Abstract
Diabetes mellitus (DM) is a chronic metabolic disease that gives rise to impaired bone remodeling. Increasing evidences have shown that miRNAs are associated with osteogenic differentiation of stem cells. However, the underlying mechanism that links DM-induced HG conditions and impaired osteogenic differentiation capacity of periodontal ligament stem cells (PDLSCs) still remains unclear. In this study, we found that diabetic mice with increased miR-31 level in periodontal ligaments exhibited greater bone loss. In vitro, the high expression of miR-31 is associated with the impaired osteogenic differentiation ability of PDLSCs in high glucose environment. Furthermore, miR-31 inhibitors increased mineralized bone matrix formation and raised Runx2, Osx and OCN expression at both mRNA and protein levels. However, PDLSCs pretreated with miR-31 mimics decreased bone matrix formation and reduced Runx2, Osx and OCN expression level in high glucose microenvironment. Moreover, Satb2 was identified as a target of miR-31 which directly binds to its 3’-untranslated region. To further elucidate the effect of Satb2 in miR-31-mediated osteogenic differentiation, PDLSCs were transfected with Satb2 siRNA and miR-31 inhibitors. The results showed that Satb2 siRNA inhibited osteogenic differentiation of PDLSCs in HG, whereas miR-31 inhibitors reversed the repression of osteogenic differentiation in Satb2 siRNA transfected PDLSCs. Taken together, these results demonstrate that miR-31 is involved in the high glucose-suppressed osteogenic differentiation of PDLSCs by targeting Satb2.
Keywords: MiR-31, high glucose, osteogenic differentiation, periodontal ligament stem cells, Satb2
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease that is characterized by hyperglycemia and results in some complications such as retinopathy, nephropathy and diabetic osteoporosis [1-4]. Periodontitis is a microbe-induced chronic inflammatory disease that leads to the supporting tissue breakdown and alveolar bone resorption. Strong evidence indicates that poorly controlled diabetes increases susceptibility to periodontitis, also known as diabetic periodontitis [5,6]. Patients with diabetic periodontitis exhibit greater alveolar bone loss and a poorer prognosis after routine treatments compared with patients who do not have diabetes [7,8].
Mesenchymal stem cells (MSCs) are a population of postnatal stem cells that have the potential to differentiate into multiple cell types and may contribute to tissue repair and regeneration. It has been reported that high glucose microenvironment influences the differentiation potential of MSCs and inhibits bone regeneration [9,10]. The recent identification of stem cells within the periodontal ligament represents an important role in there generation of alveolar bone [11,12]. However, the underlying mechanism that links DM-induced HG conditions and impaired osteogenic differentiation of periodontal ligament stem cells (PDLSCs) still remains unclear.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression at the post-transcriptional level by causing either degradation or translational repression of target mRNAs. miRNAs have been implicated in the regulation of various biological processes such as inflammation, infection, bone homeostasis and stem cell osteogenic differentiation [13-16]. More recently, several interesting studies showed that miR-31 could regulate the osteogenic differentiation of bone mesenchymal stem cells and adipose-derived stem cells [17,18]. Nevertheless, whether miR-31 could influence osteogenic differentiation of PDLSCs under high glucose conditions remains poorly understood.
In this study, we analyzed the mechanisms of high glucose-suppressed PDLSCs osteogenic differentiation. We found that miR-31 expression was upregulated both in high glucose cultured PDLSCs and periodontal ligament tissues of db/db mice. Furthermore, miR-31 was associated with the osteogenic differentiation of PDLSCs under high glucose microenvironment by targeting Satb2. In addition, we examined the effect of Satb2 downregulation on miR-31 mediated osteogenic differentiation of PDLSCs.
Materials and methods
Animals and experimental design
Male 4-week-old C57BL/KsJ type 2 diabetic db/db mice and nondiabeticdb/m littermates were originally purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were given ad libitum access to food and water and maintained at 23°C room temperature with 55% humidity and a 12-hour light/dark cycle. All animal experiments were performed according to the USA National Institute of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Ethics Committee for Experimental Research, Shanghai Stomatological Hospital, Fudan University. To induce experimental periodontitis, cotton ligatures presoaked in a medium containing porphyromonas gingivalis (108/ml) were wrapped around the cervical position of the maxillary first molars and knotted distal-buccally in the db/db mice and db/m mice. Ligatures were changed every other day. The animal experiment lasted for 4 weeks after the initial ligature application.
Alveolar bone loss measurement
At the end of the experiments, mice were sacrificed and mandibular jaws were dissected free from surrounding soft tissues. Samples were fixed with 4% paraformaldehyde and analyzed using a micro-CT system. The bone loss level of the first molars in each mouse was calculated by measuring the distance from the cementoenamel junction to the alveolar crest at six sites: mesio-buccal, mid-buccal, disto-buccal, mesio-palatal, mid-palatal and disto-palatal. The alveolar bone loss data represent the mean in millimeters of the six measured sites.
Cell culture
After obtaining approval from the Ethics Committee, intact permanent teeth were collected from healthy patients aged 13-18 years who were undergoing orthodontic treatment at the Shanghai Stomatological Hospital, Fudan University. The periodontal ligament (PDL) was gently separated from the surface of the root using a sterilized scalpel and digested in 3 mg/mL collagenase type I (Sigma, Saint Louis, MO, USA) and 4 mg/mL dispase (Roche, Mannheim, Germany) for 1 h at 37°C. Single cell-derived colonies culture was obtained using the limiting dilution technique as previously described. After 2-3 weeks in culture, the single cell-derived clones were harvested and mixed together. Multiple colony-derived hPDLSCs at passage 3 were used in our experiments. At the indicated time points, hPDLSCs were treated with 25 mmol/L glucose in high glucose group and 5.5 mmol/L glucose in control group.
miRNA microarray analysis
Total RNA was extracted from hPDLSCs cultured with 5.5 mmol/L glucose or 25 mmol/L glucose using Trizol (Invitrogen, USA). The miRNA expression profiles were established using the Human miRNA Microarray (Agilent Technologies, Santa Clara, CA, USA). All procedures were performed according to the manufacturer’s recommendations.
Osteogenic differentiation
To induce osteoblast differentiation, hPDLSCs were cultured in 25 mmol/L glucose or 5.5 mmol/L glucose supplemented with 100 nM dexamethasone, 50 ng/ml of ascorbic acid, and 5 mM β-glycerophosphate for 7-21 days. The media were changed every 2 days. Cells were washed twice in phosphate-buffered saline (PBS) after fixation in 4% paraformaldehyde for 20 min. Calcium accumulation was detected by 2% Alizarin red staining.
Cell transfection
The miR-31 oligonucleotides (mimics, inhibitors, and negative control) and small-interfering RNAs (si-Satb2) were synthesized by GenePharma Co. Ltd. (Shanghai, China). hPDLSCs were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and harvested 48 hours after transfection. For long-term detection, hPDLSCs were repeatedly transfected every 3 days.
Luciferase activity assay
Luciferase reporter constructs were used, and luciferase assays were performed as described previously. Briefly, the Satb2 3’-UTR sequence was amplified from human cDNAs by PCR. The wildtype and mutated 3’-UTR regions of Satb2 were cloned into pMIR-REPORT vector (Ambion). The reporter plasmids were co-transfected with miR-31 mimics or the control into hPDLSCs using Lipofectamine 2000 (Invitrogen). After 24 hours, luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s recommendations.
RNA extraction and quantitative real-time PCR (qRT-PCR) analysis
The total RNA was extracted from hPDLSCs using Trizol reagent (Invitrogen, USA) according to the manufacturer’s protocol. Reverse-transcription was carried out using M-MLV Reverse Transcriptase (Affymetrix). 1 μg cDNA was used for real-time PCR analysis with Fast SYBR Green qPCR Master Mix (Affymetrix) by a Bio-Rad iQ5 thermal cycler. The GAPDH was used as a control. Data were analyzed using the comparative cycle threshold (ΔΔCT) method.
Western blot analysis
The whole proteins extraction and western blot analysis were performed as previously described [19]. Briefly, cells were lysed on ice for 30 minutes with RIPA lysis buffer containing protease and phosphatase inhibitors (Sigma). Proteins were then extracted by centrifuging for 10 minutes at 10000 g at 4°C. 10 μg of each sample protein were resuspended in a 4× loading buffer and electrophoresed on 8%-10% SDS-polyacrylamide gels. Fractionated proteins were transferred onto polyvinylidenedifluoride membranes (Invitrogen). Membranes were blocked with 5% nonfat milk for 1 hour followed by overnight incubation with primary antibodies (Santa Cruz) at 4°C. The secondary antibodies (goat-anti-rabbit IgG conjugated with the horseradish peroxidase, Santa Cruz) were added on the membranes and incubated for 1 hour at room temperature. The blots were developed with enhanced chemiluminescence.
Statistical analysis
The SPSS 17.0 software was applied to complete data processing. All data were represented as mean ± SD of three independent experiments. Statistical significance was tested by Student’s t-test and one-way ANOVA. Results were considered statistically significant when P<0.05.
Results
HG increases alveolar bone loss and inhibits osteogenic differentiation of PDLSCs
To investigate the influences of hyperglycemia on periodontitis, we established experimental periodontitis models in db/m mice as control group and db/db mice as DM group. We found that there was a significant alveolar bone loss in DM group compared to the control group (Figure 1A). Several studies have suggested that HG can suppress osteogenic differentiation ability of BMSCs, we then determined whether HG could also affect the osteogenic differentiation of hPDLSCs. Alizarin red staining showed that cells treated with 25 mM glucose resulted in reduced mineralization nodule formation compared to cells treated with 5.5 mM glucose (Figure 1B). Furthermore, qRT-PCR analysis showed that the mRNA expression of osteogenic transcription factors Runx2, Osx and OCN were significantly reduced in 25 mM glucose treated cells (Figure 1B).
Figure 1.
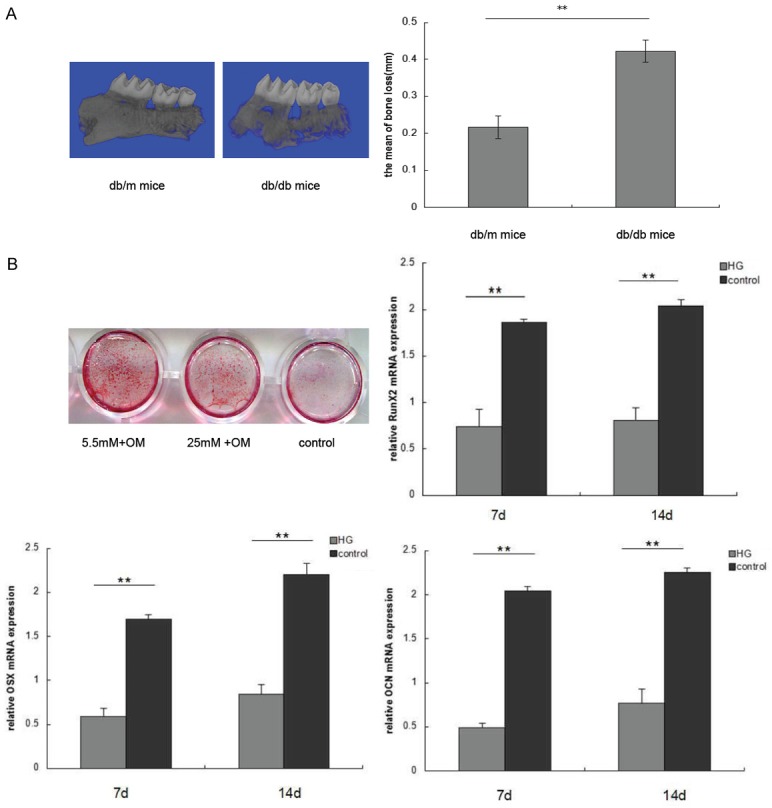
HG increases alveolar bone loss and inhibits osteogenic differentiation. A. Alveolar bone loss in db/db mice and db/m mice were determined by micro CT. The distance between the cementoenamel junction and the alveolar crest was measured at 6 sites in each mouse. B. hPDLSCs were cultured in 25 mmol/L glucose and 5.5 mmol/L glucose supplemented with 100 nM dexamethasone, 50 ng/mlof ascorbic acid, and 5 mM β-glycerophosphate for 7-14 days. Alizarin red staining and qRT-PCR were used to demonstrate mineralized bone matrix formation and Runx2, Osx and OCN expression. (**P<0.05 compared with control).
HG increases the miR-31 expression in vitro and vivo
hPDLSCs were exposed to either 5.5 mM glucose (control, normal glucose) or 25 mM glucose (high glucose: HG) for 48 hours, then the miRNA expression profiles were analyzed by miRNA microarray. Of the 700 miRNAs analysed, we found that miR-31 was the most upregulated in hPDLSCs cultured with HG (Figure 2A). The high expression of miR-31 in HG cultured hPDLSCs was validated by qRT-PCR (Figure 2B). Additionally, miR-31 was highly expressed in periodontal ligament tissues of db/db mice (n=5) compared with the db/m mice (n=5) (Figure 2C).
Figure 2.
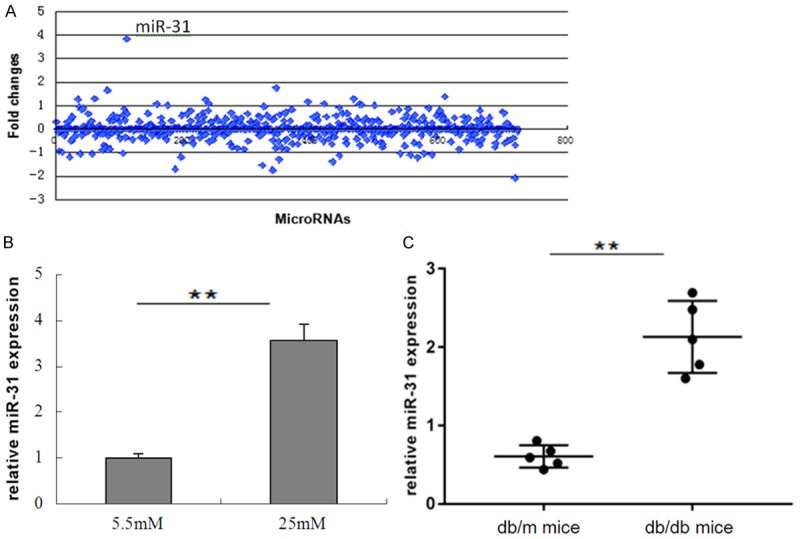
Expression of miR-31 in in vitro and vivo. hPDLSCs were treated with high glucose (25 mM) and control (5.5 mM) for 48 hours. A. Different expression of miRNAs were indicated by miRNA microarray. B. MiR-31 expression was determined by qRT-PCR. (**P<0.05 compared with normal control). C. MiR-31 expression in periodontal ligament tissues of db/db mice compared with the db/m mice was detected by qRT-PCR. (n=5 per group, **P<0.05).
MiR-31 is involved in the high glucose-suppressed osteogenic differentiation of hPDLSCs
To determine the effect of miR-31 on high glucose-induced osteogenic differentiation of hPDLSCs, cells were transfected with miR-31 inhibitors or mimics for 48 hours, and then cultured in 25 mM glucose supplemented with 100 nM dexamethasone, 50 ng/ml ascorbic acid, and 5 mM β-glycerophosphate for additional 7 or 14 days. The efficiency of transfection was measured by qRT-PCR (Figure 3A). Alizarin red staining, qRT-PCR and western blot were used to measure the osteogenic differentiation ability of hPDLSCs transfected with miR-31 inhibitors or miR-31 mimics in high glucose microenvironment. As expected, miR-31 inhibitors treatment increased mineralized bone matrix formation compared with miR-31 mimics treatment (Figure 3B). Furthermore, qRT-PCR and western blot analysis showed that miR-31 mimics reduced Runx2, Osx and OCN expression level in high glucose microenvironment. Importantly, hPDLSCs pretreated with miR-31 inhibitors in HG reversed Runx2, Osx and OCN expression at both mRNA and protein levels (Figure 3C, 3D). These results suggested that miR-31 increased the high glucose-suppressed osteogenic differentiation of hPDLSCs.
Figure 3.
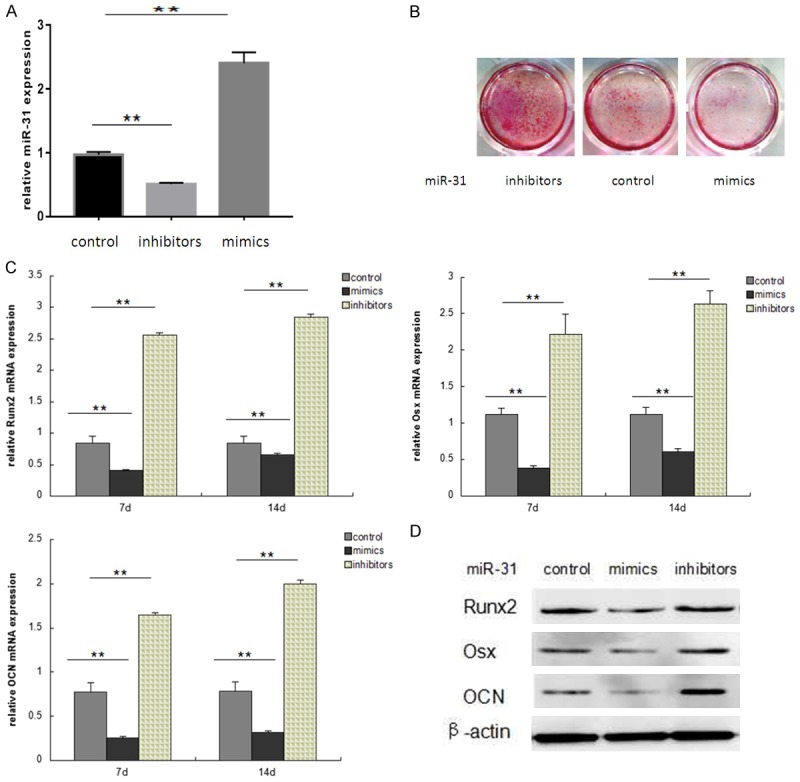
MiR-31 regulates osteogenic differentiation of hPDLSCs in high glucose. (A) hPDLSCs were treated with an miR-31 mimic, an miR-31 inhibitors or the miR-control for 48 hours, miR-31 levels were determined by qRT-PCR. (B-D) hPDLSCs were transfected with miR-31 inhibitors or mimics for 48 hours, and then cultured in 25 mM glucose supplemented with 100 nM dexamethasone, 50 ng/ml ascorbic acid, and 5 mM β-glycerophosphate for additional 7 or 14 days. Alizarin red staining (B) was used to observe mineralized bone matrix formation. qRT-PCR (C) and western blot (D) were used to measure Runx2, Osx and OCN expression. (**P<0.05 compared with control).
Satb2 is a target of miR-31 in hPDLSCs
To identify targets of miR-31, we used the online prediction algorithm TargetScan (http://www.targetscan.org) and found 2 putative binding sites of miR-31 in Satb2 (Figure 4A). Furthermore, we verified whether Satb2 is a direct miR-31 target using luciferase reporter assays. As shown in Figure 4B, hPDLSCs co-transfected with the miR-31 mimics and Satb2 3’-UTR plasmid suppressed the activity of a luciferase reporter, but did not suppress that of a reporter fused to a mutant (MUT) version of the 3’-UTR. Additionally, we examined the Satb2 protein expression in high glucose microenvironment by incubating hPDLSCs in 25 mM glucose. Our results showed a significant reduction of Satb2 expression in HG treated hPDLSCs (Figure 4C). Moreover, Satb2 expression in periodontal ligament tissues of db/db mice decreased compared with that in db/m mice (Figure 4D). To validate whether Satb2 is indeed an miR-31 target, miR-31 mimics or inhibitors were transfected into hPDLSCs. We found that the protein level of Satb2 was repressed by miR-31 mimics after 48-hour transfection. In contrast, Satb2 protein expression was dramatically upregulated by miR-31 inhibitors (Figure 4E).
Figure 4.
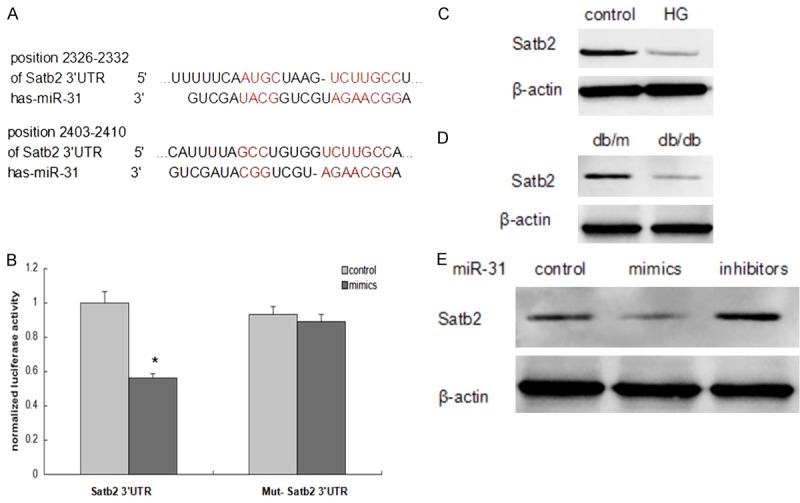
Satb2 is a direct target of miR-31. A. The sequences of miR-31 and predicted binding sites in the 3’UTRs of Satb2. B. Co-transfection of hPDLSCs with the Satb2 3’UTR or Mut-Satb2 3’UTR constructs along with the miR-31 mimic or miR-control. Luciferase activities were measured (*P<0.05 compared with the cells transfected with the miR-control plus Satb2 3’UTR). C and D. The Satb2 protein expression in periodontal ligament tissues and hPDLSCs were determined by western blot. E. Western blot analysis of Satb2 expression in hPDLSCs transfected with miR-31 mimics or inhibitors or control.
Satb2 knockdown reversed the effect of miR-31 on hPDLSCs
As we discovered that Satb2 was negatively regulated by miR-31, we examined whether Satb2 is involved in miR-31-mediated osteogenic differentiation in HG. We observed that Satb2 siRNA inhibited osteogenic differentiation of hPDLSCs in HG, whereas co-transfection with miR-31 inhibitors and Satb2 siRNA reversed the repression of osteogenic differentiation (Figure 5A, 5B). These results indicate that Satb2 is crucial in the effect of miR-31 on the high glucose-suppressed osteogenic differentiation of hPDLSCs depends on Satb2.
Figure 5.
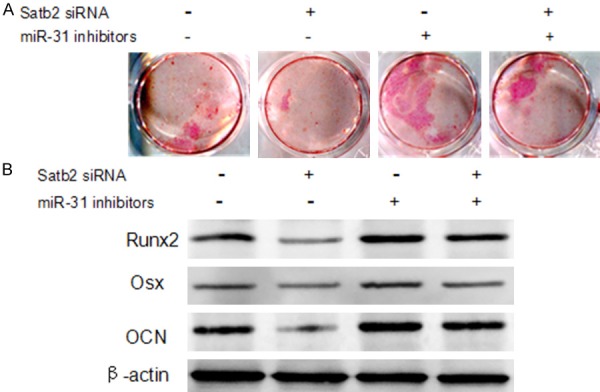
Satb2 knockdown reversed the effect of miR-31 on hPDLSCs. hPDLSCs were tranastected with Stab2 siRNA and/or an miR-31 inhibitors as indicated. Alizarin red staining (A) and western blot (B) were used to analyze the osteogenic differentiation ability of hPDLSCs.
Discussion
Diabetes mellitus is a chronic condition that is characterized by dysregulation of glucose metabolism and an increased risk for periodontal disease [20]. Recently, the relationship between periodontal disease and DM has been extensively studied. It has been reported that the periodontium and alveolar bone are targets for diabetic damage in which the regenerate function of several type of cells is severely impaired [21,22]. PDLSCs have been identified as an important factor for progression of periodontitis and regeneration of the supporting tissues of tooth. In the present study, a remarkable alveolar bone loss were observed in db/db mice induced with experimental periodontitis. Furthermore, HG also affects the osteogenic differentiation of hPDLSCs. These results demonstrate that hyperglycemia causes adverse effects on alveolar bone remodeling in patients with diabetic periodontitis compared with patients who do not have diabetes.
Recent attention has been focused on miRNAs as principal mediators of stem cells osteogenic differentiation. However, the expression of miRNAs was influenced by the microenvironment where the stem cells resided. It has been reported that inflammation microenvironment resulted in an reduction of miR-17 levels in PDLSCs, and promoted the expression of smad ubiquitin regulatory factor one (Smurf1), an important negative regulator of MSC osteogenic differentiation. So excessive inflammatory cytokine levels, miR-17, and Smurf1 are all involved in a coherent feed-forward loop [23]. Another study also showed BMSCs culture in elevated glucose down regulated miR-378 expression and miR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation [24]. Our study for the first time profiled miRNAs expression in hPDLSCs exposed to hyperglycemic conditions. We further observed that miR-31 expression was markedly higher both in hPDLSCs cultured with HG and in the periodontal ligament tissues of db/db mice.
MiR-31 is universally expressed in a variety of tissues, and has been shown to negatively regulate lymphatic vascular lineage-specific differentiation [25], to enhance vascular smooth muscle cell proliferation [26], to regulate keratinocyte proliferation [27], to regulate the osteogenic differentiation of mesenchymal stem cells [28], and to facilitate tooth eruption in cleidocranial dysplasia patients [29]. In this study, we transfected miR-31 inhibitors and miR-31 mimics into hPDLSCs to exam the effect of miR-31 on HG-induced osteogenic differentiation of hPDLSCs. Our results showed that miR-31 inhibitors increased mineralized bone matrix formation and raised Runx2, Osx and OCN expression at both mRNA and protein levels. However, hPDLSCs pretreated with miR-31 mimics decreased bone matrix formation and reduced Runx2, Osx and OCN expression level in high glucose microenvironment. These results reveal that miR-31 is involved in the high glucose-suppressed osteogenic differentiation of hPDLSCs.
Furthermore, we try to delineate the molecular mechanism by which miR-31 acts as a suppressor in osteogenic differentiation of hPDLSCs in HG. miRNAs modulate gene expression by degrading target mRNA expression or blocking protein translation at the post-transcriptional level. In our study, computational analysis predicted that Satb2 is a target gene of miR-31. Luciferase assay showed that miR-31 inhibited the expression of Satb2 by directly targeting its 3’UTR. Satb2 is a member of the family of special AT-rich binding transcription factors that interacts with nuclear matrix attachment regions and activates transcription. Satb2 physically interacts with Runx2, a transcription factor known to promote the mineralization stage of bone formation [30,31]. Several studies have shown that miR-31 negatively regulates Satb2 by targeting translational regulation in bone mesenchymal stem cells and adipose-derived stem cells. Consistent with that, our gain-and loss-of-function experiments demonstrated that miR-31 regulated Satb2 expression at the post-transcriptional level. Additionally, upregulation of miR-31 expression and a decreasion of Satb2 expression were found both in HG cultured hPDLSCs and in periodontal ligament tissues of db/db mice in our study. These results suggest that miR-31 expression negatively correlates with Satb2 levels in vitro and in vivo. To further investigate the effect of satb2 on miR-31 mediated osteogenic differentiation in HG, we co-transfected with miR-31 inhibitors and Satb2 siRNA into hPDLSCs. Interestingly, we observed that Satb2 siRNA reversed the effects of an miR-31 inhibitor on the osteogenic differentiation of hPDLSCs in HG. The above results indicate that Satb2 is an critical regulator in miR-31-mediated osteogenic differentiation suppression in high glucose microenvironment.
In conclusion, our study showed that miR-31 expression was upregulated both in high glucose cultured hPDLSCs and periodontal ligament tissues of db/db mice. The high expression of miR-31 is associated with the impaired osteogenic differentiation ability of PDLSCs in high glucose. Moreover, miR-31-mediated suppression of osteogenic differentiation in PDLSCs is by targeting Satb2 expression.
Acknowledgements
This study is supported by the Shanghai Natural Science Foundation (No. 13ZR1436300), the Young Scholars Project of the Shanghai Municipal Commission of Health and Family Planning (No. 20124y034), the Project of the Shanghai Municipal Commission of Health and Family Planning (No. 201640103).
Disclosure of conflict of interest
None.
References
- 1.Tseng ST, Chou ST, Low BH, Su FL. Risk factors associated with diabetic retinopathy onset and progression in diabetes patients: a Taiwanese cohort study. Int J Clin Exp Med. 2015;8:21507–21515. [PMC free article] [PubMed] [Google Scholar]
- 2.Katayama S, Yamada D, Nakayama M, Yamada T, Myoishi M, Kato M, Nowack C, Kolkhof P, Yamasaki Y ARTS-DN Japan Study Group. A randomized controlled study of finerenone versus placebo in Japanese patients with type 2 diabetes mellitus and diabetic nephropathy. J Diabetes Complications. 2017;31:758–765. doi: 10.1016/j.jdiacomp.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Feldbrin Z, Shargorodsky M. Bone remodelling markers in hypertensive patients with and without diabetes mellitus: link between bone and glucose metabolism. Diabetes Metab Res Rev. 2015;31:752–757. doi: 10.1002/dmrr.2668. [DOI] [PubMed] [Google Scholar]
- 4.de Paula FJ, Horowitz MC, Rosen CJ. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res Rev. 2010;26:622–630. doi: 10.1002/dmrr.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preshaw PM. Periodontal disease and diabetes. J Dent. 2009;37:S575–577. doi: 10.1016/j.jdent.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Almeida Abdo J, Cirano FR, Casati MZ, Ribeiro FV, Giampaoli V, Viana Casarin RC. Influence of dyslipidemia and diabetes mellitus on chronic periodontal disease. J Periodontol. 2013;84:1401–1408. doi: 10.1902/jop.2012.120366. [DOI] [PubMed] [Google Scholar]
- 7.Taşdemir Z, Özsarı Taşdemir F, Koçyiğit İ, Yazıcı C, Gürgan CA. The clinical and systemic effects of periodontal treatment in diabetic and non-diabetic obese patients. J Oral Sci. 2016;58:523–531. doi: 10.2334/josnusd.16-0163. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Luo G, Xuan D, Wei B, Liu F, Li J, Zhang J. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. J Periodontol. 2012;83:435–443. doi: 10.1902/jop.2011.110327. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Liu N, Shi H, Wu H, Gao Y, He H, Gu B, Liu H. High glucose microenvironments inhibit the proliferation and migration of bone mesenchymal stem cells by activating GSK3β. J Bone Miner Metab. 2016;34:140–150. doi: 10.1007/s00774-015-0662-6. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Lee JY, Park YD, Kang KL, Lee JC, Heo JS. Hesperetin alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of periodontal ligament stem cells. PLoS One. 2013;8:e67504. doi: 10.1371/journal.pone.0067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Sun J, Dong Z, Xue P, He X, Liao L, Yuan L, Jin Y. GCN5 modulates osteogenic differentiation of periodontal ligament stem cells through DKK1 acetylation in inflammatory microenvironment. Sci Rep. 2016;6:26542. doi: 10.1038/srep26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong K, Qu B, Liao D, Liu D, Wang C, Zhou J, Pan X. MiR-132 regulates osteogenic differentiation via downregulating Sirtuin1 in a peroxisome proliferator-activated receptor β/δ-dependent manner. Biochem Biophys Res Commun. 2016;478:260–267. doi: 10.1016/j.bbrc.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 14.Qadir AS, Um S, Lee H, Baek K, Seo BM, Lee G, Kim GS, Woo KM, Ryoo HM, Baek JH. MiR-124 negatively regulates osteogenic differentiation and in vivo bone formation of mesenchymal stem cells. J Cell Biochem. 2015;116:730–742. doi: 10.1002/jcb.25026. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Hao W, Wang X, Su H. miR-23b targets Smad 3 and ameliorates the LPS-inhibited osteogenic differentiation in preosteoblast MC3T3-E1 cells. J Toxicol Sci. 2016;41:185–193. doi: 10.2131/jts.41.185. [DOI] [PubMed] [Google Scholar]
- 16.Zhu G, Chai J, Ma L, Duan H, Zhang H. Downregulated microRNA-32 expression induced by high glucose inhibits cell cycle progression via PTEN upregulation and Akt inactivation in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2013;433:526–531. doi: 10.1016/j.bbrc.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Baglìo SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527:321–331. doi: 10.1016/j.gene.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Deng Y, Zhou H, Zou D, Xie Q, Bi X, Gu P, Fan X. The role of miR-31-modified adipose tissue-derived stem cells in repairing rat critical-sized calvarial defects. Biomaterials. 2013;34:6717–6728. doi: 10.1016/j.biomaterials.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Zhen L, Fan DS, Zhang Y, Cao XM, Wang LM. Resveratrol ameliorates experimental periodontitis in diabetic mice through negative regulation of TLR4 signaling. Acta Pharmacol Sin. 2015;36:221–228. doi: 10.1038/aps.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M. Diabetes and periodontal diseases: interplay and links. Curr Diabetes Rev. 2011;7:433–439. doi: 10.2174/157339911797579205. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Chen T, Sun W, Yuan Z, Yu M, Chen G, Guo W, Xiao J, Tian W. DNA demethylation rescues the impaired osteogenic differentiation ability of human periodontal ligament stem cells in high glucose. Sci Rep. 2016;6:27447. doi: 10.1038/srep27447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato H, Taguchi Y, Tominaga K, Kimura D, Yamawaki I, Noguchi M, Yamauchi N, Tamura I, Tanaka A, Umeda M. High glucose concentrations suppress the proliferation of human periodontal ligament stem cells and their differentiation into osteoblasts. J Periodontol. 2016;87:e44–51. doi: 10.1902/jop.2015.150474. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29:1804–1816. doi: 10.1002/stem.728. [DOI] [PubMed] [Google Scholar]
- 24.You L, Gu W, Chen L, Pan L, Chen J, Peng Y. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2014;7:7249–7261. [PMC free article] [PubMed] [Google Scholar]
- 25.Pedrioli DM, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, Marino D, Kälin RE, Leidel S, Cinelli P, Schulte-Merker S, Brändli AW, Detmar M. MiR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30:3620–3634. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Cheng Y, Chen X, Yang J, Xu L, Zhang C. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem. 2011;286:42371–42380. doi: 10.1074/jbc.M111.261065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng H, Kaplan N, Hamanaka RB, Katsnelson J, Blatt H, Yang W, Hao L, Bryar PJ, Johnson RS, Getsios S, Chandel NS, Lavker RM. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc Natl Acad Sci U S A. 2012;109:14030–14034. doi: 10.1073/pnas.1111292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y, Gu P, Fan X. Effects of a miR-31, Runx2, and Satb2 regulatory loop on the osteogenic differentiation of bone mesenchymal stem cells. Stem Cells Dev. 2013;22:2278–2286. doi: 10.1089/scd.2012.0686. [DOI] [PubMed] [Google Scholar]
- 29.Ge J, Guo S, Fu Y, Zhou P, Zhang P, Du Y, Li M, Cheng J, Jiang H. Dental follicle cells participate in tooth eruption via the RUNX2-MiR-31-SATB2 loop. J Dent Res. 2015;94:936–944. doi: 10.1177/0022034515578908. [DOI] [PubMed] [Google Scholar]
- 30.Hu N, Feng C, Jiang Y, Miao Q, Liu H. Regulative effect of Mir-205 on osteogenic differentiation of bone mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and ERK/MAPK pathway. Int J MolSci. 2015;16:10491–10506. doi: 10.3390/ijms160510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Fariñas I, Karsenty G, Grosschedl R. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125:971–986. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]


