Abstract
Adipose-derived mesenchymal stem cells (ADMSCs) possess immunomodulation property, yet their therapeutic potential in asthma is unclear. This study aimed to explore the effects of ADMSCs on airway hyperresponsiveness and inflammation in ovalbumin (OVA)-induced asthma models. The underlying mechanism(s) was also examined. BALB/c mice were sensitized with OVA on days 0, 7, and 14, followed by 8-week OVA challenge from day 22. ADMSCs were injected via tail vein on day 21. Animals were measured for airway responsiveness, lung pathology, IgE and cytokine levels in serum, cell composition in bronchoalveolar lavage fluid (BALF), gene expression in the lung, and regulatory T cells (Tregs). We found that delivery of ADMSCs decreased airway responsiveness and eosinophil counts in BALF and reduced infiltration of inflammatory cells and number of mucus-expressing goblet cells in the lung in OVA-challenged mice. OVA-evoked elevation of serum IgE levels and alteration of cytokine production in serum and BALF was significantly prevented by ADMSCs. In addition, administration of ADMSCs impaired the regulation of lung IL-10, Foxp3, IL-17, and RORγ expression by OVA challenge and restored the percentage of CD4+CD25+Foxp3+ Tregs in the spleen. In conclusion, ADMSCs confer protection against OVA-induced airway hyperresponsiveness and inflammation, which is associated with induction of Tregs and restoration of immune homeostasis. These findings suggest that ADMSCs may have therapeutic implications for allergic asthma.
Keywords: Allergic asthma, immune homeostasis, mesenchymal stem cells, regulatory T cells
Introduction
Allergic asthma is a common respiratory disease characterized by airway inflammation, hyperresponsiveness, and remodeling. The imbalance between T helper (Th) 1 and Th2 responses is linked to the pathogenesis of asthma [1,2]. Th2 cytokines in particular interleukin (IL)-4, IL-5 and IL-13 contribute to IgE synthesis in the progression of asthma [3]. Inhibition of Th2 cytokines has shown therapeutic benefits in the treatment of Th2 cell-driven asthma [4]. Apart from Th2 cells, regulatory T cells (Tregs) and Th17 cells are also involved in the development of asthma [5,6]. It was reported that the percentage of Tregs is significantly lower in asthmatic patients than that in normal controls [6]. Induction of Tregs has been found to alleviate airway inflammation in an ovalbumin (OVA)-induced asthma model [7]. Clinical evidence indicates that IL-17 from Th17 cells is upregulated in patients with asthma and positively correlated with disease severity [8]. Therefore, restoration of the homeostasis of immune cells is of importance in the control of asthma.
Stem cell-based therapy against asthma is attracting increasing attention [9,10]. It has been documented that delivery of bone marrow-derived mesenchymal stem cells (MSCs) significantly improves lung pathology and inflammation in OVA-induced asthma models [9]. Similarly, administration of human umbilical cord-derived MSCs can suppress the production of Th2 cytokines and lung inflammation in asthmatic mice [10]. Adipose tissues are an important source of MSCs. Adipose-derived MSCs (ADMSCs) exhibit immunosuppressive property, which is supported by the finding that allogeneic transplantation of ADMSCs can control graft-versus-host disease [11]. However, few studies have addressed the therapeutic potential of ADMSCs in asthma.
Therefore, in this study, we explored the effects of injection of ADMSCs on airway hyperresponsiveness and inflammation in OVA-induced asthma models. The effects of ADMSCs on Treg numbers and cytokine expression were also examined.
Materials and methods
Mice
Six-to-eight week-old male BALB/c mice (weighing 16-18 g) were obtained from Shanghai Silaike Laboratory Animal Co., Ltd. (Shanghai, China) and housed at 23 ± 3°C under a 12-h light/dark cycle with free access to water and lab chow. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Shanghai Jiaotong University (Shanghai, China).
Isolation and characterization of ADMSCs
Isolation of ADMSCs was performed as described previously [12]. In brief, inguinal fat pads were harvested from mice, minced into 5-mm3 pieces, and digested with 0.1% collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 40 min. After centrifugation at 300 g for 10 min, the pellet was resuspended in alpha-MEM supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) at 37°C with 5% CO2. Upon reaching 80-90% confluence, the cells were subcultured. At passages 3-8, ADMSCs were used in this study.
ADMSCs were stained with phycerythrin (PE)-conjugated anti-mouse monoclonal antibodies anti-CD90, anti-CD44, anti-CD29, anti-CD34, and anti-CD45 (1:500 dilution; BD Pharmingen, San Diego, CA, USA) for 30 min in the dark at 4°C. Stained cells were analyzed by a flow cytometer. Adipogenic and osteogenic differentiation was performed as described previously [13]. In brief, cells were grown on coverslips in 24-well plates (2 × 104 cells/well) and stimulated with osteogenic or adipogenic medium (Sigma-Aldrich). After incubation for 10 and 16 days, adipogenic differentiation was evaluated by Oil red O staining (Sigma-Aldrich) and osteogenic differentiation by Alizarin Red staining (Sigma-Aldrich).
Animal groups and experimental protocol
Mice were randomly divided into 4 groups (n = 10): control group, ADMSC group, OVA group, and OVA+ADMSC group. OVA-induced allergic asthma was generated as described previously [14]. In brief, each mouse was injected intraperitoneally with 0.1% OVA (Sigma-Aldrich) on Day 0, Day 7, and Day 14. ADMSCs (1 × 106 cells in 100 μL of PBS) were injected via tail vein on Day 21. From Day 22, mice were challenged with 2.5% OVA 3 times (30 min/time) per week for 8 consecutive weeks. In control animals, equal volume of phosphate-buffered saline (PBS) was administered intraperitoneally instead of OVA. All mice were sacrificed 24 h after the last challenge, and samples were collected for further analyses.
Airway responsiveness measurement
Mice were anesthetized with a combination of midazolam (5 mg/kg) and ketamine (100 mg/kg), and the trachea were exposed and cannulated. Airway responsiveness was determined with an EMMS system (EMMS, Hampshire, UK) by measuring lung resistance (RL) in response to inhaled methacholine (MCh; Sigma-Aldrich) at concentrations of 4 to 256 mg/mL for 20 s. The provocative challenge 100 (PC100), the MCh dose at which RL was 100% above baseline level, was calculated.
Bronchoalveolar lavage fluid (BALF)
BALF was collected by cannulating the trachea and lavaging three times with 0.8 mL of cold PBS. BALF samples were centrifuged and the supernatants were stored at -80°C for cytokine analysis. The cell pellets were resuspended. Total cell number and differential cell counts were determined after staining with Wright and Giemsa solutions.
Measurement of cytokines and IgE
The levels of IL-1β, IL-4, IL-17, IL-10, IFN-γ, and IgE in serum samples and IL-4, IL-17F, IL-10, and IFN-γ in BALF were measured using ELISA kits (eBioscience, San Diego, CA, USA), following the manufacturer’s instructions.
Lung histopathology
The left lung was fixed with 10% buffered formalin, cut into sections (5 μm), and subjected to hematoxylin and eosin (H&E) staining, periodic acid-Schiff (PAS) staining, and anti-Muc5AC immunostaining with anti-Muc5AC antibody (Abcam, Cambridge, UK).
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA from right lung tissue samples was extracted using Trizol (Invitrogen) and reverse transcribed to cDNA. PCR primers are summarized as follows: Foxp3 forward: 5’-GCCTTCAGACGAGACTTG-3’, Foxp3 reverse: 5’-CATTGGGTTCTTGTCAGAG-3’; RORγt forward: 5’-CAGGAGCAATGGAAGTCGTC-3’, RORγt reverse: 5’-CCGTGTAGAGGGCAATCTCA-3’; IL-10 forward: 5’-CCCTTTGCTATGGTGTCCT-3’, IL-10 reverse: 5’-GGATCTCCCTGGTTTCTCTT-3’; IL-17F forward: 5’-AGGGAAGAAGCAGCCATT-3’, IL-17F reverse: 5’-CCAACATCAACAGTAGCAAA-3’; β-actin forward: 5’-CAGAAGGACTCCTACGTG-3’, β-actin reverse: 5’-GCTCGGTCAGGATCTTCATG-3’. Cycling conditions were as follows: initial denaturation at 94°C for 10 min and 35 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 40 s. The relative mRNA levels were determined by normalization to the level of β-actin mRNA.
Analysis of CD4+CD25+Foxp3+ Tregs in the spleen by flow cytometry
Cells from the spleen were prepared via gentle homogenization through nylon mesh and suspended in Red Blood Cell Lysis Buffer for 5 min. After washing, spleen cells were incubated with anti-mouse CD4-FITC, CD25-APC, and Foxp3-PE antibodies. CD4+CD25+Foxp3+ Tregs were analyzed by flow cytometry.
Statistical analysis
Data are presented as mean ± standard deviation. Differences among multiple groups were determined using one-way analysis of variance followed by the Fishers least significant difference post hoc analysis. P < 0.05 was considered statistically significant.
Results
Characterization of ADMSCs
The isolated ADMSCs had a spindle shape, a typical morphology of MSCs. Immunophenotyping of them by flow cytometry revealed that the ADMSCs showed abundant expression of MSC markers CD90, CD44, and CD29, but negative for hematopoietic markers CD45 and CD34 (data not shown). To evaluate the differentiation potential of ADMSCs, they were cultured in adipogenic and osteogenic medium. As determined by Oil red O staining, ADMSCs in adipogenic medium displayed accumulation of lipid droplets (Figure 1A). In addition, Alizarin Red staining confirmed remarkable osteogenic differentiation in ADMSCs after stimulation with osteogenic medium (Figure 1B). These observations indicate that the isolated ADMSCs have multipotential differentiation capacity.
Figure 1.
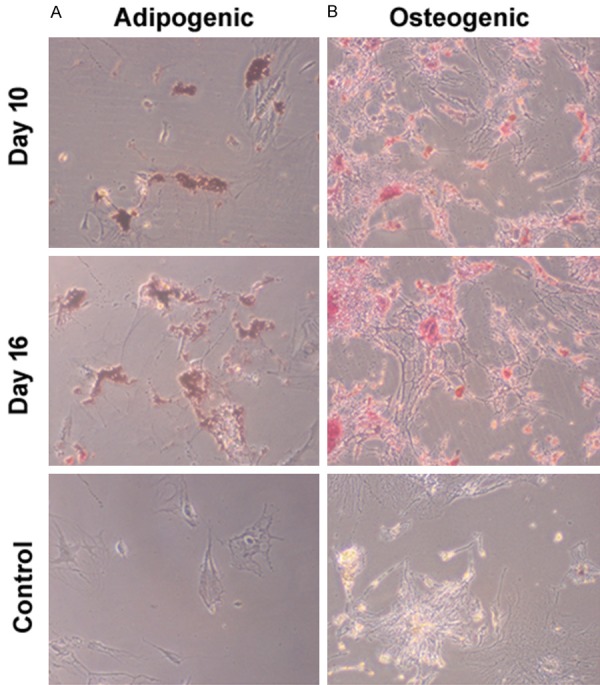
Characterization of ADMSCs. ADMSCs were cultured in adipogenic and osteogenic medium or DMEM (control) and tesed for adipogenic and osteogenic differentiation. A. Representative images of cells stained with Oil red O. B. Representative images of cells stained with Alizarin Red. Magnification, × 200.
Administration of ADMSCs attenuates OVA-induced airway hyperresponsiveness and inflammation
Next, we examined the effects of delivery of ADMSCs on OVA-induced airway hyperresponsiveness after the last challenge using inhaled MCh (4 to 256 mg/mL). OVA-challenged mice displayed significantly higher RL (Figure 2A) and lower -logPC100 (Figure 2B) than control animals, suggesting airway hyperreactivity induced by OVA. Notably, delivery of ADMSCs via tail vein resulted in a significant decline in RL and increase in -logPC100 values.
Figure 2.
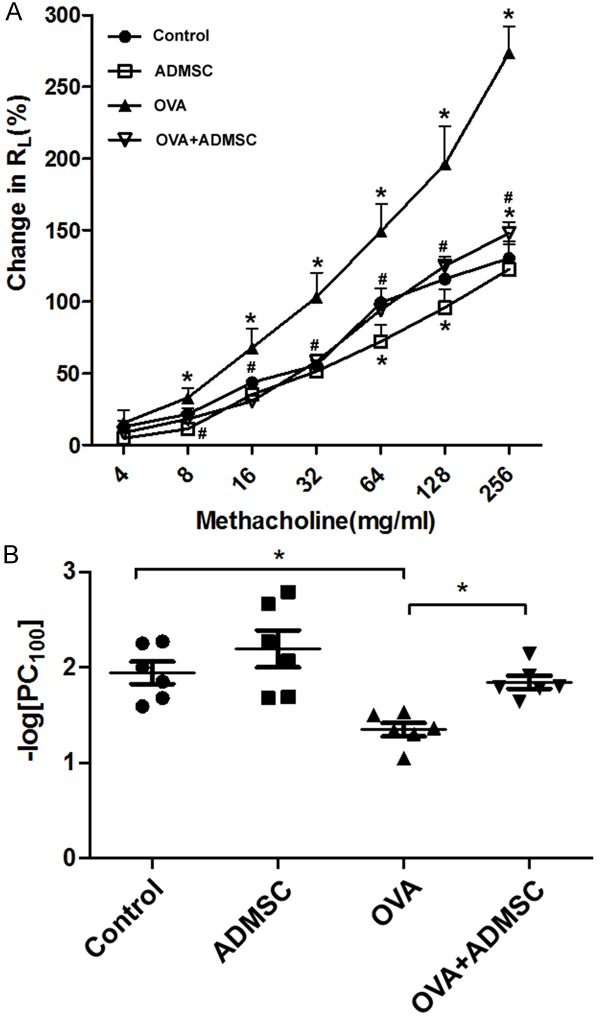
Administration of ADMSCs attenuates OVA-induced airway hyperresponsiveness. The effects of delivery of ADMSCs on OVA-induced airway hyperresponsiveness after the last challenge were determined using inhaled methacholine (MCh) at 4 to 256 mg/mL. A. Measurement of lung resistance (RL). B. Measurement of the provocative challenge 100 (PC100), the MCh dose at which RL was 100% above baseline level. *P < 0.05 vs. the control group; #P < 0.05 vs. the OVA group.
Histological analysis of lung tissues revealed that compared to the OVA group, the OVA+ADMSC treatment group had marked reductions in the number of inflammatory cells (Figure 3A) and PAS+ mucus-expressing goblet cells (Figure 3B), as wells as in the production of Muc5ac (Figure 3C). The numbers of total cells, eosinophils, and lymphocytes in BALF were significantly reduced in the OVA+ADMSC group compared to the OVA group (P < 0.05; Figure 3D).
Figure 3.
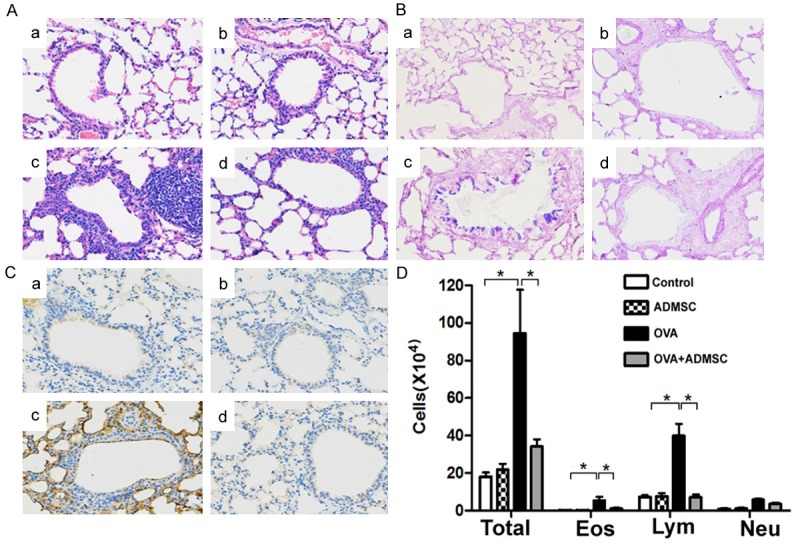
Administration of ADMSCs attenuates OVA-induced airway inflammation. (A-C) Representative lung tissue sections stained with H&E (A), periodic acid-Schiff (B), and anti-Muc5AC antibody (C). (A-C) Control, ADMSC, OVA, and OVA+ADMSC groups, respectively. Magnification, × 200. (D) Total and differential cell counts in BALF. The numbers of total cells, eosinophils (EOS), lymphocytes (Lym), and neutrophils (Neu) in BALF were significantly reduced in the OVA+ADMSC group compared to the OVA group. *P < 0.05.
Administration of ADMSCs decreases serum IgE levels and alters serum and BALF cytokine levels
OVA challenge led to a significant increase in serum IgE levels, while delivery of ADMSCs caused a significant reduction in serum IgE levels, compared to baseline values (Figure 4A). Moreover, OVA-induced elevation of IgE levels was partially rescued by injection of ADMSCs (Figure 4A). Compared to control animals, OVA-challenged mice had significantly greater levels of serum IL-1β, IL-4, and IL-17F and lower levels of serum IL-10 and IFN-γ (Figure 4B). Of note, OVA-induced alteration of cytokine levels was significantly reversed after delivery of ADMSCs. Similarly, transplantation of ADMSCs significantly reduced the induction of IL-4 and IL-17F and restored the production of IL-10 and IFN-γ in BALF after OVA challenge (Figure 4C).
Figure 4.
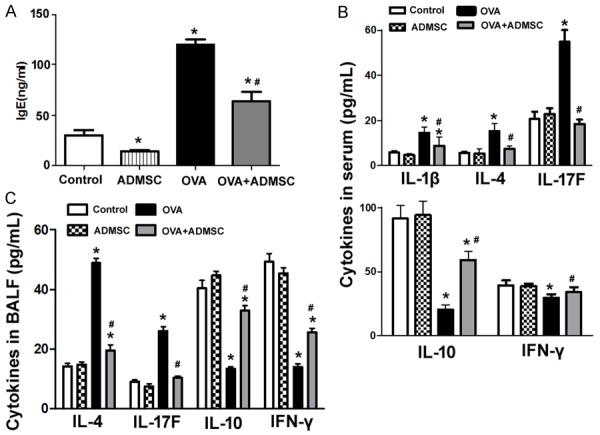
Administration of ADMSCs decreases serum IgE levels and alters serum and BALF cytokine levels. A. Measurement of IgE levels in serum samples by ELISA. Delivery of ADMSCs caused a significant reduction of serum IgE. B. Measurement of indicated cytokine levels in serum by ELISA. Compared to control animals, OVA-challenged mice had significantly greater levels of serum IL-1β, IL-4, and IL-17F and lower levels of serum IL-10 and IFN-γ. C. Measurement of indicated cytokine levels in BALF by ELISA. Transplantation of ADMSCs significantly reduced IL-4 and IL-17F production and restored the levels of IL-10 and IFN-γ in BALF after OVA challenge. *P < 0.05 vs. the control group; #P < 0.05 vs. the OVA group.
Delivery of ADMSCs modulates gene expression in the lung and restores the percentage of Tregs in the spleen
OVA challenge caused a significant reduction in IL-10 (Figure 5A) and Foxp3 (Figure 5B) transcripts and increase in IL-17 (Figure 5C) and RORγ (Figure 5D) transcripts in the lung. Such gene alteration was partially prevented by delivery of ADMSCs. In addition, administration of ADMSCs raised the percentage of CD4+CD25+Foxp3+ Tregs in the spleen in OVA-challenged mice (Figure 5E).
Figure 5.
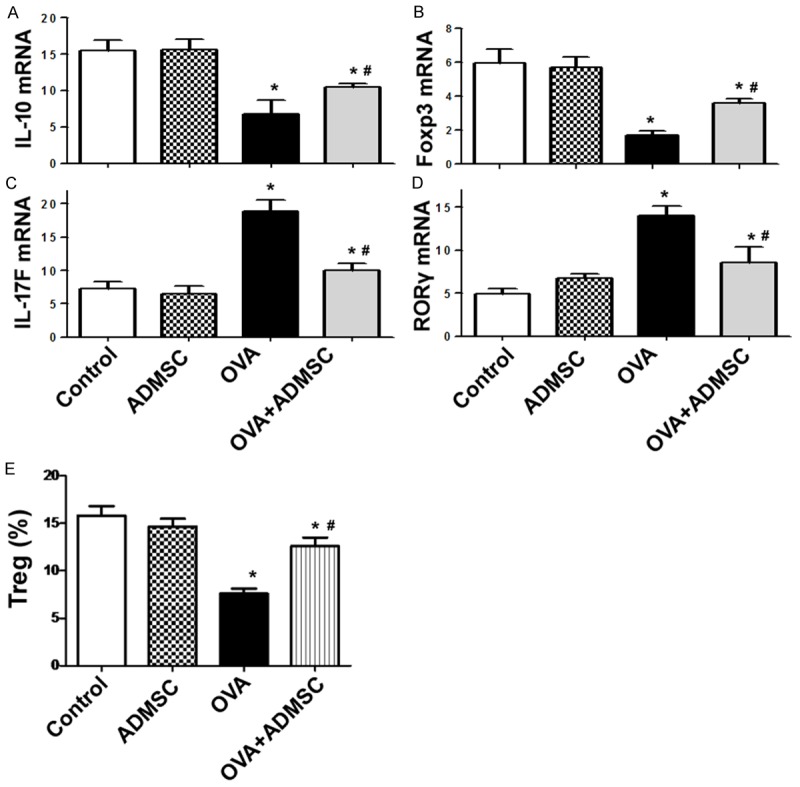
Delivery of ADMSCs modulates gene expression in the lung and restores the percentage of Tregs in the spleen. A-D. qRT-PCR analysis of indicated mRNAs in the lung from each group. E. Flow cytometric analysis of the percentage of CD4+CD25+Foxp3+ Tregs. *P < 0.05 vs. the control group; #P < 0.05 vs. the OVA group.
Discussion
Allergic asthma is a complex respiratory disorder that involves airway hyperreactivity and inflammation. Bone marrow-derived MSCs have shown anti-inflammatory properties in animal models of asthma [15,16]. Compared to bone marrow-derived MSCs, ADMSCs exhibit a higher immunomodulatory capacity [17]. Moreover, easy access and high incidence of MSCs make adipose tissue as a promising alternative source of MSCs [18]. Therefore, in this study we evaluated the potential of ADMSCs in the treatment of allergic asthma in mouse models. The isolated ADMSCs expressed MSC markers CD90, CD44, and CD29 and showed adipogenic and osteogenic differentiation capacity. When ADMSCs were injected to asthmatic mice via tail vein, OVA challenge-induced airway hyperresponsiveness and inflammation was mitigated. Pathological analysis demonstrated that OVA-induced infiltration of inflammatory cells to the lung was remarkably attenuated by ADMSCs. Eosinophils are the key inflammatory cells involved in the pathogenesis of allergic asthma [19]. It has been reported that eosinophils can promote CD4+ T cells to secrete IL-4 and IL-5 inflammatory cytokines [20]. We found that BALF from ADMSC-treated, OVA-challenged mice had significantly lower numbers of eosinophils than the counterpart from untreated OVA-challenged mice. In addition to reduction of inflammatory cells, the number of PAS+ mucus-expressing goblet cells were also decreased in the OVA+ADMSC treatment group. Consistently, the production of Muc5ac, a major mucus component in asthma, was markedly lowered by delivery of ADMSCs. Therefore, ADMSCs show the ability to suppress eosinophilic inflammation and mucus overproduction in experimental asthma.
Interestingly, we found that injection of ADMSCs significantly impaired OVA challenge-induced IgE production. Moreover, OVA-induced alteration of cytokine expression in serum and BALF was significantly reversed by ADMSCs. IL-1β, IL-4, and IL-17F are important cytokines implicated in the pathogenesis of asthma [21]. Targeting IL-4 [22] and IL-17 [23] has been suggested as a potential therapeutic strategy for asthma. IFN-γ is a major Th1 cytokine and has been found to increase airway hyperresponsiveness in severe asthma [24]. However, modulation of the Th1/Th2 cytokine balance contributes to pharmacological inhibition of asthma development [25]. Therefore, the alteration of OVA-induced cytokine profiles may provide an explanation for the protective activity of ADMSCs in asthma.
It was found that OVA challenge significantly suppressed the mRNA expression of IL-10 and Foxp3 and raised the mRNA expression of IL-17 and RORγ in the lung, suggesting an impairment of immune homeostasis. Foxp3 is an important biomarker for Tregs that play a pivotal role in controlling such Th2-biased immune responses [26]. Consistently, OVA challenge resulted in a significant reduction of CD4+CD25+Foxp3+ Tregs in the spleen. Notably, delivery of ADMSCs partially restored the expression of Foxp3 in the lung and the percentage of CD4+CD25+Foxp3+ Tregs in the spleen in OVA-challenged mice. It has been documented that IL-17-producing Th17 cells have the capacity to regulate neutrophilic and macrophage inflammation in the lung in asthma [27]. These findings suggest that the antiathmatic activity of ADMSCs is, at least in part, ascribed to stimulation of Treg and Th17 cell responses.
However, this study has several limitations. First, key molecular mediators for the attenuation of asthma by ADMSCs are not clarified. Second, the fate, distribution, and long-term consequence of ADMSCs in mice remain to be further determined.
In conclusion, our findings demonstrate that ADMSCs have the capacity to improve OVA-evoked airway hyperresponsiveness and inflammation, at least partially, through induction of Tregs and restoration of immune homeostasis. Delivery of ADMSCs may represent a promising therapeutic strategy for treatment of allergic asthma.
Acknowledgements
This work was supported by the Youth research fund of Shanghai Municipal Health Bureau of China (2012Y119) to R. Dai and the National Natural Science Foundation of China (81470218) to X. Zhou.
Disclosure of conflict of interest
None.
References
- 1.Su X, Pan J, Bai F, Yuan H, Dong N, Li D, Wang X, Chen Z. IL-27 attenuates airway inflammation in a mouse asthma model via the STAT1 and GADD45γ/p38 MAPK pathways. J Transl Med. 2016;14:283. doi: 10.1186/s12967-016-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Q, Tang Y, Hu X, Wang Q, Lei W, Zhou L, Huang J. Regulation of Th1/Th2 balance through OX40/OX40L signalling by glycyrrhizic acid in a murine model of asthma. Respirology. 2016;21:102–111. doi: 10.1111/resp.12655. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF. Asthma phenotyping: a necessity for improved therapeutic precision and new targeted therapies. J Intern Med. 2016;279:192–204. doi: 10.1111/joim.12382. [DOI] [PubMed] [Google Scholar]
- 4.Vale K. Targeting the JAK-STAT pathway in the treatment of ‘Th2-high’ severe asthma. Future Med Chem. 2016;8:405–419. doi: 10.4155/fmc.16.4. [DOI] [PubMed] [Google Scholar]
- 5.Tao B, Ruan G, Wang D, Li Y, Wang Z, Yin G. Imbalance of peripheral Th17 and regulatory T cells in children with allergic rhinitis and bronchial asthma. Iran J Allergy Asthma Immunol. 2015;14:273–279. [PubMed] [Google Scholar]
- 6.Chu M, Chu IM, Yung EC, Lam CW, Leung TF, Wong GW, Wong CK. Aberrant expression of novel cytokine IL-38 and regulatory T lymphocytes in childhood asthma. Molecules. 2016:21. doi: 10.3390/molecules21070933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Baek H, Jung KH, Lee G, Lee H, Kang GH, Lee G, Bae H. Bee venom phospholipase A2 suppresses allergic airway inflammation in an ovalbumin-induced asthma model through the induction of regulatory T cells. Immun Inflamm Dis. 2015;3:386–397. doi: 10.1002/iid3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CK, Lun SW, Ko FW, Wong PT, Hu SQ, Chan IH, Hui DS, Lam CW. Activation of peripheral Th17 lymphocytes in patients with asthma. Immunol Invest. 2009;38:652–664. doi: 10.1080/08820130903062756. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadian M, Boskabady MH, Kashani IR, Jahromi GP, Omidi A, Nejad AK, Khamse S, Sadeghipour HR. Effect of bone marrow derived mesenchymal stem cells on lung pathology and inflammation in ovalbumin-induced asthma in mouse. Iran J Basic Med Sci. 2016;19:55–63. [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CK, Lin TC, Huang YA, Chen YS, Wu CL, Lo HY, Kuo ML, Wu KH, Huang JL. The modulation of Th2 immune pathway in the immunosuppressive effect of human umbilical cord mesenchymal stem cells in a murine asthmatic model. Inflamm Res. 2016;65:795–801. doi: 10.1007/s00011-016-0961-y. [DOI] [PubMed] [Google Scholar]
- 11.Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012;21:2770–2778. doi: 10.1089/scd.2012.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng L, Kong X, Liu G, Li C, Chen H, Hong Z, Liu J, Xia J. Transplantation of adipose-derived mesenchymal stem cells efficiently rescues thioacetamide-induced acute liver failure in mice. Transplant Proc. 2016;48:2208–2215. doi: 10.1016/j.transproceed.2016.02.077. [DOI] [PubMed] [Google Scholar]
- 13.Tso GH, Law HK, Tu W, Chan GC, Lau YL. Phagocytosis of apoptotic cells modulates mesenchymal stem cells osteogenic differentiation to enhance IL-17 and RANKL expression on CD4+ T cells. Stem Cells. 2010;28:939–954. doi: 10.1002/stem.406. [DOI] [PubMed] [Google Scholar]
- 14.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolised allergen. Thorax. 1998;53:849–856. doi: 10.1136/thx.53.10.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmadi M, Rahbarghazi R, Soltani S, Aslani MR, Keyhanmanesh R. Contributory anti-inflammatory effects of mesenchymal stem cells, not conditioned media, on ovalbumin-induced asthmatic changes in male rats. Inflammation. 2016;39:1960–1971. doi: 10.1007/s10753-016-0431-2. [DOI] [PubMed] [Google Scholar]
- 16.Urbanek K, De Angelis A, Spaziano G, Piegari E, Matteis M, Cappetta D, Esposito G, Russo R, Tartaglione G, De Palma R, Rossi F, D’Agostino B. Intratracheal administration of mesenchymal stem cells modulates tachykinin system, suppresses airway remodeling and reduces airway hyperresponsiveness in an animal model. PLoS One. 2016;11:e0158746. doi: 10.1371/journal.pone.0158746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daubeuf F, Frossard N. Eosinophils and the ovalbumin mouse model of asthma. Methods Mol Biol. 2014;1178:283–293. doi: 10.1007/978-1-4939-1016-8_24. [DOI] [PubMed] [Google Scholar]
- 20.Shim EJ, Chun E, Lee HS, Bang BR, Cho SH, Min KU, Park HW. Eosinophils modulate CD4(+) T cell responses via high mobility group Box-1 in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2015;7:190–194. doi: 10.4168/aair.2015.7.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh GM. Anti-IL-4/-13 based therapy in asthma. Expert Opin Emerg Drugs. 2015;20:349–352. doi: 10.1517/14728214.2015.1050377. [DOI] [PubMed] [Google Scholar]
- 23.Lindén A, Dahlén B. Interleukin-17 cytokine signalling in patients with asthma. Eur Respir J. 2014;44:1319–1331. doi: 10.1183/09031936.00002314. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi M, Ashino S, Shiohama Y, Wakita D, Kitamura H, Nishimura T. IFN-γ elevates airway hyper-responsiveness via up-regulation of neurokinin A/neurokinin-2 receptor signaling in a severe asthma model. Eur J Immunol. 2012;42:393–402. doi: 10.1002/eji.201141845. [DOI] [PubMed] [Google Scholar]
- 25.Fang SP, Tanaka T, Tago F, Okamoto T, Kojima S. Immunomodulatory effects of gyokuheifusan on INF-gamma/IL-4 (Th1/Th2) balance in ovalbumin (OVA)-induced asthma model mice. Biol Pharm Bull. 2005;28:829–833. doi: 10.1248/bpb.28.829. [DOI] [PubMed] [Google Scholar]
- 26.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 27.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]


