Abstract
Discoidin domain receptor 1 (DDR1) is a receptor tyrosine kinase which utilizes collagen as a ligand to regulate the interaction between cancer cells and tumor stroma. However, the clinical relevance of DDR1 expression in bladder cancer as well as its molecular regulation have not been previously investigated. Here, we assessed the role of DDR1 in bladder cancer. The DDR1 levels in bladder cancer specimens were examined by Western blot, compared to the paired adhesive normal controls. The effects of DDR1 were explored on both cell migration in bladder cancer cells and tumor growth as xenograft. We detected significant higher levels of DDR1 in bladder cancer tissues. Moreover, high levels of DDR1 were correlated with poor prognosis of corresponding patients. Both the in vitro cell invasiveness and in vivo tumor xenograft growth could be promoted by the overexpressed DDR1, while both of which could be inhibited after the depletion of DDR1. Furthermore, DDR1 increased the levels of ZEB1 and Slug, based on its effects on tumor invasion. In conclusion, DDR1 may promote the aggressiveness of bladder cancer cells and drive an aggressive phenotype in bladder cancer.
Keywords: Bladder cancer, discoidin domain receptor 1 (DDR1), prognosis, cancer invasiveness, ZEB1, Slug
Introduction
Bladder cancer ranked as the third cause of cancer-related death worldwide. The mortality of bladder cancers mostly occurs to the patients with late-stage disease [1]. The recurrence of bladder cancer is currently diagnosed in approximately 25% of the total patients, who are frequently less sensitive to conventional chemotherapy [2-4]. Specifically, an invasive manner is often detected in these bladder tumors, suggesting that effective treatment of bladder cancer metastasis is critical for an optimized therapy [5].
Discoidin domain receptors (DDRs) are members of the transmembrane receptor tyrosine kinases (RTKs). There was a discoidin homology domain in extracellular region of DDRs [6-8]. Distinct from other RTKs, which are typically activated by growth factor ligands, DDRs use kinds of triple-helix collagens, independently of the integrin collagen receptors. DDR1 and DDR2 slightly varied in ligand specificities. DDR1 could be activated by most collagens such as the type I, II, III, IV, V, VIII, and XI collagen. While, DDR2 uses only collagen type I and III as its ligands [8-10]. Previous studies suggest that a collagen-activated DDR1 signaling pathway enhances the self-renewal, spreading, migration, and tubulogenesis of non-cancerous cells [11-14]. DDR1 levels are higher in solid malignant tumors than in normal tissues [15]. Moreover, elevated DDR1 levels were relevant to the poor prognosis of patients with pancreatic and lung cancers [16,17]. Bladder cancers progress through the interaction between tumor cells and stromal cells [18,19]. Therefore, DDR1 may use the stromal collagen as a ligand. However, the significance of DDR1 expression in bladder cancers is yet to be evaluated. Thus, we are prompted to study the effects of DDR1 on bladder cancer.
Here, the clinical correlation of DDR1 expression level in bladder cancer was investigated and the underlying molecular signaling pathway was explored.
Materials and methods
Patient
44 patients with bladder cancer were recruited in Ruijin Hospital (all at stage IV). All the patients have signed Informed consent. Both the bladder cancer species and corresponding paired normal tissues (NT) were resected during surgery. The species were histologically analyzed for determining the bladder cancer cells and NT cells.
Cell line culture and transfection
A human bladder cancer cell line RT4 was obtained from ATCC (USA). The cells were recovered cultured in RPMI1640 medium supplemented with 15% fetal bovine serum (Invitrogen, USA). The cells were cultured at 37°C with 5% CO2. The RT4 was derived from a 65-year-old male Caucasian [20], and expressed modest levels of DDR1. DDR1, scrambled control (scr) and short hairpin small interfering RNA for DDR1 (shDDR1, sequence: 5’-GAAUGUCGCUUCCGGCGUGUU-3’) were cloned into pCMV-luciferase-2A-GFP vector (Clontech, USA) to replace the GFP to generate pCMV-luciferase-2A-transgene. The RT4 cells were transfected with the plasmids of 50 nmol/l in Lipofectamine 2000 (Invitrogen, USA). The transfection efficiency was more than 95%.
Cell invasion assay
The transwell (BioCoatTM Invasion Chambers, BD) was involved for analyzing cell invasion. Cells with a density of 5×105 were seeded on the top layer of transwell chamber. After incubating at 37°C for 22 hours, the cells would move to below layer of chamber. The migrated and cells were fixed and stained. Cells were counted and expressed as the average number of cells in each field of view. The experiments were repeated for five times and the data were presented as mean value ± standard deviation (SD).
Scratch wound healing assay
Scratch wound healing assay was conducted according to previous reports [21]. Cells with a density of 104 cells/well was seeded in 24-well plates and incubated at 37°C until confluence. After cultured for 10 hours in DMEM without serum, the cells monolayer was scratched with pipette tip. Then the scratched cell monolayers were washed twice with PBS and cultured in DMEM at 37°C for another 24 hours. For each sample, five fields were randomly selected and pre-set. Wound image was captured at Time lapse module for 12 hours. The wound areas were analyzed with the software of Image J (NIH, USA).
RNA quantification with RT-qPCR
The RNA was quantified with RT-qPCR. The cells were digested with enzyme, concentrated by centrifuge and washed with PBS. The total RNA was extracted from the prepared cells with RNAeasy kit (Qiagen, Germany). The extracted total RNA was quantified with nanodrop (Thermo, USA). Then, complementary DNA (cDNA) was primed per 2 μg of total RNA with reverse transcription kit (Qiagen, Germany). The primers were synthesized or purchased (Qiagen, Germany). The RT-qPCR was then performed with cDNA using Commercial SYBR Green amplification system (Qiagen, Germany). Each experiment was repeated for three times. The data were collected and analyzed with 2-ΔΔCt method. All the data was normalized against the data of α-tubulin.
Protein quantification with western blot
The total proteins were analyzed with Western blot. The tissue specimens were grinded and the cultured cells were digested with enzyme, concentrated by centrifuge and washed with PBS. All the samples were lysed in RIPA lysis buffer and quantified with BCA method (Biorad, China). The lysates were diluted to 1:3 with SDS loading buffer for blotting (Biorad, China). The primary antibodies were rabbit anti-DDR1 IgG, rabbit anti-ZEB1 IgG, rabbit anti-Slug IgG and rabbit anti-α-tubulin IgG (Cell Signaling, USA). The α-tubulin was applied as a control. The 2nd antibody was HRP-labeled anti-rabbit IgG (Jackson ImmunoResearch, USA). After development with TMB substate, the blot was captured and quantified with the software of Image J (NIH, USA). The protein levels were normalized against the control. The experiments were repeated for five times and the data were presented as mean value ± standard deviation (SD).
Statistical analyses
The statistics were performed with SPSS 18.0. All the data were compared using one-way ANOVA and the Bonferroni correction was applied. The Fisher’s Exact Test was then involved in the comparison. It considered to be significant when p value <0.05. The correlation of patient survival and DDR1 levels was analyzed with Kaplan-Meier curves.
Results
The association between increased DDR1 and poor prognosis in bladder cancer
First, the DDR1 levels in bladder cancer specimens and paired non-tumor bladder tissue were determined. We detected significant increases in DDR1 in bladder cancer specimens by Western blot (Figure 1A). Next, we investigated if there was association between the DDR1 levels and overall survival of bladder patients. For the 44 patients diagnosed as Stage IV, their survival was followed for 5 years. Among the 44 cases, the median value of DDR1-level was determined as cutoff level. 44 cases were classified to 22 cases of DDR1-high level and 22 cases of DDR1-low cases. With the Kaplan-Meier curves, it indicated that patients with DDR1-high level showed a significantly poorer survival, compared to those who with DDR1-low level (Figure 1B). Thus, increased DDR1 in bladder cancer was associated with poor prognosis.
Figure 1.
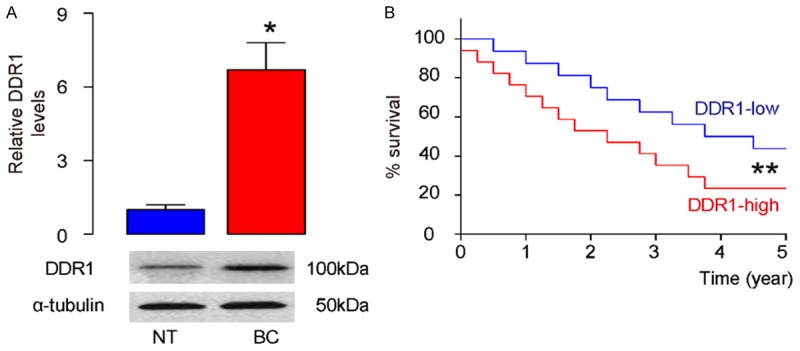
Increased DDR1 in bladder cancer is associated with poor prognosis. A: The DDR1 levels were examined by Western blot in bladder cancer specimens (BC), compared to paired non-tumor bladder tissue (NT). B: The survival of the 44 patients all diagnosed Stage IV were followed for 5 years. The median value of all 44 cases was chosen as the cutoff point for separating DDR1-high cases (n=22) from DDR1-low cases (n=22). Kaplan-Meier curves were performed. *P<0.05. **P<0.01. N=44.
Modulation of DDR1 levels in bladder cancer cells
In order to study the effects of DDR1 on bladder cancer invasiveness, a human bladder cancer cell line RT4 was involved. In this study, RT4 cells were transfected with a DDR1 overexpressing plasmid (DDR1), or a DDR1 interfering RNA (shDDR1). A scrambled sequence was transfected in RT4 to be control (scr). The modulation effects of DDR1 levels in these cells were confirmed from the levels of both mRNA (Figure 2A) and proteins (Figure 2B).
Figure 2.
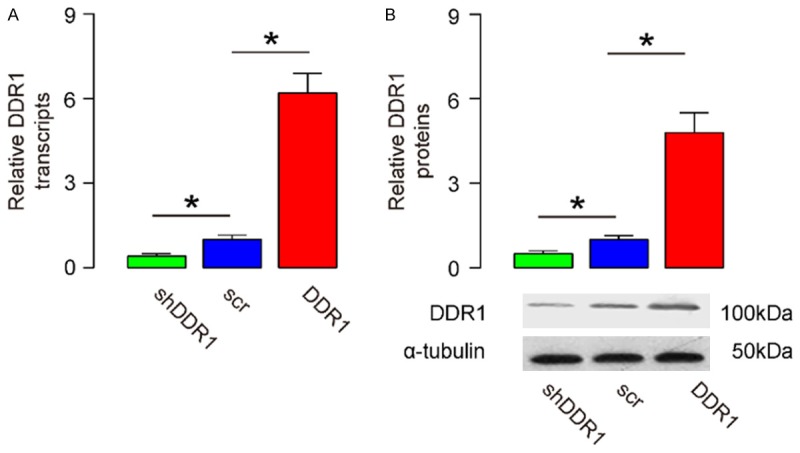
Modulation of DDR1 levels in bladder cancer cells. (A, B) A human bladder cancer cell line, RT4, was transfected with either a DDR1 overexpressing plasmid (DDR1), or a small short hairpin interfering RNA for DDR1 (shDDR1). The RT4 cells were transfected with a scrambled sequence as a control (scr). The levels of DDR1 were determined by RT-qPCR (A), and by Western blot (B). *P<0.05. N=5.
DDR1 enhances bladder cancer cell invasion in scratch wound healing assay
The scratch wound healing assay was applied to examine the effects of DDR1 on bladder cancer cell invasion. From the quantification, the overexpressed DDR1 resulted in increased cell invasion of RT4 cells (Figure 3A, 3B). Similarly, after the depletion of DDR1, the invasiveness of RT4 cells was decreased (Figure 3A, 3B). Thus, from the results of scratch wound healing assay, DDR1 enhanced bladder cancer cell invasion from the results of scratch wound healing assay.
Figure 3.
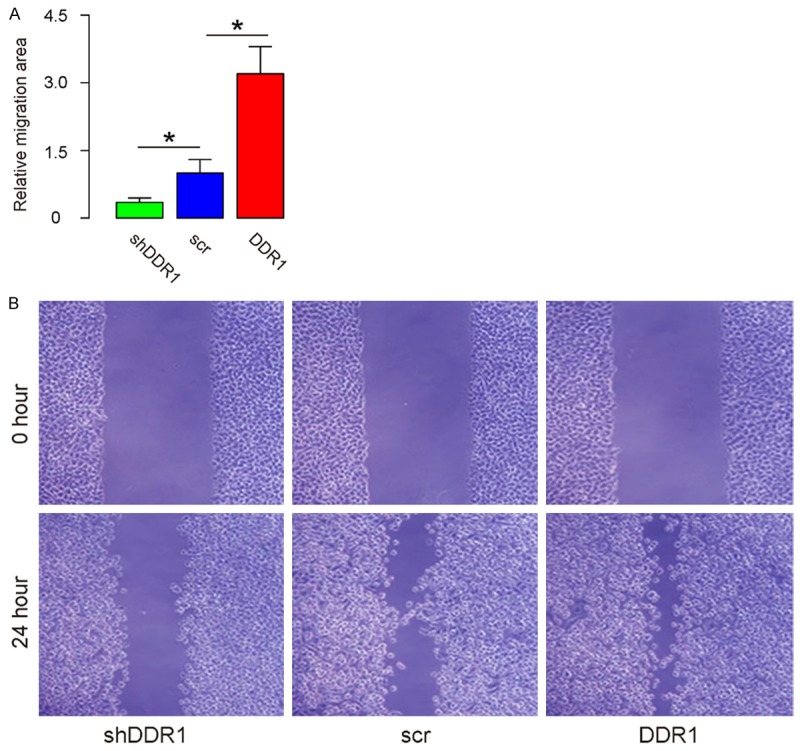
DDR1 enhances bladder cancer cell invasion in a scratch wound healing assay. (A, B) A scratch wound healing assay was applied to examine the effects of DDR1 on bladder cancer cell invasion, shown by quantification (A), and by representative images (B). *P<0.05. N=5.
DDR1 enhances bladder cancer cell invasion in a transwell cell invasion assay
The transwell cell invasion assay was also involved to examine the effects of DDR1 on bladder cancer cell invasion. From the results, the overexpressed DDR1 resulted in increased invasiveness (Figure 4A, 4B). After the depletion of DDR1, the invasiveness of RT4 cells was decreased (Figure 4A, 4B). Thus, DDR1 enhanced bladder cancer cell invasion from the results of transwell cell invasion assay.
Figure 4.
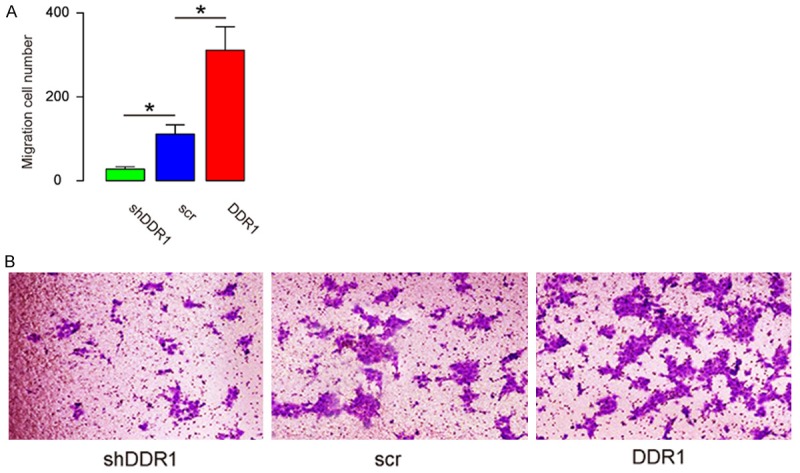
DDR1 enhances bladder cancer cell invasion in a transwell cell invasion assay. (A, B) A transwell cell invasion assay was applied to examine the effects of DDR1 on bladder cancer cell invasion, shown by quantification (A), and by representative images (B). *P<0.05. N=5.
DDR1 increases bladder cancer cell growth in vivo
Then, we used the DDR1-modified RT4 cell in a xenograft transplantation experiment into nude mice. The transfection of the cells by our plasmids allow detection of tumor implant by bioluminescence, taking advantage of their expression of luciferase. We found that two months after transplantation of 106 tumor cells, DDR1-expressing RT4 cells generated much bigger tumor, while DDR1-depleted RT4 generated much smaller tumor, compared to controls (Figure 5A, 5B). Thus, DDR1 increases bladder cancer cell growth in vivo.
Figure 5.
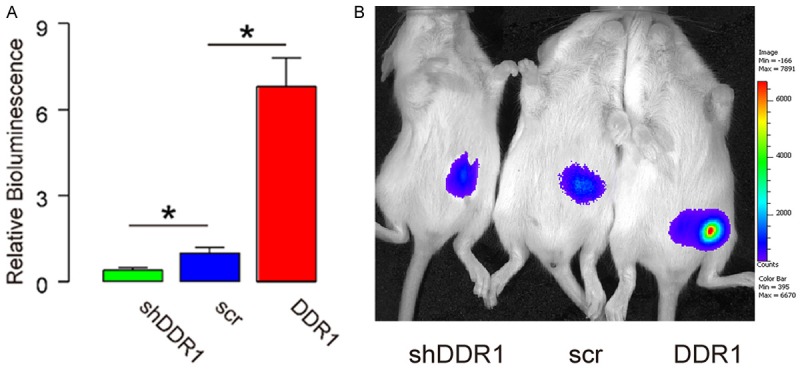
DDR1 increases bladder cancer cell growth in vivo. (A, B) 106 DDR1-modified RT4 cells were implanted s.c. into nude mice and the bioluminescence was determined after 2 months, shown by quantification (A), and by representative images (B). *P<0.05. N=5.
DDR1 enhances bladder cancer cell invasion through ZEB1 and Slug
Finally, we examined how DDR1 enhances bladder cancer invasion. We screened direct factors that increase tumor cell invasion in DDR1-modified RT4 cells. Specifically, we found that DDR1 increased the levels of ZEB1 and Slug (Figure 6). Thus, DDR1 may enhance bladder cancer cell invasion through ZEB1 and Slug.
Figure 6.
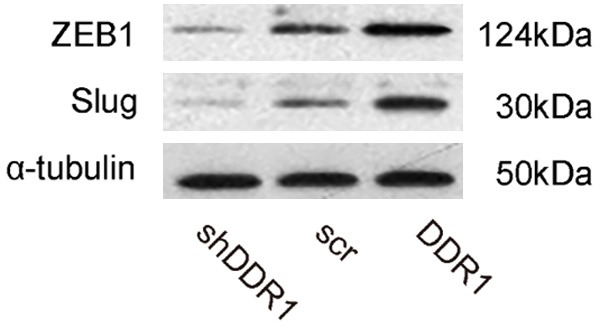
DDR1 enhances bladder cancer cell invasion through ZEB1 and Slug. Western blot for ZEB1 and Slug in DDR1-modified RT4 cells.
Discussion
In this study, we provide evidence that DDR1 expression may be correlated with poor prognosis in bladder cancer patients. Moreover, we showed that DDR1 activation enhanced aggressive tumor phenotypes in vitro and in vivo. These observations provided strong evidence that DDR1 served as a critical mediator of bladder cancer aggressiveness and that the inhibition of DDR1 can be potentially used as a novel strategy to improve the prognosis of bladder cancer patients [22-26].
Although the expression of DDR1 in bladder cancers remained unknown previously, some studies have demonstrated a positive association between DDR1 protein expression and poor prognosis in solid tumors, which supports the hypothesis that DDR1 expression may enhance the aggressiveness of malignant tumors, e.g. non-small cell lung cancer [16,27], serous ovarian cancer [28], and pancreatic ductal adenocarcinoma [17]. Like other solid tumors, bladder cancers contact non-cancerous stroma to enhance tumor growth and modulate immune responses [29,30]. Thus, the expression of DDR1 may use extracellular collagen as a ligand to enhance bladder cancer invasion and metastasis.
Here we tried to prove this hypothesis. We not only detected high DDR1 in bladder cancer, but also found an association between DDR1 levels and patients’ prognosis. We selected only Stage IV patients, since these bladder tumors are more aggressive and malignant to allow examination of the survival of the patients within a limited time course. To understand the underlying mechanisms, several human bladder cell lines were applied to examine their interactions. Since similar results were obtained in these cell lines, only data of RT4 cells were provided. It observed that overexpressed DDR1 in bladder cancer cells enhanced tumor invasion, while depletion of DDR1 in bladder cancer cells decreased cell invasion, both in vitro and in vivo. In addition, among all factors that are closely related to an aggressive manner of bladder cancer, we specifically found that ZEB1 [31-33] and Slug [34,35] were regulated by DDR1. The detailed molecular signaling should be explored in the future studies. Although we did not analyze in vitro cell growth regulated by DDR1, the adaption of the cell invasiveness may partially result from the changes in cell growth, and increases in cell invasiveness augment cell growth. These notions were supported by our in vivo data. DDR1 regulated the expression of ZEB1 and Slug, while the potential molecular mechanisms should be clarified, as well as the relationship between the control of cell growth and cell invasion. It may promote our understanding of the bladder cancer cell metastasis and may further provide basis for exploring novel therapeutic targets and strategies for treating bladder cancer.
Disclosure of conflict of interest
None.
References
- 1.Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 3.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 2006;24:2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Cho JY. Molecular diagnosis for personalized target therapy in gastric cancer. J Gastric Cancer. 2013;13:129–135. doi: 10.5230/jgc.2013.13.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- 7.Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci U S A. 1993;90:5677–5681. doi: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- 9.Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- 10.Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;13(Suppl):S77–82. doi: 10.1096/fasebj.13.9001.s77. [DOI] [PubMed] [Google Scholar]
- 11.Camara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair. 2010;3:2. doi: 10.1186/1755-1536-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226:3422–3432. doi: 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- 13.Wang CZ, Hsu YM, Tang MJ. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J Cell Physiol. 2005;203:295–304. doi: 10.1002/jcp.20227. [DOI] [PubMed] [Google Scholar]
- 14.Yeh YC, Wang CZ, Tang MJ. Discoidin domain receptor 1 activation suppresses alpha2beta1 integrin-dependent cell spreading through inhibition of Cdc42 activity. J Cell Physiol. 2009;218:146–156. doi: 10.1002/jcp.21578. [DOI] [PubMed] [Google Scholar]
- 15.Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96:808–814. doi: 10.1038/sj.bjc.6603614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huo Y, Yang M, Liu W, Yang J, Fu X, Liu D, Li J, Zhang J, Hua R, Sun Y. High expression of DDR1 is associated with the poor prognosis in Chinese patients with pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2015;34:88. doi: 10.1186/s13046-015-0202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi H, Sakai R. Direct Interaction between Carcinoma Cells and Cancer Associated Fibroblasts for the Regulation of Cancer Invasion. Cancers (Basel) 2015;7:2054–2062. doi: 10.3390/cancers7040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yashiro M, Hirakawa K. Cancer-stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron. 2010;3:127–135. doi: 10.1007/s12307-010-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rigby CC, Franks LM. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970;24:746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 22.Ding G, Yu S, Cheng S, Li G, Yu Y. Androgen receptor (AR) promotes male bladder cancer cell proliferation and migration via regulating CD24 and VEGF. Am J Transl Res. 2016;8:578–587. [PMC free article] [PubMed] [Google Scholar]
- 23.Long JD, Sullivan TB, Humphrey J, Logvinenko T, Summerhayes KA, Kozinn S, Harty N, Summerhayes IC, Libertino JA, Holway AH, Rieger-Christ KM. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am J Transl Res. 2015;7:2500–2509. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, He W, Li J, Huang S, Wan X, Luo H, Wu D. MiRNA-125b inhibits proliferation and migration by targeting SphK1 in bladder cancer. Am J Transl Res. 2015;7:2346–2354. [PMC free article] [PubMed] [Google Scholar]
- 25.Wilz SW, Liu D, Liu C, Yang J. Development of a test to identify bladder cancer in the urine of patients using mass spectroscopy and subcellular localization of the detected proteins. Am J Transl Res. 2015;7:1458–1466. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Li J, Huang S, Wan X, Luo H, Wu D. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am J Transl Res. 2015;7:1382–1389. [PMC free article] [PubMed] [Google Scholar]
- 27.Yang SH, Baek HA, Lee HJ, Park HS, Jang KY, Kang MJ, Lee DG, Lee YC, Moon WS, Chung MJ. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung carcinomas. Oncol Rep. 2010;24:311–319. doi: 10.3892/or_00000861. [DOI] [PubMed] [Google Scholar]
- 28.Quan J, Yahata T, Adachi S, Yoshihara K, Tanaka K. Identification of receptor tyrosine kinase, discoidin domain receptor 1 (DDR1), as a potential biomarker for serous ovarian cancer. Int J Mol Sci. 2011;12:971–982. doi: 10.3390/ijms12020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno S, Tachibana M, Fujii T, Ueda S, Kubota H, Nagasue N. Role of stromal collagen in immunomodulation and prognosis of advanced gastric carcinoma. Int J Cancer. 2002;97:770–774. doi: 10.1002/ijc.10144. [DOI] [PubMed] [Google Scholar]
- 30.Nie XC, Wang JP, Zhu W, Xu XY, Xing YN, Yu M, Liu YP, Takano Y, Zheng HC. COL4A3 expression correlates with pathogenesis, pathologic behaviors, and prognosis of gastric carcinomas. Hum Pathol. 2013;44:77–86. doi: 10.1016/j.humpath.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Chung VY, Tan TZ, Tan M, Wong MK, Kuay KT, Yang Z, Ye J, Muller J, Koh CM, Guccione E, Thiery JP, Huang RY. GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci Rep. 2016;6:19943. doi: 10.1038/srep19943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Zhao Y, Zhang J, Lu Y, Liu X, Geng P, Huang B, Zhang Y, Lu J. The dual function of PRMT1 in modulating epithelial-mesenchymal transition and cellular senescence in breast cancer cells through regulation of ZEB1. Sci Rep. 2016;6:19874. doi: 10.1038/srep19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ungewiss C, Rizvi ZH, Roybal JD, Peng DH, Gold KA, Shin DH, Creighton CJ, Gibbons DL. The microRNA-200/Zeb1 axis regulates ECM-dependent beta1-integrin/FAK signaling, cancer cell invasion and metastasis through CRKL. Sci Rep. 2016;6:18652. doi: 10.1038/srep18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan Y, Li J, Zhang Y, Wang N, Liang H, Liu Y, Zhang CY, Zen K, Gu H. Slug-upregulated miR-221 promotes breast cancer progression through suppressing E-cadherin expression. Sci Rep. 2016;6:25798. doi: 10.1038/srep25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Zhang J, Yang H, Wu C, Dang X, Liu Y. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci Rep. 2015;5:12410. doi: 10.1038/srep12410. [DOI] [PMC free article] [PubMed] [Google Scholar]


