Abstract
Background and objective: MicroRNAs play an important role in cell proliferation, apoptosis, differentiation, and invasion by regulating the expression of various genes. For example, the downregulation of microRNA-199a-3p (miR-199a-3p) that is noted in numerous human malignancies, including hepatocellular carcinoma (HCC), results in a poor prognosis in patients with HCC. This finding suggests that miR-199a-3p overexpression in HCC could provide a new treatment approach. We explored this possibility by examining the effects of miR-199a-3p on the growth and apoptosis of HCC cells in vitro and vivo. Methods: The miR-199a-3p signaling pathway was examined using ZHX1 (zinc-fingers and homeoboxes-1) or PUMA (a p53 upregulated modulator of apoptosis) siRNA transfection to determine the effects of miR-199a-3p on growth and apoptosis of HepG2 cells in vitro. A subcutaneously implanted tumor model of HepG2 cells in nude mice was used to assess the effects of miR-199a-3p on the signaling pathway and tumorigenesis development in vivo. Results: miR-199a-3p inhibited growth and induced apoptosis of HepG2 cells in vitro. These effects were accompanied by upregulation of ZHX1 and PUMA. Targeting ZHX1 inhibited upregulation of PUMA after miR-199a-3p transfection. In addition, miR-199a-3p inhibited Bcl2 expression, but increased Bax and cleaved caspase-3 expression. Targeting PUMA or ZHX1 reversed the effect of miR-199a-3p, followed by upregulation of Bcl2 and downregulation of Bax and cleaved caspase-3, respectively. Furthermore, miR-199a-3p inhibited tumorigenesis of xenografts in nude mice. Conclusions: miRNA-199a-3p could effectively prevent primary tumor formation. The ability of this therapy to decrease tumorigenesis may be related toZHX1-dependent PUMA signals.
Keywords: Hepatocellular carcinoma, apoptosis, miRNA-199a-3p, ZHX1, PUMA
Introduction
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer-related death globally [1]. The incidence of HCC is increasing in Asian countries because of the high prevalence of chronic hepatitis and liver diseases [2]. There is also a high recurrence rate of HCC in patients following principal treatment modalities that include surgical liver resection, local ablation, and liver transplantation. Unfortunately, this high recurrence rate and poor response to medical treatments can result in a grave prognosis for the patient. There are no effective adjuvant therapies for HCC, so research into ways to effectively treat patients with HCC who do not respond to currently available medical treatments is critical.
MicroRNAs (miRNAs), which have rapidly emerged as modulators of gene expression in cancer, may have great diagnostic and therapeutic importance. miRNAs are actively involved in carcinogenesis and mediate fundamental cellular processes like cell invasion, proliferation, differentiation, and apoptosis [3]. MicroRNA-199a-3p (miR-199a-3p) is downregulated in several human malignancies, such as ovarian carcinoma [4], osteosarcoma [5], and prostate cancer [6]. Clinical and pathological surveys of patients with these types of cancer indicate that low levels of miR-199a-3p correlate with poor patient prognosis [4-6]. Conversely, administration of miR-199a-3p inhibits proliferation and invasion of osteosarcoma [7], ovarian carcinoma [4], and papillary thyroid carcinoma cells both in vitro and in vivo [8]. Low levels of miR-199a-3p are also present in tissue and serum samples of HCC patients [9,10], whereas overexpression of miR-199a-3p is found in normal liver tissue [9]. Fornari et al. reported that patients with lower miR-199a-3p levels have a shorter time before recurrence of disease after HCC resection than those with high miR-199a-3p levels [11]. In addition, they found that restoring miR-199a-3p in HCC cells leads to G1-phase cell cycle arrest and reduced invasive capability in vitro [11]. Taken together, these findings suggest that miR-199a-3p could provide a new approach for treatment of HCC.
The inhibitory role of miR-199a-3p in tumor progression may result from an ability of miR-199a-3p to interfere with the function of various cellular anti-oncogenes. Zinc-fingers and homeoboxes-1 (ZHX1), a type of zinc-finger protein that contains two C2H2 zinc-finger motifs and five homeodomains, is downregulated in gastric cancer and HCC [12,13]. In addition, ZHX1 inhibits growth and enhances apoptosis via downregulation of Bcl2 and upregulation of Bax and cleaved caspase-3 in gastric cancer cells [12]. Wang et al. demonstrated that miR-199a-3p promotes cell proliferation and suppresses apoptosis in gastric cancer cells via targeting ZHX1 for RNA degradation [14]. Furthermore, restoration of ZHX1 expression abolishes oncogenicity in gastric cancer cells [14]. However, in HCC cells, miR-199a-3p leads to G1-phase cell cycle arrest and induces cell apoptosis [10]. We theorized that the anti-tumor effect of miR-199a-3p in HCC cells could be due to upregulation of ZHX1 expression.
PUMA, a p53-upregulated modulator of apoptosis, is a pro-apoptotic member of the BH3-only subgroup of the Bcl-2 family [15,16]. PUMA induces death signals primarily to the mitochondria, where it acts indirectly on the Bcl-2 family members Bax and/or Bak by relieving the inhibition imposed by antiapoptotic members and inducing mitochondrial dysfunction and caspase activation [15]. ZHX1 inducesmitochondrial dysfunction and caspase activation, resulting in inhibition of growth and enhancement of apoptosis [12]. Whether PUMA is regulated by ZHX1 needs further investigation.
In the present study, we assessed the effect of miR-199a-3p on apoptosis and growth of HepG2 cells in vitro and vivo to shed light on the molecular mechanisms involved and potential usefulness of this miRNA as an anticancer therapeutic agent. Our findings demonstrate that miR-199a-3p inhibited growth and induced apoptosis of HepG2 cells at least in part via activation of the ZHX1/PUMA signaling pathway. This study reveals a novel mechanism for the effect of miR-199a-3p on growth and apoptosis in HepG2 cells and provides data indicating that miR-199a-3p may be a novel therapeutic target for the treatment of HCC.
Materials and methods
Cell line and culture
The HepG2 cell line was purchased from the Institute of Biochemistry and Cell Biology (Shanghai, China). The cell line was maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) at 37°C in a humid atmosphere of 5% CO2.
siRNA transfection
ZHX1 siRNA, PUMA siRNA, and control siRNA were purchased from Santa Cruz Biotechnology (Shanghai, China). ZHX1 and PUMA silencing was accomplished by transfecting HepG2 cells with 10-nM specific siRNAs for 48 h using Lipofectamine™ LTX and Plus™ Reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s reverse transfection protocol. Cells were distributed into 96-well plates (104/well) and then subjected to 2 μg/ml of puromycin-containing medium 48 h after siRNA transfection to achieve stable transfection. All transfection experiments were performed at least three times.
Oligonucleotide transfection
A chemically synthesized miRNA-199a-3p that mimics mature endogenous miRNA-199a-3p (miR-199a-3p mimic) and scrambled oligonucleotides (NS) were purchased from Genecopoeia (Shanghai, China). To achieve miR-199a-3p over-expression, HepG2 cells were transfected with 0.1 μM miR-199a-3p mimic for 72 h using Lipofectamine™ LTX and Plus™ Reagent (Invitrogen) according to the manufacturer’s instructions. The transfection efficiency was determined by green fluorescence. Cells were distributed into 96-well plates (104/well) and then subjected to 2 μg/ml of puromycin-containing medium 48 h after miR-199a-3p mimic or control NS transfection to achieve stable transfection. All transfection experiments were performed at least three times.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Expression levels of the miR-199a-3p mimic were quantified by two-step qRT-PCR. RNA (50 ng) was reverse transcribed using a TaqManMicroRNA RT Kit and quantified using a TaqManmiRNA assay (Applied Biosystems, Inc., Hangzhou, China). U6-snRNA was selected as the endogenous control for the normalization of RNA input. miR-199a-3p mimic expression levels were calculated by relative quantitation using ABI 7900HTRT-PCR SDS (Sequence Detection System) 1.2 software (Applied Biosystems Inc., Foster City, CA). The qRT-PCR reactions were performed in triplicate. Data were analyzed with SDS 2.2.2 software using the 2-ΔΔCt method with a relative quantification RQmin/RQmax confidence set at 95%.
Cell viability assay
Cell viability was detected by lactate dehydrogenase (LDH) release using an LDH cytotoxicity assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol. LDH activity was quantified by measuring absorbance at 490 nm with a microplate reader (Hangzhou, China). The ratio of released LDH to total LDH was calculated and presented as relative LDH release compared to LDH release in non-treated cells. All experiments were performed with triplicate wells and repeated three times.
Colony formation assay
Stable miR-199a-3p mimic-transfected HepG2 cells were seeded at a density of 300 cells/ml on 35-mm dishes. Colonies were allowed to grow for 14 days. The cells were fixed in methanol for 15 min and stained with crystal violet for 20 min. Positive colony formation (more than 50 cells/colony) was counted.
Flow cytometry
Annexin V/PI double staining followed by flow cytometry was used to detect cell apoptosis using an apoptosis detection kit (Biovision, Mountain View, CA) according to the manufacturer’s instructions. Briefly, transfected cells were gently detached and washed with ice-cold phosphate-buffered saline (PBS). Cells were suspended in 300 µL of binding buffer containing Annexin V and propidium iodide, and incubated for 5 min at room temperature. Early apoptotic cells were identified as Annexin V positive/PI negative cells and late apoptotic/necrotic cells were identified as Annexin V positive/PI positive cells using a BD LSR II cell analyzer.
Western blot assay
Cells were washed once with PBS and lysed with RIPA buffer (Hangzhou, China) for 30 min on ice to extract total protein. Protein concentrations were quantified with a Coomassie Plus (Bradford) Protein Assay Reagent according to the manufacturer’s instructions. Then, 40 μg ofextract was resolved using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to Hybond-C Extra nitrocellulose membrane (GE Healthcare; Shanghai, China). Membranes were probed with primary antibodies against ZHX1, PUMA, Bcl2, Bax, and cleaved caspase-3 (Cell signal, Shanghai, China) followed by incubation for 1 h at room temperature with the appropriate HRP-conjugated anti-rabbit IgG or anti-goat IgG secondary antibodies. Immunoblotting for β-actin served as the protein loading control. All experiments were performed at least three independent times.
Xenograft model in nude mice
A xenograft model in nude mice was created by injecting 3 × 105 cells (miR-199a-3p mimic/HepG2 or HepG2/NS) in 100 μl of PBS into the right hind limbs of 4-6-week-old Balb/C athymic nude mice. The mice were sacrificed 6 weeks after tumor inoculation. Tumor size was measured using a slide caliper. Tumor volume was determined using the formula 0.44 × A × B2, where A is the diameter of the base of the tumor and B is the corresponding perpendicular value. After mice were euthanized, the tumors were excised, fixed in 10% neutral buffered formalin, and embedded in paraffin before preparing 4-μm sections that were stained with hematoxylin.
Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assay
TUNEL was performed using an In situ Cell Death Detection Kit (Roche, Shanghai, China). Cell apoptosis was quantified by calculating the percent of positively stained cells for all nuclei in 20 randomly chosen fields/section at 200 × magnification. Two independent reviewers quantified slides of the apoptosis studies in a blind manner at two different times.
Statistical analysis
Statistical analyses were performed using SPSS.21 Software. All data are presented as mean ± standard error of the mean (S.E.). Significance was determined by Student’s t test or one-way analysis of variance with Bonferroni post-tests where applicable. Experiments were performed in triplicate. P<0.05 was considered statistically significant.
Results
The miR-199a-3p mimic induces apoptosis and inhibits growth of HepG2 cells in vitro
To investigate the function of miR-199a-3p on HepG2 cells in vitro, we transiently transfected HepG2 cells with the miR-199a-3p mimic to for 72 h. As shown in Figure 1A, there was a significant increase in the percentage of apoptotic cells in the miR-199a-3p mimic-transfected cells compared with control cells. Cell viability (Figure 1B) was inhibited compared to the controls. Furthermore, there were fewer stable miR-199a-3p mimic/HepG2 colonies compared to controls (Figure 1C). These results support the hypothesis that miR-199a-3p is necessary to inhibit HepG2 cells growth.
Figure 1.
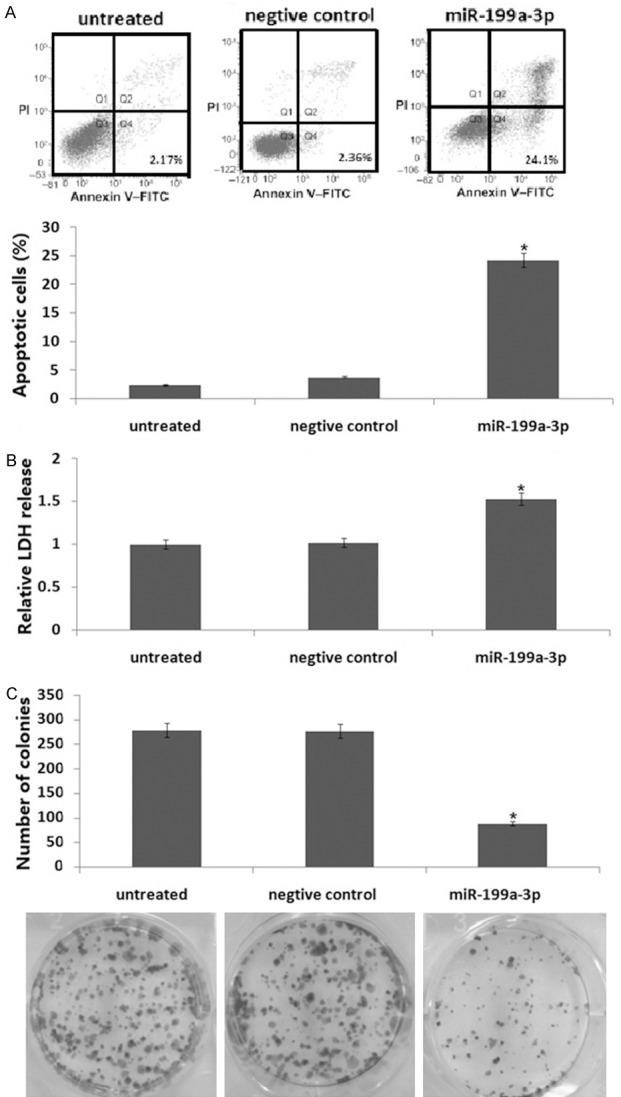
The miR-199a-3p mimic induces apoptosis and inhibits growth and colony formation of HepG2 cells in vitro. HepG2 cells were transiently transfected with miR-199a-3p mimic for 72 h. A. Apoptosis was analyzed by annexin V/PI staining followed by flow cytometry. B. Cell viability was analyzed with a LDH toxicology assay. C. Cell growth was analyzed with a colony formation assay. Data are shown as the mean ± SD. The experiments were all repeated at least three times to confirm the reproducibility of the results. *P<0.01 versus the control or untreated groups.
Expression of the miR-199a-3p mimic correlates with apoptosis induction and growth inhibition by upregulation of PUMA
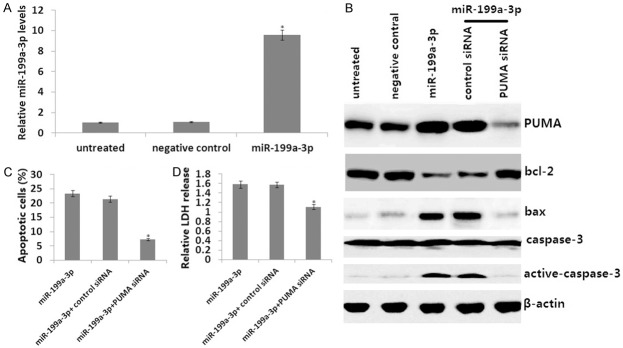
HepG2 cells that were transiently transfected with the miR-199a-3p mimic for 72 h had markedly increased levels of miR-199a-3p mimic compared to the controls as determined by qRT-PCR assays (Figure 2A). PUMA protein was also markedly induced by miR-199a-3p mimic transfection (Figure 2B). In addition, miR-199a-3p mimic inhibited Bcl2 expression, but increased Bax and cleaved caspase-3 expression (Figure 2B).
Figure 2.
PUMA mediates the apoptotic effects of the miR-199a-3p mimic through the mitochondrial pathway. A. HepG2 cells were transiently transfected with the miR-199a-3p mimic for 72 h. miR-199a-3p mimic mRNA content was detected by qRT-PCR assay. B. HepG2 cells were stably transfected with either a control scrambled siRNA or a PUMA siRNA and then transiently transfected with the miR-199a-3p mimic for 72 h. PUMA, active caspase-3, bcl-2, and BAX were detected by Western blot analysis. C. HepG2 cells were stably transfected with either a control scrambled siRNA or a PUMA siRNA and then transiently transfected with miR-199a-3p mimic for 72 h. Apoptosis was analyzed by annexin V/PI staining followed by flow cytometry. D. Cell viability was analyzed by LDH toxicology assay. *P<0.01 versus the control.
To investigate whether PUMA is essential for miR-199a-3p mimic-induced apoptosis and growth inhibition, PUMA siRNA was stably transfected into HepG2 cells and then the miR-199a-3p mimic was transiently transfected into the PUMA-expressing HepG2 cells for 72 h. The results show targeting PUMA reversed the effect of the miR-199a-3p mimic (Figure 2C and 2D), which was followed by upregulation ofBcl2 and downregulation of Bax and cleaved caspase-3 (Figure 2B). Therefore, PUMA is necessary for the apoptotic effect of miR-199a-3p mimic in HepG2 cells.
PUMA activation by the miR-199a-3p mimic is mediated by ZHX1
We analyzed the mechanism of PUMA induction by the miR-199a-3p mimic in HepG2 cells. HepG2 cells transiently transfected with themiR-199a-3p mimic for 72 h markedly induced ZHX1 (Figure 3A) and PUMA (Figure 2B) protein expression. However, targeting ZHX1 abrogated PUMA induction by the miR-199a-3p mimic in HepG2 cells (Figure 3A), which was followed by upregulation of Bcl2 and downregulation of Bax and cleaved caspase-3 (Figure 3A).
Figure 3.
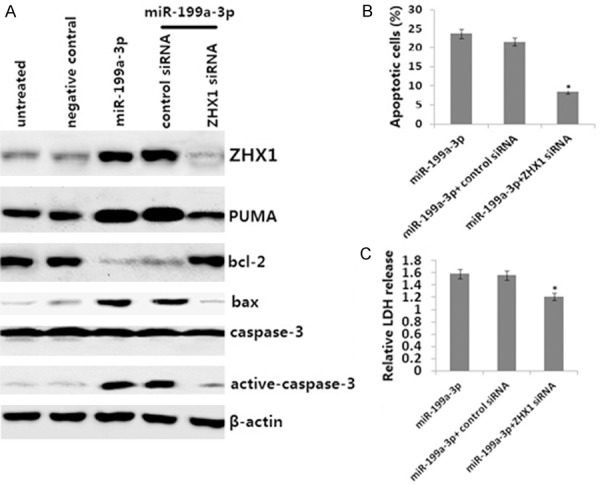
Activation of ZHX1 mediates PUMA induction in response to miR-199a-3p mimic overexpression. HepG2 cells were stably transfected with either control siRNA or ZHX1 siRNA and then transiently transfected with the miR-199a-3p mimic for 72 h. A. ZHX1, PUMA cleaved, bcl-2, BAX, and caspase-3 protein were detected by Western blot assay. B. Apoptosis was analyzed by annexin V/PI staining followed by flow cytometry. C. Cell viability was analyzed by an LDH toxicology assay. *P<0.01 versus the control.
To study the effects of targeting ZHX1, ZHX1 siRNA was stably transfected into the HepG2 cells and the miR-199a-3p mimic was transiently transfected into HepG2 cells for 72 h. Targeting ZHX1 reversed the effect of the miR-199a-3p mimic (Figure 3B and 3C). Therefore, ZHX1-dependent PUMA upregulation is necessary for the apoptotic effect of the miR-199a-3p mimic in HepG2 cells.
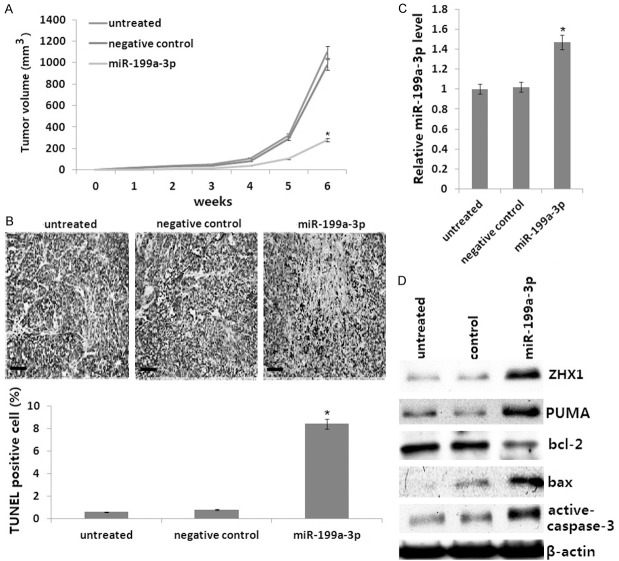
The miR-199a-3p mimic inhibits tumorigenesis of HCC in vivo
Given that the miR-199a-3p mimic inhibited the proliferation and induced apoptosis of HepG2 cells in vitro, we investigated the effect of miR-199a-3p on the tumorigenicity of HepG2 cells using an in vivo mouse model. As shown in Figure 4A, the tumors formed by miR-199a-3p mimic-transfected HepG2 cells grew less and were smaller in size than the tumors formed by control cells. Moreover, the tumors established using miR-199a-3p mimic-transfected HepG2 cells showed increased TUNEL-positive cells (Figure 4B). We next analyzed the expression of ZHX1, PUMA, Bcl2, BAX, and cleaved caspase-3 in the tumor tissues in vivo. The results showed that the miR-199a-3p mimic (Figure 4C), ZHX1, PUMA, Bax, and cleaved caspase-3 were overexpressed, and bcl-2 was downregulated in the miR-199a-3p mimic-transfected tumors compared to the controls (Figure 4D).
Figure 4.
Effect of the miR-199a-3p mimic onxenografted HepG2 tumor growth in vivo. A. Representative growth curves of tumor volume. B. Tissue apoptotic cells measured by TUNEL assay. C. miR-199a-3p mimic mRNA was detected by qRT-PCR. D. ZHX1, PUMA cleaved, bcl-2, BAX, and active-caspase-3 protein were detected by Western blot assay. Data represent the mean and the standard deviation (n=6). The p values were calculated with the Student’s t-test. *P<0.01.
Discussion
Increasing evidence demonstrates that miRNAs play important roles in human cancers and, therefore, are promising therapeutic targets [17]. The fact that numerous miRNAs are dramatically downregulated in HCC cells suggests that miRNAs are involved in the initiation and progression of this disease [18]. Specifi-cally, miR-199a-3p is downregulated in colon cancer [19] and HCC [11]; restoring the expression of miR-199a-3p leads to an increased sensitivity to chemotherapeutic drugs and reduced invasive capability in HCC cells [11]. Thus, the exogenous transfection of a specific miR-199a-3p mimic into HCC cells may open new avenues for the effective treatment of HCC.
The present study shows that overexpression of a miR-199a-3p mimic inhibited HepG2-cell proliferation and induced apoptosis in vitro. In addition, the tumors formed by miR-199a-3p mimic-transfected HepG2 cells grew less and were smaller in size than those formed by non-transfected HepG2 cells, suggesting apotential therapeutic application of miR-199a-3p mimics for the treatment of patients with HCC.
Although miR-199a-3p inhibits the growth of HepG2 cells, little is known about the mechanism of this effect. Numerous studies have found that miR-199a-3p functions via the protection of mitochondria in association with miR-199a-3p downregulation [20,21]. PUMA induces death signals primarily to the mitochondria. Whether or not the antitumor effects of miR-199a-3p involve PUMA signaling is not clear. We performed most in vitro experiments by transfection of a miR-199a-3p mimic into HepG2 cells for 72 h to substantially induce PUMA expression and apoptosis. Importantly, the results of these in vitro experiments were confirmed by those from xenograft tumor experiments. Our results demonstrate for the first time that tumor suppression by a miR-199a-3p mimic is dependent on PUMA induction and onset of mitochondria-mediated apoptosis.
Although apoptosis provoked by the miR-199a-3p mimic requires PUMA upregulation, the specific miR-199a-3p regulated genes that upregulate PUMA remain unknown. ZHX1 is generally downregulated in HCC tissues and overexpression of ZHX1 inhibits the growth of SMMC-7721 cells [13], suggesting that the ZHX family may be involved in the carcinogenesis of human HCCs. A previous study [12] demonstrated that miR-199a-3p inhibits ZHX1 expression in SGC-7901 cells, whereas reduction in miR-199a-3p enhances ZHX1 expression in NCI-N87 cells. Moreover, restoring ZHX1 expression in SGC-7901/miR-199a-3p cells inhibits proliferation induced by miR-199a-3p. These findings suggest that miR-199a-3p may function as a novel tumor promoter in gastric cancer and its oncogenic activity may involve the direct targeting and inhibition of ZHX1 [12]. In our present study, the miR-199a-3p mimic also induced ZHX1-dependent apoptosis in HepG2 cells in vitro and in vivo, indicating that ZHX1 is possibly a downstream target of miR-199a-3p.
To further investigate whether the enhanced cell growth inhibition and apoptosis induced by the miR-199a-3p mimic was mediated through the ZHX1/PUMA pathway, we conducted ZHX1 and PUMA siRNA transfection studies. ZHX1 siRNA transfection inhibited the miR-199a-3p mimic-induced upregulation of PUMA expression and apoptosis in the HepG2 cells. However, targeting PUMA did not affect miR-199a-3p mimic-induced upregulation of ZHX1 expression and did inhibit miR-199a-3p mimic-induced apoptosis in the HepG2 cells. Therefore, miR-199a-3p-mediated cell growth inhibition and induction of apoptosis could be mediated via activation of ZHX1/PUMA signals.
Conclusion
In this study, we demonstrated that a miR-199a-3p mimichadstrong antitumor effects in HepG2 cells in vitro and in vivo. miR-199a-3p could potentially be an effective therapeutic agent for the activation of ZHX1 and its downstream target gene PUMA, resulting in the inhibition of cell growth. Our study suggests that miR-199a-3p represents a promising gene target for the treatment of HCC.
Acknowledgements
This study was supported a grant from the National Natural Scientific Research Foundation of China (No. 81372632).
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- 4.Kinose Y, Sawada K, Nakamura K, Sawada I, Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Takahashi K. The hypoxia-related microRNA miR-199a-3p displays tumor suppressor functions in ovarian carcinoma. Oncotarget. 2015;6:11342–11356. doi: 10.18632/oncotarget.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Y, Feng Y, Shen JK, Lin M, Choy E, Cote GM, Harmon DC, Mankin HJ, Hornicek FJ, Duan Z. CD44 is a direct target of miR-199a-3p and contributes to aggressive progression in osteosarcoma. Sci Rep. 2015;5:11365. doi: 10.1038/srep11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu Y, Huang X, Li Z, Liu J, Wu J, Chen D, Zhao F, Mu D. miR-199a-3p inhibits aurora kinase A and attenuates prostate cancer growth: new avenue for prostate cancer treatment. Am J Pathol. 2014;184:1541–9. doi: 10.1016/j.ajpath.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Lyer AK, Yang X, Kobayashi E, Guo Y, Mankin H, Hornicek FJ, Amiji MM, Duan Z. Polymeric nanoparticle-based delivery of microRNA-199a-3p inhibits proliferation and growth of osteosarcoma cells. Int J Nanomedicine. 2015;10:2913–2924. doi: 10.2147/IJN.S79143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minna E, Romeo P, De Cecco L, Dugo M, Cassinelli G, Pilotti S, Degl’Innocenti D, Lanzi C, Casalini P, Pierotti MA, Greco A, Borrello MG. miR-199a-3p displays tumor suppressor functions in papillary thyroid carcinoma. Oncotarget. 2014;5:2513–2528. doi: 10.18632/oncotarget.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin J, Hou P, Wu Z, Wang T, Nie Y. Circulating miR-375 and miR-199a-3p as potential biomarkers for the diagnosis of hepatocellular carcinoma. Tumour Biol. 2015;36:4501–4507. doi: 10.1007/s13277-015-3092-0. [DOI] [PubMed] [Google Scholar]
- 11.Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Huang M, Wang Z, Liu B, Zhu Z, Li C. ZHX1 inhibits gastric cancer cell growth through inducing cell-cycle arrest and apoptosis. J Cancer. 2016;7:60–68. doi: 10.7150/jca.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Liu D, Liang X, Gao L, Yue X, Yang Y, Ma C, Liu J. Construction of a recombinant eukaryotic human ZHX1 gene expression plasmid and the role of ZHX1 in hepatocellular carcinoma. Mol Med Rep. 2013;8:1531–1536. doi: 10.3892/mmr.2013.1700. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Ma X, Cai Q, Wang X, Yu B, Cai Q, liu B, Zhu Z, Li C. MiR-199a-3p promotes gastric cancer progression by targeting ZHX1. FEBS Lett. 2014;588:4504–4512. doi: 10.1016/j.febslet.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Hikisz P, Kiliańska ZM. PUMA, a critical mediator of cell death--one decade on from its discovery. Cell Mol Biol Lett. 2012;17:646–669. doi: 10.2478/s11658-012-0032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher JI, Huang DC. Controlling the cell death mediators Bax and Bak: puzzles and conundrums. Cell Cycle. 2008;7:39–44. doi: 10.4161/cc.7.1.5178. [DOI] [PubMed] [Google Scholar]
- 17.Cho WC. Exploiting the therapeutic potential of microRNAs in human cancer. Expert Opin Ther Targets. 2012;16:345–350. doi: 10.1517/14728222.2012.663354. [DOI] [PubMed] [Google Scholar]
- 18.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Huang HJ, He CN, Wang KY. MicroRNA-199a-3p regulates endometrial cancer cell proliferation by targeting mammalian target of rapamycin (mTOR) Int J Gynecol Cancer. 2013;23:1191–1197. doi: 10.1097/IGC.0b013e31829ea779. [DOI] [PubMed] [Google Scholar]
- 20.Lee CG, Kim YW, Kim EH, Meng Z, Huang W, Hwang SJ, Kim SG. Farnesoid X receptor protects hepatocytes from injury by repressing miR-199a-3p, which increases levels of LKB1. Gastroenterology. 2012;142:1206–1217. doi: 10.1053/j.gastro.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunavat TR, Cheng L, Kim DK, Bhadury J, Jang SC, Lässer C, Sharples RA, López MD, Nilsson J, Gho YS, Hill AF, Lötvall J. Small RNA deep sequencing discriminates subsets of extracellular vesicles released by melanoma cells--Evidence of unique microRNA cargos. RNA Biol. 2015;12:810–823. doi: 10.1080/15476286.2015.1056975. [DOI] [PMC free article] [PubMed] [Google Scholar]