Abstract
Hyperglycemia-mediated damage to retinal pigment epithelial (RPE) cells plays a central role in the pathogenesis of diabetic retinopathy. Dysregulation of microRNA (miR)-383 modulates pancreatic beta cell survival in diabetes; however, its role in diabetic retinopathy remains unclear. In this study, we examined the expression of miR-383 in ARPE-19 human RPE cell lines after high glucose treatment and investigated its functions in high glucose-induced reactive oxygen species (ROS) generation and apoptotic responses. The downstream target gene that mediated the action of miR-383 was functionally characterized. It was found that high glucose induced a 2.4-fold increase in miR-383 abundance, compared to ARPE-19 cells treated with normal glucose. Overexpression of miR-383 inhibited cell viability and promoted apoptosis and ROS formation in ARPE-19 cells, which was coupled with deregulation of Bcl-2 and Bax. Peroxiredoxin 3 (PRDX3) expression was repressed by miR-383 in ARPE-19 cells. Restoration of PRDX3 counteracted miR-383-induced ROS generation and apoptosis, while silencing of PRDX3 phenocopied the detrimental effects of miR-383 on ARPE-19 cells. Delivery of anti-miR-383 inhibitors led to an increase of PRDX3 expression and prevented high glucose-elicited ROS formation and apoptosis in ARPE-19 cells. Overall, miR-383 upregulation accounts for high glucose-induced oxidative stress and apoptosis in RPE cells by repressing PRDX3 expression. Targeting miR-383 may have therapeutic potential in the treatment of diabetic retinopathy.
Keywords: Apoptosis diabetic retinopathy, microRNA, oxidative stress, target gene
Introduction
Diabetic retinopathy is a serious complication of diabetes, leading to vision loss and even blindness [1]. Retinal pigment epithelial (RPE) cells are an important component of the outer blood-retina barrier (BRB) that selectively regulates the flux of molecules into and out of the retina [2]. It has been suggested that in the setting of diabetes, sustained hyperglycemia triggers apoptosis in RPE cells [3,4], thereby contributing to the progression of diabetic retinopathy. Induction of reactive oxygen species (ROS) generation is causally linked to high glucose-mediated toxicity to different types of cells [5,6]. Attenuation of ROS production via activation of glucagon-like peptide-1 receptor can confer protection against high glucose-induced apoptosis in RPE cells [4]. Therefore, understanding the mechanisms for regulation of ROS formation is of importance in developing effective therapeutic approaches for diabetic complications.
microRNAs (miRNAs) are a class of endogenous, small non-coding RNAs implicated in a broad range of biological processes [7]. They regulate gene expression by binding to the 3’-untranslated region (UTR) of target mRNAs, causing mRNA degradation and/or translational suppression. Numerous miRNAs have been identified to modulate the pathogenesis of diabetic retinopathy [8,9]. For example, miR-15a is downregulated in diabetic retina and shows anti-inflammatory and anti-angiogenic activity [8]. miR-200b was found to prevent vascular endothelial growth factor (VEGF)-mediated permeability and angiogenesis in diabetic retinopathy [10]. Despite these reports, many miRNAs that are dysregulated in diabetes have not been functionally characterized. It has been documented that miR-383 is deregulated before the onset of diabetes and can regulate pancreatic beta cell apoptosis [11]. This miRNA also shows apoptosis-regulatory activity in several types of malignant cells [12,13]. For instance, the presence of miR-383 induces apoptosis in breast cancer cells by increasing cellular sensitivity to DNA damage [12]. However, the role of miR-383 in hyperglycemia-induced RPE cell apoptosis is still unclear.
In this study, we examined the expression of miR-383 in RPE cells in response to high glucose and determined the function of miR-383 in high glucose-induced apoptosis and oxidative stress in RPE cells. Furthermore, the target genes mediating the function of miR-383 in RPE cells were identified.
Materials and methods
Cell culture and high glucose treatment
A human PRE cell line ARPE-19 was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in Dulbecco’s Modification of Eagle’s Medium (DMEM)/F12 medium supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis, MO, USA). For high glucose treatment, ARPE-19 cells were exposed to 33 mM (HG group) or 5 mM (control group) D-glucose for 48 h [14] and then examined for gene expression, apoptosis, and reactive oxygen species (ROS) production.
Real-time PCR analysis of miR-383 expression
Total RNA was extracted from cells using TRIzol (Invitrogen, Frederick, MD, USA). Reverse transcription was achieved using the RevertAid first strand cDNA synthesis kit (Thermo Scientific, Waltham MA, USA) with a stem-loop primer specific for miR-383. Real-time PCR was performed using the Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). PCR primers are as follows [12]: miR-383 forward: 5’-GTGCAGGGTCCGAGGT-3’, miR-383 reverse: 5’-AGATCAGAAGGTGATTGTGGCT-3’, and U6 forward: 5’-CTCGCTTCGGCAGCACA-3’, U6 reverse: 5’-AACGCTTCACGAATTTGCGT-3’. Cycling conditions were as follows: initial denaturation at 95°C for 5 min and 36 cycles of denaturation at 95°C for 10 s, annealing at 58°C for 10 s, and extension at 72°C for 20 s. miR-383 levels were normalized to U6 levels.
Oligonucleotides and plasmids
Mature miR-383 mimic, locked nucleic acid (LNA)-modified anti-miR-383 inhibitor, and corresponding negative controls were synthesized by Exiqon (Vedbaek, Denmark). Small interfering RNA (siRNA) targeting Prdx3 and scrambled control siRNA were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). For generation of PRDX3-expressing plasmid, human Prdx3 cDNA (SinoBiol. Co. Inc., Beijing, China) lacking the 3’-UTR was amplified by PCR and cloned into pcDNA3.1(+) vector.
Cell transfection
ARPE-19 cells were seeded 24 h before transfection and transiently transfected with miR-383 mimic, miR-383 inhibitor, and Prdx3 siRNA (50 nM for each) using Lipofectamine 2000 (Invitrogen). Transfected cells were cultured for 48 h before gene expression, apoptosis, and ROS measurement. In some experiments, cells were pretreated with N-acetyl-l-cysteine (NAC, Sigma; 10 mM) for 1 h prior to transfection. In rescue experiments, cells were seeded at a density of 2 × 105 cells/well in 24-well plates and co-transfected with miR-383 mimic (50 nM) together with the pcDNA3.1/Prdx3 plasmid (1 μg).
Measurement of cell viability
Cells (6 × 103/well) were cultured in 96-well plates for 48 h, and viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, 0.5 mg/ml MTT (Sigma) was added to the cell culture and incubated for 4 h. Dimethyl sulfoxide (DMSO) was then added to dissolve the formazan. Absorbance was measured at 570 nm.
Apoptosis analysis
Cell apoptosis was assessed using the Annexin-V/propidium iodide (PI) Apoptosis Detection Kit (KeyGEN, Nanjing, China). In brief, cells were collected 48 h after transfection and suspended in 1 × binding buffer. Fluorescein isothiocyanate-conjugated Annexin-Vand PI were added into the cell suspension and incubated for 15 min in the dark. Stained cells were analyzed by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
Whole cell lysates were prepared using ice-cold RIPA buffer containing protease inhibitors (Pierce, Rockford, IL, USA). Protein samples were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were probed with the following primary antibodies: rabbit anti-Bcl-2 monoclonal antibody (ab32124, Abcam, Cambridge, UK), rabbit anti-Bax monoclonal antibody (ab32503, Abcam), rabbit anti-PRDX3 polyclonal antibody (AV52341, Sigma), rabbit anti-PRDX6 polyclonal antibody (AV48268, Sigma), and rabbit anti-β-actin polyclonal antibody (ab8227, Abcam). After further incubation with peroxidase-conjugated goat anti-rabbit IgG (Sigma), immunoreactive bands were visualized by enhanced chemiluminescence (ECL) reagents (Pierce, Rockford, IL, USA). Signal intensity was quantified using Quantity One software (Bio-Rad, Hercules, CA, USA).
Measurement of ROS production
Intracellular reactive oxygen species (ROS) levels were quantified using the cell permeant reagent 2’,7’-dichlorofluorescin diacetate (DCF-DA), as described previously [15]. In brief, cells were incubated with 25 μM of DCF-DA (Sigma) for 15 min at 37°C in the dark. Cells were collected and analyzed by flow cytometry.
Statistical analysis
Data are expressed as mean ± standard deviation. Multiple group comparisons were conducted by analysis of variance (ANOVA) followed by the Tukey’s test. Differences were considered statistically significant at P < 0.05.
Results
Upregulation of miR-383 in response to high glucose triggers apoptosis in ARPE-19 cells
Compared to control cells cultured in normal glucose-containing medium, ARPE-19 cells exposed to high glucose displayed a 2.4-fold increase in the abundance of miR-383 (Figure 1A). To determine the biological significance of upregulation of miR-383, we transfected miR-383 mimic to ARPE-19 cells. Overexpression of miR-383 was found to suppress the viability of ARPE-19 cells by 46%, compared to transfection with negative control miRNA (P < 0.05; Figure 1B). The percentage of apoptosis was significantly greater in the miR-383-overexpressing ARPE-19 cells than in the control cells (22.6 ± 3.5% vs. 5.2 ± 2.4%; P < 0.05; Figure 1C). Western blot analysis revealed that miR-383 overexpression increased the expression of Bax and decreased the expression of Bcl-2 (Figure 1D). Collectively, miR-383 shows pro-apoptotic activity in ARPE-19 cells.
Figure 1.
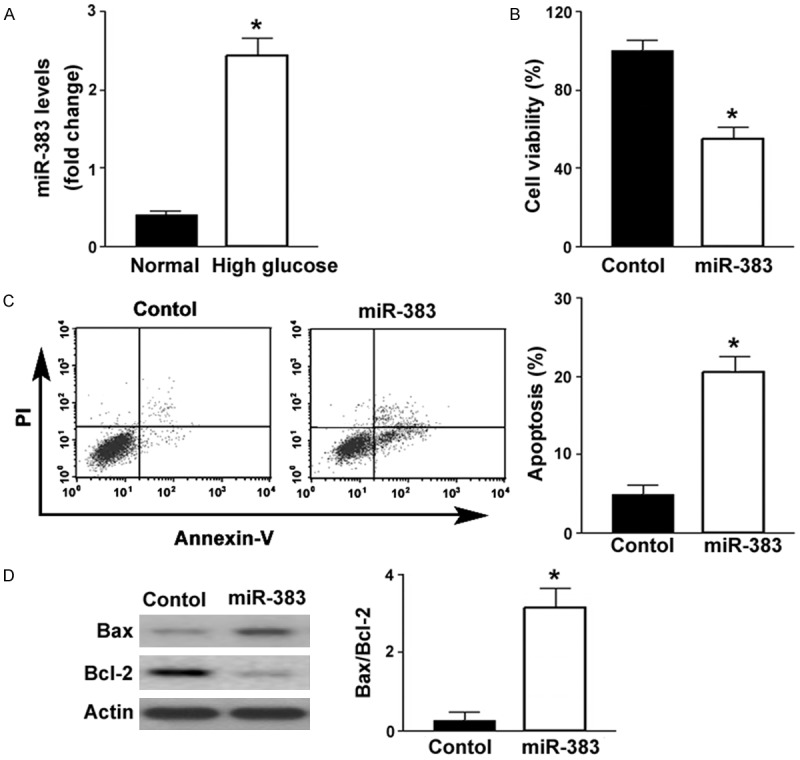
Upregulation of miR-383 in response to high glucose triggers apoptosis in ARPE-19 cells. A. qRT-PCR analysis of miR-383 levels in ARPE-19 cells exposed to normal or high glucose. High glucose treatment increased the expression of miR-383. *P < 0.05 vs. cells under normal glucose conditions. B. ARPE-19 cells were transfected with control miRNA (control) or miR-383 mimic and tested for viability at 48 h posttransfection by MTT assay. miR-383 overexpression caused a reduction of cell viability. C. Flow cytometric analysis of apoptosis after Annexin-V/PI staining. Ectopic expression of miR-383 induced apoptosis in ARPE-19 cells. Left, representative dot plots of apoptotic cells. D. Western blot analysis of Bcl-2 and Bax protein levels. Right, quantification of the Bax/Bcl-2 protein ratio. *P < 0.05 vs. cells transfected with control miRNA.
Promotion of ROS release is involved in miR-383-induced apoptosis in ARPE-19 cells
Induction of oxidative stress is an important mechanism underlying high glucose-induced apoptosis in RPE cells [16]. Thus, we tested the impact of miR-383 upregulation on ROS generation in ARPE-19 cells. The delivery of miR-383 mimic resulted in a 7.8-fold increase in ROS produced by ARPE-19 cells (Figure 2A). Using the antioxidant NAC, miR-383-induced ROS formation was almost completely blocked. Notably, NAC pretreatment impaired the apoptotic response induced by miR-383 in ARPE-19 cells, leading to a 64% reduction in apoptosis (Figure 2B). Therefore, generation of ROS is required for miR-383-induced apoptosis in RPE cells.
Figure 2.
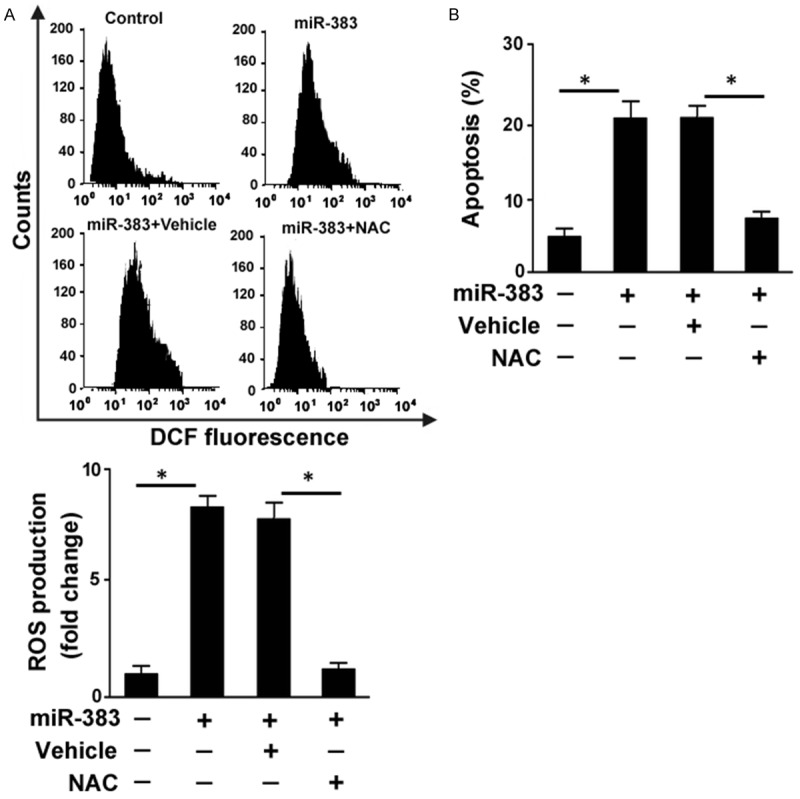
Promotion of ROS release is involved in miR-383-induced apoptosis in ARPE-19 cells. ARPE-19 cells were transfected with control miRNA or miR-383 mimic with or without pretreatment with NAC or vehicle. ROS production and apoptosis were assessed 48 h posttransfection. A. Measurement of intracellular ROS generation using the DCFH-DA probe by flow cytometry. Top, representative flow cytometric histogram of DCF fluorescent levels. Bottom, quantification of intracellular ROS levels from three independent experiments. B. Quantification of apoptotic death by flow cytometry after Annexin-V/PI staining. *P < 0.05.
miR-383 promotes ROS formation and apoptosis in ARPE-19 cells by targeting Prdx3
Next, we asked how miR-383 affected ROS generation and cell survival in RPE cells. It has been reported that miR-383 exerts its growth-suppressive effects on medulloblastoma cells by repressing PRDX3 expression [17]. PRDX3 is known as an antioxidant enzyme that can protect cells from oxidative stress [18]. Therefore, we examined whether the pro-apoptotic activity of miR-383 in RPE cells is mediated through downregulation of PRDX3. To this end, we performed rescue experiments with a miRNA-resistant variant of Prdx3. Delivery of miR-383 mimic was found to suppress endogenous expression of PRDX3 in ARPE-19 cells, which was reversed by co-transfection with a PRDX3-expressing plasmid (Figure 3A). The PRDX6 protein expression was also examined to validate the specificity to PRDX3. In contrast to PRDX3, PRDX6 protein levels remained unchanged after transfection with miR-383 mimic or the PRDX3-expressing plasmid (Figure 3A). Notably, restoration of PRDX3 reduced ROS amounts by 62% (Figure 3B) and apoptosis by 40% (Figure 3C) in miR-383 mimic-transfected cells. The deregulation of Bcl-2 and Bax by miR-383 mimic was also reversed by co-transfection with the PRDX3-expressing plasmid (Figure 3D).
Figure 3.
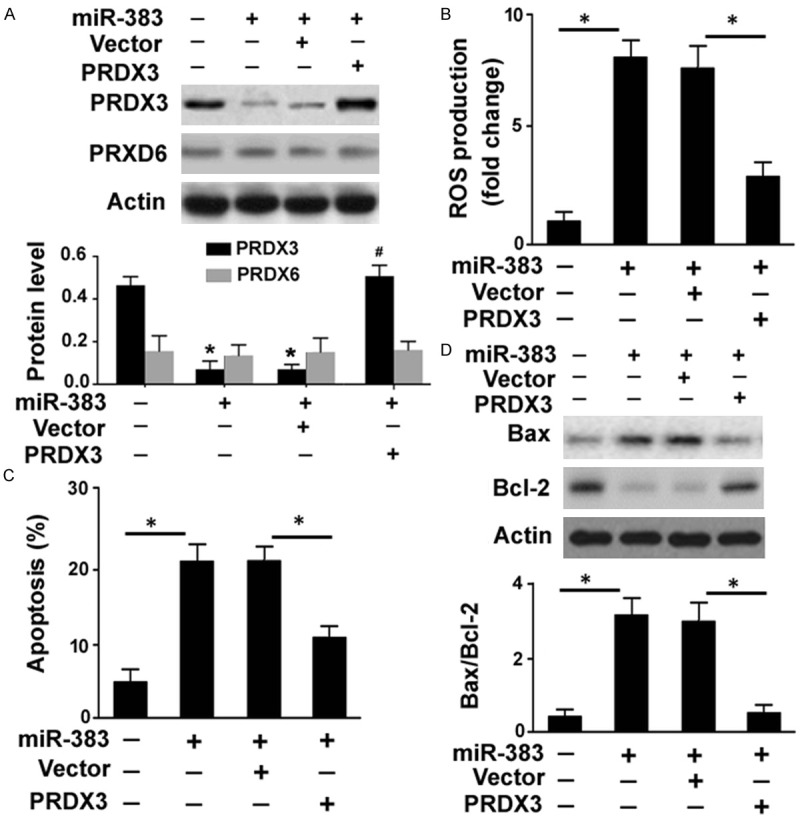
Overexpression of PRDX3 reverses miR-383-mediated ROS formation and apoptosis. A. Western blot analysis of PRDX3 and PRDX6 protein levels in ARPE-19 cells transfected with indicated constructs. *P < 0.05 vs. control cells; #P < 0.05 vs. cells co-transfected with miR-383 mimic and PRDX3-expressing plasmid. B. Intracellular ROS generation was determined using the DCFH-DA probe by flow cytometry. C. Detection of apoptotic death by flow cytometry after Annexin-V/PI staining. D. Western blot analysis of Bax and Bcl-2 protein levels. Bottom, quantification of the Bax/Bcl-2 protein ratio. *P < 0.05.
To confirm the role of PRDX3 in the survival of RPE cells, siRNA-mediated downregulation of PRDX3 was achieved (Figure 4A). Similar to the findings with miR-383 mimic, transfection with Prdx3 siRNA was found to enhance ROS generation (Figure 4B) and trigger apoptotic death (Figure 4C) in ARPE-19 cells. Moreover, silencing of PRDX3 increased the expression of Bax and decreased the expression of Bcl-2 (Figure 4D). Taken together, miR-383-mediated ROS formation and apoptosis in RPE cells are partially ascribed to repression of PRDX3.
Figure 4.
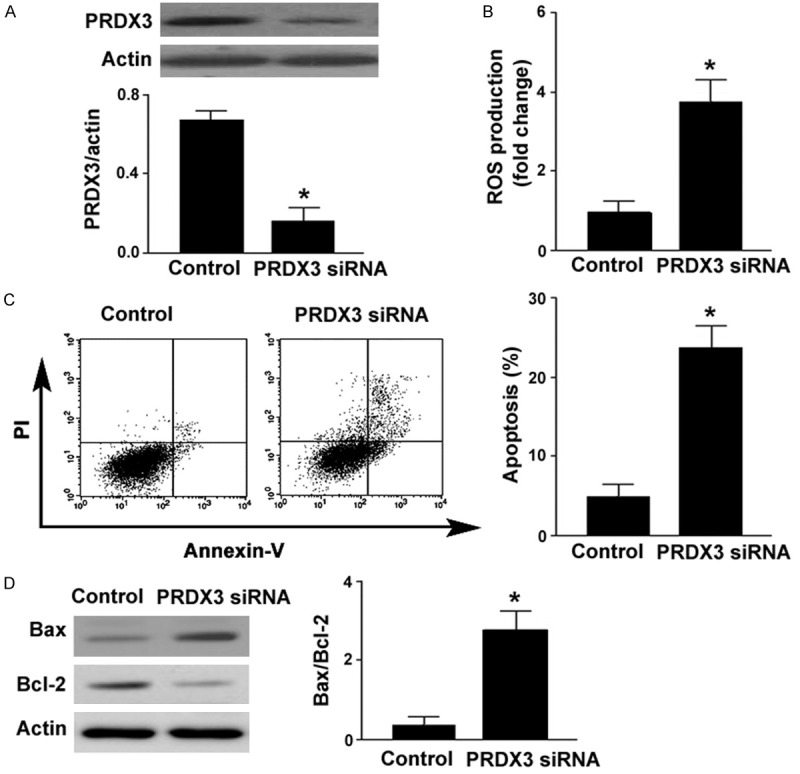
PRDX3 knockdown promotes ROS formation and apoptosis. A. Western blot analysis of PRDX3 protein levels in ARPE-19 cells transfected with control siRNA or PRDX3 siRNA. B. Intracellular ROS generation was determined using the DCFH-DA probe by flow cytometry. C. Detection of apoptotic death by flow cytometry after Annexin-V/PI staining. D. Western blot analysis of Bax and Bcl-2 protein levels. Right, quantification of the Bax/Bcl-2 protein ratio. *P < 0.05 vs. cells transfected with control siRNA.
Targeting miR-383 blocks high glucose-induced oxidative stress and apoptosis
Finally, we investigated the protective potential of targeting miR-383 against high glucose-induced oxidative injury in RPE cells. LNA-modified miR-383 inhibitors were transfected to ARPE-19 cells to inhibit the gene-regulatory activity of miR-383. As expected, the level of PRDX3 was increased by 1.8-fold after transfection with miR-383 inhibitors (Figure 5A). Most importantly, delivery of miR-383 inhibitors significantly prevented ROS formation (Figure 5B) and apoptotic death (Figure 5C) in ARPE-19 cells after treatment with high glucose. In contrast, transfection with negative control inhibitors did not affect ROS generation and apoptosis in high glucose-exposed ARPE-19 cells. Taken together, targeting miR-383 is a beneficial approach to improve RPE cell survival in response to high glucose.
Figure 5.

Targeting miR-383 blocks high glucose-induced oxidative stress and apoptosis. A. Western blot analysis of PRDX3protein levels in ARPE-19 cells transfected with control inhibitor (control) or anti-miR-383 inhibitor. B and C. ARPE-19 cells were pretransfected with control inhibitor or anti-miR-383 inhibitor 24 h before exposure to high glucose. B. Intracellular ROS formation was determined by flow cytometry. C. Detection of apoptotic death by flow cytometry after Annexin-V/PI staining. *P < 0.05.
Discussion
miR-383 is implicated in multiple pathological processes such as tumorigenesis [13,19] and cerebral ischemia [20]. In the present study, we showed that miR-383 was aberrantly induced in ARPE-19 cells upon exposure to high glucose. Moreover, ectopic expression of miR-383 led to reduced viability and increased apoptosis in ARPE-19 cells, suggesting an involvement of miR-383 in hyperglycemia-induced RPE cell damage. The Bcl-2 family members are key regulators of apoptosis in various types of cells [21]. It has been documented that high glucose-induced apoptosis in ARPE-19 cells involves the downregulation of Bcl-2 [22]. Consistently, miR-383 overexpression inhibited the expression of Bcl-2 and induced the expression of Bax in ARPE-19 cells, which provides an explanation for the pro-apoptotic activity of miR-383.
It has been suggested that high glucose-induced apoptotic response in RPE cells is, in part, a consequence of ROS overproduction [16]. In this study, we confirmed that ectopic expression of miR-383 significant promoted ROS formation in ARPE-19 cells, which is similar to the effect elicited by high glucose [16]. Our data further demonstrated that blockade of ROS generation by NAC significantly prevented apoptotic response in miR-383-overexpressing ARPE-19 cells. These observations suggest that induction of ROS accumulation contributes to miR-383-mediated apoptosis in RPE cells. It has been reported that the antioxidant enzyme PRDX3 is a direct target gene of miR-383 [17]. In line with this finding, we showed that enforced expression of miR-383 significantly repressed the expression of PRDX3 in ARPE-19 cells. However, PRDX6 expression levels were not altered by miR-383, indicating the specific targeting of PRDX3 by miR-383. Rescue experiments provided evidence that miR-383-mediated ROS generation and apoptosis in ARPE-19 cells were causally linked to downregulation of PRDX3. Accumulating evidence underscores PRDX3 as a positive regulator of cell survival [18,23,24]. It has been reported that PRDX3 overexpression protects prostate cancer cells from oxidative stress-induced apoptosis [18]. Another study reported that PRDX3 downregulation promotes ROS generation and apoptosis in hepatocellular carcinoma Hep3B cells [23]. In agreement with these studies, we found that silencing of PRDX3 induced ROS formation and apoptosis in ARPE-19 cells. At the molecular level, PRDX3 downregulation increased the level of Bax and reduced the level of Bcl-2 in ARPE-19 cells. Therefore, it is suggested that the pro-survival activity of PRDX3 is associated with modulation of the Bcl-2 family members. However, the signaling pathway(s) involved in such regulation is not clear. A previous study has shown that targeting PRDX3 can enhances cisplatin-induced apoptosis in ovarian cancer cells via the NF-κB pathway [24]. There is a close relationship between NF-κB and Bcl-2 family members in the regulation of apoptotic response [25], which suggests a possibility that PRDX3 coordinates the expression of Bcl-2 and Bax through the NF-κB pathway.
To explore the potential of miR-383 as a therapeutic target for hyperglycemia-induced RPE cell death, we performed miR-383 knockdown experiments. As expected, silencing of miR-383 significantly elevated the expression of PRDX3 in ARPE-19 cells. Furthermore, miR-383 downregulation attenuated ROS production and apoptotic death induced by high glucose. These results provide a rationale to investigate the significance of targeting miR-383 in animal models of diabetic retinopathy.
In conclusion, we provide evidence that miR-383 functions as a key mediator for high glucose-induced oxidative stress and apoptosis in RPE cells, which is, in part, ascribed to downregulation of PRDX3 and subsequent modulation of Bax and Bcl-2. Our data suggest that miR-383 represents a promising target in the treatment of diabetic retinopathy.
Acknowledgements
This work was supported by the Shanghai Natural Science Foundation of China (No: 14ZR1433600) to Qinghua Qiu and by the Foundation of Scientific Research Program of Health Bureau of Shanghai City of China (No: 20134041) to Yanzhi Sang.
Disclosure of conflict of interest
None.
References
- 1.Stewart MW. Treatment of diabetic retinopathy: recent advances and unresolved challenges. World J Diabetes. 2016;7:333–341. doi: 10.4239/wjd.v7.i16.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21(Suppl 6):S3–S9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 3.Kim DI, Park MJ, Lim SK, Choi JH, Kim JC, Han HJ, Kundu TK, Park JI, Yoon KC, Park SW, Park JS, Heo YR, Park SH. High-glucose-induced CARM1 expression regulates apoptosis of human retinal pigment epithelial cells via histone 3 arginine 17 dimethylation: role in diabetic retinopathy. Arch Biochem Biophys. 2014;560:36–43. doi: 10.1016/j.abb.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Kim DI, Park MJ, Choi JH, Lim SK, Choi HJ, Park SH. Hyperglycemia-induced GLP-1R downregulation causes RPE cell apoptosis. Int J Biochem Cell Biol. 2015;59:41–51. doi: 10.1016/j.biocel.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, Wang J, Qin Y, Liu Y, Tang C, He L, Greka A, Zhou Z, Liu F, Dong Z, Sun L. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2016;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YF, Zhang Y, Liu CX, Huang J, Ding GH. microRNA-125b contributes to high glucose-induced reactive oxygen species generation and apoptosis in HK-2 renal tubular epithelial cells by targeting angiotensin-converting enzyme 2. Eur Rev Med Pharmacol Sci. 2016;20:4055–4062. [PubMed] [Google Scholar]
- 7.Chen Y, Pan R, Pfeifer A. Regulation of brown- and beige fat by microRNAs. Pharmacol Ther. 2016;170:1–7. doi: 10.1016/j.pharmthera.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Navitskaya S, Chakravarthy H, Huang C, Kady N, Lydic TA, Chen YE, Yin KJ, Powell FL, Martin PM, Grant MB, Busik JV. Dual anti-inflammatory and anti-angiogenic action of mir-15a in diabetic retinopathy. EBioMedicine. 2016;11:138–150. doi: 10.1016/j.ebiom.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silambarasan M, Tan JR, Karolina DS, Armugam A, Kaur C, Jeyaseelan K. MicroRNAs in hyperglycemia induced endothelial cell dysfunction. Int J Mol Sci. 2016;17:518. doi: 10.3390/ijms17040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, Prentki M, Regazzi R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56:2203–2212. doi: 10.1007/s00125-013-2993-y. [DOI] [PubMed] [Google Scholar]
- 12.Zhao L, Gu H, Chang J, Wu J, Wang D, Chen S, Yang X, Qian B. MicroRNA-383 regulates the apoptosis of tumor cells through targeting Gadd45g. PLoS One. 2014;9:e110472. doi: 10.1371/journal.pone.0110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma H, Liu B, Wang S, Liu J. MicroRNA-383 is a tumor suppressor in human lung cancer by targeting endothelial PAS domain-containing protein 1. Cell Biochem Funct. 2016;34:613–619. doi: 10.1002/cbf.3237. [DOI] [PubMed] [Google Scholar]
- 14.Chang KC, Snow A, LaBarbera DV, Petrash JM. Aldose reductase inhibition alleviates hyperglycemic effects on human retinal pigment epithelial cells. Chem Biol Interact. 2015;234:254–260. doi: 10.1016/j.cbi.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whibley CE, McPhail KL, Keyzers RA, Maritz MF, Leaner VD, Birrer MJ, Davies-Coleman MT, Hendricks DT. Reactive oxygen species mediated apoptosis of esophageal cancer cells induced by marine triprenyl toluquinones and toluhydroquinones. Mol Cancer Ther. 2007;6:2535–2543. doi: 10.1158/1535-7163.MCT-06-0760. [DOI] [PubMed] [Google Scholar]
- 16.Miranda S, González-Rodríguez á, García-Ramírez M, Revuelta-Cervantes J, Hernández C, Simó R, Valverde ÁM. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J Cell Physiol. 2012;227:2352–2362. doi: 10.1002/jcp.22970. [DOI] [PubMed] [Google Scholar]
- 17.Li KK, Pang JC, Lau KM, Zhou L, Mao Y, Wang Y, Poon WS, Ng HK. MiR-383 is downregulated in medulloblastoma and targets peroxiredoxin 3 (PRDX3) Brain Pathol. 2013;23:413–425. doi: 10.1111/bpa.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitaker HC, Patel D, Howat WJ, Warren AY, Kay JD, Sangan T, Marioni JC, Mitchell J, Aldridge S, Luxton HJ, Massie C, Lynch AG, Neal DE. Peroxiredoxin-3 is overexpressed in prostate cancer and promotes cancer cell survival by protecting cells from oxidative stress. Br J Cancer. 2013;109:983–993. doi: 10.1038/bjc.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Tian H, Duan Z, Cao Y, Zhang XS, Sun F. microRNA-383 impairs phosphorylation of H2AX by targeting PNUTS and inducing cell cycle arrest in testicular embryonal carcinoma cells. Cell Signal. 2014;26:903–911. doi: 10.1016/j.cellsig.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Pei L, Meng S, Yu W, Wang Q, Song F, Ma L. Inhibition of microRNA-383 ameliorates injury after focal cerebral ischemia via targeting PPARγ. Cell Physiol Biochem. 2016;39:1339–1346. doi: 10.1159/000447838. [DOI] [PubMed] [Google Scholar]
- 21.Delbridge AR, Grabow S, Strasser A, Vaux DL. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Wang W, Ma J, Ye P, Wang K. High glucose induces mitochondrial dysfunction and apoptosis in human retinal pigment epithelium cells via promoting SOCS1 and Fas/FasL signaling. Cytokine. 2016;78:94–102. doi: 10.1016/j.cyto.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang YG, Li L, Liu CH, Hong S, Zhang MJ. Peroxiredoxin 3 is resistant to oxidation-induced apoptosis of Hep-3b cells. Clin Transl Oncol. 2014;16:561–566. doi: 10.1007/s12094-013-1117-y. [DOI] [PubMed] [Google Scholar]
- 24.Duan J, Lang Y, Song C, Xiong J, Wang Y, Yan Y. siRNA targeting of PRDX3 enhances cisplatin-induced apoptosis in ovarian cancer cells through the suppression of the NF-κB signaling pathway. Mol Med Rep. 2013;7:1688–1694. doi: 10.3892/mmr.2013.1370. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Wu W, Huang W, Hu G, Yuan W, Li W. NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm Res. 2013;62:387–397. doi: 10.1007/s00011-013-0590-7. [DOI] [PubMed] [Google Scholar]


