Abstract
Severe burns may lead to intestinal inflammation and oxidative stress resulting in intestinal barrier damage and gut dysfunction. In the management of severe burns, therapies are needed to attenuate whole-body inflammatory responses and control the burden of oxidative stress. In this study, we evaluated the effects of oral glutamine (Gln) with probiotics on burn-induced intestinal inflammation and oxidative stress using a Wistar rat burn injury model. We then explored potential molecular mechanisms for the effects of glutamine and probiotics on intestinal tissue inflammation and oxidative stress. We found that glutamine and probiotics together significantly inhibited nitric oxide (NO) content; reduced levels of the inflammatory factors TNF-α, IL-6, and IL-8; and altered expression of oxidative stress factors including reactive oxygen species and superoxide dismutase. We found that the apoptotic proportion of intestinal epithelial cells in severely burned subjects was notably decreased following treatment with glutamine plus probiotics. We also found that glutamine and probiotics given together markedly reduced NO content by down-regulating the expression of iNOS in blood and intestinal tissue. These findings indicate that regulation of the iNOS gene plays a pivotal role in inflammation and oxidative stress in the response to severe burns in the Wistar rat. We then further investigated the mechanism by which combined therapy with glutamine and probiotics might reduce expression of iNOS and found that this treatment resulted in increased methylation of the iNOS gene. The methylation level of the iNOS gene was found to be regulated via differential expression of DNMT1 and Tet1. Collectively, our results suggest that combined therapy with glutamine and probiotics can markedly reduce the synthesis of NO, suppressing intestinal inflammation and oxidative stress in the Wistar rat burn injury model.
Keywords: Glutamine, probiotics, NO, iNOS, DNA methylation
Introduction
Severe burns can be associated with intestinal inflammation and oxidative stress which may then lead to intestinal barrier damage and further organ dysfunction [1-3]. The intestinal tract serves as the largest immune system organ in the body. Thus, following severe burn injury, the intestinal tract is the first organ to be damaged by associated systemic stress and inflammation [4,5]. Severe burns can directly and indirectly lead to intestinal injury by contributing to overgrowth of intestinal bacteria, compromising the integrity of the intestinal wall, and reducing immune system responsiveness. In the setting of ongoing inflammation, apoptosis and necrosis may occur among intestinal epithelial cells, leading to increased permeability of the mucosa and thereby permitting bacteria and other macromolecular substances to pass into the bloodstream, causing additional oxidative stress and provoking further inflammation. This cycle can cause progressive damage to the intestinal mechanical and immune barriers, eventually leading to worsening systemic infection and inflammation if not controlled [6-8].
A significant increase in intestinal tract nitric oxide (NO) following burn injury has been previously reported in a rat severe burn injury model [9]. Under physiological conditions, NO is widely distributed in gastrointestinal tract tissues, where this substance plays roles in peristalsis and mucosal protection [10,11]. Following severe burn injury, NO has been proposed to play an important role in the intestinal tract inflammatory response to injury [12,13]. NO is generated from L-arginine through a reaction catalyzed by nitric oxide synthase (NOS). NOS is known to have the forms neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS) [14]. In response to a range of stimuli, including pro-inflammatory factors, foreign antigens, lipopolysaccharide (LPS), and cytokine stimulation, the expression of iNOS is upregulated in macrophages, T lymphocytes, liver cells, muscle cells, and endothelial cells; abundant NO production can induce a severe inflammatory response [15-17].
Previous studies have found significantly decreased glutamine in muscle tissues and plasma after severe burn injury [18]. Glutamine supplementation has been found to promote biological processes necessary for intestinal repair, intestinal injury relief, maintenance of intestinal barrier functions, and prevention of intestinal bacterial and endotoxin translocation [19-21]. In addition, probiotics, microbial preparations containing physiologically active bacteria and their byproducts, have also been found to inhibit the growth of pathogenic bacteria, reduce intestinal inflammation, and inhibit signaling pathways including the NF-κB pathway; these activities may result in suppression of inflammatory reactions, protection of intestinal epithelial cells, and enhancement of the intestinal mechanical barrier [22-24]. To achieve synergistic benefits in the treatment of intestinal injury following severe burns, glutamine and probiotics can be used together. However, the degree of synergy and the specific mechanisms accounting for the combined effects of glutamine and probiotics remain to be studied.
In this investigation we evaluated the effects of glutamine combined with probiotics on intestinal injury using a Wistar rat severe burn model. The objectives of this study were to identify and characterize treatment effects and mechanisms for further exploration in subsequent clinical trials in burn patients.
Materials and methods
Materials
L-glutamine was purchased from Jiangsu Shenhua Pharmaceutical Co., Ltd (Z320210, Jiangsu, China). Probiotics were purchased from Beijing Tongrentang Co., Ltd (X12-110, Beijing, China).
Wistar rat burn injury model: model creation, study groups, and data collection
120 healthy male Wistar rats (4-6 weeks) from local experimental animal center in Southwest hospital were used in this study. Rats were obtained and then fed for one week. Subjects were randomly selected for inclusion in the severe burn group (n = 80). All rats were weighed prior to burn injury creation or control warm water treatment. Burn injuries were created by scalding the skin of the back using boiling water. Prior to burn creation, each rat received an intraperitoneal injection of 3% pentobarbital sodium (40 mg/kg) as an anesthetic. The hair on each subject’s back was then removed using scissors. The analgesic buprenorphine (1 mg/kg) was injected to provide pain relief. A custom-made positioning apparatus was used to hold subjects while a scald burn injury was created on the back of the animal. To create the burn injury, the test area on the back was completely immersed in boiling water (100°C) for 15 seconds (s), creating a 20% body surface area full thickness burn. Rats in the normal control group were treated with warm water (25°C, 15 s) and otherwise received the same treatment as rats in the burn group. Following burn injury creation, animals were caged individually for 1 day, then returned to their cages and permitted to eat and drink freely.
Oral gavage was used to administer the following treatments to burn injury and control rats: control group (n = 15), 1 ml normal saline; burn model group (n = 15), 1 ml normal saline; glutamine treatment group (Gln group; n = 15), 1.5 g/kg glutamine + 1 ml normal saline; probiotics treatment group (probiotics group; n = 15), 300 mg/kg probiotic + 1 ml normal saline; glutamine and probiotics combined treatment group (n = 15), 1.5 g/kg + 300 mg/kg glutamine + 1 ml normal saline. Treatments were given once per day for seven days. Wounds were treated with iodophor (Mosen disinfection technology Co. Ltd, Shandong, China) each day. Wounds were kept dry and clean. And rats were treated according to the following methods: subjects in the control group (n = 15) and the burn model group (n = 15) were administered 1 ml saline by tail intravenous injection immediately after injury. Rats in the L-NAME (N-nitro-L-arginine methyl ester, NOS-specific inhibitor, S0006, Beyotime Biotechnology, Shanghai, China) group (n = 15) received L-NAME HCL (100 mg/kg) by tail intravenous injection immediately after burn injury creation.
Blood and tissue samples were collected after three and seven days of treatment. Subjects were weighed and then anesthetized using an intraperitoneal injection of sodium pentobarbital (40 mg/kg). Each rat was then placed on a dissecting table tray, the abdominal cavity was opened using scissors, the position of the heart was observed, and 2 ml of blood was collected by inserting a 1 ml needle (G2100573425002, Zhengkang instruments Co., Ltd, Jiangsu, China) into the heart. The collected blood was kept in room temperature for 20 minutes (min) and then centrifuged at 4°C at 4000 rpm for 15 min. Then, the collected blood was divided into two parts, to be frozen in liquid nitrogen and stored at -20°C, Samples of intestinal tissue were surgically resected and immediately immersed in 10% formaldehyde, and rats were sacrificed.
Examination of intestinal tissue sections using hematoxylin and eosin (HE) staining
Intestinal tissue specimens were removed from 10% formaldehyde solution after 24 h and rinsed with saline. Surface liquid was gently and carefully wiped using filter paper (Yiyuan machine tool accessory Co., Ltd, Yangtai, China). Specimens were dehydrated overnight using 95-100% alcohol in an. automatic hydroextractor (TP1020, Leica, Wetzlar, Germany). A pathological tissue embedding machine (HistoCore Arcadia, Leica, Wetzlar, Germany) was used to section intestinal tissue into slices of 3 μm. Using forceps, slices were gently placed onto glass slides and any folds were stretched. The slices were then de-waxed twice with xylene, each time for 10 min. Afterwards, the slices were rinsed with saline. Sections were then stained for 5 min with hematoxylin, rinsed, and stained for 30 s with 0.5% eosin. Slices were then dehydrated with alcohol and de-alcoholized with xylene. Neutral gum and coverslips were applied. Sections were examined using light microscopy with ×400 magnification (Leica DM1000, Leica, Wetzlar, Germany) and photographed.
Blood sample biochemical index determination
IL-6, IL-4, IL-8 and TNF-α (ASS3189, ASS3182, KHC0081 and KHC3012, Abgent, San Diego, CA, USA) were measured using ELISA assays. ROS (MAK144-1KT, Sigma-Aldrich, St. Louis, MO, USA) and SOD (19160-1KT-F, Sigma-Aldrich, St. Louis, MO, USA) were measured using chemiluminescent assays. NO was measured using a NO detection kit (23479-1KT-F, Sigma-Aldrich, St. Louis, MO, USA). All tests were performed by one technologist. All operations were carried out according to kit instructions.
Flow cytometry analysis of cellular apoptosis proportion
Intestinal epithelial cells were isolated from rats in the control group, burn model group, glutamine treatment group, probiotics treatment group, and combined glutamine and probiotics group. 1×106 cells from each rat were harvested following normal protocols, and these were double-stained with Tali® Apoptosis Kit (A10788, Invitrogen, Carlsbad, CA, USA) containing annexin V and PI. Percentages of apoptotic cells were detected after staining using flow cytometry (FACSCalibur, Becton and Dickinson Company, Franklin Lakes, NJ, USA). The experiment was repeated three times, and representative images were shown.
Quantitative real-time polymerase chain reaction (PCR) assays
Total ribonucleic acids (RNAs) were isolated from intestinal epithelial cells using TRIzol® Reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. NanoDrop-1000 (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine the concentration and purity of total RNAs. 1 μg total RNA was synthesized as the first strand cDNA according to the instructions from the reverse transcription kit (A5001, Promega, Madison, WI, USA). RT-PCR was used to detect mRNA expression of iNOS, eNOS, nNOS, DNMT1, and Tet1 (ab15323, ab76198 and ab102087 ab2849 and ab191698, Abcam, Cambridge, MA, USA). GAPDH mRNA (ab70699, Abcam, Cambridge, MA, USA) served as an inner control of other mRNAs.
Western blot analysis
Intestinal epithelial cells were collected and total proteins were extracted using ProteoPrep® Total Extraction Sample Kit (PROTTOT-1KT, Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. Then supernatant was collected for detection of protein concentration using a BCA protein assay kit (P0012, Beyotime Biotechnology, Shanghai, China). A 20 μg protein sample was used for SDS-PAGE electrophoresis and then this was transferred to a nitrocellulose (NC) membrane. Afterwards 5% skimmed milk powder/Phosphate Buffer Solution (PBST) was used for sealing at room temperature for 2-4 h. Anti-iNOS (1:500), eNOS (1:500), nNOS (1:500), DNMT1 (1:400), Tet1 (1:1000), and GADPH (1:1000) primary antibodies were incubated overnight at 4°C. Horseradish Peroxidase (HRP)-labeled secondary antibodies were then incubated with specimens at room temperature for 2 h. Electrochemical luminescence (ECL) luminescent liquid was applied prior to analysis using a chemiluminescence imaging system (Leica M205 FCA, Leica, Wetzlar, Germany).
Bisulfite sequencing PCR (BSP) and methylation-specific PCR (MSP)
Intestinal tissue samples were collected from the control group, burn model group, glutamine group, probiotics group, and combined glutamine and probiotics group. Genomic DNA were extracted from intestinal tissues using a DNA extraction kit (10503027, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Recovery of DNA was performed using a Promega DNA purification kit (A1620, Promega, Madison, WI, USA) for PCR detection. The methods for these procedures have been previously published [25].
Statistical analysis
All data in the manuscript are presented as mean ± standard error of the mean (SEM). GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA) was used for graphical analyses and data processing. Statistical significance was determined by one-way or two-way analyses of variance, depending on the concentration of different factors or the level of gene expression. P < 0.05 was considered to be a statistically significant difference.
Results
Rats in the severe burn group demonstrated aggravated inflammatory reactions with an increase in the apoptosis proportion among intestinal epithelial cells
In order to assess whether combined supplementation with glutamine and probiotics can effectively reduce inflammation and oxidative stress in the intestinal tract following burn injury, a rat severe burn injury model was created. Examination of intestinal tissue samples at three and seven days after injury was performed using HE staining. Inflammatory changes were observed in intestinal tissues from rats in the severe burn group on days 3 and 7 after burn injury creation, compared with the control group (Figure 1).
Figure 1.
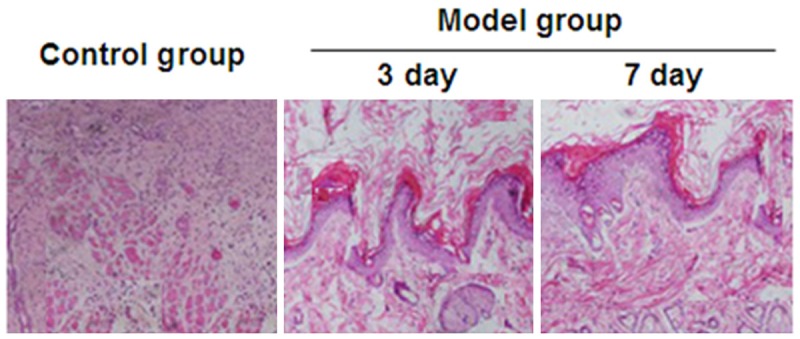
Damage to the intestinal tissue following burn injury on the third and seventh day in the burn model group compared to a control tissue section. Sections were stained with hematoxylin and eosin (HE) and photographed at 40× magnification.
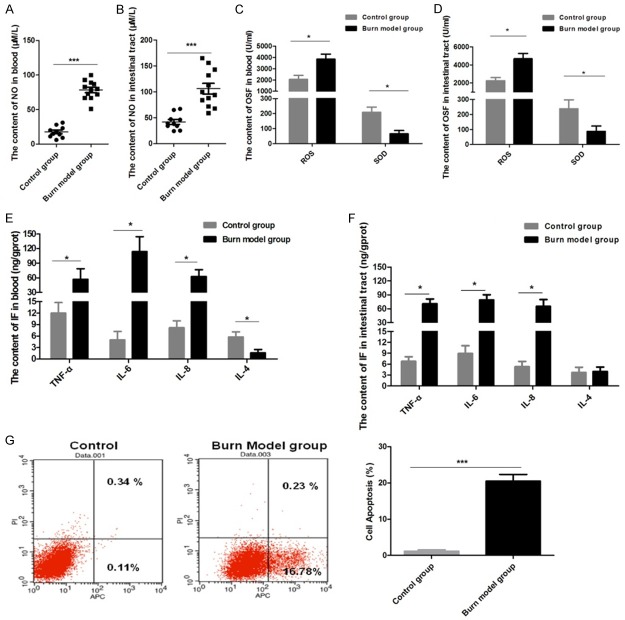
It has been reported that synthesis and release of NO within the intestinal tract following severe burn injury may be a major factor in the stress response contributing to subsequent intestinal injury [26]. Levels of NO in the blood and intestinal tissues from burned rats were assessed using chemiluminescence, and we observed that NO content was significantly higher in blood and intestinal tissue samples from subjects in the burn group compared to controls (Figure 2A, 2B). Furthermore, our data also revealed that ROS was markedly increased in blood and intestinal tissues from the burn group, while SOD activity was significantly inhibited (Figure 2C, 2D). We also found that blood levels of TNF-α, IL-6, and IL-8 in the burn group were higher than those in the control group, but that the level of IL-4 was significantly lower in the burn group (Figure 2E). Except for IL-4, these trends for intestinal tissue samples were consistent with those observed in blood (Figure 2F).
Figure 2.
In blood and intestinal tissue samples, the content of nitric oxide (NO), oxidative stress factors, and inflammatory factors (IF) were significantly increased and the apoptosis rate of the intestinal epithelial cells was increased in the burn model group: A: NO content detected in blood. B: NO content detected in intestinal tissue. C: OSFs were detected in blood. D: OSFs were detected in intestinal tissue. E: IFs were detected in blood. F: IFs were detected in intestinal tissue. G: The apoptosis ratio of intestinal epithelial cells was determined by flow cytometry. Data are presented as the mean ± standard error of measurement (SEM) (n = 3) with *P < 0.05 or ***P < 0.001, analyzed by two-way ANOVA.
Apoptosis and necrosis of intestinal epithelial cells after intestinal tissue injury were then assayed. We found that the apoptosis ratio for intestinal epithelial cells in the burn group was markedly increased (Figure 2G). These results characterize the Wistar rat model of severe burn injury which was found to have measurable associated systemic and gastrointestinal tract inflammation markers. These measured outcomes reflect burn-injury-induced oxidative stress and inflammatory responses.
Combined application of glutamine and probiotics inhibits the synthesis of NO, effectively reducing oxidative stress and inflammation in the blood and intestinal tissues in the Wistar rat burn model
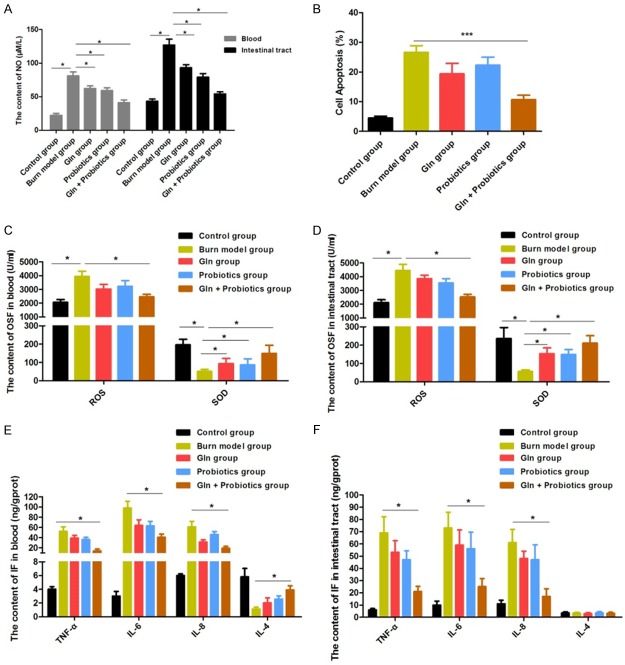
In order to further study the effects of combined application of glutamine and probiotics on intestinal inflammation and oxidative stress, subjects were divided into five groups: control group, burn model group, glutamine group, probiotics group, and combined treatment group (Gln + probiotics group). Since NO is a critical stress factor and a main contributor to intestinal injury following severe burn injury, we first investigated the effects of glutamine and probiotics, alone and in combination with each other, on the content of NO. We observed that the increase in NO after burn injury was sharply depressed when burned rats received either glutamine or probiotics single treatment, slightly alleviating the effects of the burn injury (Figure 3A). Levels of NO were significantly inhibited in blood and intestinal tissue samples compared to controls (P < 0.01) when receiving combined treatment with both glutamine and probiotics (Figure 3A). Apoptosis and necrosis of intestinal epithelial cells was then further assessed using flow cytometry. The apoptosis ratio for intestinal epithelial cells was significantly lower in the glutamine with probiotics group compared to the Gln and Probiotics single treatment groups (P < 0.05, P < 0.05) (Figure 3B). In addition, levels of ROS in the combined treatment group compared with the Gln or Probiotics single treatment groups were decreased (P < 0.05, P < 0.05) in the blood and intestinal tissue, while SOD activity was remarkably increased (P < 0.01, P < 0.01) (Figure 3C, 3D). These results suggest that the combination of glutamine with probiotics can significantly reduce oxidative stress, which can then effectively attenuate stress injury to the intestines in the setting of severe burn injury.
Figure 3.
The oxidative stress and inflammation in the blood and intestine were effectively reduced, and increased NO was markedly inhibited, by treatment of burned rats with combined glutamine and probiotics: A: NO levels in intestinal tissue and blood samples were measured for different treatment groups. B: The apoptosis rate of intestinal cells for different treatment groups was determined by flow cytometry. C: Oxidative stress factors (OSFs) were measured in blood. D: OSFs were measured in intestinal tissue. E: Inflammatory factors (IFs) were measured in blood. F: IFs were measured in intestinal tissue. Data are presented as the mean ± standard error of measurement (SEM) (n = 3) with *P < 0.05 or ***P < 0.001, analyzed by two-way ANOVA.
Levels of inflammatory factors were also assayed after treatment. Our data showed that treating burned rats with glutamine or probiotics alone rarely controlled increases in the levels of TNF-α, IL-6, and IL-8 after injury, but levels of TNF-α, IL-6, and IL-8 were markedly decreased after treatment with glutamine and probiotics combined. Blood levels of IL-4 were also significantly increased in the combined treatment group than Gln or Probiotics single treatment group (P < 0.05, P < 0.05) (Figure 3E, 3F), indicating a remarkable and pronounced anti-inflammatory effect. Taken together, these results suggest that the combination of glutamine with probiotics can significantly reduce oxidative stress and inflammation in the blood and intestine in the setting of severe burn injury.
Combined application of glutamine and probiotics downregulates expression of iNOS and reduces the synthesis of NO
In order to further explore how the combined application of glutamine and probiotics can reduce NO synthesis, inhibit intestinal oxidative stress and inflammation, and reduce intestinal cell apoptosis after severe burn injury, we assayed the expression and activity of iNOS, eNOS, and nNOS following the study treatment protocols. Compared with controls, expression and activity of iNOS were both significantly increased in the burn model group (P < 0.05). The expression and activity of iNOS was significantly reduced by treatment of the burned rats with glutamine and probiotics alone (P < 0.05, P < 0.05). Our data also showed that the expression and activity of iNOS was decreased in combined treatment group compare to burn model group, Gln group and probiotics single treatment group (P < 0.01, P < 0.05, P < 0.05), compared with the burn model group, glutamine group, or probiotics group. Of note, the expression and activity levels of eNOS and nNOS were rarely found to differ from other groups (P > 0.05) (Figure 4A, 4B). The expression of iNOS, eNOS, and nNOS proteins were then further examined by western blot, and these results were consistent with the above findings (Figure 4C). Accordingly, our results suggest that abnormal expression of iNOS in the intestine is the main factor accounting for the increase in NO following severe burn injury. This abnormal expression of iNOS was clearly inhibited by treatment of the severely burned rats with combined gavage doses of glutamine and probiotics. Combined treatment resulted in a measurable and significant decrease in the abnormal production of NO, thereby inhibiting intestinal inflammation and reducing intestinal injury.
Figure 4.
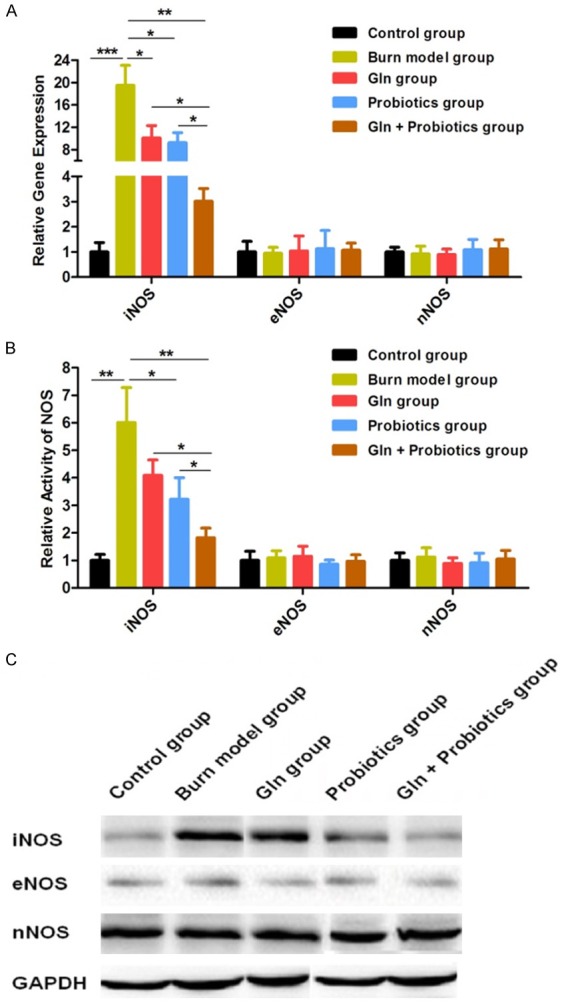
Expression of iNOS was down-regulated and synthesis of NO was decreased by treatment of severely burned rats with combined glutamine and probiotics. A: The relative gene expression of iNOS, eNOS and nNOS were detected for the different treatment groups by RT-PCR. B: The relative gene activities of iNOS, eNOS, and nNOS were detected for the different treatment groups. C: The expression of iNOS, eNOS, and nNOS were detected for the different treatment groups by western blot. Data are presented as the mean ± standard error of measurement (SEM) (n = 3) with *P < 0.05, **P < 0.01, or ***P < 0.001, analyzed by two-way ANOVA.
iNOS plays a critical role in intestinal inflammation and oxidative stress reactions in severely burned rats
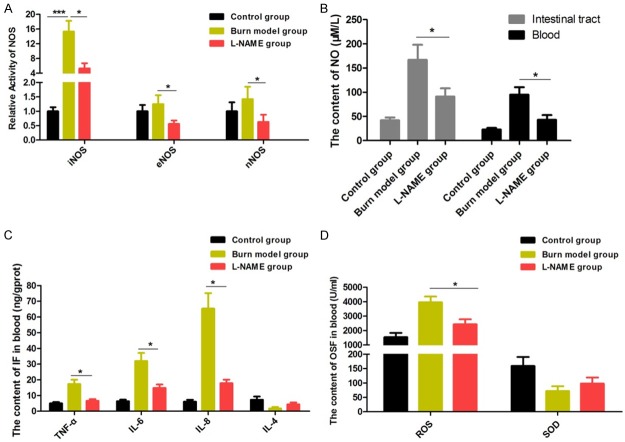
To further study how NO synthesis is impacted by regulation of iNOS expression, rats that had received severe burns were treated with the NOS-specific inhibitor L-NAME in order to block the activity of NOS. We then measured changes oxidative stress markers and expression of inflammatory factors in the blood and intestinal tissues. While the activities of iNOS, eNOS, and nNOS were all obviously inhibited by L-NAME (Figure 5A), only the activity of iNOS in the burn model group was significantly improved compared with the control group (Figures 4A, 5A). Changes in eNOS and nNOS activity were rarely observed. In addition, NO content was significantly inhibited by L-NAME in the blood and intestinal tissues, compared with the burn model group (Figure 5B). These results indicate that the inhibitory effect of L-NAME on NOS activity is achieved mainly by depressing iNOS activity to decrease NO synthesis. Moreover, the significant increases in inflammatory factors (TNF-α, IL-6, IL-8) and oxidative stress factors in the burn model group all declined markedly when iNOS activity was inhibited by L-NAME (Figure 5C, 5D). Taken together, the finding that repression of NO synthesis by L-NAME results in reduced levels of oxidative stress and inflammatory response markers provides further support for the roles of NO and iNOS in secondary intestinal tract injury following severe burns.
Figure 5.
iNOS plays a critical role in inflammation and oxidative stress processes in severely burned rats. A: The relative gene activity of iNOS, eNOS, and nNOS were assayed after treatment with L-NAME. B: The content of NO was measured after treatment with L-NAME. C: Inflammatory factors (Ifs) were measured after treatment with L-NAME. D: Oxidative stress factors (OSFs) were measured after treatment with L-NAME. Data are presented as the mean ± standard error of measurement (SEM) (n = 3) with *P < 0.05, **P < 0.01, or ***P < 0.001, analyzed by two-way ANOVA.
The combination of glutamine and probiotics decreases iNOS expression and NO synthesis by increasing methylation of the iNOS gene
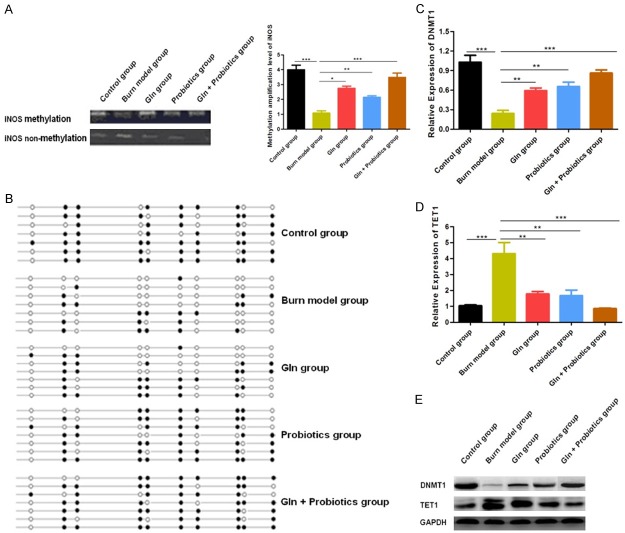
We then explored the molecular mechanisms by which glutamine and probiotics might regulate iNOS expression in our rat severe burn injury model. Hori et al. have previously reported that the promoter region of the iNOS gene is hyper-methylated under non-pathological conditions, blocking transcription of the iNOS gene. However, various stimulating factors, including burn injury and oxidative stress, are known to change the DNA methylation status of the iNOS gene in vivo [27]. Accordingly, the methylation state of the iNOS gene in our rat model was first probed by MS-PCR and BS-PCR, and we found that the methylation level of the iNOS gene was decreased significantly following burn injury creation. The methylation level of the iNOS gene was then found to improve after treatment of with combined glutamine and probiotics (Figure 6A, 6B). The level of DNA methylation is controlled by two processes: DNA methylation and demethylation. Therefore, expression levels of methyltransferase and demethylase were then further assessed by western blot and RT-PCR. We observed that expression of methyltransferase DNMT-1 was significantly down-regulated and that the expression of demethylase Tet1 was significantly increased in rats following burn injury. Following treatment of burned rats with glutamine and probiotics, the expression of DNMT-1 improved and the expression of Tet1 was reduced (Figure 6C-E). These findings suggest that the methylation level of the iNOS gene was impacted by changes in the activities of DNA methyltransferase DNMT1 and demethylase Tet1, thus leading to altered synthesis of NO, intestinal cell apoptosis, and tissue capability for repair and maintenance of the intestinal barrier.
Figure 6.
Expression of iNOS and synthesis of NO was clearly decreased by increasing the methylation level of the iNOS gene after treatment of severely burned rats with combined glutamine and probiotics. A: The methylation status of the iNOS gene was detected for the different treatment groups by MS-PCR. B: The methylation status of the iNOS gene was detected for the different treatment groups by BSP-PCR. C: The relative gene expression of DNMT1 was determined by RT-PCR. D: The relative gene expression of TET1 was determined by RT-PCR. E: The expression of DNMT1 and TET1 were determined by western blot. Data are presented as the mean ± standard error of measurement (SEM) (n = 3) with *P < 0.05, **P < 0.01, or ***P < 0.001, analyzed by two-way ANOVA.
Discussion
Severe burns can cause oxidative stress reactions and inflammation throughout the body, contributing to reduced immunity and a variety of burn complications, including sepsis and possible death [28-30]. In our study, we observed measurable increases in ROS and NO in the plasma of Wistar rats following the creation of severe scald burn injuries. At the same time, our results showed excessive consumption of SOD anti-oxidant capacity. Oxidative stress can induce the production of inflammatory mediators such as leukotrienes and prostaglandins, which can mediate further inflammatory reactions. In this investigation, consistent with previous reports, we found that levels of TNF-α, IL-6, and IL-8 were markedly increased in the blood and intestinal tissues of rats in our burn injury model.
Previous studies have shown that glutamine contributes to the regulation of immune responses in the body, enhances the proliferation of immune cells, reduces inflammation, and promotes the expression and secretion of anti-inflammatory cytokines. Moreover, results from animal experiments and clinical trials have also reported that probiotics can regulate oxidative stress and improve anti-oxidant activity [31]. In a study by de Oliveira, probiotics were found to mediate the expression of SOD and GSH-Px, increasing the activity of SOD and decreasing levels of ROS and IL-6 in vivo [32]. In this study, our results confirmed that oral glutamine and probiotics, alone or in combination, can reduce oxidative stress and inflammation in the intestinal tract following severe burn injury in a rat model. Following glutamine and/or probiotic oral therapy, levels of NO, ROS, IL-6, TNF-α, and IL-8 were effectively controlled, while SOD levels were found to improve in blood and intestinal tissue samples. Of further note, our results also demonstrated that combined treatment with glutamine and probiotics achieved superior outcomes compared to treatment with either substance alone. These synergistic effects of glutamine and probiotics mean that they can be used to complement each other to achieve more effective anti-inflammatory and anti-oxidant control of intestinal complications following severe burn injury, particularly to effectively reduce ongoing burn-induced oxidative stress and inflammation.
Aberrant increases in ROS are important markers of oxidative stress. Moreover, NO is a ROS component, which has been reported to be an important biological messenger and the only substance that can compete with SOD for superoxide anion; therefore, NO plays crucial roles in immune and digestive physiological processes, especially in the intestines where it is essential for the maintenance of intestinal functions [33]. However, Zhang has suggested that stress responses can lead to excessive production of NO in the intestines [34]. In this investigation, when rats with severe burns were treated with the NOS-specific inhibitor L-NAME, we observed significantly decreased NO and ROS levels, markedly increased SOD activity, and significant reductions in production of the inflammatory factors TNF-α and IL-6 in the blood and intestinal tissues. In addition, we found that expression levels of eNOS and nNOS were rarely increased following severe burn injury in our model, which provides further indication that iNOS functions as the main enzyme contributing to increase NO production after a severe burn. Targeted regulation of iNOS therefore appears to offer a main strategy for dealing with inflammatory responses after severe burns.
How iNOS is regulated as part of the intestinal stress response following severe burn injury has not been clearly established. Hai has previously reported that various types of stimuli, such as burns and oxidative stress, can change DNA methylation status in vivo [35]. It has also been reported that O6-methylguanine produced by ROS oxidation of DNA, which can be paired with thymine, eventually results in reduced DNA methylation. Moreover, it has been found that ROS can regulate the expression and activity of methyltransferases, such as Tet1 [36]. The stability of DNA methylation patterns is maintained by a balance between DNA methylation and demethylation. Neri has reported that Tet1 protein can mediate demethylation reactions by the base excision repair pathway, and accordingly, it is necessary to understand the effects of severe burns on the expression of Tet1 in the intestinal tract [36]. Since severe burns can immediately trigger oxidative stress and rapid increases in ROS, such increases in ROS might be expected to influence relative methylase and demethylase activities, including the activity of DNMT-1 which may ultimately result in altered expression of iNOS. Abnormal expression of iNOS can then be expected to further aggravate the inflammatory state [37]. In this study, our data reveal decreased expression of DNMT-1 and increased expression of Tet1 in Wistar rats with severe burns. Our data also showed that the application of glutamine and probiotics in combination effectively enhanced expression of DNMT-1 and inhibited expression of Tet1, efficiently restoring the high-methylation status of the iNOS gene. This restoration of the methylation status of the iNOS gene silenced the expression of NOS and would be expected to eventually lead to a reduced inflammatory reaction. Taken together, our findings demonstrate that the combination of glutamine and probiotics can regulate the production of NO via altered iNOS gene methylation, and these effects were found to reduce oxidative stress and inflammatory response in our Wistar rat severe burn injury model.
Conclusion
In this study, we found that glutamine and probiotics given together following burn injury can reduce expression of iNOS by restoring its high-methylation state, leading to decreased synthesis of NO. Reduction in NO production was found to be associated with reduced systemic and intestinal tissue markers of inflammation and oxidative stress in the Wistar rat burn injury model. Moreover, we further demonstrated that methylation of iNOS in this model was regulated by the DNA methyltransferase DNMT1 and demethylase Tet1.
Acknowledgements
This work was supported by a grant from the Key Program of Logistics Scientific Research of the PLA (BWS11J039).
Disclosure of conflict of interest
None.
Authors’ contribution
ZY Gong and YZ Peng participated in the design of the study, data acquisition, and analysis, as well as drafting the manuscript. ZY Gong was responsible for the laboratory assays and troubleshooting. ZQ Yuan and ZW Dong participated in data analysis, interpretation, and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.O’Dea KP, Porter JR, Tirlapur N, Katbeh U, Singh S, Handy JM, Takata M. Circulating microvesicles are elevated acutely following major burns injury and associated with clinical severity. PLoS One. 2016;11:e0167801. doi: 10.1371/journal.pone.0167801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Chen R, Zhu Z, Zhang X, Wang S. Effects of insulin combined with ethyl pyruvate on inflammatory response and oxidative stress in multiple-organ dysfunction syndrome rats with severe burns. Am J Emerg Med. 2016;34:2154–2158. doi: 10.1016/j.ajem.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Dickerson RN, Van Cleve JR, Swanson JM, Maish GO 3rd, Minard G, Croce MA, Brown RO. Vitamin D deficiency in critically ill patients with traumatic injuries. Burns Trauma. 2016;17:28. doi: 10.1186/s41038-016-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Ren HB, Chen XL, Wang F, Wang RS, Zhou B, Wang C, Sun YX, Wang YJ. Puerarin attenuates severe burn-induced acute myocardial injury in rats. Burns. 2015;41:1748–1457. doi: 10.1016/j.burns.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Saraf MK, Herndon DN, Porter C, Toliver-Kinsky T, Radhakrishnan R, Chao T, Chondronikola M, Sidossis LS. Morphological changes in subcutaneous white adipose tissue after severe burn injury. J Burn Care Res. 2016;37:e96–103. doi: 10.1097/BCR.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahemia IA, Muganza A, Moore R, Sahid F, Menezes CN. Microbiology and antibiotic resistance in severe burns patients: a 5 year review in an adult burns unit. Burns. 2015;41:1536–42. doi: 10.1016/j.burns.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Tao K, Bai X, Jia W, Liu Y, Zhu X, Han J, Dong M, Li J, Chen D, Hu D. Effects of resveratrol on the treatment of inflammatory response induced by severe burn. Inflammation. 2015;38:1273–1280. doi: 10.1007/s10753-014-0097-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Yu P, YongYang , Liu X, Jiang J, Liu D, Xue G. Hydrogen-rich saline resuscitation alleviates inflammation induced by severe burn with delayed resuscitation. Burns. 2015;41:379–385. doi: 10.1016/j.burns.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Lu XM, Tompkins RG, Fischman AJ. Nitric oxide activates intradomain disulfide bond formation in the kinase loop of Akt1/PKBα after burn injury. Int J Mol Med. 2013;31:740–750. doi: 10.3892/ijmm.2013.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Lan D, Li T, Yang G, Liu L. Angiopoietins regulate vascular reactivity after haemorrhagic shock in rats through the Tie2-nitric oxide pathway. Cardiovasc Res. 2012;96:308–319. doi: 10.1093/cvr/cvs254. [DOI] [PubMed] [Google Scholar]
- 11.Dunn JL, Hunter RA, Gast K, Maile R, Cairns BA, Schoenfisch MH. Direct detection of blood nitric oxide reveals a burn-dependent decrease of nitric oxide in response to Pseudomonas aeruginosa infection. Burns. 2016;42:1522–1527. doi: 10.1016/j.burns.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farina Junior JA, Celotto AC, da Silva MF, Evora PR. Guanylate cyclase inhibition by methylene blue as an option in the treatment of vasoplegia after a severe burn. A medical hypothesis. Med Sci Monit. 2012;18:HY13–7. doi: 10.12659/MSM.882718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel T, Alexander M, Hubbard WJ, Chaudry IH, Choudhry MA, Schwacha MG. Nitric oxide contributes to the development of a post-injury Th2 T-cell phenotype and immune dysfunction. J Cell Physiol. 2006;208:418–427. doi: 10.1002/jcp.20677. [DOI] [PubMed] [Google Scholar]
- 14.Filippou D, Papadopoulos VP, Triga A, Filippou G, Rizos S, Skandalakis P, Manolis E. Nitric oxide, antioxidant capacity, nitric oxide synthase and xanthine oxidase plasma levels in a cohort of burn patients. Burns. 2007;33:1001–1007. doi: 10.1016/j.burns.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Yu Y, Li M, Su N, Zhang Z, Zhao H, Yu H, Xu Y. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol Med Rep. 2016;13:1353–1360. doi: 10.3892/mmr.2015.4660. [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Kim YH, Park G. Cucurbitacins attenuate microglial activation and protect from neuroinflammatory injury through Nrf2/ARE activation and STAT/NF-κB inhibition. Neurosci Lett. 2015;609:129–36. doi: 10.1016/j.neulet.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Abbasi Habashi S, Sabouni F, Moghimi A, Ansari Majd S. Modulation of lipopolysaccharide stimulated nuclear factor kappa B mediated iNOS/NO production by bromelain in rat primary microglial cells. Iran Biomed J. 2016;20:33–40. doi: 10.7508/ibj.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu K, Feng L, Jiang W, Liu Y, Jiang J, Li S, Zhou X. Oxidative damage repair by glutamine in fish enterocytes. Fish Physiol Biochem. 2014;40:1437–1445. doi: 10.1007/s10695-014-9938-3. [DOI] [PubMed] [Google Scholar]
- 19.Donmez R, Oren D, Ozturk G, Kisaoglu A, Ozogul B, Atamanalp SS. The combined effects of glutamine and growth hormone on intestinal anastomosis in the rat intra-abdominal sepsis model. J Surg Res. 2013;182:142–145. doi: 10.1016/j.jss.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Noé JE. L-glutamine use in the treatment and prevention of mucositis and cachexia: a naturopathic perspective. Integr Cancer Ther. 2009;8:409–415. doi: 10.1177/1534735409348865. [DOI] [PubMed] [Google Scholar]
- 21.Eyarefe OD, Emikpe BO, Arowolo OA. Small bowel responses to enteral honey and glutamine administration following massive small bowel resection in rabbit. Afr J Med Med Sci. 2008;37:309–314. [PubMed] [Google Scholar]
- 22.Navarro F, Liu Y, Rhoads JM. Can probiotics benefit children with autism spectrum disorders? World J Gastroenterol. 2016;22:10093–10102. doi: 10.3748/wjg.v22.i46.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastings CN, Sheridan H, Pariante CM, Mondelli V. Does diet matter? The use of polyunsaturated fatty acids (PUFAs) and other dietary supplements in inflammation-associated depression. Curr Top Behav Neurosci. 2017;31:321–338. doi: 10.1007/7854_2016_31. [DOI] [PubMed] [Google Scholar]
- 24.Cenit MC, Olivares M, Codoñer-Franch P, Sanz Y. Intestinal microbiota and celiac disease: cause, consequence or co-evolution? Nutrients. 2015;7:6900–6923. doi: 10.3390/nu7085314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Mao R, Guo H, Zhang J. Detection of HBV cccDNA methylation from clinical samples by bisulfite sequencing and methylation-specific PCR. Methods Mol Biol. 2017;1540:73–84. doi: 10.1007/978-1-4939-6700-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira DW, Naquet P, Manautou JE. Influence of vanin-1 and catalytic products in liver during normal and oxidative stress conditions. Curr Med Chem. 2015;22:2407–2416. doi: 10.2174/092986732220150722124307. [DOI] [PubMed] [Google Scholar]
- 27.Gatson JW, Liu MM, Rivera-Chavez FA, Minei JP, Wolf SE. Oxidative stress and DNA hypermethylation status in renal cell carcinoma arising in patients on dialysis. J Pathol. 2007;212:218–226. doi: 10.1002/path.2176. [DOI] [PubMed] [Google Scholar]
- 28.Gatson JW, Liu MM, Rivera-Chavez FA, Minei JP, Wolf SE. Serum levels of neurofilament-H are elevated in patients suffering from severe burns. J Burn Care Res. 2015;36:545–50. doi: 10.1097/BCR.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 29.Zu H, Li Q, Huang P. Expression of Treg subsets on intestinal T cell immunity and endotoxin translocation in porcine sepsis after severe burns. Cell Biochem Biophys. 2014;70:1699–1704. doi: 10.1007/s12013-014-0116-0. [DOI] [PubMed] [Google Scholar]
- 30.Fullerton JN, O’Brien AJ, Gilroy DW. Lipid mediators in immune dysfunction after severe inflammation. Trends Immunol. 2014;35:12–21. doi: 10.1016/j.it.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sikalidis AK. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol Oncol Res. 2015;21:9–17. doi: 10.1007/s12253-014-9860-0. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira GP, Silva JD, de Araújo CC, Prota LF, Abreu SC, Madeira C, Morales MM, Takiya CM, Diaz BL, Capelozzi VL, Panizzutti R, Pelosi P, Rocco PR. Intravenous glutamine administration reduces lung and distal organ injury in malnourished rats with sepsis. Shock. 2014;41:222–232. doi: 10.1097/SHK.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 33.Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL. From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J Dent Res. 2016;95:1452–1456. doi: 10.1177/0022034516673019. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F, Wu W, Deng Z, Zheng X, Zhang J, Deng S, Chen J, Ma Q, Wang Y, Yu X, Kang S, Wang X. High altitude increases the expression of hypoxia-inducible factor 1α and inducible nitric oxide synthase with intest-inal mucosal barrier failure in rats. Int J Clin Exp Pathol. 2015;8:5189–5195. [PMC free article] [PubMed] [Google Scholar]
- 35.Hai Z, Zuo W. Aberrant DNA methylation in the pathogenesis of atherosclerosis. Clin Chim Acta. 2016;456:69–74. doi: 10.1016/j.cca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Neri F, Dettori D, Incarnato D, Krepelova A, Rapelli S, Maldotti M, Parlato C, Paliogiannis P, Oliviero S. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene. 2015;34:4168–4176. doi: 10.1038/onc.2014.356. [DOI] [PubMed] [Google Scholar]
- 37.Palsamy P, Bidasee KR, Shinohara T. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp Eye Res. 2014;121:26–34. doi: 10.1016/j.exer.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]