Abstract
Rheumatoid arthritis is a chronic and systemic autoimmune disease characterized by inflammatory cell infiltration and joint erosion. Human adipose-derived mesenchymal stem cells (hASCs) have shown the capacity of suppressing effector T cell activation and inflammatory cytokine expression. We investigated whether hASCs play a therapeutic role in collagen-induced arthritis (CIA) by administering a single dose of hASCs in mice with established CIA. In vivo, a beneficial effect was observed following hASC infusion as shown by a marked decrease in the severity of arthritis. Human ASCs were detectable in the joints, and reduced levels of pro-inflammatory cytokines and increased levels of anti-inflammatory cytokines were observed in the sera of the hASC-treated mice. Furthermore, hASC treatment induced the expansion of regulatory T cells (Tregs) both in the peripheral blood and in the spleen tissues. In vitro, hASCs downregulated the production of proinflammatory cytokines TNF-α, IL-1β, and IL-6 in mouse macrophages stimulated with lipopolysaccharide and inhibited the proliferation of human primary T cells in response to mitogens. Thus hASCs represent a novel and effective therapeutic strategy for RA.
Keywords: hASC, rheumatoid arthritis, immune, T cell, collagen-induced arthritis, therapy
Introduction
Rheumatoid arthritis (RA) is a chronic and systemic autoimmune disease characterized by inflammatory cell infiltration and synovial hyperplasia that leads to joint and cartilage erosion [1,2]. It is generally accepted that RA is a multifactorial disease of which both genetic and environmental factors play important roles in disease development [3]. Genetic and environmental factors modulate the aberrant activation of the innate and adaptive immune cells and contribute to disease pathogenesis. Anti-rheumatic drugs and biologic agents have greatly enhanced the management of the symptoms, but they also exhibit limitations including incomplete treatment, adverse effects, and high cost [4-6]. Therefore, it is of considerable importance to develop new and more effective therapies for RA.
TNF-α produced by innate immune cells plays a central role in joint inflammation. TNF-α induces a pro-inflammatory cytokine cascade involving IL-1, IL-6, and IFN-γ, as well as several proinflammatory chemokines [7]. CD4+ T cells are also closely related to the pathogenesis of RA; their main subsets such as Th1, Th2, and Th17 cells play an important role in the pathogenesis of RA [8]. In addition, clinical findings have demonstrated alterations in the phenotypes and numbers of Treg cells in patients with RA, and that the sensitivity of effector T cells to Treg cells is also reduced [9].
Mesenchymal stem cells (MSCs), derived from the mesoderm, are multipotent progenitor cells that reside in all adult tissues including the bone marrow, umbilical cord blood, dermis, and adipose tissue. These cells can differentiate into mesenchymal tissues including bone, cartilage, fat, tendon, muscle, and marrow stroma under appropriate culture conditions [10]. MSCs are weakly immunogenic but mediate immunosuppressive reactions both in vitro and in vivo [11,12]. Bone marrow (BM)-MSCs have been used in animal models to control acute graft-versus-host disease, and alleviate experimental autoimmune encephalomyelitis, collagen-induced arthritis (CIA), and autoimmune myocarditis [13-15].
In addition to bone marrow, other major sources of human MSCs include umbilical cord blood, peripheral blood, and adipose tissue. Large amounts of adipose tissue can be derived from lipoaspirates, which contain high numbers of MSCs that can be readily cultured in vitro [16]. Human adipose-derived MSCs (hASCs) share similar immunomodulatory properties with BM-MSCs and can exert profound immunomodulatory properties on animal models of acute graft versus host disease and experimental arthritis [17]. Notably, hASCs also exert profound suppressive effects on collagen-reactive T cells from patients with RA [18]. In this study, we isolated MSCs derived from adipose tissues and investigated their immunomodulatory effects in an experimental arthritis model.
Materials and methods
Isolation and culture of hASCs
Liposuction aspirates from subcutaneous adipose tissue samples were obtained from the waists and thighs of healthy donors (n=15) undergoing elective plastic surgical procedures. This study was approved by Guangdong Provincial People’s Hospital (Guangzhou, China) and all patients provided informed consent. The obtained samples were digested with 0.25% collagenase type Iunder shaking for 45 min at 37°C and filtered with a 200-mesh screen to remove the undigested fragments. The suspension was neutralized with fetal bovine serum and centrifuged at 300 g for 5 min. The supernatant was aspirated and the cell pellet was washed three times with phosphate buffered saline. All acquired cells were cultured overnight at 37°C in 5% CO2 inbasal medium for human adipose-derived MSCs (Cyagen Biosciences, Santa Clara, CA, USA). Once adherent cells were observed, the medium was changed for the first time. The obtained cell population was passaged over 3-5 days until reaching 70-80% confluence, which was considered as passage 1. The hASCs were cultured and expanded in basal medium, and passages 3-5 were generally utilized in the subsequent experiments.
Flow cytometric characterization and differentiation of hASCs
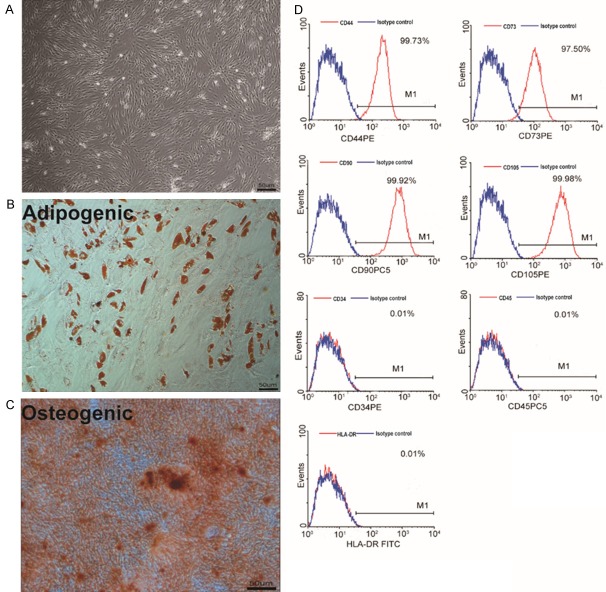
hASCs were characterized by fluorescence activated cell sorting (FACS) analysis for surface antigens; i.e., surface expression of CD44, CD73, CD90, and CD105 and the absence of surface expression of CD45, CD34, and human leukocyte antigen (HLA)-DR (all antibodies were obtained from BD Biosciences, San Jose, CA, USA). Flow cytometry was performed as described [19]. A total of 1-2 × 106 cells were incubated with FcR blocking reagent and then stained with unconjugated monoclonal antibodies; the labeled cells were subsequently analyzed by flow cytometry (FACS Calibur, Beckton Dickinson, Bedford, MA, USA). To test the multipotency of the cells, hASCs were treated in adipogenic or osteogenic media for three weeks. Medium changes were carried out every 3 days. The adipogenic medium consisted of MEM-a supplemented with 0.5 mM 3-isobutyl-1-methylxanthine (Sigma Aldrich, St. Louis, MO, USA), 10 μg insulin, 1 μM dexamethasone (Sigma), 200 μM indomethacin (INDO, Sigma), and 10% bovine serum (Sigma). The osteogenic medium consisted of MEM-a supplemented with 0.1 μM dexamethasone, 10 mM β-glycerol phosphate (Sigma), and 0.2 mM ascorbic acid (Sigma). Oil red O (applied after adipogenic differentiation; Sigma) or Alizarin Red stains (after osteogenic differentiation, Sigma) were used according to manufacturer protocol.
Induction and treatment of CIA
To generate the experimental arthritis model, DBA/1 mice (six to eight weeks old; obtained from the SLAC Laboratory Animal Center, Shanghai, China) were injected with 200 μg bovine type II collagen (CII) (Chondrex Inc., Redmond, WA, USA) emulsified with equal amounts of Freund’s complete adjuvant (Sigma) into the subcutaneous tissue of their tails, and a week later were administered a booster injection at the base of the tail with Freund’s incomplete adjuvant. On day 21, the mice received an intraperitoneal booster injection of lipopolysaccharide (LPS). Paw swelling was monitored by measuring the mean thickness of hind paws and ankles with calipers every other day [20], and was scored on a scale of 0-4, where 0 = no swelling and erythema, 1 = mild swelling and erythema, 2 = joint swelling, 3 = severe swelling and joint rigidity, and 4 = maximal swelling with ankylosis. Each limb was graded, with a maximum possible score of 16 for each animal [21].
Treatment was initiated on day 28 after the arthritis had become established. Mice with CIA were injected intravenously via the tail vein each day for five days with saline alone, 2 × 106 hASCs, or 2 μg dexamethasone as a positive control. Animals were sacrificed 50 days after immunization with CII and their joints were examined in serial sections as described below. Animal experiments were carried out according to institutional guidelines for the care and use of laboratory animals and were approved by the Animal Ethics Committee of the Chinese Academy of Medical Sciences.
Detection of hASCs in vivo
The hASCs used in the animal injection experiments were previously transduced with a lentiviral vector carrying red fluorescent protein (RFP) [22]. As control, mice were injected with saline alone at the same time. General anesthesia was induced with 5% isoflurane (IsoSol; Medeva Pharmaceuticals, Inc., Rochester, NY, USA) and the mouse was placed in a chamber; anesthesia was continued during the procedure with 2% isoflurane introduced via a nose cone. The mice were imaged for fluorescence detection of the location of the RFP-hASCs at 1, 7, 14, and 21 days after hASC injection using fluorescence reflectance imaging with an Xtreme in vivo imaging system (Bruker, Karlsruhe, Germany). After the mice were sacrificed, the ankle joints were also harvested for imaging.
Cytokine quantification in CIA mouse serum
Sera were obtained from the peripheral blood by centrifugation at 600 g. The levels of the inflammatory cytokines TNF-α, IL-6, IL-1β, IL-10, and the anti-CII IgG and anti-CII IgG2a antibodies were assayed in mouse serum samples using ELISA kits (MultiSciences Biotech Co., Ltd., Suzhou, China). The ELISA plates were measured at 450 nm according to the manufacturer’s instructions. The levels of TNF-α, IL-6, IL-1β, IL-10, and anti-CII antibodies in the sera were determined from standard curves.
Histologic analysis of CIA mice
After the mice were sacrificed, the ankle joints were fixed in 4% paraformaldehyde for 48 h. The joints were then decalcified in 10% EDTA for 4 weeks, embedded in paraffin, and sectioned. Each joint section (5 μm) was stained with hematoxylin and eosin (H&E) prior to observation by light microscopy. The severity of arthritis in the joints was scored on a scale of 3 different parameters: cell infiltration, synovial hyperplasia, and bone erosion. Scoring was based on a previously described scoring system and performed by 3 independent observers: grade 0, normal; grade 1, slight cell infiltration and synovial cell hyperplasia; grades 2-4, severe inflammation and synovial lining hyperplasia, with pannus formation and cartilage erosion [23]. The qualitative scoring system used to assess the severity of arthritis in the joints is presented in Table 1.
Table 1.
Qualitative scoring system used to assess the severity of arthritis in the joints
| Score | Condition |
|---|---|
| 0 | Normal |
| 1 | Slight cell infiltration and synovial cell hyperplasia |
| 2-4 | Severe inflammation and synovial lining hyperplasia, with pannus formation and cartilage erosion |
Flow cytometric analysis of CIA mouse mononuclear cells
Mononuclear cell suspensions were isolated from the spleen and peripheral blood of the CIA mice. These were washed with FACS buffer and were stained using anti-CD4-FITC or anti-CD25-APC antibodies (eBioscience, San Diego, CA, USA) for 30 min. Subsequently, the cells were stained using anti-Foxp3-PE antibodies (eBioscience) in FACS buffer after being fixed and permeabilized for 30 min. Isotype control antibodies were used in all FACS analyses. Cells were analyzed on a FACS Calibur flow cytometer using Cell Quest software (Beckman Coulter, Brea, CA, USA).
Quantitative real time RT-PCR
Total RNAs were extracted from xxx? using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA. qRT-PCR analyses for the mRNA expression of IL-1β, TNF-α, IL-6, and GAPDH were performed using SsoFast Eva-Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and analyzed with a C1000 Thermal Cycler (CFX96 Real-Time System, Bio-Rad). The mRNA level of GAPDH was measured as an internal control. The following PCR conditions were used for all primer pairs: 95°C for 3 min, 59°C for 5 s, followed by 39 cycles of 95°C for 5 s in a 10-μL reaction volume. Three experiments from 3 independent RNA samples were performed. The primer sequences for quantitative RT-PCR are listed in Table 2.
Table 2.
Oligonucleotide primers used in this study
| Gene Name | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| GAPDH | GTCATTGAGAGCAATGCCAG | GTGTTCCTACCCCCAATGTG |
| IL-1β | GAGCCTGTGTTTCCTCCTTG | CAAGTGCAAGGCTATGACCA |
| TNF-α | GGGAGCAAAGGTTCAGTGAT | CCTGGCCTCTCTACCTTGTT |
| IL-6 | CTGACAATATGAATGTTGGG | TCCAAGAAACCATCTGGCTAGG |
In vitro suppression assay
Peripheral blood mononuclear cells (PBMCs), obtained from healthy donors following provision of informed consent, were isolated using Ficoll density gradient centrifugation. The PBMCs were stained with the carboxyfluoresceinsuccinimidyl ester (CFSE), cultured in the presence of hASCs at ratio of 10:1, and stimulated with phytohemagglutinin (PHA) (Sigma) at a 10 μg/mL final concentration. After 72 h, the proliferation of T cells was quantified by measuring the corresponding decrease in cell fluorescence by flow cytometry. Raw264.7 macrophages (ATCC; Rockville, MD, USA) were cocultured with hASCs at a ratio of 10:1 directly, followed by stimulation with 100 ng/mL LPS (Sigma) for 24 h. Total RNAs were isolated and subjected to reverse transcription, followed by quantitative real-time polymerase chainreaction analysis.
Statistical analysis
Statistical analyses were performed using Excel and GraphPad Prism6.0 software analysis, and the experimental results presented using mean ± SD values. Statistical analyses of the quantitative results were carried out using t-tests. For between group comparisons, one-way ANOVA followed by Dunnett’s test for statistical analysis was performed, with significance set at *P<0.05 or **P<0.01.
Results
Isolation and identification of hASCs in vitro
hASCs were isolated and expanded from healthy human adipose tissues (Table 3). hASCs exhibited a fibroblast-like morphology (Figure 1A), and they maintained multipotency and differentiated into adipocytes and osteocytes (Figure 1B, 1C). In addition, they were uniformly negative for CD45, CD34, and HLA-DR, but positive for CD44, CD73, CD90, and CD105 (Figure 1D), in accordance with accepted phenotypic markers for hASCs [24].
Table 3.
Donor profiles: age, gender, BMI, and cell viability
| Lot.no | Age | Gender | BMI | Viability (%) |
|---|---|---|---|---|
| 1 | 49 | F | 22.1 | 93.9 |
| 2 | 56 | F | 24.9 | 94.5 |
| 3 | 32 | F | 23.2 | 97.6 |
| 4 | 47 | F | 22.5 | 93.6 |
| 5 | 58 | F | 27.6 | 96.5 |
| 6 | 36 | F | 28.4 | 92.1 |
| 7 | 43 | F | 20.6 | 94.3 |
| 8 | 54 | F | 24.7 | 94.8 |
| 9 | 28 | F | 22.6 | 97.9 |
| 10 | 31 | F | 26.1 | 96.9 |
| 11 | 29 | F | 25.3 | 85.3 |
| 12 | 50 | F | 26.5 | 94.2 |
| 13 | 35 | F | 24.2 | 86.7 |
| 14 | 27 | M | 26.1 | 95.2 |
| 15 | 60 | M | 25.8 | 92.0 |
| Mean | 42.3 | 24.7 | 93.7 | |
| SD | 11.8 | 2.2 | 3.5 |
Figure 1.
Characteristics of hASCs. A: Cell culture of passage 3. Original magnification × 100. B: Oil red O staining of hASCs after the induction of adipogenic differentiation for 21 days. Original magnification × 100. C: Osteogenic differentiation of hASCs as shown by staining with Alizarin Red. Original magnification × 100. D: Flow cytometric analysis of the surface-marker expression on hASCs.
Treatment with hASCs ameliorates arthritis severity in CIA mice
CIA was induced by injecting DBA/1 with bovine type II collagen (CII) emulsified in CFA, followed by boosting 7 days later with CII emulsified in incomplete Freund’s adjuvant (IFA). On day 21, DBA/1 received an injection of LPS. Daily injections of ASCs, dex, or saline were administered intravenously at day 28 (Figure 2A). The development of arthritis was monitored until day 50 and the arthritis score was evaluated everyday. Photographs of the paws taken at day 50 after the induction of RA are shown in Figure 2B, hASC treatment markedly inhibited the edema and erythema of the arthritic joints compared with CIA mice. No weight loss was observed in mice receiving treatment with hASCs (Supplementary Figure 1A). As shown in Figure 2C, 2D, the administration of hASCs to mice with established arthritis (arthritis score >2) significantly reduced disease severity and hind paw thickness as compared to saline administration. We also found that hASCs decreased the swelling of the ankle joint (Supplementary Figure 1B).
Figure 2.
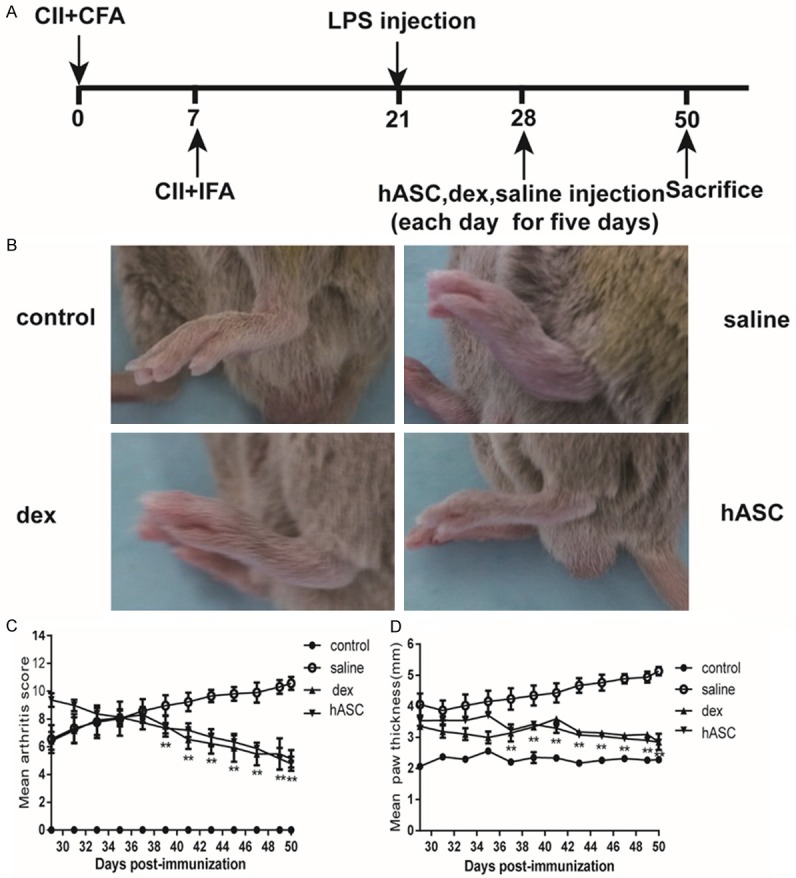
Therapeutic effects of hASCs in CIA mice. Mice with established CIA were injected intravenously with saline or with 107 h ASCs daily for 5 days starting on day 28. A: Schematic showing the immune injection procedures. B: Photographs showing the paw swelling in normal saline-, dexamethasone-, or hASC-treated mice (n=10/group). C: Arthritis severity was assessed by clinical scoring. **P<0.01. All the data are expressed as the means ± SD. D: Hind paw thickness of the mice in each group was assessed with caliper measurements. **P<0.01. All the data are expressed as the means ± SD.
hASC treatment reduces histological damage and alleviates cartilage destruction in CIA mice
After the mice were sacrificed at day 50, the ankle joints of the experimental mice were removed, fixed in paraformaldehyde, and stained with H&E. The vehicle-treated CIA mice showed severe synovial cell proliferation, numerous inflammatory cell infiltrates, and cartilage destruction. In contrast, the ASC-treated group showed markedly reduced joint destruction and had normal-looking synovial tissue with only few infiltrated immune cells (Figure 3A). Histopathological evaluation by three independent investigators demonstrated that hASCs resulted in a statistically significant reduction in inflammatory cell infiltration, synovial hyperplasia, and bone and cartilage erosion (Figure 3B).
Figure 3.
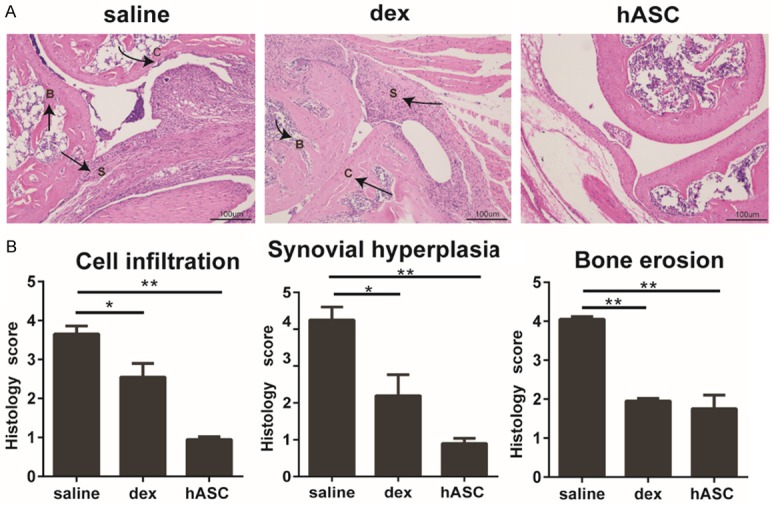
hASC treatment reduces synovial cell proliferation, inflammatory cell infiltration, and joint erosion in CIA. A: Representative H&E-stained joint tissue sections from DBA/1 mice from a CIA prevention study. C indicates the site of cartilage damage, B indicates the site of bone erosion, and S indicates the site of synovium inflammation. Original magnification, 100 ×. B: Histopathological scores of cell infiltration, synovial hyperplasia, and bone erosion in DBA/1 mice from a CIA prevention study. n=5, **P<0.01, vs. the saline controls. All the data are expressed as the means ± SD.
hASCs were detected in the ankle joints of CIA mice
To follow the biodistribution of hASCs in a homeostatic host, RFP-hASCs were intravenously injected into CIA mice [25]. The mice were imaged for fluorescence detection to determine the hASC location over time. hASCs transduced with RFP in vitro are shown in Figure 4A. After 24 h, fluorescence could be detected predominantly in the ankle joints of the imaged mice. The fluorescence signal decreased by threefold and remained localized to the joints at 21 days post-injection (Figure 4B). At 21 days, following removal of the joints from the mice, we also detected this lower level of fluorescence in vitro (Figure 4C).
Figure 4.
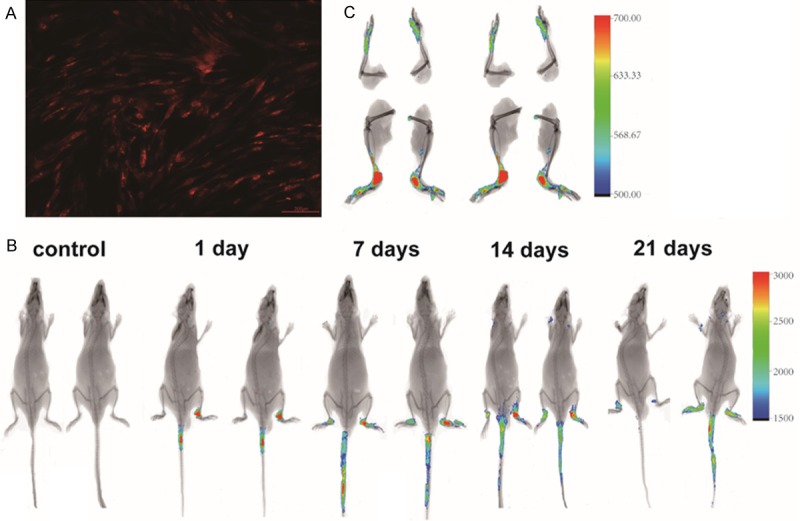
Biodistribution of hASCs in CIA mice. hASCs were intravenously injected into CIA mice (n=3) and imaged on days 1, 7, 14, and 21 post injection. A: hASCs transduced with RFP in vitro. B: The fluorescence signal quantified over time was detected in the ankle joints on days 1, 7, 14, and 21. C: Following mouse sacrifice, the fluorescence activity can be seen to be localized to the ankle joints.
hASCs reduced the inflammatory responses in CIA
To evaluate the mechanisms underlying the observed decrease in the severity of CIA following administration of hASCs, we first investigated their effects on the production of mediators of inflammation that are primary linked to joint inflammation. hASC injection significantly decreased the expression of the proinflammatory cytokines IL-1β, TNF-α, and IL-6, whereas it upregulated the expression of the anti-inflammatory cytokine IL-10 in the serum of mice with CIA (Figure 5A). Antibodies directed against collagen-rich joint tissue are thought to drive CIA progression through their activation of FcγR- and complement-dependent pathways [18,26]. The administration of hASCs led to reduced serum levels of CII-specific IgGs, and in particular of the autoreactive IgG2a antibodies (Figure 5B).
Figure 5.
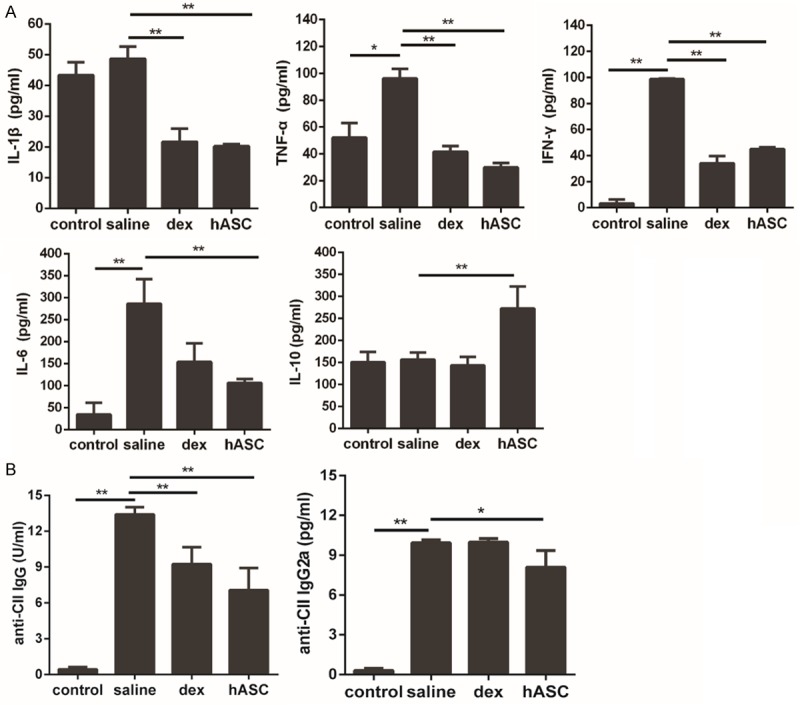
Administration of hASCs reduced the inflammatory responses in CIA. A: IL-1β, TNF-α, IFN-γ, and IL-6 were significantly reduced in dex-treated mice and in all hASC-treated mice. IL-10 levels were significantly increased in all hASC-treated, but not index-treated mice. B: Serum concentrations of anti-CII IgG and anti-CII IgG2a antibodies were significantly decreased after treatment with hASCs. All data are expressed as Means ± SD, **P<0.01, *P<0.05.
Promotion of Treg cells in CIA following treatment with hASCs
It is known that Tregs play a critical role in the maintenance of self-immune tolerance in RA [22]. Several studies have shown that the IL-10 produced by Tregs exerts significant protection against CIA by inhibiting the activation of Th1 cells [27]. As shown in Figure 6A, 6B, mice with CIA that were treated with hASCs exhibited significantly higher numbers of CD4+CD25+FoxP3+ Treg cells in both their splenocytes and peripheral blood mononuclear cells than did control mice with CIA. The results suggest that treatment with hASCs induces the expansion of Tregs, which may exert immunosuppressive effects on inflammatory cells.
Figure 6.
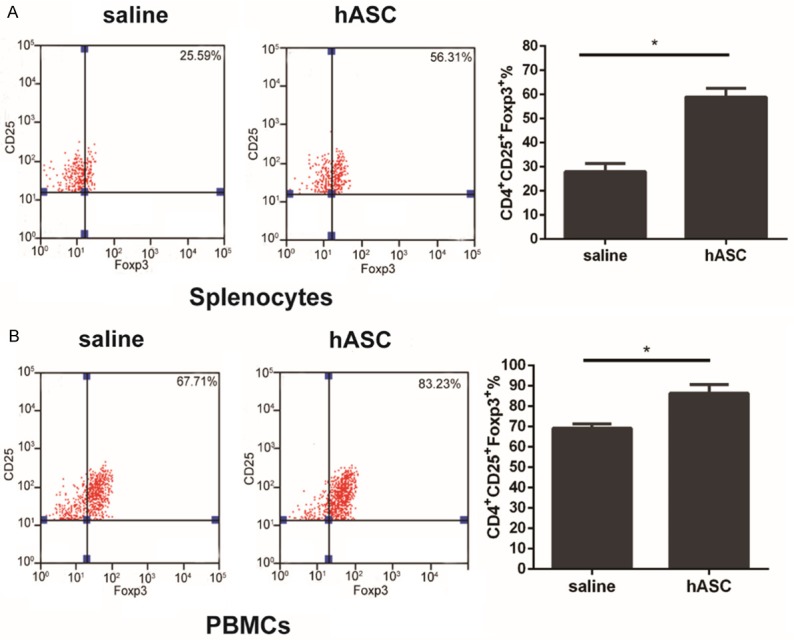
Induction of Tregs by hASCs in mice with CIA. Cells were activated with PMA and BFA for 4 h in the experimental groups. The splenocytes and PBMCs were stained with CD4, CD25, and Foxp3. A: Expression of Tregs was significantly increased in the spleen as analyzed by flow cytometry. B: Percentages of CD4+CD25+Foxp3+ cells in the peripheral blood in the hASC-treated group were higher than those in the saline control group. *P<0.05. Data are expressed as Means ± SD.
Effects of hASCs on T cell proliferation and cytokine production
The immunoregulatory properties of hASCs were investigated in a proliferative assay using phytohemagglutinin (PHA)-stimulated PBMCs as responder T cells [28]. The addition of hASCs at ratio of 10:1 significantly inhibited the proliferation of PHA-stimulated T cells at day 3 (Figure 7A). Furthermore, hASCs significantly decreased IL-1β, TNF-α, and IL-6 expression in macrophages stimulated with 100 ng/mL LPS at day 3 (Figure 7B).
Figure 7.
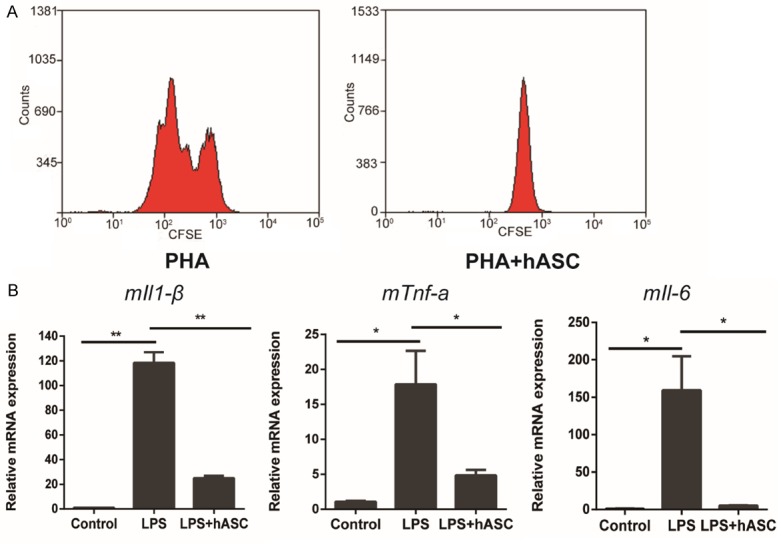
hASCs display immune suppressive capacities in vitro. A: Allogeneic activated PBMCs, labeled with CFSE, were co-cultured with hASCs at a 10:1 ratio. B: hASCs inhibited proinflammatory factor mRNAs in LPS-stimulated mouse macrophages (RAW264.7). **P<0.01, *P<0.05. All data are presented as the means ± SD. Abbreviations: m, mouse.
Discussion
The hASCs obtained in our study demonstrated a phenotype that was positive for CD73, CD44, CD90, and CD105, and negative for CD34, CD45, and HLA-DR. They retained the capability of self-renewal and differentiation intoadipocytes and osteocytes. The systemic infusion of hASCs significantly decreased the severity of arthritis, ameliorated the symptoms, and prevented joint damage in CIA in mice. The immune-protective effects of hASCs are likely due to the profound effects as we demonstrated on the inhibition of effector T cell proliferation and inflammatory cytokine expression, and increases in Treg cell expansion and IL-10 production.
The pathophysiology of RA is complex and both innate and adaptive immunity contributes to the pathology of the disease. A number of cytokines and chemokines including TNF-α, IL-6, IL-1β, IL-10, IL-4, IL-17, and IFN-γ are abundantly expressed in patients with RA [29,30]. Anti-TNF-α monoclonal antibodies or soluble TNF-α receptors can greatly alleviate symptoms [31]. Our data demonstrated that hASCs significantly diminished the production of a wide range of inflammatory mediators in sera and were similar in efficacy to the most potent immunosuppressive drugs. Previous data have shown that the immunosuppressive function of hASCs is not MHC restricted; specifically, the therapeutic effect of hASCs on CIA was not restricted to a xenogeneic system, since both syngeneic and allogeneic murine ASCs were found to be as efficient as hASCs at ameliorating the clinical signs of arthritis [12,32,33]. Given that recent clinical trials have shown that infusion of hBM-MSCs into patients with severe RA is safe [34], our data further supported that hASCs might represent a promising therapy for RA.
T cells are believed to play a critical role in orchestrating the inflammatory response in RA. Accordingly, the suppression of T cell responses is of great importance in RA treatment, as evidenced by the facts that allogeneic BM-MSCs and umbilical cord-MSCs both suppress the responses of CII-reactive T cells in RA [6,34,35]. It is well known that Tregs play an important role in the prevention of autoimmunity, and it has been demonstrated that they are able to modulate the severity of CIA [36]. Our results demonstrated that the administration of hASCs could upregulate regulatory T cell populations both in the peripheral blood and in spleen tissues of CIA mice, suggesting that the immunosuppressive activity of hASCs might be prolonged by the participation of Tregs. Furthermore, hASCs increased the levels of the anti-inflammatory cytokine IL-10. Aside from its role as an anti-inflammatory factor [37], IL-10 is a signature cytokine for Tregs, and plays a key role in the control of self-antigen-reactive T cells in vivo [27]. Our results are consistent with those of a recent study showing that the injection of murine BM-MSCs into mice with CIA prevented disease progression by inducing the emergence of antigen-specific Treg cells [6]. With respect to the in vitro immunoregulatory properties of hASCs, our data are also consistent with previous studies showing that BM-MSCs do not induce the proliferation of allogeneic T cells [11,38] but suppress the proliferation of T cells induced by mitogens [12,38]. The detailed immunomodulatory roles of hASCs will be addressed in future studies.
Current therapy for RA is directed toward diminishing the inflammatory response and treating the sequelae of uncontrolled inflammation. However, these treatments have not been able to prevent the disease or to completely arrest the disease process. In this study, we present the development of an effective new therapeutic approach to target the pathogenic mechanism of autoimmune arthritis using adult stem cells. However, the detailed mechanisms of hASC-mediated immune regulation were not elucidated. Future studies should investigate the immune modulatory mechanisms of ASCs from the perspective of their interactions with other immune cells.
Conclusions
We demonstrated that the administration of hASCs in mice with CIA attenuated systemic inflammation, as shown by decreased pro-inflammatory cytokines expression and increased anti-inflammatory cytokine IL-10 production and Treg expansion. Together, these data suggest that hASCs might represent a new and effective therapeutic perspective in RA.
Acknowledgements
This study was supported by Grants from the National Natural Science Foundation of China (no. 81500227, 81673670), the Fundamental Research Funds for the Central Universities (no. 21615410), the Natural Science Foundation for Guangdong Province (no. 2014A030310434), the Science Technology Project for Guangdong Province (no. 2013B090600144 and no. 2015A050502028), and the Science and Technology Program of Guangzhou (no. 201610010108) and Jinan Double Hundred Talents Plan.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Fournier C. Where do T cells stand in rheumatoid arthritis? Joint Bone Spine. 2005;72:527–532. doi: 10.1016/j.jbspin.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhou B, Yuan J, Zhou Y, Ghawji M, Deng YP, Lee AJ, Lee AJ, Nair U, Kang AH, Brand DD. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol. 2011;141:328–337. doi: 10.1016/j.clim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 4.Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43:617–627. doi: 10.1002/1529-0131(200003)43:3<617::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 6.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Li S, Liu Y, Tian G, Yuan Q, Bai F, Wang W, Zhang Z, Ren G, Zhang Y. Fibroblast growth factor 21 (FGF21) ameliorates collagen-induced arthritis through modulating oxidative stress and suppressing nuclear factor-kappa B pathway. Int Immunopharmacol. 2015;25:74–82. doi: 10.1016/j.intimp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Martens PB, Goronzy JJ, Schaid D, Weyand CM. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997;40:1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- 9.Boissier MC, Assier E, Biton J, Denys A, Falgarone G, Bessis N. Regulatory T cells (Treg) in rheumatoid arthritis. Joint Bone Spine. 2009;76:10–14. doi: 10.1016/j.jbspin.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 12.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 13.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Z, Li X, Ding J, Jia J, Zhu P. Allogeneic mesenchymal stem cell and mesenchymal stem cell-differentiated chondrocyte suppress the responses of type II collagen-reactive T cells in rheumatoid arthritis. Rheumatology. 2008;47:22–30. doi: 10.1093/rheumatology/kem284. [DOI] [PubMed] [Google Scholar]
- 16.Yanez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 17.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Rey E, Gonzalez MA, Varela N, O’Valle F, Hernandez-Cortes P, Rico L, Büscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 20.Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- 21.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 22.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 23.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11:1066–1072. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 24.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilar E, Bagó JR, Soler-Botija C, Alieva M, Rigola MA, Fuster C, Vila OF, Rubio N, Blanco J. Fast-proliferating adipose tissue mesenchymal-stromal-like cells for therapy. Stem Cells Dev. 2014;23:2908–2920. doi: 10.1089/scd.2014.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellsworth JL, Hamacher N, Harder B, Bannink K, Bukowski TR, Byrnes-Blake K, Underwood S, Oliver C, Waggie KS, Noriega C. Recombinant soluble human FcγR1A (CD64A) reduces inflammation in murine collagen-induced arthritis. J Immunol. 2009;182:7272–7279. doi: 10.4049/jimmunol.0803497. [DOI] [PubMed] [Google Scholar]
- 27.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+ CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005;7:R402–R415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luz-Crawford P, Torres MJ, Noël D, Fernandez A, Toupet K, Alcayaga-Miranda F, Tejedor G, Jorgensen C, Illanes SE, Figueroa FE. The immunosuppressive signature of menstrual blood mesenchymal stem cells entails opposite effects on experimental arthritis and graft versus host diseases. Stem Cells. 2016;34:456–69. doi: 10.1002/stem.2244. [DOI] [PubMed] [Google Scholar]
- 29.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 30.Mitani Y, Honda A, Jasin HE. Polymorphonuclear leukocyte adhesion to articular cartilage is inhibited by cartilage surface macromolecules. Rheumatol Int. 2001;20:180–185. doi: 10.1007/s002960000098. [DOI] [PubMed] [Google Scholar]
- 31.Yuan H, Qian H, Liu S, Zhang X, Li S, Wang W, Li Z, Jia J, Zhao W. Therapeutic role of a vaccine targeting RANKL and TNF-α on collagen-induced arthritis. Biomaterials. 2012;33:8177–8185. doi: 10.1016/j.biomaterials.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 32.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 33.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 34.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–1186. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 35.Park KH, Mun CH, Kang MI, Lee SW, Lee SK, Park YB. Treatment of collagen-induced arthritis using immune modulatory properties of human mesenchymal stem cells. Cell Transplant. 2016;25:1057–72. doi: 10.3727/096368915X687949. [DOI] [PubMed] [Google Scholar]
- 36.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 37.Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Tim YY, Friedline RH, Kurt-Jones E, Finberg R, Fischer MA. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maitra B, Szekely E, Gjini K, Laughlin M, Dennis J, Haynesworth S, Koc O. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.