Abstract
The clinical translation of tissue engineering methods is confined by the limited external cell sources, which is hopefully to be addressed by the cell guidance approach as cytokine-induced homing and differentiation of the patients’ autologous cells. Synovium-derived stem cells (SDSCs) are a potent cell source for cartilage restoration due to its intrinsic proximity and tissue-specific chondrogenic capacity. In this study, stromal cell-derived factor-1α (SDF-1α) in combination with transforming growth factor β1 (TGF-β1) were used to induce SDSCs migration and chondrogenesis in vitro. The migration capacity was evaluated by transwell assay and for chondrogenic evaluation, the expression of Sox9, ACAN and COL2A1 were assessed by quantitative RT-PCR while the expression of sulfated GAG and collagen II were evaluated by Alcian Blue stain and immunohistochemistry respectively. Our data showed that SDF-1α/CXC chemokine receptor 4 (CXCR4) was involved in SDSCs migration through phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway. Exogenous TGF-β1 enhanced SDF-1α-induced SDSCs migration in a concentration and time-dependent manner through CXCR4, evidenced as complete blockage by AMD3100, the CXCR4 antagonist and this effect was mediated by extracellular regulated protein kinases (ERK) activation. Moreover, the addition of SDF-1α augmented the TGF-β1-induced SDSCs chondrogenesis, evidenced by the increased pellet sizes and the expressions of COL 2A1, ACAN and Sox9. This effect was related to c-Jun N-terminal kinase (JNK) activation. Collectively, these results suggest that SDF-1α and TGF-β1 interacts with each other and synergistically enhance the SDSCs migration and chondrogenesis through MAPK pathways.
Keywords: Stromal cell-derived factor-1α, transforming growth factor-β1, synovium-derived stem cells, migration, chondrogenesis
Introduction
Although great strides have been made in the field of tissue engineering in last few decades, the requirement of external donor cells still remains as one notable limitation. In clinical practice, cell harvest procedure would be translated into a biopsy or additional surgery that may result in the donor site morbidity. Cell guidance presents to be an appealing alternative, the concept of which is based on the site-specific homing of the desired host cells to the transplanted scaffold that constantly delivering cytokines for tissue regeneration [1].
Regarding to the application of this technique for articular cartilage regeneration, synovium-derived stem cells (SDSCs) present to be an ideal cell source. Due to the intrinsic proximity, SDSCs are recognized as a kind of tissue specific stem cells for cartilage restoration [2,3]. Endogenous SDSCs could migrate into the injured cartilage area and undergo chondrogenic differentiation [4]. Stromal cell-derived factor-1α (SDF-1α) acts as a potent stimulus in mesenchymal stem cells (MSCs) homing through the recruitment of CXC chemokine receptor 4 (CXCR4) positive progenitors and SDF-1α/CXCR4 axis has been involved in the repair of various tissue injuries including kidney injury [5], myocardial infarction [6], and fracture repair [7]. If SDF-1 signaling pathway is destroyed, the repair effect would be attenuated [8]. The phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) activation acts downstream CXCR4, participating in the chemotaxis of MSCs. AMD3100 could downregulate the level of phospho-Akt during MSCs migration induced by SDF-1α [6]. However, it is not clear whether SDF-1α/PI3K/Akt axis plays a role in SDSCs migration.
Transforming growth factor β (TGF-β) is a widely-acknowledged chondrogenic induction factor for MSCs including SDSCs [9-11]. Treatment with TGF-β1 or TGF-β3 induced the expression of Sox9 and other cartilage-related extracellular matrix markers in chondrogenic pellets [12].
In previous studies, TGF-β1 was reported to interplay with SDF-1α, subsequently affecting the chemotaxis of MSCs to SDF-1α in ischemia reperfusion-injured kidney model and in periodontal ligament [5,13]. Moreover, SDF-1α played a regulatory role in bone morphogenetic protein 2 (BMP-2) induced chondrogenic and osteogenic differentiation in vitro and these effects could be inhibited by the blockage of SDF-1/CXCR4 axis [14]. It raises the possibility that despite their well-recognized role of migration and chondrogenic induction, SDF-1α and TGF-β1 may cross-talk with each other and further facilitate their counterpart in SDSCs-based in situ cartilage restoration.
Mitogen-activated protein kinase (MAPK) signaling cascade, mainly including extracellular regulated protein kinases (ERK), c-Jun N-terminal kinase (JNK) and P38, are known to involved in various biological responses including cell migration and differentiation, whereas playing different roles under different situations. While ERK signal was activated in SDSCs condensation procedure, it was downregulated during chondrogenesis [15]. Meanwhile, the inhibition of P38 signal pathway promoted the SDSCs chondrogenic capacity [11].
In this study, we hypothesized that SDF-1α interacts with TGF-β1 and synergistically facilitats the SDSCs migration and chondrogenesis. Meanwhile, we explored the potential involvement of MAPK signaling pathways underlying both processes.
Material and methods
Cell isolation, culture and identification
Synovium was harvested from primary knee osteoarthritis patients who underwent total knee arthroplasty in accordance with the guidelines approved by the Ethics Committee of Zhongshan Hospital, Fudan University. Written informed consent was obtained from all patients. Cell isolation and identification procedures were described in our previous study [16]. Briefly, the collected synovial tissue was rinsed three times with phosphate buffered solution (PBS) solution containing 100 U/ml penicillin and 100 U/ml streptomycin before minced finely. Afterward, the tissue was digested with trypsin-EDTA (0.1% trypsin, 0.4 mM EDTA) and 0.1% collagenase II (Sigma-Aldrich, St. Louis, MO, USA) for 0.5 h and 2 h at 37°C sequentially. Digested cells were filtered through a 70-μm nylon filter (Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged at 1000 rpm for 5 min to obtain a cell pellet, which was then resuspended and seeded in culture flasks consisting of alpha minimum essential medium (αMEM) containing 10% fetal bovine serum (FBS) (Gibco, Invitrogen, Carlsbad, CA, USA). The cells were cultured at 37°C in a humidified atmosphere of 5% CO2 with the medium changed after 48 h. When the attached cells reached 90% confluence, they were passaged at a dilution rate of 1:4 for subculture. For flow cytometry analysis, SDSCs were harvested and aliquots of 2×105 cells were resuspended before directly stained with 5 μL fluorescein isothiocyanate (FITC)-labeled antibodies against CD29, CD45 and CD105 and phycoerythrin (PE)-labeled anti-CD44 (eBioscience, San Diego, CA, USA) and incubated at 4°C for 30 min.
For osteogenic and adipogenic differentiation, SDSCs were seeded at 10,000 cells/cm2. Once the cells reached confluence, the culture medium was switched to adipogenic induction medium or osteogenic induction medium (Cyagen Biosciences, Santa Clara, CA, USA) for an additional 21 days. Adipogenic differentiation was assessed with Oil Red O staining while osteogenic differentiation was assessed using alkaline phosphatese (ALP) and Alizarin Red S staining as described before. Reverse transcription polymerase chain reaction (RT-PCR) was used to evaluate the relative gene expressions and the primers sequences used are listed in Table 1.
Table 1.
Primer list
| Genes | Forward primer sequence (5’ to 3’) | Reverse primer sequence (5’ to 3’) |
|---|---|---|
| β-actin | CCCCAAGGCCAACCGCGAGAAGATG | AGGTCCCGGCCAGCCAGGTCCAG |
| SOX9 | TATGACTGGACCCTGGTG | TGTGGCTTGTTCTTGCTGG |
| COL2A1 | CAACACTGCCAAACGTCCAGAT | CTGCTTCGTCCAGATAGGCAAT |
| AGG | GCAGAGACGCATCTAGAAATTG | GGTAATTGCAGGGAACATCATT |
| RUNX2 | ATTCCTGTAGATCCGAGCACC | GCTCACGTCGCTCATTTTGC |
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| PPARγ | CCTATTGACCCAGAAAGCGATT | CATTACGGAGAGATCCACGGA |
| LPL | TCATTCCCGGAGTAGCAGAGT | GGCCACAAGTTTTGGCACC |
| CXCR4 | AAACTGAGAAGCATGACGGACAA | GCCAACATAGACCACCTTTTCAG |
Cell migration assay
Chemotaxis assay was conducted in a 24-well plate with 8.0-μm pore-size transwell inserts (Millipore, Billerica, MA, USA). SDSCs were pretreated with or without TGF-β1 of different concentration (5, 10, 20, 40 ng/mL) for 30 min. Then SDSCs were dissociated into single cells and resuspended at a density of 106 cell/mL and 200 μL of cell suspension was transferred into the upper chamber. For the inhibition experiment, SDSCs were preincubated with an inhibitor (10 μM AMD3100 for CXCR4, 10 μM LY294002 for PI3K, or 10 μM PD98059 for ERK, Abcam) for 30 min prior to seeding. Afterwards, 600 μL αMEM medium without (control) or with 20, 50, 100 and 200 ng/mL SDF-1α (Pepro Tech, Rocky Hill, NJ, USA) were added to the lower chambers. After incubation at 37°C for certain time, SDSCs remaining on the top of the membrane were removed with a cotton swab and those migrated to the bottom of the membrane were harvested, fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet for 10 min. Cell numbers from 5 random visual fields were counted under a microscope (Nikon Eclipse, Nikon Instruments, Inc, Melville, NY, USA). The chemotaxis index (CI) was calculated.
CI = [Stimulated migration (SDSC number per high power field (HPF))]/[Random migration (SDSC number per HPF)]
Chondrogenic differentiation assay
For chondrogenic differentiation, aliquots of 1×106 SDSCs were centrifuged at 500 g for 5 min in a 15-ml polypropylene tube to form a pellet. The pellets were cultured in serum-free chondrogenic medium (Cyagen Biosciences) with the supplementation of 10 ng/mL TGF-β1 (Pepro Tech, Rocky Hill, NJ, USA). The medium was changed every 3 days with SDF-1α added into the medium of corresponding group. The pellets were cultured for 21 days and collected for the following evaluations.
Histology and immunohistochemistry
For in vitro histology, the representative pellets (n = 3) were fixed in 4% paraformaldehyde, embedded in paraffin blocks, and cut into 5-μm-thick sections. To detect sulfated glycosaminoglycans (GAGs), sections were stained with Alcian blue (Sigma-Aldrich). For immunohistochemistry, the sections were probed with primary antibody against collagen II (ab34712, 1:100, Abcam), followed by the usage of immunohischemistry kit for rabbit primary antibody (Yeasen, Shanghai, China) for the following detection.
Western blot assay
Cell lysates were extracted using RIPA lysis buffer (Beyotime, Institute of Biotechnology, Haimen, China) with PMSF at 4°C and equal amount of protein was loaded onto 10% SDS-PAGE gels. The protein samples were separated and transferred onto polyvinylidenedifluoride (PVDF) membranes (Millipore, Germany) for 1.5 h at 310 mA. After blocking the membrane with 5% milk solution in 0.1% TBS-Tween 20 for 1 h at room temperature, the membranes were incubated overnight at 4°C with the following primary antibodies: anti-CXCR4 (ab124824, 1:75, Abcam, Cambridge, UK), anti-phospho-Akt (ab183758, 1:1000, Abcam), anti-Akt antibodies (ab179463, 1:10000, Abcam), anti-phospho-ERK (ab201015, 1:1000, Abcam), anti-ERK (ab184699, 1:10000, Abcam), anti-phospho-JNK (ab124956, 1:1000, Abcam), anti-JNK (ab179461, 1:1000, Abcam), anti-phospho-p38 (ab4822, 1:1000, Abcam), anti-p38 (ab170099, 1:1000, Abcam), anti-vimentin (ab92547, 1:1000, Abcam) and anti-β-actin (ab8226, 1:2500, Abcam). Membranes were washed in 0.1% TBS-Tween 20 and incubated for 1 h with appropriate HRP-conjugated secondary antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and protein bands were visualized using the enhanced chemiluminescence method (Pierce, IL). The protein expressions were quantified using densitometry with ImageJ software (National Institutes of Health, USA) and normalized to relative β-actin expression. All experiments were performed in triplicates.
Quantitative real-time polymerase chain reaction assay
To analyze the expression of CXCR4, type II collagen α1 (COL 2A1) and ACAN, markers for chondrogenic differentiation as well as Sox9, the key chondrogenic transcription factor, total RNA was extracted using TRIzol® (Invitrogen) and then reverse transcribed with PrimeScript RT Reagent Kit (Takara-bio, Otsu, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed in an EPPENDORF Master cycler ep realplex machine (Eppendorf, Germany). HieffTMqPCRSYBR® Green Master Mix (YEASEN, China) was used according to the manufacturer’s instruction. Relative RNA expression were calculated using χ = 2-ΔΔCt method [17]. β-actin was employed as the housekeeping gene and the primers sequences used are listed in Table 1. All experiments were performed in triplicates.
Statistical analysis
All data were presented as the mean ± standard deviation (SD). Student’s t-test was used to assess between-group differences. All statistical analyses were performed with SPSS 20.0 statistical software (SPSS Inc., Chicago, IL). A p value less than 0.05 was considered statistically significant.
Results
Identification of SDSCs
The identification was demonstrated in our previous study [16]. After 21 days of culture, SDSCs were successfully differentiated into chondrocyte pellets, adipocytes and calcified nodules. RT-PCR showed the significantly increased chondrogenic genes (SOX9, COL2A1 and ACAN), adipogenic marker genes (PPARγ and LPL) and osteogenic marker genes (RUNX2 and ALP) expressions in the differentiated SDSCs respectively, indicating SDSCs have the characteristics of MSCs.
SDF-1α induces the SDSCs migration through PI3K/Akt signaling pathway
A transwell-based migration assay was established to quantitatively identify the contribution of SDF-1α on SDSCs migration in vitro. SDF-1α augmented the SDSCs migration in a dose (20, 50, 100, 200 ng/mL) and time (6, 12, 18, 24 h)-related manner with the average number of migrated SDSCs increased significantly compared with that in the control group (P<0.05) and reached a peak at 100 ng/mL and 24 h respectively (Figure 1A). The number of migrated cells was significantly reduced when preincubated with AMD3100, the CXCR4 antagonist (P<0.05) (Figure 1B).
Figure 1.
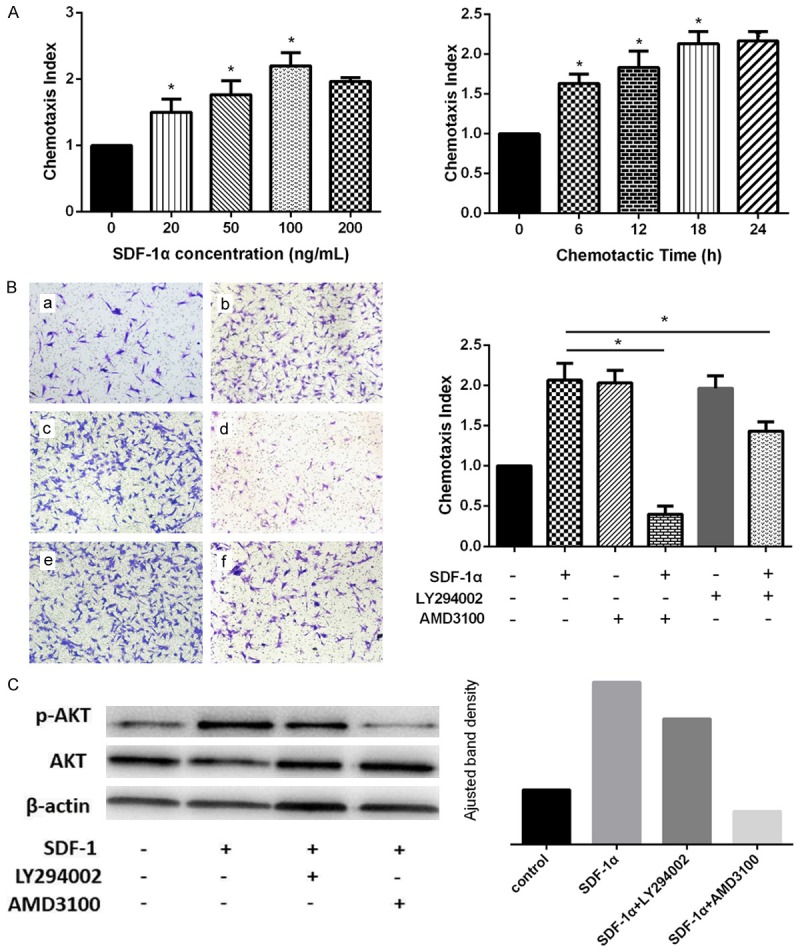
Effect of SDF-1α on SDSCs migration and Akt expression. (A) Effect of SDF-1α on SDSCs migration with different doses and time. *P<0.05 versus control group. (B) Effect of SDF-1α on SDSCs were detected by transwell migration assay. a) Control group, b) SDF-1α (100 ng/ml) group, c) AMD3100 group, d) SDF-1α+AMD3100 group, e) LY294002 group, f) SDF-1α+LY294002 group. *P<0.05. Magnification: 100×. (C) With LY294002 and AMD3100, effect of SDF-1α on phospho-Akt expression was detected by western blot analysis at both phosphorylation and total protein levels. ImageJ software was used to quantifiy the bands.
To further elucidate whether SDF-1α induces SDSCs migration via PI3K/Akt pathways, the PI3K/Akt selective inhibitor, LY294002 was used. After preincubated with LY294002 (10 μM) for 30 minutes, SDSCs were added to the upper chambers. The application of LY294002 attenuated the influence of SDF-1α on SDSCs migration as the average number of migrated SDSCs was significant decreased whereas still remained observably more than that in control group (P<0.05) (Figure 1B). Western blot analysis showed the significantly increased phospho-Akt expression in SDF-1α group, which was not totally blocked by the pretreatment of LY294002 whereas was almost blocked with the pretreatment of AMD3100 (Figure 1C). The above results indicate that SDF-1α induce the SDSCs migration through CXCR4 and downstream activation of PI3K/Akt pathway.
TGF-β1 augments the SDF-1α-induced migration through the activation of CXCR4
To determine whether TGF-β1 has an effect on SDF-1α-induced migration, a transwell-based migration assay was set up with the pre-treatment of TGF-β1. The administration of TGF-β1 resulted in a significant increase in the average number of migrated SDSCs, which was also dose-dependent (5, 10, 20, 40 ng/mL) and culminated at 20 ng/mL (Figure 2A). To further investigate whether the SDF-1α-driven migration is CXCR4 dependent, the cells were preincubated with AMD3100 (10 μM). The number of migrated cells was significantly reduced with AMD3100 treatment (P<0.05) (Figure 2B).
Figure 2.
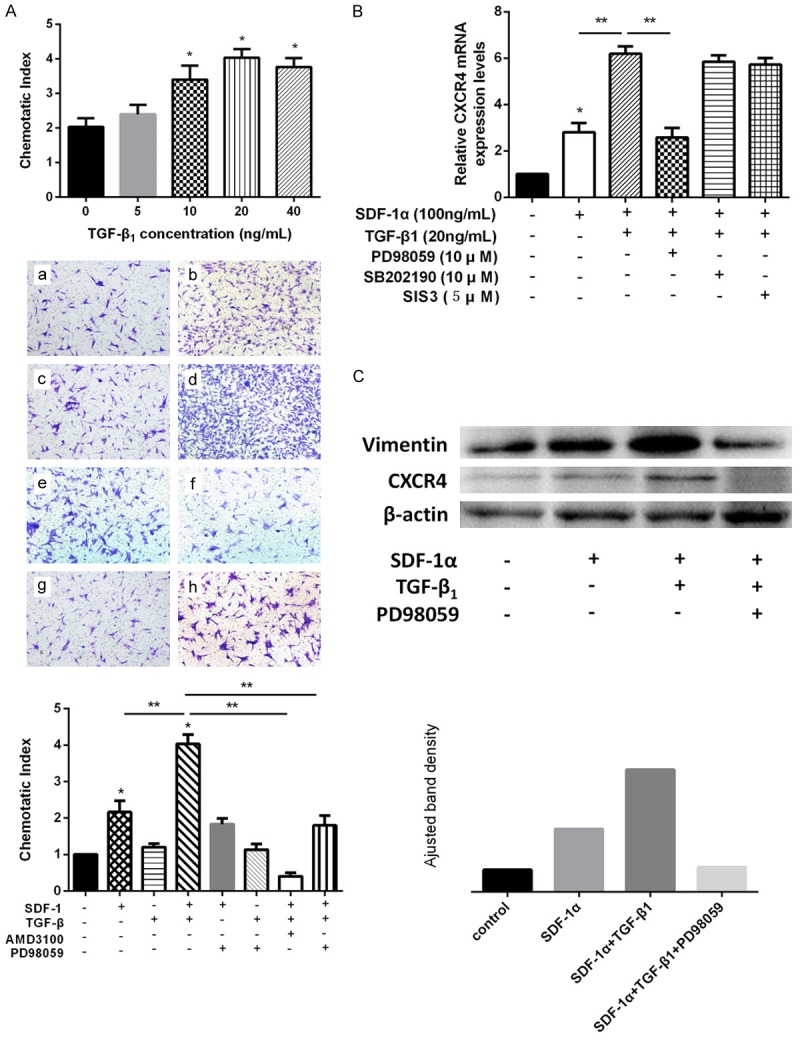
Effect of TGF-β1 on SDF-1α-induced SDSCs migration and Vimentin and CXCR4 expression. (A) Effects of different concentrations of TGF-β1 on SDF-1α (100 ng/ml)-induced SDSCs migration and with ERK inhibitor PD98059, effect of TGF-β1 on SDF-1α-induced SDSCs migration were detected by transwell migration assay. a) control group, b) SDF-1α (100 ng/ml) group, c) TGF-β1 (20 ng/ml) group, d) SDF-1α+TGF-β1 group, e) SDF-1α+PD98059 group, f) TGF-β1+PD98059 group, g) SDF-1α+TGF-β1+AMD3100 group, h) SDF-1α+TGF-β1+PD98059 group. *P<0.05 compared with control group. **P<0.05. Magnification: 100×. (B) With PD98059, SB202190 or SIS3, effect of TGF-β1 on SDF-1α-induced SDSCs migration was detected by qRT-PCR *P<0.05 compared with the control group. **P<0.05. (C) With PD98059, effects of SDF-1α and TGF-β1 on Vimentin and CXCR4 expression were detected by western blot analysis. ImageJ software was used to quantifiy bands.
To further confirm the role of ERK signal on the TGF-β1-enhanced migration, SDSCs were pre-treated with PD98059 (10 μM) before added to the upper chamber for migration assay. The administration of this ERK inhibitor significantly suppressed the effect of TGF-β1 on the migration of SDSCs whereas the migrated cell number was still more than that in SDF-1α group (Figure 2A). qRT-PCR analysis showed that PD98059 significantly inhibited the CXCR4 expression (P<0.05). In contrast, this effect was not observed with other inhibitors including Smad3 inhibitor (SIS3) and p38 MAPK inhibitor (SB202190) (Figure 2B). The western blot analysis confirmed that TGF-β1 remarkably augmented the expression of CXCR4 compared with that in SDF-1α group, which however, was significantly attenuated by the ERK inhibitor PD98059 (Figure 2C). These results indicate that TGF-β1 drives SDSCs migration through the activation of CXCR4, which is ERK-dependent.
SDF-1α enhances the TGF-β1-induced chondrogenesis through the activation of JNK pathway
To determine whether SDF-1α has an effect on TGF-β1-induced chondrogenesis, different concentrations (20, 50 and 100 ng/mL) of SDF-1α were added into the chondrogenic culture medium. After 21 days of culture, the addition of SDF-1α resulted in an enhanced chondrogenesis in a concentration-dependent manner, evidenced by the increase in pellet sizes, sulfated GAGs and collagen II deposition by Alcian Blue and immunohistological staining respectively compared with the only TGF-β1 group (Figure 3A). Meanwhile, qRT-PCR results confirmed the significant increase in the expression of COL 2A1, ACAN, as well as Sox9. (P<0.05) (Figure 3B).
Figure 3.
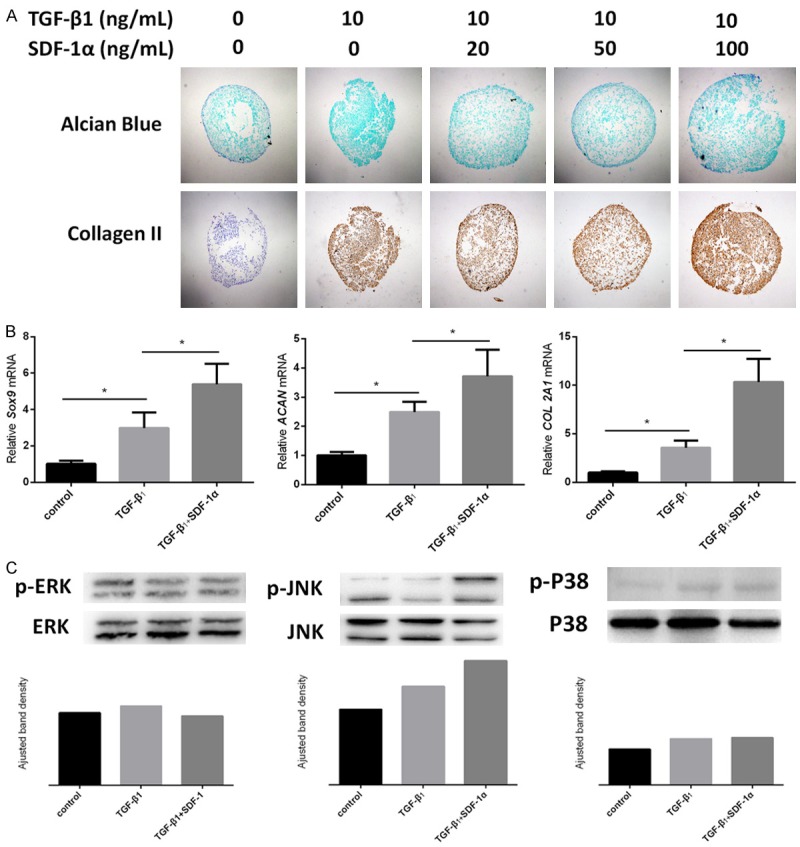
Effect of SDF-1α on TGF-β1-induced SDSCs chondrogenic differentiation. (A) Effects of different concentrations of SDF-1α on TGF-β1 (10 ng/ml)-induced SDSCs chondrogenesis were detected by Alcian blue for sulfated GAGs and immunohistochemical staining for collagen II. Magnification: 40×. (B) qRT-PCR was used to evaluate chondrogenic marker gene expressions (Sox9, ACAN and COL2A1) *P<0.05. (C) Western blot was used to detect the MAPK signals (ERK, JNK and P38) at both phosphorylation and total protein levels. ImageJ software was used to quantifiy bands.
Meanwhile, to explore the involvement of MAPK pathways in this SDF-1α-enhanced chondrogenesis, western blot assay was conducted and the results showed the phosphorylation of JNK instead of ERK or P38 in TGF-β1 group, the expression of which was further increased with the addition of SDF-1α (Figure 3C). These results indicate that SDF-1α enhances TGF-β1-induced chondrogenesis through the activation of JNK pathway. The underlying mechanisms are illustrated in Figure 4.
Figure 4.
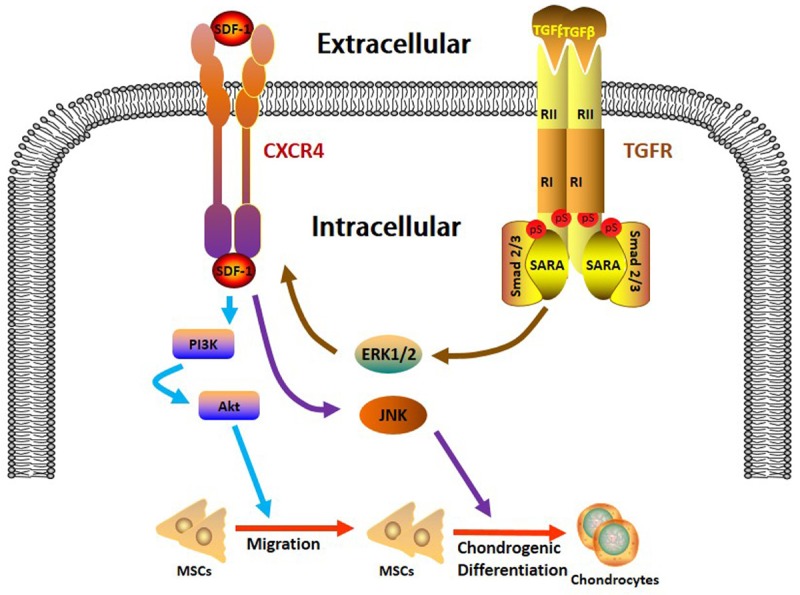
The schematic diagram of the cross-talk between SDF-1α and TGF-β1 during SDSCs migration and chondrogenesis. SDF-1α/CXCR4 axis induces the SDSCs migration through PI3K/Akt signaling pathway (blue arrows). TGF-β1 augments the SDF-1α-driven migration by targeting CXCR4 through the activation of ERK pathway (brown arrows) while SDF-1α facilitates the TGF-β1-induced chondrogenesis through JNK activation (purple arrows).
Discussion
Cell guidance is an attractive approach to tissue engineering. This technique seeks to induce progenitor cells in circulation to cytokine-impregnated scaffolds and facilitate tissue formation without causing additional donor site injury [1]. In this study, SDSCs were used as the target cell for cartilage regeneration in vitro. Synovium exists intra-articularly and has the intimate relationship with articular cartilage both in development and in function. Synovial cells share common properties with chondrocytes including cartilage oligomeric matrix, link protein, and sulfated GAGs [18]. Hence, SDSCs possess a better chondrogenic capacity than cells derived from bone marrow, adipose tissue and periosteum [19].
Under appropriate stimulatory conditions, synovial cells are able to migrate into articular cartilage defects. In our study, SDSCs migration was observably driven by SDF-1α, as the migrated cell number in SDF-1α treated group was significantly increased than that in control group. In neural-like cells and heart myocardial infarction model, SDF-1/CXCR4 mediated cell migration toward injured areas through the activation of PI3K/Akt pathway [6,20]. Similarly in our study, PI3K/Akt pathway was found downstream CXCR4 in SDF-1α-induced migration. Interestingly, the number of migrated cells in group pretreated with LY294002, a PI3K inhibitor, was still significantly more than that in control group, implying the involvement of other signaling pathways downstream CXCR4 to facilitate the SDF-1α-driven migration.
After cell recruitment, chondrogenic differentiation is another pivotal step for functional cartilage tissue engineering. TGF-β1 is the traditional induction factor for chondrogenesis. In our study, TGF-β1-pretreated SDSCs exhibited a further augmented migration capacity as evidenced by the increased number of chemo-attracted cells, which was nearly 4 folds and 2 folds compared with that in control group and SDF-1α alone group. It is in accordance with the previous study demonstrating TGF-β1 in combination with SDF-1α led to a more efficient BMSCs migration [21]. Additionally, the expression of CXCR4 in TGF-β1 pretreated group was significantly higher than that in only SDF-1α treated group and AMD3100 administration almost completely abolished the TGF-β1 effect, indicating CXCR4 a target downstream TGF-β1 and SDF-1α interaction. TGF-β1-enhanced CXCR4 expression was also reported in choroid-retinal endothelial cells [22] and in bone marrow-derived stem cells (BMSCs) [23]. The expression of CXCR4 is quite limited (2-17%) and diminishes in culture-expanded MSCs, given the mobilization of this homing receptor a therapeutic method to facilitate progenitor cells migration [24,25]. Cytokines released during inflammation, including the tumor necrosis factor, vascular endothelial growth factor and insulin-like growth factor 1 were capable of inducing CXCR4 expression and facilitating the following repair process [26,27]. Thus, in this tissue repair process, it is possible for TGF-β1, this multi-potent factor to assist in the SDF-1α-driven migration by targeting CXCR4. Meanwhile, the expression of Vimentin, a typical diagnostic maker for tumor metastasis was elevated after TGF-β1 stimulation in our study. This type III intermediate filament protein was recently demonstrated to induce tumor cell invasion [28]. Moreover, Vimentin was highly-expressed in MSCs and was proved to be positively associated with MSC migration. The overexpression of Vimentin in umbilical cord mesenchymal stem cells (UC-MSCs) promoted migration while the knockdown of Vimentin in BM-MSCs limited their migration ability [29]. In this scenario, the increased expression of Vimentin may be related with the increased migration capacity of TGF-β1 pretreated SDSCs. Furthermore, we first elucidate that the TGF-β1-augmented CXCR4 expression is mediated by the phosphorylation of ERK pathway. The inhibition of ERK by PD98059 significantly reduced the migrated SDSCs in TGF-β1 combined SDF-1α group, the number of which was even lower than that in SDF-1α group. These results are in accordance with the western blot and qRT-PCR results that the suppression of ERK almost abolished the positive effect of SDF-1α and TGF-β1 on CXCR4 and Vimentin expressions. Interestingly, PD98059 administration even partially abrogated the effect of SDF-1α on SDSCs migration as the number of migrated cells was reduced compared with SDF-1α group, implying the possible role of ERK in SDF-1α-induced CXCR4 expression. In studies referring to oncology, the downregulation of ERK signaling pathway has been the target for controlling cancer cell migration and invasion including in human colon cancer cells [30], pantreatic cancer cells [31], and human melanoma A375 cells [32]. It is reasonable for ERK to present as an indispensable point in TGF-β1-enhanced SDSCs migration so as to benefit the subsequent cartilage restoration.
More interestingly, we firstly demonstrate that SDF-1α could also facilitate the TGF-β1-induced chondrogenesis. The chondrogenic pellets treated with TGF-β1 in combination with SDF-1α demonstrated larger sizes, more sulfated GAGs and collagen II deposition and higher chondrogenic marker genes expression. This finding subverts the conventional role of SDF-1α as a chemotaxis factor. In a previous study, a scaffold loaded with SDF-1α and TGF-β1 was transplanted into a rat model for the purpose of site-directed cartilage tissue engineering. Similar to our results, the combination of SDF-1α and TGF-β1 delivery led to the enhanced chondrogenesis as the higher density of cellular infiltrate and formation of differentiated tissue compared with specimen treated with only SDF-1α [33]. However, whether TGF-β1 or both SDF-1α and TGF-β1 were responsible for the enhanced chondrogenesis was not clear as the underlying mechanism was not further explored. In our study, we show that SDF-1α contributes to the TGF-β1-induced chondrogenesis, making it more reasonable to apply the combination of these two cytokines together for functional cartilage tissue engineering. However, in a study of in situ recruitment of human BMSCs for cartilage regerentaion, neither macrophage inflammatory protein-3 (MIP-3α) nor interleukin-8 (IL-8), two chemokines proven to be more effective in chemotaxis than SDF-1 in the same study, influenced the osteoblast differentiation or chondrogenesis of BMSCs [34]. This discrepancy may probably due to the diffenrent cell types and chemokine used. Regarding to the downstream signaling pathway of SDF-1α, we have found that JNK, instead of ERK or p38 was involved. JNK phosphorylation acted downstream cytoskeletal regulatory proteins RhoA/Rac1 pathway in the pressure enhanced chondrogenic and osteogenic differentiation of BMSCs [35]. Meanwhile, JNK was also related to the reorganization of the actin cytoskeleton, which is essential for chondrogenic differentiation [36]. In this scenario, the upregulation of p-JNK in our pellets upon SDF-1α stimulation may be associated with the cytoskeletal rearrangement during chondrogenic differentiation.
Conclusion
In conclusion, our work elucidates that SDF-1α/CXCR4 axis promotes SDSCs migration through PI3K/Akt signaling pathway. Meanwhile, TGF-β1 enhances this SDF-1α-driven migration by targeting CXCR4 through ERK activation while SDF-1α facilitates the TGF-β1-induced chondrogenesis through JNK activation. These information could help us better understand the mechanisms of SDSCs-based cell guidance therapy before the performance of further in vivo study for the purpose of achieving functional cartilage tissue engineering. However, it is worth noticing that the optimal TGF-β1 concentration for SDF-1α-induced migration is not exactly the same as the traditional recommended dose for chondrogenesis, which needs to be further studied and optimized in the future.
Acknowledgements
All of the authors thank Dr. Yuan Chu for his help in the experiment. This work is supported by grants from the National Natural Science Foundation of China (No. 81672142).
Disclosure of conflict of interest
None.
References
- 1.Schantz JT, Chim H, Whiteman M. Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue Eng. 2007;13:2615–2624. doi: 10.1089/ten.2006.0438. [DOI] [PubMed] [Google Scholar]
- 2.Ando W, Kutcher JJ, Krawetz R, Sen A, Nakamura N, Frank CB, Hart DA. Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage. Cytotherapy. 2014;16:776–788. doi: 10.1016/j.jcyt.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin Orthop Relat Res. 2015;473:2316–2326. doi: 10.1007/s11999-015-4324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurth TB, Dell’accio F, Crouch V, Augello A, Sharpe PT, De Bari C. Functional mesenchymal stem cell niches in adult mouse knee joint synovium in vivo. Arthritis Rheum. 2011;63:1289–1300. doi: 10.1002/art.30234. [DOI] [PubMed] [Google Scholar]
- 5.Si XY, Li JJ, Yao T, Wu XY. Transforming growth factor-beta1 in the microenvironment of ischemia reperfusion-injured kidney enhances the chemotaxis of mesenchymal stem cells to stromal cell-derived factor-1 through upregulation of surface chemokine (C-X-C motif) receptor 4. Mol Med Rep. 2014;9:1794–1798. doi: 10.3892/mmr.2014.1989. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Li M, Qu Z, Yan D, Li D, Ruan Q. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55:496–505. doi: 10.1097/FJC.0b013e3181d7a384. [DOI] [PubMed] [Google Scholar]
- 7.Yellowley C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. Bonekey Rep. 2013;2:300. doi: 10.1038/bonekey.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu C, Yong X, Li C, Lu M, Liu D, Chen L, Hu J, Teng M, Zhang D, Fan Y, Liang G. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res. 2013;183:427–434. doi: 10.1016/j.jss.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood. 2006;107:4589–4596. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Li J, Davis ME, Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater. 2015;20:39–50. doi: 10.1016/j.actbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Pizzute T, Li J, He F, Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment - A feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials. 2015;64:88–97. doi: 10.1016/j.biomaterials.2015.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater. 2011;22:302–319. doi: 10.22203/ecm.v022a23. discussion 319-320. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T, Chosa N, Asakawa T, Yoshimura Y, Fujihara Y, Kitamura T, Tanaka M, Ishisaki A, Mitome M. Differential effects of TGF-beta1 and FGF-2 on SDF-1alpha expression in human periodontal ligament cells derived from deciduous teeth in vitro. Int J Mol Med. 2012;30:35–40. doi: 10.3892/ijmm.2012.957. [DOI] [PubMed] [Google Scholar]
- 14.Guang LG, Boskey AL, Zhu W. Regulatory role of stromal cell-derived factor-1 in bone morphogenetic protein-2-induced chondrogenic differentiation in vitro. Int J Biochem Cell Biol. 2012;44:1825–1833. doi: 10.1016/j.biocel.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35:642–653. doi: 10.1016/j.biomaterials.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 16.Fan W, Li J, Wang Y, Pan J, Li S, Zhu L, Guo C, Yan Z. CD105 promotes chondrogenesis of synovium-derived mesenchymal stem cells through Smad2 signaling. Biochem Biophys Res Commun. 2016;474:338–344. doi: 10.1016/j.bbrc.2016.04.101. [DOI] [PubMed] [Google Scholar]
- 17.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 18.Zhou C, Zheng H, Seol D, Yu Y, Martin JA. Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res. 2014;32:981–988. doi: 10.1002/jor.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18:301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Deng L, Gong L, Bian H, Dai Y, Wang Y. Upregulation of CXCR4 favoring neural-like cells migration via AKT activation. Neurosci Res. 2010;67:293–299. doi: 10.1016/j.neures.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Wobus M, List C, Dittrich T, Dhawan A, Duryagina R, Arabanian LS, Kast K, Wimberger P, Stiehler M, Hofbauer LC, Jakob F, Ehninger G, Anastassiadis K, Bornhauser M. Breast carcinoma cells modulate the chemoattractive activity of human bone marrow-derived mesenchymal stromal cells by interfering with CXCL12. Int J Cancer. 2015;136:44–54. doi: 10.1002/ijc.28960. [DOI] [PubMed] [Google Scholar]
- 22.Feng YF, Yuan F, Guo H, Wu WZ. TGF-beta1 enhances SDF-1-induced migration and tube formation of choroid-retinal endothelial cells by up-regulating CXCR4 and CXCR7 expression. Mol Cell Biochem. 2014;397:131–138. doi: 10.1007/s11010-014-2180-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SJ, Song XY, He M, Yu SB. Effect of TGF-beta1/SDF-1/CXCR4 signal on BM-MSCs homing in rat heart of ischemia/perfusion injury. Eur Rev Med Pharmacol Sci. 2016;20:899–905. [PubMed] [Google Scholar]
- 24.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 25.Uchida D, Onoue T, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H, Miyamoto Y. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. Eur J Cancer. 2011;47:452–459. doi: 10.1016/j.ejca.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, Ito A, Misener S, Tanaka T, Klyachko E, Kobayashi K, Tongers J, Roncalli J, Tsurumi Y, Hagiwara N, Losordo DW. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Ye C, Wang G, Gao Y, Tan K, Zhuo Z, Liu Z, Xia H, Yang D, Li P. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. Biomed Res Int. 2013;2013:526367. doi: 10.1155/2013/526367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013;25:600–612. doi: 10.1016/j.ceb.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L, Niu C, Willard B, Zhao W, Liu L, He W, Wu T, Yang S, Feng S, Mu Y, Zheng L, Li K. Proteomic analysis of porcine mesenchymal stem cells derived from bone marrow and umbilical cord: implication of the proteins involved in the higher migration capability of bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6:77. doi: 10.1186/s13287-015-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han S, Bui NT, Ho MT, Kim YM, Cho M, Shin DB. Dexamethasone inhibits TGF-beta1-induced cell migration by regulating the ERK and AKT pathways in human colon cancer cells via CYR61. Cancer Res Treat. 2016;48:1141–1153. doi: 10.4143/crt.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao L, Chen X, Xiao X, Ma Q, Li W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int J Oncol. 2016;49:735–743. doi: 10.3892/ijo.2016.3559. [DOI] [PubMed] [Google Scholar]
- 32.Mao XH, Chen M, Wang Y, Cui PG, Liu SB, Xu ZY. MicroRNA-21 regulates the ERK/NF-kappaB signaling pathway to affect the proliferation, migration, and apoptosis of human melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol Carcinog. 2017;56:886–894. doi: 10.1002/mc.22542. [DOI] [PubMed] [Google Scholar]
- 33.Chim H, Miller E, Gliniak C, Alsberg E. Stromal-cell-derived factor (SDF) 1-alpha in combination with BMP-2 and TGF-beta1 induces site-directed cell homing and osteogenic and chondrogenic differentiation for tissue engineering without the requirement for cell seeding. Cell Tissue Res. 2012;350:89–94. doi: 10.1007/s00441-012-1449-x. [DOI] [PubMed] [Google Scholar]
- 34.Park MS, Kim YH, Jung Y, Kim SH, Park JC, Yoon DS, Kim SH, Lee JW. In situ recruitment of human bone marrow-derived mesenchymal stem cells using chemokines for articular cartilage regeneration. Cell Transplant. 2015;24:1067–1083. doi: 10.3727/096368914X681018. [DOI] [PubMed] [Google Scholar]
- 35.Zhao YH, Lv X, Liu YL, Zhao Y, Li Q, Chen YJ, Zhang M. Hydrostatic pressure promotes the proliferation and osteogenic/chondrogenic differentiation of mesenchymal stem cells: the roles of RhoA and Rac1. Stem Cell Res. 2015;14:283–296. doi: 10.1016/j.scr.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Kim D, Song J, Kim S, Park HM, Chun CH, Sonn J, Jin EJ. MicroRNA-34a modulates cytoskeletal dynamics through regulating RhoA/Rac1 cross-talk in chondroblasts. J Biol Chem. 2012;287:12501–12509. doi: 10.1074/jbc.M111.264382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


