Abstract
Polycystic ovarian syndrome (PCOS), with a prevalence of 5-8%, is the most common endocrine disorder in women of reproductive age. Although cryptotanshinone (CRY) has been shown to effectively reverse reproductive disturbances, it remains poorly understood whether it can be used to treat PCOS. Here we used an established rat model of PCOS. Prepubertal female Sprague-Dawley rats were subcutaneously injected with dehydroepiandrosterone (DHEA) 60 mg/kg/day for 3 weeks. Those in the DHEA group were given normal saline, while the CRY group was given 27 mg/kg by gastrogavage for 3 weeks. DHEA-treated rats had irregular estrous cycles and significantly increased ovarian weight compared to control rats (P < 0.05), and 3 weeks of treatment with CRY in DHEA-treated rats significantly decreased ovarian weight (P < 0.05). CRY decreased the levels of testosterone, estradiol, and luteinizing hormone (LH) as well as the LH/follicle stimulating hormone ratio (P < 0.05). CRY decreased inhibin B and follistatin mRNA and protein expression but upregulated activin A mRNA and protein expression in the ovarian tissue of rats with PCOS (P < 0.05). These results suggest that CRY treatment could reverse reproductive disturbances in rats with PCOS.
Keywords: Cryptotanshinone, reproductive disturbances, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic dysfunction causing infertility in 3-10% of reproductive-aged women [1]. PCOS prevents follicles within the ovary from producing and releasing mature eggs. This results in polycystic ovaries, infertility and hormonal imbalances. Neuroendocrine abnormalities are reflected by an imbalance in gonadotropin secretion characterized by elevated levels of luteinizing hormone (LH) and low levels of follicle-stimulating hormone (FSH) [2]. The basic clinical and pathological features of PCOS are chronic persistent anovulation and hyperandrogenism, bilateral cystic enlargement of the ovaries, and significantly increased androgen levels with relatively insufficient estradiol in the peripheral blood [3]. Many studies have found that hyperandrogenism is the primary manifestation of PCOS, and this excess androgen exposure appears to be the cause of the PCOS morphology. Local ovarian hyperandrogenism is caused by abnormalities in enzymatic activity or in the expression of ovarian granular cell layer P450arom and theca cell P450c17a [4].
Inhibins A and B are peptides produced in the ovary that can regulate pituitary FSH secretion by an endocrine mechanism and ovarian steroidogenesis. Activins A and B stimulate FSH secretion by the pituitary, stimulate follicular development and function, and inhibit thecal cell androgen production. Follistatin is known to inhibit FSH secretion and regulate folliculogenesis and ovarian steroidogenesis, primarily by modulating activin activity. Accordingly, increases in follistatin may arrest follicular development and increase ovarian androgen production, both of which are fundamental in PCOS [5-7].
Salvia miltiorrhiza Bunge has been often prescribed for cardiovascular disorders, and some endocrine metabolic diseases such as diabetes. Cryptotanshinone, traditionally known as tanshinone, was originally isolated from the dried roots of Salvia miltiorrhiza Bunge. CRY is the main and active component of Salvia miltiorrhiza Bunge and previous studies have shown that it can effectively ameliorate androgen excess and insulin resistance in a PCOS rat model induced by dehydroepiandrosterone (DHEA) [8,9].
Here we aimed to test the hypothesis that CRY reverses reproductive disturbances in rats with DHEA-induced PCOS. We also examined the effects of CRY on ovarian quotiety, reproductive hormone levels, and gene expression related to inhibin B, activin A, and follistatin in rat ovarian tissues. In addition, the estrous cycle changes and protein expression levels in ovarian tissues involved in sex steroidogenesis were investigated.
Materials and methods
Animals
A total of 30 adult Sprague-Dawley female rats (age 21 days weighing 35-40 g) were supplied by Shanghai Laboratory Animal Center of Chinese Academy of Sciences (animal license no. SCXK-Shanghai 02569). The rats were housed in standard cages at a constant temperature of 22 ± 2°C and humidity of 55 ± 5% and allowed free access to food and water with a 12-hour light/dark cycle. All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China.
Agents
CRY (> 98% pure) was purchased from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China). DHEA was purchased from Sigma (no. 723266). Sesame oil was purchased from Sigma (no. 1612404).
Establishment of animal model of PCOS and treatments
The PCOS rat model was prepared by a modified method as described previously [10,11]. At 21 days of age, the rats were subcutaneously injected with DHEA 60 mg/kg/day (dissolved into 0.2 mL of sesame oil) to induce PCOS, while the control group was subcutaneously injected with sesame oil 0.2 mL/day only. DHEA-induced rats were injected for 3 weeks, after which time the vaginal exfoliated cytology was examined for 10 continuous days. The successful PCOS model rats were selected and randomly divided into two groups: DHEA, given normal saline; and CRY, given equal volume of CRY (27 mg/kg) orally. Both groups were subjected to administration once daily between 9:00 and 10:00 A.M. for 3 weeks.
Vaginal cytology
Estrous cycle stage was determined using vaginal smears. The animals were subjected to vaginal cytology examinations daily starting from 3 weeks of age until the end of the study. One drop of the vaginal lavage was placed on a glass slide and air-dried. The smears were stained with methylene blue [12]. Estrous cyclicity was evaluated as the percentage of the time in each phase (diestrous, proestrous, estrous, and metestrous) 1 week after the start of the treatment.
Determination of serum FSH, LH, T, P, E2, inhibin B, activin A and follistatin
At the end of the study, blood samples were collected and centrifuged at 3,000 rpm (1,500 × g) for 15 min to obtain serum. Serum FSH, LH, T, P, E2, inhibin B, activin A and follistatin levels were measured by a rat enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions. Absorbance was measured at 450 nm.
Measurement of mRNA expression in rat ovarian tissues
At the end of the study, all rats were killed by rapid decapitation. The brains were dissected on ice and the prefrontal cortexes were quickly isolated by dissection and immediately stored at -80°C for determination of biochemical measurements. The total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and the concentration was determined using a BioPhotometer (Eppendorf, Hamburg, Germany). Complementary DNA was synthesized from 2.5 μg of total RNA using a PrimeScript RT reagent kit (TaKaRa, Dalian, China). Real-time polymerase chain reaction was performed in triplicate and detected by a SYBR Premix Ex Taq™ Kit (TaKaRa) using a Light Cycler 2.0 System (Roche, Basel, Switzerland). The relative mRNA expression levels were normalized to β-actin. The primers were designed by Oligo Perfect™ Designer (Invitrogen, see Table 1). Fold changes in gene expression were estimated using the CT comparative method normalizing β-actin CT values and relative to control samples as follows: ΔCt = Ct assayed samples-Ct β-actin; ΔΔCt = ΔCt-ΔCt control; fold difference = 2-(ΔΔCT).
Table 1.
Sequences of primer pairs for quantitative real-time PCR
| Gene name | Forward primer sequences [5’-3’] | Reverse primer sequences [5’-3’] | Length |
|---|---|---|---|
| Inhibin B | GAACCAGAGGAGGAAGATGTC | CCAGATGATAGCACCAGAAGA | 260 |
| Activin A | AAGTTGGCGTTCGCCGTCTTT | CACAGGTCCACATCAACACTG | 813 |
| Follistatin | AGTCCAGTACCAAGGCAGATGT | GGTCACACAGTAGGCATTATTGG | 618 |
| β-actin | CTGAGAGGGAAATCGTGCGT | CCACAGGATTCCATACCCAAGA | 208 |
Measurement of protein expression in rat ovarian tissues
The rat brains were rinsed with cold phosphate buffered saline and then scraped into ice-cold lysis buffer with protease and phosphatase Inhibitor Cocktail (Roche, Meylan, France). The protein concentration was assessed using a BCA protein assay (Pierce, Rockford, IL, USA). An equal amount of protein was separated using 4-20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Hybond; Amersham, Arlington Heights, IL, USA). The blots were incubated with the appropriate antibodies, including anti-inhibin B, anti-activin A, and anti-follistatin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The signals were detected using Super Signal Immobilon Western Chemiluminescent HRP Substrate (Millipore, France) and quantified by densitometry using an image analysis system (Bio-Tanon, Shanghai, China). The data were normalized using β-actin to ensure equal loading and are expressed as a ratio of the experimental group to the control group.
Statistical analysis
All values are presented as mean ± standard error of the mean. The differences among multiple groups were compared using one-way analysis of variance (ANOVA). Individual differences derived from the one-way ANOVA were analyzed using the Bonferroni post hoc test after demonstrating significant intergroup differences using ANOVA. Values of P < 0.05 were considered significant.
Results
Effects of CRY on ovarian quotiety in rats with PCOS
The ovaries were heavier in DHEA rats than in controls (190.1 ± 8.2 mg vs. 150.3 ± 6.9 mg, P < 0.05). The average ovarian weight of the CRY rats was significantly lower than that of the DHEA rats (160.7 ± 10.4 mg, P < 0.05) (Figure 1A). The average body weight of the DHEA rats (275.2 ± 10.2 g) was significantly higher than that of the control (223.2 ± 12.1 g, P < 0.05) and CRY rats (252.5 ± 9.4 g, P < 0.05) (Figure 1B). The average ovarian quotiety of the DHEA rats (0.78 ± 0.04 mg/g) was significantly increased compared to that of the control rats (0.63 ± 0.03 mg/g, P < 0.05), but statistically decreased compared to that of the DHEA rats (0.64 ± 0.05 mg/g, P < 0.05) (Figure 1C).
Figure 1.
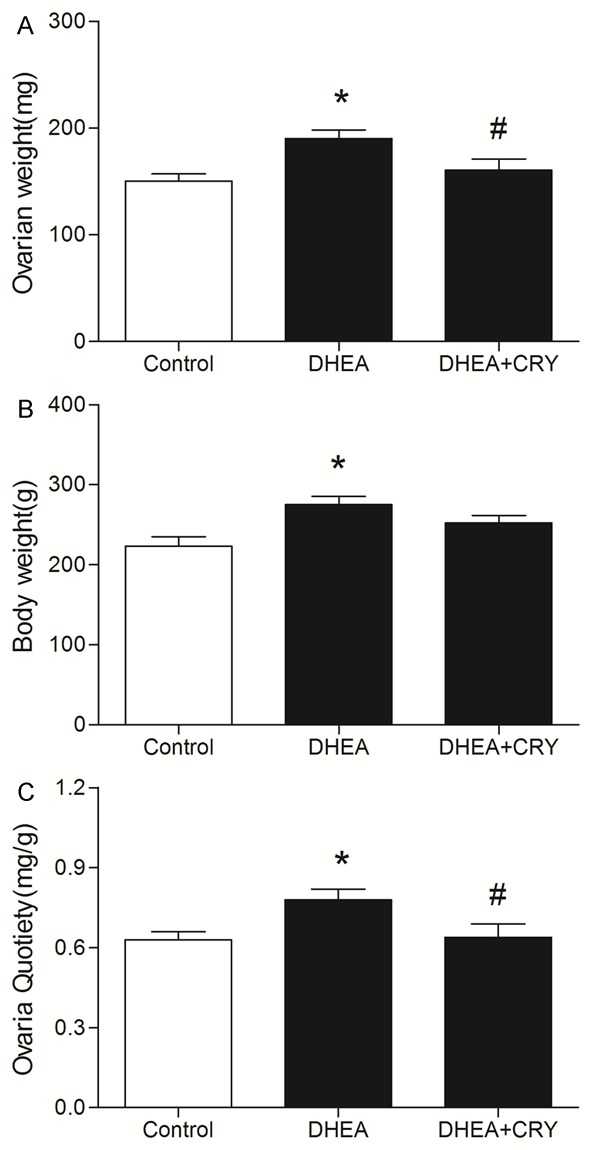
The effects of CRY on (A) ovarian weight, (B) body weight and (C) ovaries quotiety in rats with DHEA-induced PCOS in the following groups: control, DHEA and DHEA + CRY group. Ovaries Quotiety = ovarian weight/body weight (mg/100 g). The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus DHEA.
Effects of CRY on estrous cycle in rats with PCOS
The control rats had a normal estrous cycle of 4-5 days. Rats exposed to DHEA were acyclic, and leukocytes were predominant in their vaginal smears, indicating pseudo-diestrous. On the other hand, after 3 weeks of CRY treatment, DHEA-exposed rats showed changed estrous cycles in which three animals per group were in the estrous phase at least once during a 6-day period, whereas only one animal in the DHEA group was in estrous during the same period (Figure 2).
Figure 2.
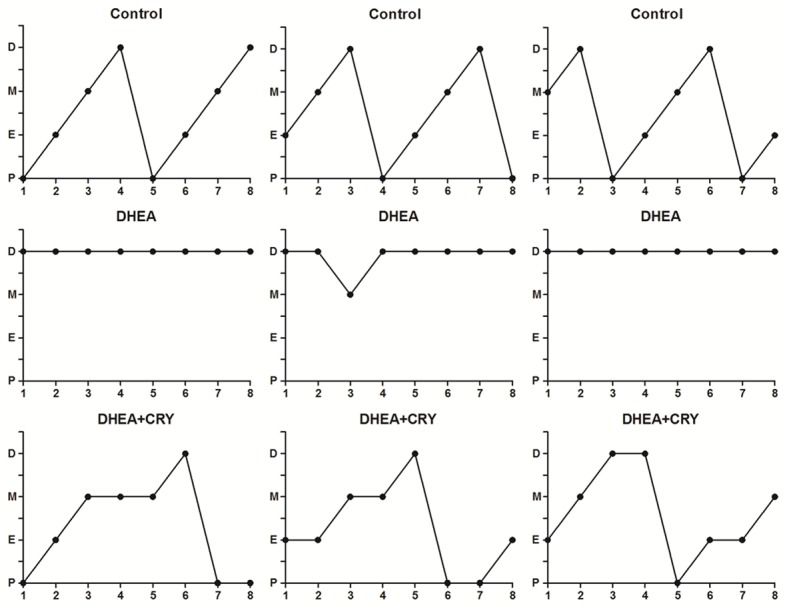
Estrous cycle pattern in the three groups. Vaginal cytology was done every day starting at 3 weeks of life till the end of the study. Data presented are from the last week of the study. The figure shows pattern of three representative rats in each group including control, DHEA and DHEA + CRY. Estrous cyclicity was evaluated as the percentage of the time in each phase. P: proestrus; E: estrus; M: metestrus; and D: diestrus.
Effects of cryptotanshinone on serum levels of P, T, E2, FSH and LH in rats with PCOS
Circulating progesterone concentrations did not differ among the control, DHEA, and CRY groups (P > 0.05) (Figure 3A). The circulating T and E2 levels were higher in DHEA-exposed rats than in controls (P < 0.05) (Figure 3B, 3C). Treatment with CRY decreased circulating T and E2 levels to concentrations than those in the DHEA group (P < 0.05). Circulating LH levels were higher and FSH levels were lower, resulting in a higher LH/FSH ratio, in rats exposed to DHEA than in controls (P < 0.05) (Figure 3D-F). CRY decreased LH and increased FSH in DHEA rats (P < 0.05). Further, the LH/FSH ratio was lower in the CRY group than in the DHEA group (P < 0.05).
Figure 3.
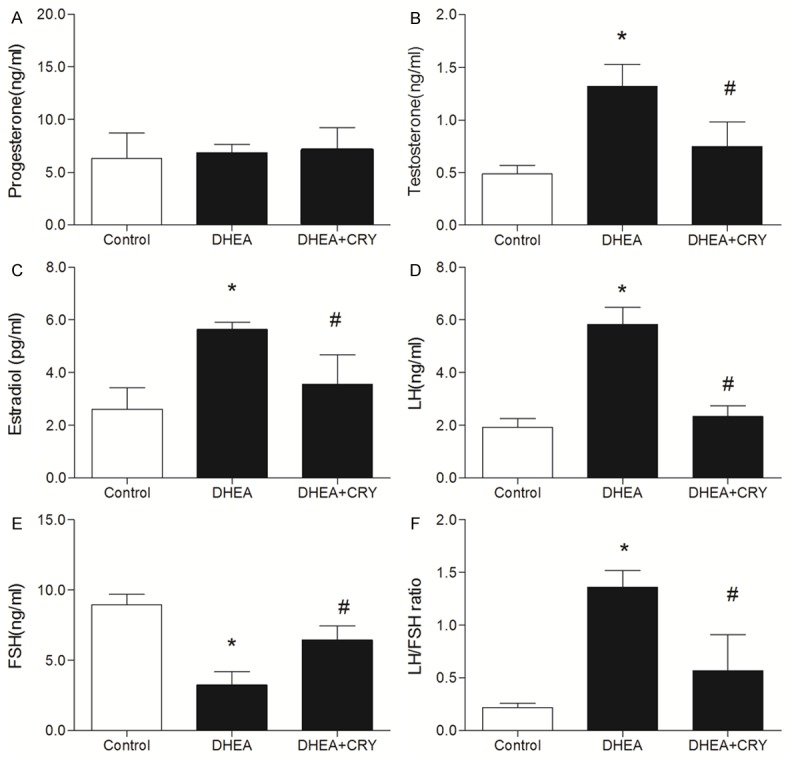
A-F. Serum progesterone, testosterone, estradiol, LH, FSH and LH/FSH ratio in each group including control, DHEA and DHEA + CRY. Testosterone and estradiol level was higher in DHEA group than in controls. CRY group decreased testosterone and estradiol to concentrations than those in DHEA group. LH levels were higher and FSH levels were lower, resulting in a higher LH/FSH ratio, in rats exposed to DHEA than in controls. CRY group decreased LH and increased FSH in DHEA, while LH/FSH ratio was lower in CRY group than in DHEA group. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus DHEA.
Effects of CRY on serum concentration of inhibin B, activin A, and follistatin in rats with PCOS
The serum concentrations of inhibin B, activin A, and follistatin are presented in Figure 4. Rats exposed to DHEA had significantly reduced activin A concentrations compared to those in the control group (P < 0.05), while the serum levels of inhibin B and follistatin significantly increased in comparison to control group (P < 0.05). The chronic administration of CRY significantly increased serum activin A levels relative to those in the DHEA group (P < 0.05). CRY significantly decreased serum inhibin B and follistatin levels compared to those in the DHEA group (P < 0.05).
Figure 4.
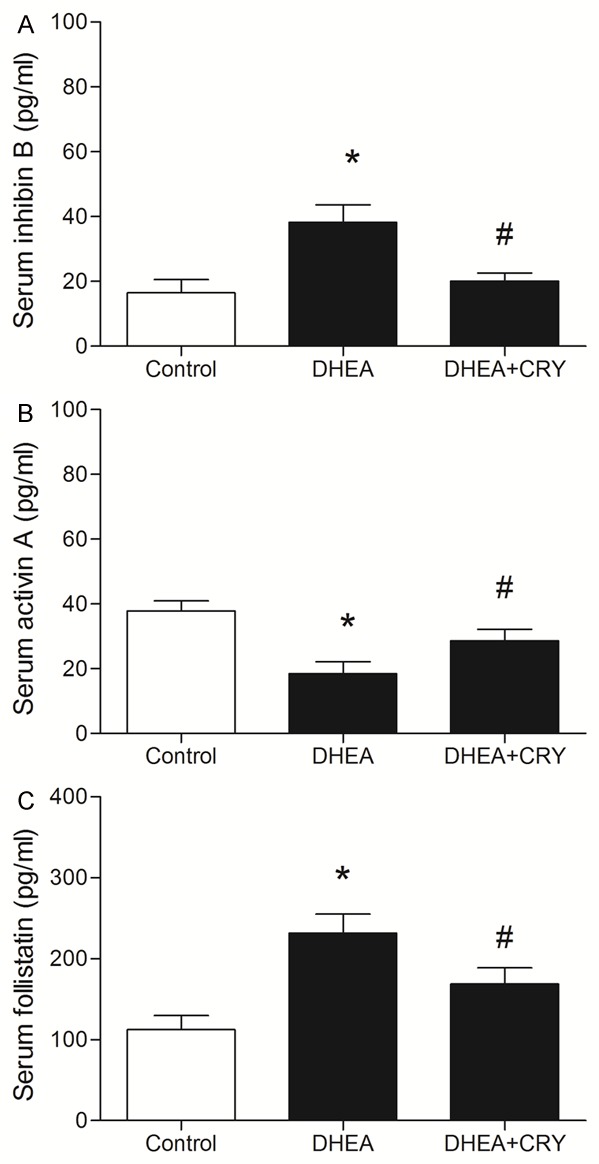
A-C. Serum inhibin B, activin A and follistatin concentrations assayed by ELISA in each group including control, DHEA and DHEA + CRY. Rats exposed to DHEA reduced significant activin A, while inhibin B and follistatin significant increased in comparison to control group. DHEA + CRY group increased activin A, while significantly decreased inhibin B and follistatin compared with DHEA group. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus DHEA.
Effects of CRY on mRNA expressions of inhibin B, activin A, and follistatin in the ovaries of rats with PCOS
As shown in Figure 5, inhibin B and follistatin mRNA expressions in rat ovaries of the DHEA group increased significantly compared to those in the control group (P < 0.05), while inhibin B and follistatin mRNA expressions in ovaries of the CRY group were significantly decreased comparison to those in the DHEA group (P < 0.05). These results indicate that CRY decreased inhibin B and follistatin mRNA expressions in rat ovarian tissues.
Figure 5.
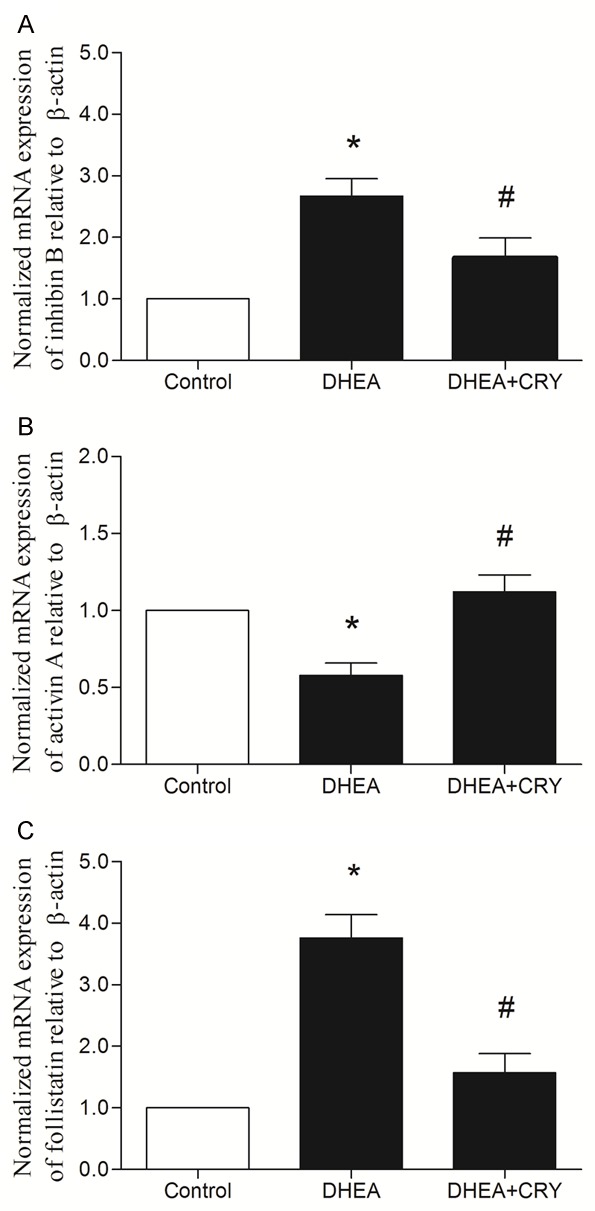
The genes expression of ovarian tissues analyzed by real-time PCR. The effects of CRY on inhibin B, activin A and follistatin mRNA expression in rats with DHEA-induced PCOS in the following groups: control, DHEA and DHEA + CRY group. The expression of inhibin B mRNA (2.63 ± 0.23), and follistatin mRNA (3.77 ± 0.29) in DHEA group was increased significantly, while activin A mRNA (0.59 ± 0.17) was decreased significantly than in the control group (1.00 ± 0.00). After drug intervention, the expression of inhibin B mRNA (1.64 ± 0.31) and follistatin mRNA (1.35 ± 0.34) was statistically decreased, while activin A mRNA (1.17 ± 0.21) was increased significantly. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus DHEA.
Activin A mRNA expression decreased significantly in rat ovaries of the DHEA group compared with that in the control group (P < 0.05), while activin A mRNA expression was significantly increased in the ovaries of the CRY group compared to the DHEA group (P < 0.05). These results indicate that CRY increased the activin A expression in rat ovarian tissues.
Effects of CRY on protein expression of inhibin B, activin A, and follistatin in the ovaries of rats with PCOS
As shown in Figure 6, inhibin B and follistatin protein expressions increased significantly in the rat ovaries in the DHEA group compared to those in the control group (P < 0.05), while inhibin B and follistatin protein expressions were significantly decreased in ovaries of the CRY group compared to those in ovaries of the DHEA group (P < 0.05). These results indicate that CRY decreased inhibin B and follistatin expressions in rat ovarian tissues.
Figure 6.
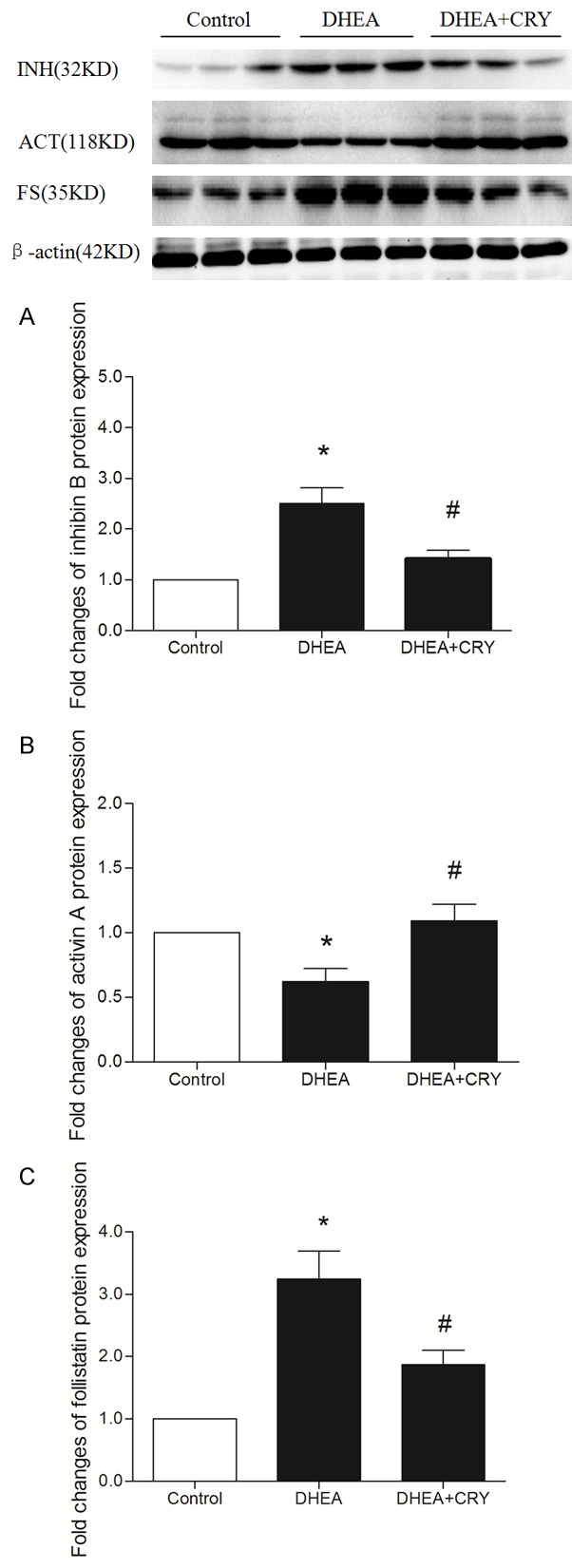
The protein expression of ovarian tissues was analyzed by western blot. The effects of CRY on inhibin B, activin A and follistatin protein expression in rats with DHEA-induced PCOS in the following groups: control, DHEA and DHEA + CRY group. As is shown above, the expression of inhibin B and follistatin significantly increased in DHEA group than that of control group, while in DHEA + CRY group were decreased according to statistics. The expression of activin A significantly decreased in DHEA group than that of control group, while in DHEA + CRY group were increased according to statistics. The data are expressed as the mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus DHEA.
Activin A protein expression decreased significantly in rat ovaries of the DHEA group compared to that in rat ovaries of the control group (P < 0.05), while activin A protein expression was significantly increased in ovaries of rats in the CRY group compared to those in the DHEA group (P < 0.05). These results indicate that CRY increased activin A expression in rat ovarian tissues.
Discussion
PCOS is a common endocrine disorder characterized by typical ovarian morphology, follicular arrest, anovulation, and hyperandrogenism. PCOS is difficult to treat because of its complex etiology and pathogenesis as well as its heterogeneous or polymorphic clinical manifestations. The treatment effects suggest that regulating abnormal androgen and/or estrogen is an effective method of treating PCOS [13].
In recent years, clinical studies have demonstrated the effectiveness of Salvia miltiorrhiza Bunge treatment for PCOS. It is a member of the genus Salvia of the mint family, known as Danshen in Chinese. As one of the best-known Chinese traditional herbs, its root has been clinically used for more than 2000 years. CRY, a lipophilic diterpenoid, was originally isolated from the dried roots of Salvia miltiorrhiza Bunge and is its main and active component [14,15]. Pharmacological studies revealed that CRY has anti-coronary heart disease, anti-bacterial, and anti-inflammatory activity [16]. CRY has recently been used to treat endocrine metabolic diseases. Kim et al. reported that it could reverse the disturbances in prenatally androgenized rats. Huang et al. showed that it could improve androgen excess and insulin resistance in a PCOS rat model induced by DHEA [17-19].
Here we successfully prepared a PCOS rat model by subcutaneously injecting DHEA, an exogenous androgen. The rat model recapitulated the reproductive and metabolic features of human PCOS, including increased body weight and ovarian quotiety. After the CRY intervention, ovarian weight, body weight, and ovarian quotiety of the PCOS model rats had improved. In addition, the irregular estrous cycles of the model rats were obviously restored. Improved estrous cyclicity was found in the CRY group in which four to five rats per group were in the estrous phase at least once during a 6-day period, although it must be noted that they did not have regular cycles. One possible explanation for the improved cyclicity is that decreased sympathetic activity will directly impact the ovaries and affect the sex steroid synthesis pathways. Even more noteworthy is that the serum biochemical indicators including P, T, E2, LH, and LH/FSH were significantly reversed, a finding that is consistent with previous research results [20,21].
Studies have shown that inhibins, activins, and follistatin play very important roles in the onset of ovarian dysfunction, particularly in PCOS. Inhibins are primarily produced by the gonads and the placenta and have been initially characterized by their ability to exert negative feedback on pituitary FSH synthesis and release [22]. Inhibins are also produced in the ovary and can regulate pituitary FSH secretion by an endocrine mechanism and ovarian steroidogenesis. Hillier et al. reported that increased inhibin levels could suppress circulating FSH concentrations and increase LH-stimulated androgen production [23]. Inhibins are dimeric glycoproteins that consist of an α-subunit covalently joined by disulfide links to the βA (inhibin A) or βB (inhibin B) subunit. In addition to these mature bioactive forms, inhibins exist in the circulation as both unprocessed or partially processed high molecular weight forms and free α subunits [24]. Pigny et al. demonstrated that serum inhibin B levels are obviously increased in patients with PCOS. In women with PCOS and normal controls, serum inhibin B and E2 levels exhibit similar and simultaneous dose-responsiveness to FSH stimulation. During recovery from ovarian suppression, basal and stimulated inhibin B release appears to be restored earlier than E2 release in women with PCOS [25].
Follistatin is a monomeric glycosylated polypeptide chain that was isolated from follicular fluid according to its ability to inhibit pituitary FSH secretion. It also can bind activin, a protein also found in the ovary that is partly responsible for follicular growth [26]. It is known to inhibit FSH secretion and regulate folliculogenesis and ovarian steroidogenesis, primarily by modulating activin activity [27]. Accordingly, increases in follistatin may arrest follicular development and increase ovarian androgen production, both of which are fundamental in PCOS [28].
Activins A and B belong to the transforming growth factor-β superfamily. They share 65% homology and overlapping biological activities but display some divergent functions due to differential expression and receptor utilization and affinities [29]. Activin A is produced by follicles in culture and has been proposed to promote the proliferation of granulosa cells, enhance FSH receptor expression, decrease LH-induced androgen production, increase pituitary FSH secretion, and generally promote follicle growth [30]. Norman et al. recently published their findings that a decreased activin concentration or functional activity as well as an increased follistatin concentration might encourage characteristic features of PCOS [31].
Our study confirmed that the mRNA and protein expression levels of inhibin B and follistatin were higher in the DHEA-induced PCOS rats than in control group rats; on the contrary, the mRNA and protein expression levels of activin A were significantly lower than those of the control group consistent with previous research results [32]. We found that CRY could increase the mRNA and protein expression levels of activin A in model rats, while those of inhibin B and follistatin were decreased. The above results showed that the mechanism of CRY in the treatment of PCOS not only corrects the sex steroid synthesis disorder but also adjusts the mRNA and protein expression levels of activin A, inhibin B, and follistatin in rats.
Conclusions
We have shown that 3-week CRY treatment can reverse the reproductive and metabolic disturbances in DHEA-induced PCOS rats by downregulating inhibin B and follistatin expressions and may indirectly increase activin A expression. Therefore, it could be a potential therapeutic agent for treating PCOS.
Acknowledgements
This work was supported by the project of Shanghai Science and Technology Committee (No. 074119624, 12ZR1432700) and “Three-year” Development Project of Shanghai Traditional Chinese Medicine (No. ZYSNXD-CC-HPGC-JD-008, ZYSNXD-CC-ZDYJ051).
Disclosure of conflict of interest
None.
References
- 1.Azziz R. Androgen excess is the key element in polycystic ovary syndrome. Fertil Steril. 2003;80:252–254. doi: 10.1016/s0015-0282(03)00735-0. [DOI] [PubMed] [Google Scholar]
- 2.Homer MV, Rosencrantz MA, Shayya RF, Chang RJ. The effect of estradiol on granulose cell responses to FSH in women with polycystic ovary syndrome. Reprod Biol Endocrinol. 2017;15:13. doi: 10.1186/s12958-017-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guttmann-Bauman I. Approach to adolescent polycystic ovary syndrome (PCOS) in the pediatric endocrine community in the U. S.A. J Pediatr Endocrinol Metab. 2005;18:499–506. doi: 10.1515/jpem.2005.18.5.499. [DOI] [PubMed] [Google Scholar]
- 4.Qin KN, Rosenfield RL. Role of cytochrome P450c17 in polycystic ovary syndrome. Mol Cell Endocrinol. 1998;145:111–121. doi: 10.1016/s0303-7207(98)00177-4. [DOI] [PubMed] [Google Scholar]
- 5.Rosencrantz MA, Wachs DS, Coffler MS, Malcom PJ, Donohue M, Chang RJ. Comparison of inhibin B and estradiol responses to intravenous FSH in women with polycystic ovary syndrome and normal women. Hum Reprod. 2010;25:198–203. doi: 10.1093/humrep/dep373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teede H, Ng S, Hedger M, Moran L. Follistatin and activins in polycystic ovary syndrome: relationship to metabolic and hormonal markers. Metabolism. 2013;62:1394–400. doi: 10.1016/j.metabol.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen MJ, Han DS, Yang JH, Yang YS, Ho HN, Yang WS. Myostatin and its association with abdominal obesity, androgen and follistatin levels in women with polycystic ovary syndrome. Hum Reprod. 2012;27:476–83. doi: 10.1093/humrep/des209. [DOI] [PubMed] [Google Scholar]
- 8.Ji XY, Tan BK, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin. 2000;21:1089–1094. [PubMed] [Google Scholar]
- 9.Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 10.Anderson E, Lee GY, O’Brien K. Polycystic ovarian condition in the dehydroepiandrosterone treated rat model: hyperandrogenism and the resumption of meiosis are major initial events associated with cystogenesis of antral follicles. Anat Rec. 1997;249:44–53. doi: 10.1002/(SICI)1097-0185(199709)249:1<44::AID-AR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Tessaro I, Modina SC, Franciosi F, Sivelli G, Terzaghi L, Lodde V, Luciano AM. Effect of oral administration of low-dose follicle stimulating hormone on hyperandrogenized mice as a model of polycystic ovary syndrome. J Ovarian Res. 2015;8:64. doi: 10.1186/s13048-015-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirivelu MP, Shin AC, Perez GI, MohanKumar PS, MohanKumar SM. Effect of L-dopa on interleukin-1 beta-induced suppression of luteinizing hormone secretion in intact female rats. Hum Reprod. 2009;24:718–725. doi: 10.1093/humrep/den434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1–13. doi: 10.2147/CLEP.S37559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hur JM, Shim JS, Jung HJ, Kwon HJ. Cryptotanshinone but not tanshinone IIA inhibits angiogenesisin vitro. Exp Mol Med. 2005;37:133–137. doi: 10.1038/emm.2005.18. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 16.Jin DZ, Yin LL, Ji XQ, Zhu XZ. Cryptotanshinone inhibits cyclooxygenase-2 enzyme activity but not its expression. Eur J Pharmacol. 2006;549:166–172. doi: 10.1016/j.ejphar.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Kim EJ, Jung SN, Son KH, Kim SR, Ha TY, Park MG, Jo IG, Park JG, Choe W, Kim SS, Ha J. Antidiabetes and antiobesity effect of cryptotanshinone via activation of AMP-activated protein kinase. Mol Pharmacol. 2007;72:62–72. doi: 10.1124/mol.107.034447. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Zhai D, Hao L, Zhang D, Bai L, Cai Z, Yu C. Cryptotanshinone reverses reproductive and metabolic disturbances in PCOS model rats via regulating the expression of CYP17 and AR. Evid Based Complement Alternat Med. 2014;2014:670743. doi: 10.1155/2014/670743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Li W, Wang CC, Wu X, Zheng J. Cryptotanshinone reverses ovarian insulin resistance in mice through activation of insulin signaling and the regulation of glucose transporters and hormone synthesizing enzymes. Fertil Steril. 2014;102:589–596. doi: 10.1016/j.fertnstert.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Zhang Y, Wu X, Bae CS, Hou L, Kuang H, Wang Y, Stener-Victorin E. Cryptotanshinone reverses reproductive and metabolic disturbances in prenatally androgenized rats via regulation of ovarian signaling mechanisms and androgen synthesis. Am J Physiol Regul Integr Comp Physiol. 2011;300:R869–875. doi: 10.1152/ajpregu.00334.2010. [DOI] [PubMed] [Google Scholar]
- 21.Li XH, Yang XM, Wu XK. Effects of cryptotanshinone in lowering androgens synthesis for the prenatally androgenized male rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28:1001–1004. [PubMed] [Google Scholar]
- 22.Sharkey DJ, Schjenken JE, Mottershead DG, Robertson SA. Seminal fluid factors regulate activin A and follistatin synthesis in female cervical epithelial cells. Mol Cell Endocrinol. 2015;417:178–190. doi: 10.1016/j.mce.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant activin on androgen synthesis in cultured human thecal cells. J Clin Endocrinol Metab. 1991;72:1206–1211. doi: 10.1210/jcem-72-6-1206. [DOI] [PubMed] [Google Scholar]
- 24.Chinya A, Ratan SK, Aggarwal SK, Garg A, Mishra TK. Association of levels of serum inhibin B and follicle-stimulating hormone with testicular vascularity, volume, and echotexture in children with undescended testes. J Indian Assoc Pediatr Surg. 2017;22:3–8. doi: 10.4103/0971-9261.194609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pigny P, Cortet-Rudelli C, Decanter C, Deroubaix D, Soudan B, Duhamel A, Dewailly D. Serum levels of inhibins are differentially altered in patients with polycystic ovary syndrome: effects of being overweight and relevance to hyperandrogenism. Fertil Steril. 2000;73:972–977. doi: 10.1016/s0015-0282(00)00421-0. [DOI] [PubMed] [Google Scholar]
- 26.Knight PG, Satchell L, Glister C. Intra-ovarian roles of activins and inhibins. Mol Cell Endocrinol. 2012;59:53–65. doi: 10.1016/j.mce.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Sang Q, Zhang S, Zou S, Wang H, Feng R, Li Q, Jin L, He L, Xing Q, Wang L. Quantitative analysis of follistatin promoter methylation in peripheral blood of patients with polycystic ovary syndrome. Reprod Biomed Online. 2013;26:157–163. doi: 10.1016/j.rbmo.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Chen MJ, Yang WS, Chen HF, Kuo JJ, Ho HN, Yang YS, Chen SU. Increased follistatin levels after oral contraceptive treatment in obese and non-obese women with polycystic ovary syndrome. Hum Reprod. 2010;25:779–785. doi: 10.1093/humrep/dep459. [DOI] [PubMed] [Google Scholar]
- 29.Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011;85:255–297. doi: 10.1016/B978-0-12-385961-7.00013-5. [DOI] [PubMed] [Google Scholar]
- 30.Teede H, Ng S, Hedger M, Moran L. Follistatin and activins in polycystic ovary syndrome: relationship to metabolic and hormonal markers. Metabolism. 2013;62:1394–1400. doi: 10.1016/j.metabol.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Hum Reprod. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- 32.Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]


