Abstract
Hypoxia is common in solid tumors and results in the activation of hypoxia-response genes. Hypoxia-inducible factor-1α (HIF-1α) is thought to reflect major cellular adaptation to hypoxia and contributes to chemoresistance in various tumors including hepatocellular carcinoma (HCC). N1-guanyl-1,7-diaminoheptane (GC7) is an inhibitor which suppresses the active eukaryotic translation initiation factor 5A-2 (eIF5A2), preventing epithelial-mesenchymal transition (EMT) in chemoresistance. In this study, we investigated the role of GC7 in the therapeutic effect of doxorubicin in hypoxia in HCC. We utilized four types of HCC cell line (Huh7, Hep3B, SNU387 and SNU449) in this study. Western blot and immunofluorescence were used to detect expression of epithelial/mesenchymal markers for EMT evaluation and HIF-1α was knocked down using HIF-1α-siRNA. Hypoxia-induced EMT contributed to doxorubicin chemoresistance in HCC cells. Low concentrations of GC7 sensitized Huh7 and Hep3B to doxorubicin by reversing EMT. Knockdown of HIF-1α attenuated hypoxia-induced EMT and abolished the unique feature of GC7. GC7 enhanced sensitivity to doxorubicin in HCC by reversing hypoxia-induced EMT via the HIF-1α-mediated signaling pathway. We suggest a new method of enhancing cytotoxicity of chemotherapy and improving the long-term survival rate in HCC.
Keywords: GC7, doxorubicin, hepatocellular carcinoma, hypoxia, HIF-1α
Introduction
As one of the most common malignant tumor types, HCC has both high mortality and morbidity and is a severe threat to human health worldwide [1]. There are approximately 75,000 incident cases and over 600,000 deaths every year attributable to this type of cancer [2]. Chemotherapy is one of the most important treatment regimens in HCC. However, the low cure rates partly a result of chemoresistance to traditional drugs [3]. Hypoxia is common in solid tumors resulting in an imbalance in homeostasis in normal tissue while affecting angiogenesis, invasion, metastasis and tumor cell survival [4]. Recent studies have indicated that hypoxia plays an important role in chemoresistance against chemotherapy drugs including irinotecan and sorafenib [5,6]. Both of these are widely used in the treatment of HCC but have poor therapeutic effect [7,8]. HIF-1 mediates the hypoxia response pathway [9] and it is universally acknowledged that HIF-1α reflects major cellular adaptation to hypoxia [10,11], thereby contributing to chemoresistance in various tumors including HCC [12,13].
Recently, Tariq et al. indicated that the expression of eIF5A2 was crucially important for HIF-1α expression during hypoxia [14]. As a novel oncogene, eIF5A2 is involved in the chemoresistance in many malignant tumors because of EMT [15,16]. GC7, a novel inhibitor of deoxyhypusine synthase (DHS), enhances the therapeutic efficacy of doxorubicin and prevents EMT in HCC cells. These findings indicated that GC7 may be able to attenuate chemoresistance induced by hypoxia in HCC.
Recent studies have suggested that abnormal expression of genes contributes to the aforementioned molecular biological characteristics [17]. The abnormally expressed genes could disturb molecular signaling pathways in the proliferation, apoptosis, metabolism and cell cycle. Consequently, tumor cells display significant drug resistance [18]. Tumor cells exhibit special molecular and morphological characteristics with EMT being one of the representative forms [19,20]. EMT is a process whereby invasive mesenchymal cells replace the epithelial cells which lose apical-basal polarity and cell adhesion. Although the role of EMT in cancer metastasis is controversial, the relationship between EMT and drug resistance has been increasingly emphasized [21]. Doxorubicin can induce EMT in HCC contributing to chemoresistance to doxorubicin [22].
In this study, we aimed to investigate the role of hypoxia in the progress of chemoresistance in HCC, and to ascertain whether GC7 could attenuate hypoxia-induced chemoresistance via the HIF1α-mediated signaling pathway in HCC.
Materials and methods
Cell lines and cell culture
Four types of HCC cell line (Huh7, Hep3B, SNU387 and SNU449) were selected and cultured in this study. Huh7 cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Hep3B cells were cultured in MEM supplemented with 10% FBS and 1% penicillin/streptomycin. SNU449 and SNU387 cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. All cells were maintained at 37°C in a 5% carbon dioxide incubator. Doxorubicin and GC7 were purchased from Sigma-Aldrich (St. Louis, MO, USA). The HIF-1α-siRNA and negative control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
CCK-8 assay
GC7 and doxorubicin were used in this study. HCC cells or siRNA-transfected HCC cells were plated in 96-well plates at the appropriate density. Then, the medium was replaced with corresponding serum-free medium. Twenty-four hours later, cells were treated with indicated concentrations of drugs for another 48 h. Then, 10 μL/well CCK8 solution (Dojindo, Kumamoto, Japan) was added to plates, they were incubated for 3 h, and absorbance was measured at 450 nm using a MRX II microplate reader (Dynex, Chantilly, VA, USA). Finally, the percentage of the untreated control was applied to calculate cell viability.
Western blotting
Cells were lysed with RIPA buffer (Cell Signaling, Danvers, MA, USA), which contains protease inhibitors (Sigma, St. Louis, MO, USA). A BCA Protein Kit (Thermo, Rockford, IL, USA) was used to quantify the protein concentration. Cell lysates were separated by 10% SDS-PAGE and the proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Then the blots were incubated with diluted primary antibodies (E-cadherin, Vimentin or eIF5A2, diluted 1:1000 in TBS/T; all obtained from Abcam( Abcam, Cambridge, USA) overnight at 4°C. The membranes were washed three times with TBS/T and then incubated with the appropriate HRP-conjugated secondary antibodies for 1 h at room temperature. The expression of protein was detected by chemiluminescence (GE Healthcare, Piscataway, NJ, USA) and visualized by autoradiography (Kodak, Rochester, NY, USA).
Immunofluorescence
HCC cells and siRNA-transfected cells were seeded into 48-well plates at the appropriate density. After treatment for the indicated times, the cells were fixed with 4% formaldehyde for 15 min, washed with PBS, treated with 5% BSA for 30 min at room temperature, and incubated with mouse anti-human Vimentin or anti-human E-cadherin primary antibodies (Cell Signaling, Danvers, MA, USA) overnight at 4°C. The cells were incubated with the secondary antibody conjugated with goat anti-mouse Cy5 (E-cadherin; Abcam) or goat anti-mouse FITC (Vimentin; Abcam) at 4°C for two hours. Then, the cells were incubated with 4’,6-diamidino-2-phenylindole (DAPI; Sigma) for 2 min at room temperature, washed twice with PBS, and observed using an inverted fluorescence microscope (Olympus, Tokyo, Japan).
Cell transfection
HIF-1α-siRNA or negative control siRNA were transfected into HCC cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The transfection medium (Opti-MEM) was replaced with complete medium 6 h after transfection, and the efficiency of transfection was measured by Western blotting at 48 h after transfection.
Statistical analysis
Experimental data was presented as the mean ± SD. Prism 5 (GraphPad, San Diego, CA, USA) was used for statistical analysis. Two-way ANOVA and Bonferroni’s post hoc test were applied to assess the effects of doxorubicin and the combined treatment. Other analysis for comparing two groups was performed using Student’s t-tests and a P-value less than 0.05 was considered to be statistically significant.
Results
Hypoxia induces chemoresistance to doxorubicin and low concentrations of GC7 sensitizes HCC cells to doxorubicin
To assess the role of hypoxia in chemotherapy sensitivity, we used the CCK8 assay to detect cell viability of HCC cells in different conditions. Huh7, Hep3B, SNU387 and SNU449 cells were more sensitive to doxorubicin under conditions of hypoxia compared with normoxia (Figure 1E-H). Hence, hypoxia can induce chemoresistance to doxorubicin in these four types of HCC cell line.
Figure 1.
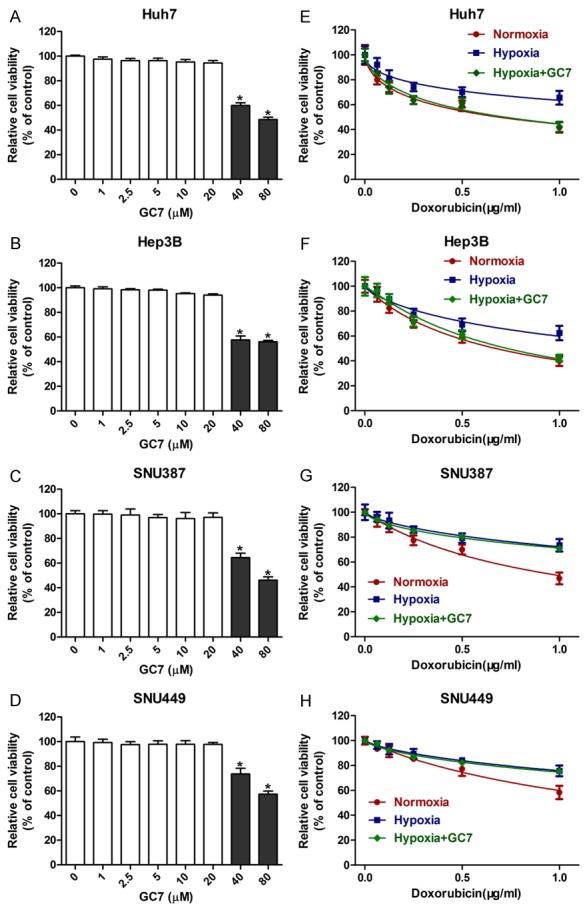
Hypoxia induces chemoresistance to doxorubicin. A-D: The cytotoxicity of GC7 in HCC cells. HCC cells were incubated with different concentrations of GC7 for 48 h. The value of CCK8 was the treated HCC cells that were normalized to the control group treated in the absence of GC7; *P < 0.05 vs GC7 0 μM. E-H. Hypoxia can induce chemoresistance to doxorubicin in Huh7, Hep3B, SNU387 and SNU449 cell lines. Low concentrations of GC7 (20 μM) sensitized Huh7 and Hep3B cell lines to doxorubicin in hypoxia.
In a recent study, Tariq et al. indicated that hypusinated eIF5A was indispensible for the expression of HIF-1α in hypoxia [23]. As an inhibitor of active eIF5A2, GC7 has been widely used in recent studies and can reverse doxorubicin-induced EMT by inhibiting activation of eIF5A2 [22]. Thus, GC7 was applied throughout our study. As GC7 is cytotoxic to HCC cells, we used the CCK8 assay to initially detect an appropriate concentration of GC7. The cytotoxicity of GC7 in all four HCC cell lines was rare when a dose of 0 to 20 μM was employed. However, the viability of the HCC cell lines was significantly inhibited at higher concentrations of GC7 (40-100 μM) (Figure 1A-D). Finally, the preferred concentration (20 μM) was used in our study. Interestingly, co-treatment with GC7 significantly enhanced the sensitivity of Huh7 and Hep3B cell lines to doxorubicin in hypoxic conditions (Figure 1E-H). Therefore, low concentrations of GC7 can enhance the sensitivity to doxorubicin in both Huh7 and Hep3B cell lines.
GC7 enhanced sensitivity to doxorubicin and regulated doxorubicin-induced EMT in epithelial phenotype HCC cells
To evaluated whether hypoxia induced doxorubicin resistance in HCC epithelial phenotype cells, we speculated whether EMT contributed to drug resistance [24]. Western blot analysis showed that doxorubicin treatment led to significant down-regulation of E-cadherin and up-regulation of Vimentin in HCC cells (Figure 2A). These results suggested that doxorubicin could induce EMT in HCC cells. Interestingly, doxorubicin treatment with GC7 reversed doxorubicin-induced EMT in epithelial phenotype HCC cells, but not in HCC mesenchymal phenotype cells (Figure 2A). Immunofluorescent staining also showed similar results that were consistent with the Western blot analysis (Figure 2B). These data suggested that GC7 could reverse doxorubicin-induced EMT in HCC cells typical of an epithelial phenotype. Furthermore, the CCK-8 assay was used to measure cell viability in cells treated with doxorubicin alone or doxorubicin plus GC7, and the results showed that the sensitivity of doxorubicin was reduced in HCC epithelial cells after co-treatment with GC7 (Figure 2C, 2D). In contrast, there was no significant difference between the two groups (Figure 2E, 2F).
Figure 2.
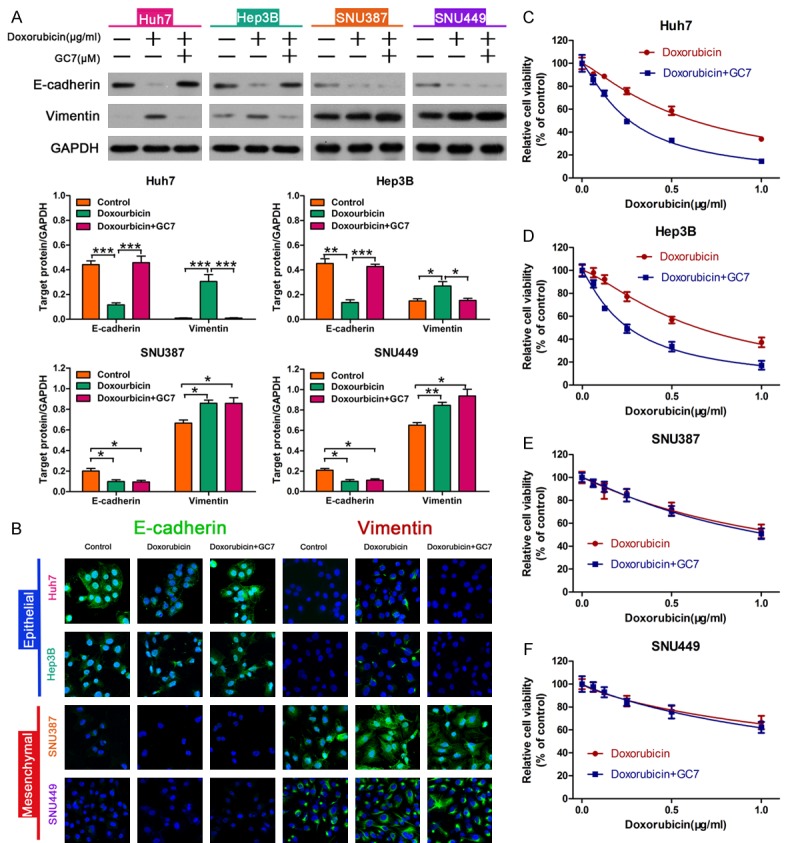
GC7 enhanced sensitivity to doxorubicin and regulated doxorubicin-induced EMT in epithelial phenotype HCC cells. A: GC7 reversed the expression of doxorubicin-induced EMT-markers in HCC cells. Western blotting was used to examine the expression of E-cadherin and Vimentin compared with control, treated with doxorubicin alone or with doxorubicin plus GC7 (*P < 0.05; **P < 0.01; ***P < 0.001). B: Immunofluorescence was performed to analysis the expression of E-cadherin and Vimentin in HCC cells. C-F: Cell viability was measured using a CCK-8 assay. GC7 (20 μM) significantly enhanced the cytotoxicity of doxorubicin in Huh7, Hep3B but had little synergistic effect in SNU387 and SNU449 cells.
EMT is induced during hypoxia and GC7 prevents EMT in HCC cells with an epithelial phenotype
From previous experience, we observed that hypoxia could induce chemoresistance to doxorubicin and GC7 significantly sensitized two of the four HCC cell lines to doxorubicin. However, the exact mechanism was not clear. To investigate the role of phenotype in combined therapy, we applied Western blotting to detect the expression of epithelial/mesenchymal markers including E-cadherin and Vimentin, which both mutually transform during EMT. The E-cadherin/Vimentin ratio is typically used for identification of cell phenotype. Huh7 and Hep3B cell lines exhibit an epithelial phenotype with a high E-cadherin/Vimentin ratio while the other two cell lines exhibit a mesenchymal phenotype (Figure 3A). Immunofluorescent staining showed the same results (Figure 3B). Consequently, GC7 enhanced the cytotoxicity of doxorubicin in HCC cells with an epithelial phenotype.
Figure 3.
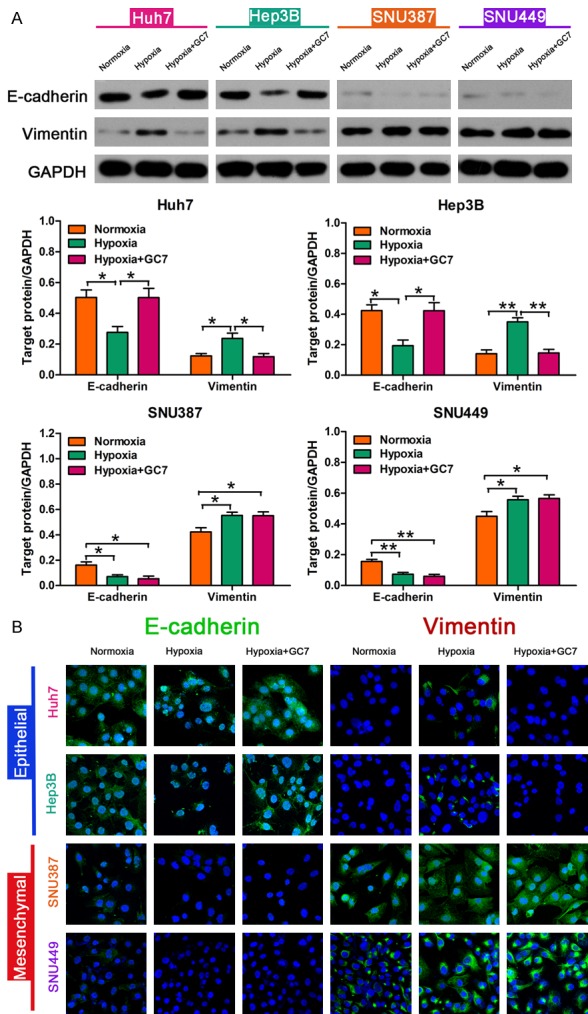
GC7 prevents EMT in HCC cells during hypoxia. A: Western blotting was applied to examine the expression of epithelial/mesenchymal markers including E-cadherin and Vimentin. GC7 enhanced the cytotoxicity of doxorubicin in HCC cells with an epithelial phenotype. Hypoxia contributed to EMT in Huh7, Hep3B, SNU387 and SNU449 cell lines; *P < 0.05, **P < 0.01. GC7 prevented hypoxia-induced EMT- in Huh7 and Hep3B cell lines with an epithelial phenotype; *P < 0.05, **P < 0.01. B: Immunofluorescent staining showing the expression of E-cadherin and Vimentin.
To confirm whether EMT was induced by hypoxia in HCC cells, we examined the EMT markers in HCC cells during hypoxia. After that, a decrease in E-cadherin expression and an increase in Vimentin expression were detected during hypoxia compared to normoxic conditions in all four HCC cell lines (Figure 3A). Our data revealed that hypoxia contributed to EMT in HCC cells.
To further ascertain if GC7 reversed hypoxia-induced EMT in HCC cells, we examined the expression of EMT markers in HCC cells treated with GC7 for 48 h. The expression of E-cadherin in Huh7 and Hep3B cell lines was significantly down-regulated under the action of GC7, while the mesenchymal marker Vimentin was highly expressed simultaneously. Interestingly, SNU387 and SNU449 did not significantly alter the expression of their phenotypic marker after 48 h incubation of hypoxia plus GC7, compared to the control group treated in the absence of GC7 (Figure 3A). Hence, the increase in E-cadherin expression and decrease in Vimentin expression caused by GC7 suggest that GC7 prevented EMT induced by hypoxia in both Huh7 and Hep3B cell lines, which exhibit an epithelial phenotype.
Knock down of HIF-1α attenuates hypoxia-induced EMT and abolishes the unique feature of GC7
DHS is one of two key enzymes in the activation process of eIF5A2. As a specific inhibitor of DHS, GC7 has caused extensive concerns [25]. Nevertheless, the relationship between GC7 and hypoxia is ambiguous. The data from our study suggest that GC7 exerted a remarkable characteristic in hypoxia-induced EMT. To clarify whether GC7 reversed the hypoxia-induced EMT via HIF-1α-mediated signaling pathway, we applied RNAi to knock down the expression of HIF-1α in HCC cells. Si-RNA transfected HCC cells during hypoxia were incubated with doxorubicin or doxorubicin plus GC7 for 48 h. After knocking down HIF-1α, the HCC cells in hypoxia did not show chemoresistance to doxorubicin and the E-cadherin/Vimentin ratio did not alter under hypoxic conditions either (Figure 4A, 4B). The CCK8 assay showed that HIF-1α-siRNA attenuated the ability of GC7 to sensitize HCC cells to doxorubicin during hypoxia in four HCC cell lines and the viability of the HCC cells was not significantly different between the two groups.
Figure 4.
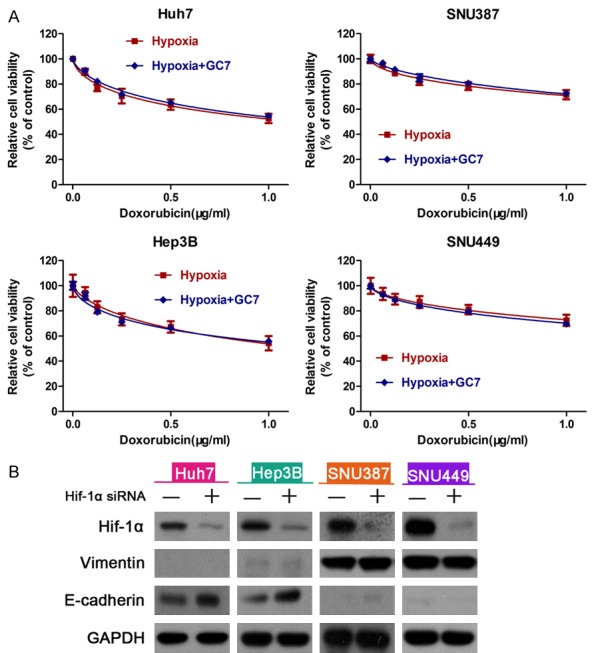
Inhibiting of HIF-1α attenuates hypoxia-induced EMT. A: There was no significant difference in the viability of cells between those treated with doxorubicin and doxorubicin plus GC7 under hypoxic conditions after the knock down of HIF-1α. B: Western blot showing HIF-1α, E-cadherin and Vimentin expression in HCC cells transfected with HIF-1α siRNA or a negative control siRNA.
GC7 enhanced the effect of doxorubicin by inhibiting activation of eIF5A2
eIF5A1/eIF5A2 are the only substrates containing the unusual amino acid hypusine, which is derived from spermidine. Hence, we used SDS-PAGE fluorography to detect cell lysate hypusine content after incubation of HCC cells in the presence of [3H]-spermidine. The results showed that 20 μM GC7 significantly decreased the activity of incorporated [3H]-spermidine compared to control HCC cells (Figure 5A). Furthermore, we transfected eIF5A2 siRNA into these cells and conducted CCK-8 assays to determine cell viability of cells treated with doxorubicin alone, or doxorubicin combined with GC7 in HCC cells. The results showed that there were barely detectable changes in the doxorubicin sensitivity between the two groups (Figure 5B-E), suggesting that GC7 was involved in the chemosensitivity of HCC cells. Western blot analysis indicated that the expression of E-cadherin was increased and Vimentin was reduced by knock down of eIF5A2 (Figure 5F). In summary, these findings demonstrated that GC7 enhanced HCC cell line sensitivity to doxorubicin by reversing hypoxia-induced EMT and doxorubicin-induced EMT.
Figure 5.
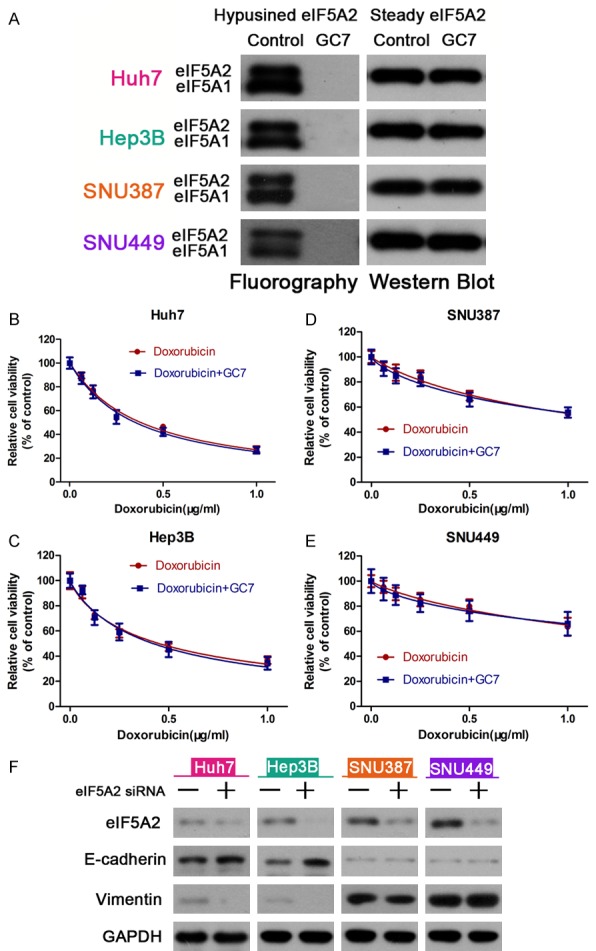
GC7 enhanced the effect of doxorubicin by inhibiting activation of eIF5A2. A: Fluorogram of SDS-PAGE separating hypusinated-eif5a1/eIF5A2 protein (Hpu eIF5A isoforms) in HCC cell protein lysates after incubation with or without GC7 (20 μM) in the presence of [3H]-spermidine. B-E: After transfection with eIF5A2 siRNA, CCK-8 cell viability assays were performed to determine the chemosensitivity of HCC cells treated with doxorubicin alone or doxorubicin plus GC7. F: Western blot was used to detect the expression of eIF5A2 siRNA or a negative control siRNA.
Discussion
Chemoresistance induced by hypoxia is strongly associated with a poor prognosis in many tumors. Therefore, it is important to clarify the critical regulatory factors for hypoxia-induced chemoresistance in order to improve prognosis of cancer. HIF-1α is considered to be the master regulatory factor of various cellular processes in hypoxia. HIF-1α is involved in the expression of more than 70 genes and regulates invasion, angiogenesis, metastasis and chemoresistance [26]. Iovine et al. proposed that the increase in HIF-1α expression decreased the sensitivity of several anticancer drugs, on account of its unique influence on DNA repair mechanisms, the expression of drug efflux transporters, and the balance between pro- and anti-apoptotic factors. Subsequently, that researcher indicated that HIF-1α should be a potential target to treat cancer and improve survival rate in their study [27]. Recently, concern has increased over the inhibition of HIF-1α, alleviating hypoxic drug resistance and arresting tumor xenograft growth [28-30]. In this study, we investigated the sensitivity of HCC cells to doxorubicin under conditions of hypoxia compared to normoxic conditions, and applied a new inhibitor to attenuate chemoresistance induced by hypoxia.
EMT is described as a morphogenetic process in which epithelial patterns transform into mesenchymal characteristics, including motility and invasiveness [21]. This morphogenetic process is involved in the regulation of invasion, angiogenesis, metastasis and chemoresistance [31,32]. Our data showed that HCC cell lines appeared insensitive to doxorubicin and EMT under hypoxic conditions. Furthermore, the reversion of EMT was associated with improved sensitivity to doxorubicin. Hence, we can attribute hypoxia-induced chemoresistance to EMT.
EIF5A2, one of two isoforms in the eIF5A family, was first isolated by a chromosome microdissection-hybrid selection technique in 2001 [33]. It is considered to be an oncogene and is highly expressed in many cancers including non-small cell lung cancer, colon cancer, and ovarian cancer [33-35]. A series of in vitro and in vivo studies revealed that eIF5A2 could stimulate tumor formation, enhance cancer cell growth, induce chemoresistance to traditional chemotherapy drugs, and increase cancer cell motility and invasiveness by inducing EMT [25]. As a novel inhibitor of DHS, GC7 suppresses the activation of DHS which is the key enzyme in the activation of eIF5A2 and contributes to inhibition of eIF5A2 [36]. In recent years, GC7 has been postulated as having antitumor activities in regulating tumor cell sensitivity to chemotherapy drugs in many malignant tumors such as breast cancer, bladder cancer and HCC [22,37,38]. In a previous study, researchers showed that hypoxia increased expression of eIF5A2 by as much as 4- to 8-fold in esophageal squamous cell carcinoma (ESCC) cell lines, and observed bidirectional regulation between eIF5A2 and HIF1α [39]. Further research indicated that over-expressed eIF5A2 increased angiogenesis and metastasis in ESCC via the HIF1α-mediated signaling pathway [14]. Thus, we hypothesized that GC7 has a critical role in hypoxia-induced chemoresistance via a signaling pathway mediated by HIF1α. Our data revealed that GC7 attenuated chemoresistance induced by hypoxia in the HCC cells with an epithelial phenotype. We also observed that EMT was reversed under the influence of GC7 by Western blotting. To further ascertain the role of GC7 in hypoxia-induced EMT, we transfected HCC cells with HIF-1α-siRNA. After transfection, hypoxia-induced chemoresistance vanished and the remarkable feature of GC7 was also abolished.
As described above, GC7 enhanced sensitivity to doxorubicin under hypoxic condition in HCC cells exhibiting an epithelial phenotype via a HIF-1α-mediated signaling pathway. The mechanism of why this does not work with HCC cells that have a mesenchymal phenotype is unclear. A detailed study is needed to illuminate how it is possible to enhance the therapeutic effect of HCC cells with a mesenchymal phenotype. Our data showed that GC7 sensitized HCC cells to doxorubicin via a HIF-1α-mediated signaling pathway. Our novel aim for the future is to clarify the relationship between eIF5A2 and HIF-1α and confirm the HIF-1α-mediated signaling pathway which plays a critical role in increased sensitivity to doxorubicin by GC7. In addition, HIF-1α is a crucial regulator of invasion, angiogenesis, metastasis and chemoresistance under hypoxic conditions. This molecule is expected to be a potential target in improving sensitivity to chemotherapy and improvement in the prognosis of HCC.
In conclusion, the present study demonstrated that GC7 enhanced sensitivity to doxorubicin in HCC by reversing hypoxia-induced EMT via the HIF-1α-mediated signaling pathway. It suggests that a new method of enhancing the cytotoxicity of chemotherapy is possible, which may translate to an improvement in the long-term survival rate of those affected by HCC.
Acknowledgements
This study was supported by Zhejiang Province of Medical and Health Technology Plan projects (2015KYB454) and Zhejiang Province of Lishui Key Research and Development Plan projects (2016zdyf01).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13:2140–2151. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 4.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, Jiang H, Liu J, Liu L. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1alpha inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 6.Zhao D, Zhai B, He C, Tan G, Jiang X, Pan S, Dong X, Wei Z, Ma L, Qiao H, Jiang H, Sun X. Upregulation of HIF-2alpha induced by sorafenib contributes to the resistance by activating the TGF-alpha/EGFR pathway in hepatocellular carcinoma cells. Cell signal. 2014;26:1030–1039. doi: 10.1016/j.cellsig.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Chintala S, Toth K, Cao S, Durrani FA, Vaughan MM, Jensen RL, Rustum YM. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1alpha. Cancer Chemother Pharmacol. 2010;66:899–911. doi: 10.1007/s00280-009-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai TY, Liu XW, Zhu H, Cao J, Zhang J, Ding L, Lou JS, He QJ, Yang B. Tirapazamine sensitizes hepatocellular carcinoma cells to topoisomerase I inhibitors via cooperative modulation of hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2014;13:630–642. doi: 10.1158/1535-7163.MCT-13-0490. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–30. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 12.Tong Y, Li QG, Xing TY, Zhang M, Zhang JJ, Xia Q. HIF1 regulates WSB-1 expression to promote hypoxia-induced chemoresistance in hepatocellular carcinoma cells. FEBS Lett. 2013;587:2530–5. doi: 10.1016/j.febslet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Jiao M, Nan KJ. Activation of PI3 kinase/Akt/HIF-1alpha pathway contributes to hypoxia-induced epithelial-mesenchymal transition and chemoresistance in hepatocellular carcinoma. Int J Oncol. 2012;40:461–468. doi: 10.3892/ijo.2011.1197. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X, Qian C, Cai Z, Xie D, Huang P, Guan XY. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146:1701–13. e9. doi: 10.1053/j.gastro.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 15.Liu RR, Lv YS, Tang YX, Wang YF, Chen XL, Zheng XX, Xie SZ, Cai Y, Yu J, Zhang XN. Eukaryotic translation initiation factor 5A2 regulates the migration and invasion of hepatocellular carcinoma cells via pathways involving reactive oxygen species. Oncotarget. 2016;7:24348–24360. doi: 10.18632/oncotarget.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Du F, Chen W, Yao M, Lv K, Fu P. EIF5A2 is a novel chemoresistance gene in breast cancer. Breast cancer. 2015;22:602–607. doi: 10.1007/s12282-014-0526-2. [DOI] [PubMed] [Google Scholar]
- 17.Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, Park MH, Johansson HE. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270:4254–4263. doi: 10.1046/j.1432-1033.2003.03806.x. [DOI] [PubMed] [Google Scholar]
- 18.Tong Y, Park I, Hong BS, Nedyalkova L, Tempel W, Park HW. Crystal structure of human eIF5A1: insight into functional similarity of human eIF5A1 and eIF5A2. Proteins. 2009;75:1040–1045. doi: 10.1002/prot.22378. [DOI] [PubMed] [Google Scholar]
- 19.Josson S, Sharp S, Sung SY, Johnstone PA, Aneja R, Wang R, Gururajan M, Turner T, Chung LW, Yates C. Tumor-stromal interactions influence radiation sensitivity in epithelial-versus mesenchymal-like prostate cancer cells. J Oncol. 2010:2010. doi: 10.1155/2010/232831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 21.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Lou B, Fan J, Wang K, Chen W, Zhou X, Zhang J, Lin S, Lv F, Chen Y. N1-guanyl-1,7-diaminoheptane (GC7) enhances the therapeutic efficacy of doxorubicin by inhibiting activation of eukaryotic translation initiation factor 5A2 (eIF5A2) and preventing the epithelial-mesenchymal transition in hepatocellular carcinoma cells. Exp Cell Res. 2013;319:2708–2717. doi: 10.1016/j.yexcr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Tariq M, Ito A, Ishfaq M, Bradshaw E, Yoshida M. Eukaryotic translation initiation factor 5A (eIF5A) is essential for HIF-1alpha activation in hypoxia. Biochem Biophys Res Commun. 2016;470:417–424. doi: 10.1016/j.bbrc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Drasin DJ, Robin TP, Ford HL. Breast cancer epithelial-to-mesenchymal transition: examining the functional consequences of plasticity. Breast Cancer Res. 2011;13:226. doi: 10.1186/bcr3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang FW, Guan XY, Xie D. Roles of eukaryotic initiation factor 5A2 in human cancer. Int J Biol Sci. 2013;9:1013–1020. doi: 10.7150/ijbs.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–7. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 27.Iovine B, Guardia F, Irace C, Bevilacqua MA. l-carnosine dipeptide overcomes acquired resistance to 5-fluorouracil in HT29 human colon cancer cells via downregulation of HIF1-alpha and induction of apoptosis. Biochimie. 2016;127:196–204. doi: 10.1016/j.biochi.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Lou S, Liu G, Shimada H, Yang X, He Q, Wu L. The lost intrinsic fragmentation of MAT1 protein during granulopoiesis promotes the growth and metastasis of leukemic myeloblasts. Stem cells. 2013;31:1942–1953. doi: 10.1002/stem.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ao Q, Su W, Guo S, Cai L, Huang L. SENP1 desensitizes hypoxic ovarian cancer cells to cisplatin by up-regulating HIF-1alpha. Sci Rep. 2015;5:16396. doi: 10.1038/srep16396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco FF, Jimbo M, Wulfkuhle J, Gallagher I, Deng J, Enyenihi L, Meisner-Kober N, Londin E, Rigoutsos I, Sawicki JA. The mRNA-binding protein HuR promotes hypoxia-induced chemoresistance through posttranscriptional regulation of the proto-oncogene PIM1 in pancreatic cancer cells. Oncogene. 2016;35:2529–2541. doi: 10.1038/onc.2015.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, Baba H, Mori M. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–299. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C, Moran MS, Shao C, Yang Q. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011;102:1151–1157. doi: 10.1111/j.1349-7006.2011.01919.x. [DOI] [PubMed] [Google Scholar]
- 33.Guan XY, Sham JS, Tang TC, Fang Y, Huo KK, Yang JM. Isolation of a novel candidate oncogene within a frequently amplified region at 3q26 in ovarian cancer. Cancer Res. 2001;61:3806–3809. [PubMed] [Google Scholar]
- 34.He LR, Zhao HY, Li BK, Liu YH, Liu MZ, Guan XY. Overexpression of eIF5A-2 is an adverse prognostic marker of survival in stage I non-small cell lung cancer patients. Int J Cancer. 2011;129:143–150. doi: 10.1002/ijc.25669. [DOI] [PubMed] [Google Scholar]
- 35.Xie D, Ma NF, Pan ZZ, Wu HX, Liu YD, Wu GQ, Kung HF, Guan XY. Overexpression of EIF-5A2 is associated with metastasis of human colorectal carcinoma. Hum Pathol. 2008;39:80–86. doi: 10.1016/j.humpath.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M, Brassat U, Priemer M, Hauber I, Wilhelm T, Schwarz G, Kanz L, Bokemeyer C, Hauber J, Holyoake TL, Nordheim A, Brümmendorf TH. Hypusination of eukaryotic initiation factor 5A (eIF5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach. Blood. 2007;109:1701–1711. doi: 10.1182/blood-2005-03-037648. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Liu R, Fu P, Du F, Hong Y, Yao M. N1-guanyl-1,7-diaminoheptane sensitizes estrogen receptor negative breast cancer cells to doxorubicin by preventing epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2 activation. Cell Physiol Biochem. 2015;36:2494–2503. doi: 10.1159/000430209. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Yu H, Shen M, Wei W, Xia L, Zhao P. N1-guanyl-1,7-diaminoheptane sensitizes bladder cancer cells to doxorubicin by preventing epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2 activation. Cancer Sci. 2014;105:219–227. doi: 10.1111/cas.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146:1701–1713. doi: 10.1053/j.gastro.2014.02.029. [DOI] [PubMed] [Google Scholar]


