ragno P, Selleri C, Montuori N, Lavecchia A. Discovery of new small molecules inhibiting 67 kDa laminin receptor interaction with laminin and cancer cell invasion. Oncotarget. 2015;6:18116–18133. doi: 10.18632/oncotarget.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boccafoschi F, Fusaro L, Mosca C, Bosetti M, Chevallier P, Mantovani D, Cannas M. The biological response of poly (L-lactide) films modified by different biomolecules: role of the coating strategy. J Biomed Mater Res A. 2012;100:2373–2381. doi: 10.1002/jbm.a.34180. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Suzuki Y, Tanihara M, Kakimaru Y, Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25:1407–1414. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- 12.Grant DS, Kinsella JL, Fridman R, Auerbach R, Piasecki BA, Yamada Y, Zain M, Kleinman HK. Interaction of endothelial cells with a laminin A chain peptide (SIKVAV) in vitro and induction of angiogenic behavior in vivo. J Cell Physiol. 1992;153:614–625. doi: 10.1002/jcp.1041530324. [DOI] [PubMed] [Google Scholar]
- 13.Gerente C, Lee VKC, Le Cloirec P, McKay G. Application of chitosan for the removal of metals from wastewaters by adsorption-Mechanisms and models review. Crit Rev Environ Sci Technol. 2007;37:41–127. [Google Scholar]
- 14.Harkins AL, Duri S, Kloth LC, Tran CD. Chitosan-cellulose composite for wound dressing material. Part 2. Antimicrobial activity, blood absorption ability, and biocompatibility. J Biomed Mater Res B Appl Biomater. 2014;102:1199–1206. doi: 10.1002/jbm.b.33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernkop-Schnurch A, Dunnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81:463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274:1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 19.Dragostin OM, Samal SK, Dash M, Lupascu F, Panzariu A, Tuchilus C, Ghetu N, Danciu M, Dubruel P, Pieptu D, Vasile C, Tatia R, Profire L. New antimicrobial chitosan derivatives for wound dressing applications. Carbohydr Polym. 2016;141:28–40. doi: 10.1016/j.carbpol.2015.12.078. [DOI] [PubMed] [Google Scholar]
- 20.Lefler A, Ghanem A. Development of bFGF-chitosan matrices and their interactions with human dermal fibroblast cells. J Biomater Sci Polym Ed. 2009;20:1335–1351. doi: 10.1163/092050609X12457417534295. [DOI] [PubMed] [Google Scholar]
- 21.Chen SX, Zhang M, Shao XB, Wang X, Zhang L, Xu PC, Zhong W, Zhang L, Xing M, Zhang L. A laminin mimetic peptide SIKVAV-conjugated chitosan hydrogel promoting wound healing by enhancing angiogenesis, re-epithelialization and collagen deposition. Journal of Materials Chemistry B. 2015;3:6798–6804. doi: 10.1039/c5tb00842e. [DOI] [PubMed] [Google Scholar]
- 22.Ju HW, Lee OJ, Lee JM, Moon BM, Park HJ, Park YR, Lee MC, Kim SH, Chao JR, Ki CS, Park CH. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int J Biol Macromol. 2016;85:29–39. doi: 10.1016/j.ijbiomac.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 23.Kunz-Schughart LA, Schroeder JA, Wondrak M, van Rey F, Lehle K, Hofstaedter F, Wheatley DN. Potential of fibroblasts to regulate the formation of three-dimensional vessel-like structures from endothelial cells in vitro. Am J Physiol Cell Physiol. 2006;290:C1385–1398. doi: 10.1152/ajpcell.00248.2005. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhuri V, Zhou L, Karasek M. Inflammatory cytokines induce the transformation of human dermal microvascular endothelial cells into myofibroblasts: a potential role in skin fibrogenesis. J Cutan Pathol. 2007;34:146–153. doi: 10.1111/j.1600-0560.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 25.Madigan MC, McEnaney RM, Shukla AJ, Hong G, Kelley EE, Tarpey MM, Gladwin M, Zuckerbraun BS, Tzeng E. Xanthine oxidoreductase function contributes to normal wound healing. Mol Med. 2015;21:313–322. doi: 10.2119/molmed.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu ZP, Xie HX, Li L, Zhang X, Liu F, Yu XX. Application of strontium-doped calcium polyphosphate scaffold on angiogenesis for bone tissue engineering. J Mater Sci Mater Med. 2013;24:1251–1260. doi: 10.1007/s10856-013-4891-8. [DOI] [PubMed] [Google Scholar]
- 27.Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2016;164:379–385. doi: 10.1016/j.jsbmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol. 2010;19:777–783. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 29.Arancibia R, Maturana C, Silva D, Tobar N, Tapia C, Salazar JC, Martinez J, Smith PC. Effects of chitosan particles in periodontal pathogens and gingival fibroblasts. J Dent Res. 2013;92:740–745. doi: 10.1177/0022034513494816. [DOI] [PubMed] [Google Scholar]
- 30.Moore K, Thomas A, Harding KG. Iodine released from the wound dressing Iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int J Biochem Cell Biol. 1997;29:163–171. doi: 10.1016/s1357-2725(96)00128-8. [DOI] [PubMed] [Google Scholar]
- 31.Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219:172–178. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro MP, Espiga A, Silva D, Baptista P, Henriques J, Ferreira C, Silva JC, Borges JP, Pires E, Chaves P, Correia IJ. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009;17:817–824. doi: 10.1111/j.1524-475X.2009.00538.x. [DOI] [PubMed] [Google Scholar]