Summary
Background
Post-partum haemorrhage is the leading cause of maternal death worldwide. Early administration of tranexamic acid reduces deaths due to bleeding in trauma patients. We aimed to assess the effects of early administration of tranexamic acid on death, hysterectomy, and other relevant outcomes in women with post-partum haemorrhage.
Methods
In this randomised, double-blind, placebo-controlled trial, we recruited women aged 16 years and older with a clinical diagnosis of post-partum haemorrhage after a vaginal birth or caesarean section from 193 hospitals in 21 countries. We randomly assigned women to receive either 1 g intravenous tranexamic acid or matching placebo in addition to usual care. If bleeding continued after 30 min, or stopped and restarted within 24 h of the first dose, a second dose of 1 g of tranexamic acid or placebo could be given. Patients were assigned by selection of a numbered treatment pack from a box containing eight numbered packs that were identical apart from the pack number. Participants, care givers, and those assessing outcomes were masked to allocation. We originally planned to enrol 15 000 women with a composite primary endpoint of death from all-causes or hysterectomy within 42 days of giving birth. However, during the trial it became apparent that the decision to conduct a hysterectomy was often made at the same time as randomisation. Although tranexamic acid could influence the risk of death in these cases, it could not affect the risk of hysterectomy. We therefore increased the sample size from 15 000 to 20 000 women in order to estimate the effect of tranexamic acid on the risk of death from post-partum haemorrhage. All analyses were done on an intention-to-treat basis. This trial is registered with ISRCTN76912190 (Dec 8, 2008); ClinicalTrials.gov, number NCT00872469; and PACTR201007000192283.
Findings
Between March, 2010, and April, 2016, 20 060 women were enrolled and randomly assigned to receive tranexamic acid (n=10 051) or placebo (n=10 009), of whom 10 036 and 9985, respectively, were included in the analysis. Death due to bleeding was significantly reduced in women given tranexamic acid (155 [1·5%] of 10 036 patients vs 191 [1·9%] of 9985 in the placebo group, risk ratio [RR] 0·81, 95% CI 0·65–1·00; p=0·045), especially in women given treatment within 3 h of giving birth (89 [1·2%] in the tranexamic acid group vs 127 [1·7%] in the placebo group, RR 0·69, 95% CI 0·52–0·91; p=0·008). All other causes of death did not differ significantly by group. Hysterectomy was not reduced with tranexamic acid (358 [3·6%] patients in the tranexamic acid group vs 351 [3·5%] in the placebo group, RR 1·02, 95% CI 0·88–1·07; p=0·84). The composite primary endpoint of death from all causes or hysterectomy was not reduced with tranexamic acid (534 [5·3%] deaths or hysterectomies in the tranexamic acid group vs 546 [5·5%] in the placebo group, RR 0·97, 95% CI 0·87-1·09; p=0·65). Adverse events (including thromboembolic events) did not differ significantly in the tranexamic acid versus placebo group.
Interpretation
Tranexamic acid reduces death due to bleeding in women with post-partum haemorrhage with no adverse effects. When used as a treatment for postpartum haemorrhage, tranexamic acid should be given as soon as possible after bleeding onset.
Funding
London School of Hygiene & Tropical Medicine, Pfizer, UK Department of Health, Wellcome Trust, and Bill & Melinda Gates Foundation.
Introduction
Primary post-partum haemorrhage, usually defined as a blood loss of more than 500 mL within 24 h of giving birth, is the leading cause of maternal death worldwide, responsible for about 100 000 deaths every year.1, 2, 3 Most of the deaths occur soon after giving birth and almost all (99%) occur in low-income and middle-income countries.4, 5
Tranexamic acid reduces bleeding by inhibiting the enzymatic breakdown of fibrinogen and fibrin by plasmin.6 Findings of a systematic review of clinical trials of tranexamic acid in surgery showed that the drug reduces blood loss by about one third.7, 8 Tranexamic acid reduces death due to bleeding in patients with trauma. The CRASH-2 trial,9 which recruited 20 211 adults with acute traumatic bleeding, showed that tranexamic acid reduced death due to bleeding, with no apparent increase in vascular occlusive events. Planned subgroup analysis of the effect of tranexamic acid by time from injury to the start of treatment showed that early treatment is essential. In patients given treatment within 3 h of injury, tranexamic acid reduced death due to bleeding by nearly one third. However, when given after 3 h, there was no benefit.10 Early activation of fibrinolysis is common after trauma and is associated with increased mortality.11 Trauma triggers the release of tissue plasminogen activator, the enzyme that converts plasminogen to the fibrinolytic enzyme plasmin.12, 13
Research in context.
Evidence before this study
Before the WOMAN trial, there was evidence that antifibrinolytics reduced surgical blood loss and re-operation to control bleeding. To assess the effects of anti-fibrinolytics in post-partum haemorrhage, we did a systematic review of randomised trials published in any language. We searched the following databases up to November, 2008: MEDLINE, PubMed, Embase, Cochrane Central Register of Controlled trials, Web of Science, metaRegister of controlled trials, LILACS, Reproductive Health Library, African Health-line, POPLINE, MedCarib, CINAHL, Clinicaltrials.gov, and the reference lists of eligible trials. Search terms have been published previously (Ferrer P, 2009). We found no trials of antifibrinolytics in post-partum haemorrhage. In 2010, while the WOMAN trial was underway, findings of an international multicentre randomised trial of 20 211 bleeding trauma patients (the CRASH-2 trial) showed that tranexamic acid reduced death due to bleeding with no apparent increase in vascular occlusive events. Subgroup analyses showed that tranexamic acid was only effective when given within 3 h of injury. In 2012, WHO guidelines recommended that tranexamic acid should be used for the treatment of post-partum haemorrhage when uterotonics fail to control the bleeding or when the bleeding is thought to be due to trauma. The evidence for this recommendation was extrapolated from trials in surgery and trauma showing that tranexamic acid was a safe option for the treatment of trauma-related bleeding.
Added value of this study
The WOMAN trial results show that the effect of tranexamic acid in post-partum haemorrhage is consistent with the effects recorded in surgery and trauma. There was a significant reduction in death due to bleeding and laparotomy to control bleeding with tranexamic acid and no evidence of any increased risk of thromboembolic events. With regards to time to treatment, when set in the context of results from trauma, early treatment also seems to be more effective. There is no evidence that the effect of tranexamic acid varies by cause of bleeding or type of birth. Tranexamic acid did not prevent hysterectomy possibly because this is done so soon after the onset of primary post-partum haemorrhage that there is little time for tranexamic acid to have an effect.
Implications of all the available evidence
Our results support the inclusion of tranexamic acid in WHO treatment guidelines for primary post-partum haemorrhage but suggest that treatment should be given as soon as possible after onset. Future research should assess the bioavailability of tranexamic acid after alternative (non-intravenous) routes of administration because this might facilitate its use in primary health-care settings.
Early activation of fibrinolysis is also recorded after childbirth. Within 1 h of giving birth, the serum concentration of tissue plasminogen activator doubles, possibly because of tissue damage during childbirth;14 thereafter, the concentration falls.14 On the basis of results of clinical trials in surgery and trauma, tranexamic acid is recommended for the treatment of primary post-partum haemorrhage if uterotonics fail to control the bleeding or if the bleeding is thought to be due to trauma.1 However, further trials of tranexamic acid in primary post-partum haemorrhage are needed.1 Here we aimed to address this research gap and assess the effects of early administration of tranexamic acid on death, hysterectomy, and other relevant outcomes in women with post-partum haemorrhage.
Methods
Study design and participants
The WOMAN (World Maternal Antifibrinolytic) trial is an international, randomised, double-blind placebo-controlled trial of women aged 16 years and older with a clinical diagnosis of post-partum haemorrhage after a vaginal birth or caesarean section done in 193 hospitals in 21 countries. Although the diagnosis was clinical, we specified that diagnosis of primary post-partum haemorrhage could be based on clinically estimated blood loss of more than 500 mL after vaginal birth or 1000 mL after caesarean section or any blood loss sufficient to compromise haemodynamic stability. The fundamental eligibility criterion was the clinician's uncertainty about whether to use tranexamic acid in a particular woman with post-partum haemorrhage. Patients received all usual care but were also randomly allocated to receive tranexamic acid or placebo.
The trial was done in accordance with the good clinical practice guidelines by the International Conference on Harmonisation.15 The consent procedures are described in detail in the protocol.16 The procedure at each site was approved by the relevant ethics committee and regulatory agencies. In summary, consent was obtained from women if their physical and mental capacity allowed (as judged by the treating clinician). If a woman was unable to give consent, proxy consent was obtained from a relative or representative. If a proxy was unavailable, then if permitted by local regulation, consent was deferred or waived. When consent was deferred or given by a proxy, the woman was informed about the trial as soon as possible, and consent was obtained for ongoing data collection, if needed.
Randomisation and masking
After eligibility was confirmed and consent procedures completed, baseline information was collected on the entry form. Patients were then randomly allocated to receive tranexamic acid or placebo by selection of the lowest numbered treatment pack from a box containing eight numbered packs that were identical apart from the pack number. The randomisation codes were generated and held by an independent statistical consultant from Sealed Envelope Ltd (UK). The codes were given to the drug packers so that treatment packs could be prepared in accordance with the randomisation list. Once the treatment pack was opened and the ampoules were confirmed as intact, the patient was considered to be randomly assigned. After randomisation, outcome data were obtained for every participant even if the treatment was not given. Participants, caregivers, and study staff (site investigators and trial coordinating centre staff) were masked to treatment allocation. An emergency un-blinding service was available via Sealed Envelope Ltd.
The tranexamic acid (cyklokapron injection) used in the trial was manufactured by Pfizer Ltd, Sandwich, UK. The matching placebo (sodium chloride 0·9%) was prepared by South Devon Healthcare NHS Trust, Devon, UK. Ampoules and packaging were identical in appearance. The masking was done by Brecon Pharmaceuticals Limited, Hereford, UK and involved the removal of the original manufacturer's label and replacement with the clinical trial label bearing the randomisation number, which was used as the pack identification. Apart from the randomisation number, all pack label texts were identical for tranexamic acid and placebo. Correct masking and coding of ampoules was checked by independent random testing of each batch by high-performance liquid chromatography to confirm the contents of the ampoules.
Procedures
Patients were randomly allocated to receive 1 g tranexamic acid or placebo by slow intravenous injection. Investigators were advised to give 1 g (100 mg/mL) of tranexamic acid intravenously at an approximate rate of 1 mL per min. If bleeding continued after 30 min or stopped and restarted within 24 h of the first dose, a second dose of 1 g of tranexamic acid or placebo could be given. Every patient was assigned a uniquely numbered treatment pack, containing four ampoules of 500 mg tranexamic acid or placebo, two syringes and needles, stickers with the trial details and randomisation number (for attaching to data forms and medical records) and instructions. Every box had information leaflets for participants and their representatives, consent forms, and data collection forms. The stickers, instructions, leaflets, and forms were translated into local languages. Outcome data were collected at death, discharge or 6 weeks (42 days) after randomisation (whichever occurred first). Adverse events were reported up to day 42.
Outcomes
The primary outcome was a composite of death from all causes or hysterectomy within 42 days of randomisation. Death was also assessed separately. Participating clinicians were requested to record the immediate cause of death (the final pathophysiological process leading to death) rather than the underlying cause of death and were trained accordingly. In the event that there was more than one cause, clinicians were asked to record the main cause. Because there was no reason to believe that tranexamic acid can reduce deaths from causes unrelated to bleeding, we planned to assess the effect of tranexamic acid on cause-specific mortality with death due to bleeding as the key secondary outcome. Other secondary outcomes were thromboembolic events (deep-vein thrombosis, pulmonary embolism, myocardial infarction, and stroke), surgical interventions (intrauterine tamponade, embolisation, brace sutures, arterial ligation, hysterectomy, and laparotomies done after randomisation to control bleeding and achieve haemostasis), complications (renal failure, cardiac failure, respiratory failure, hepatic failure, sepsis, and seizures), other untoward medical events (adverse events), quality of life measured using the EQ5D and status of any thromboembolic events in breastfed babies (assessed as per normal clinical practice with no special tests done). Outcomes were measured at hospital discharge or on day 42 if still in hospital. Data were sent to the trial coordinating centre by direct entry into an electronic database or by using encrypted data forms (which were sent by fax, email, or uploaded to a secure server). We monitored data quality using a combination of centralised consent monitoring, statistical data checking, and site visits at which patient data forms were compared with clinical case notes.
Statistical analysis
We published a statistical analysis plan before the allocation was unblinded.18 This plan included details of a protocol amendment to increase the sample size. Before the trial started, we anticipated a baseline event rate of 2·5% for death and 2·5% for hysterectomy. Assuming a control group event rate of 2·5% for death and 2·5% for hysterectomy and that 1% of women die after hysterectomy, we originally estimated that a trial with 15 000 women would have 90% power to detect a 25% reduction (from 4–3%) in the composite primary endpoint death or hysterectomy at the 5% significance level. However, during the trial it became apparent that the decision to conduct a hysterectomy was often made at the same time as randomisation. For example, in response to life-threatening bleeding during caesarean section, a clinician might decide to do a hysterectomy, and while the hysterectomy is underway, the woman is enrolled into the trial. Although tranexamic acid could affect the risk of death in these cases, it could not affect the risk of hysterectomy. To protect against the possibility that the effect of tranexamic acid on death and hysterectomy was different, the sample size was increased from 15 000 to 20 000 women. We estimated that a trial with 20 000 women should have sufficient power to detect a 25% reduction in mortality at the 5% significance level (3–2·25%). We hoped that the increased sample size might compensate for the dilution of the treatment effect from hysterectomies that were done at the same time as randomisation. We also refined the study hypothesis in view of new evidence that had become available since the trial was initiated. In particular, findings of the CRASH-2 trial10 had shown that tranexamic acid reduces death due to bleeding in trauma patients and that early treatment was more effective, with strong evidence of an interaction by time to treatment. In response, we pre-specified an analysis of cause-specific mortality with death due to bleeding as the main outcome. We also pre-specified subgroup analyses by time to treatment. These changes were made before un-blinding and without any knowledge of the trial results.
All analyses were done on an intention-to-treat basis. For each binary outcome, we calculated risk ratios and 95% CIs and two-sided p values. We did a complete case analysis with no imputation for missing data. To mitigate the risk that a chance imbalance in prognostic factors could affect the results, for the primary endpoint (death or hysterectomy) and the most important secondary endpoint (death due to bleeding), we pre-specified an analysis adjusted for baseline risk. The safety of trial participants was overseen by an independent data safety and monitoring committee, which reviewed seven un-blinded interim analyses.
We planned to report the effects of treatment on the primary outcome subdivided by three baseline characteristics: hours from giving birth to randomisation (<1, 1–3, >3 h); type of birth (vaginal or caesarean section); and primary cause of haemorrhage (uterine atony vs all others). To examine the hypothesis that tranexamic acid would be most effective when given soon after birth and less effective (possibly even harmful) when given several hours after giving birth, we pre-specified a subgroup analysis of the effect of tranexamic acid on death due to bleeding according to the time interval between giving birth and tranexamic acid treatment. The main analysis for the pre-specified subgroups was an unadjusted test of interaction in a logistic regression model to assess evidence for whether the effect of treatment differs across subgroup categories. Unless there was strong evidence against the null hypothesis of homogeneity of effects (ie, p<0·001), the overall relative risk was regarded as the most reliable guide to the approximate relative risks in all subgroups. However, because there was strong prior evidence to expect a time to treatment interaction, we pre-specified that we would set the results of this analysis in the context of all available data on the time to treatment interaction.18
After publication of the planned primary and secondary analyses, the trial data will be made available via our data sharing portal, The Free Bank of Injury and Emergency Research Data (freeBIRD) website. This will allow for maximum utilisation of the data to improve patient care and advance medical knowledge.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
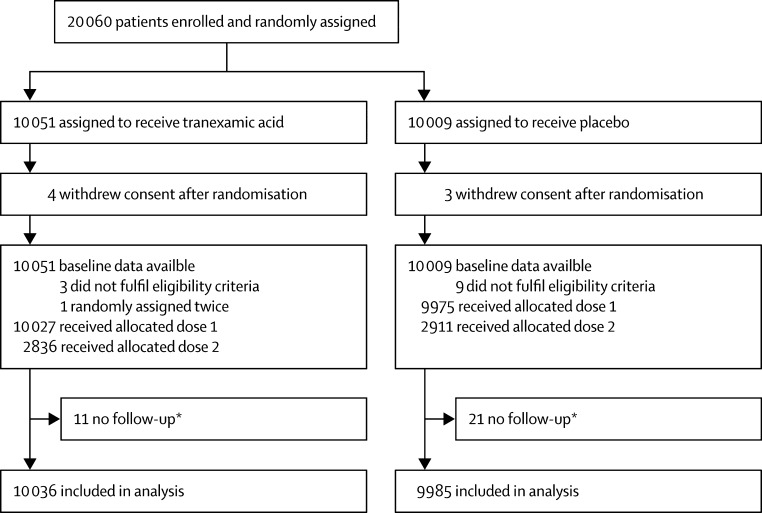
Between March, 2010, and April, 2016, 20 060 women were enrolled and randomly assigned to receive tranexamic acid (n=10 051) or placebo (n=10 009), of whom 20 002 (99·7%) received the first dose of the allocated treatment (10 037 received tranexamic acid and 9975 received placebo; figure 1). Seven women withdrew their consent after randomisation and we excluded their data from the analyses (four in the tranexamic acid group and three in the placebo group). We were unable to obtain primary outcome data for 32 women and 12 patients did not fulfil the trial eligibility criteria. One patient in the tranexamic acid group was randomly assigned twice. The primary analysis includes data for 20 021 (99·8%) women. The baseline characteristics were similar between the treatment groups (table 1).
Figure 1.
Trial profile
*Patients for whom there is no information about the primary endpoint.
Table 1.
Baseline characteristics of participants before randomisation
| Tranexamic acid group (n=10 051) | Placebo group (n=10 009) | |
|---|---|---|
| Age at randomisation (years) | ||
| <16 | 1 (<1%) | 3 (<1%) |
| 16–25 | 3445 (34%) | 3407 (34%) |
| 26–33 | 4580 (46%) | 4608 (46%) |
| ≥34 | 2022 (20%) | 1987 (20%) |
| Unknown | 3 (<1%) | 4 (<1%) |
| Baby delivered in the randomising hospital | ||
| Yes | 8869 (88%) | 8756 (88%) |
| No | 1181 (12%) | 1251 (13%) |
| Unknown | 1 (<1%) | 2 (<1%) |
| Type of delivery | ||
| Vaginal | 7093 (71%) | 7126 (71%) |
| Caesarean section | 2957 (29%) | 2879 (29%) |
| Unknown | 1 (<1%) | 4 (<1%) |
| Time between delivery and randomisation (h) | ||
| ≤1 | 4852 (48%) | 4733 (47%) |
| >1 to ≤3 | 2678 (27%) | 2691 (27%) |
| >3 | 2517 (25%) | 2574 (26%) |
| Unknown | 4 (<1%) | 11 (<1%) |
| Placenta fully delivered | ||
| Yes | 9089 (90%) | 9016 (90%) |
| No | 962 (10%) | 990 (10%) |
| Primary cause of haemorrhage | ||
| Uterine atony | 6437 (64%) | 6347 (63%) |
| Placenta praevia or accreta | 943 (9%) | 935 (9%) |
| Surgical trauma or tears | 1834 (18%) | 1857 (19%) |
| Other | 720 (7%) | 737 (7%) |
| Unknown | 117 (1%) | 133 (1%) |
| Systolic blood pressure (mm Hg) | ||
| ≥90 | 8138 (81%) | 8065 (81%) |
| <90 | 1908 (19%) | 1929 (19%) |
| Unknown | 5 (<1%) | 15 (<1%) |
| Estimated volume of blood lost (mL) | ||
| ≤500 | 295 (3%) | 313 (3%) |
| >500 to ≤1000 | 4949 (49%) | 4861 (49%) |
| >1000 to ≤1500 | 2832 (28%) | 2882 (29%) |
| >1500 | 1973 (20%) | 1953 (20%) |
| Unknown | 2 (<1%) | 0 |
| Uterotonic prophylaxis given | ||
| Yes | 9687 (96%) | 9618 (96%) |
| No | 131 (1%) | 139 (1%) |
| Unknown | 233 (2%) | 252 (3%) |
| Clinical signs of haemodynamic instability | ||
| Yes | 5961 (59%) | 5898 (59%) |
| No | 4090 (41%) | 4110 (41%) |
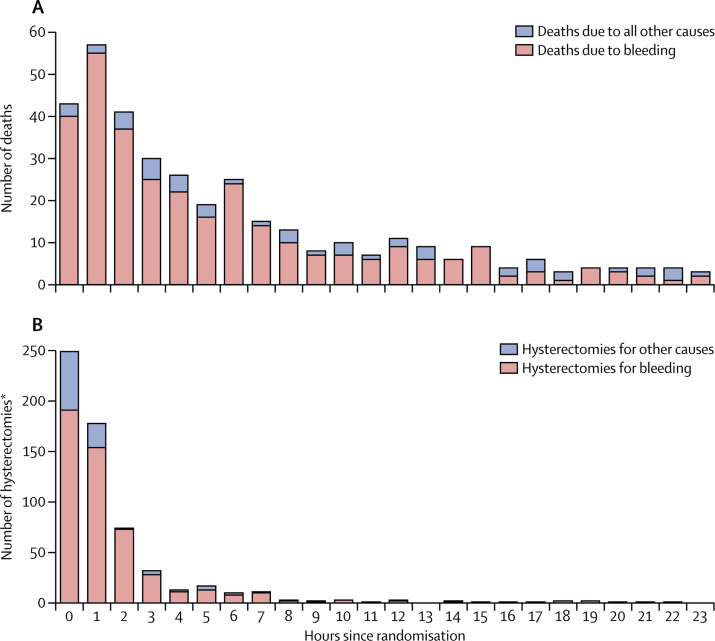
There were 483 maternal deaths of which 374 (77%) were within 24 h of randomisation and 43 (9%) were within 1 h of randomisation (figure 2). The appendix shows the distribution of deaths from hours since childbirth (appendix p 1). 346 (72%) deaths were due to bleeding. Table 2 shows the effect of tranexamic acid on maternal death. The risk of death due to bleeding was significantly reduced in patients who received tranexamic acid (155 [1·5%] of 10 036 vs 191 [1·9%] in the placebo group; risk ratio [RR] 0·81, 95% CI 0·65–1·00; p=0·045). After adjusting for baseline risk, the risk ratio for death due to bleeding with tranexamic acid was 0·78 (95% CI 0·62–0·98; p=0·03). Deaths from pulmonary embolism, organ failure, sepsis, eclampsia and other causes did not differ significantly between the tranexamic acid and the placebo group (table 2). We recorded fewer deaths from all causes with tranexamic acid but the reduction was not significant (227 [2·3%] in the tranexamic acid group vs 256 [2·6%] in the placebo group; RR 0·88, 95% CI 0·74–1·05; p=0·16).
Figure 2.
Cause of death by hours since randomisation (A) and cause of hysterectomy by hours since randomisation (B)
*Excludes data for 311 women who had a hysterectomy before randomisation.
Table 2.
Effect of tranexamic acid on maternal death
| Tranexamic acid group (n=10 036) | Placebo group (n=9985) | RR (95% CI) | p value (two-sided) | |
|---|---|---|---|---|
| Bleeding | 155 (1·5%) | 191 (1·9 %) | 0·81 (0·65–1·00) | 0·045 |
| Pulmonary embolism | 10 (0·1%) | 11 (0·1) | 0·90 (0·38–2·13) | 0·82 |
| Organ failure | 25 (0·3%) | 18 (0·2%) | 1·38 (0·75–2·53) | 0·29 |
| Sepsis | 15 (0·2%) | 8 (0·1%) | 1·87 (0·79–4·40) | 0·15 |
| Eclampsia | 2 (0·02%) | 8 (0·1%) | 0·25 (0·05–1·17) | 0·057 |
| Other | 20 (0·2%) | 20 (0·2%) | 0·99 (0·54–1·85) | 0·99 |
| Any cause of death | 227 (2·3%) | 256 (2·6%) | 0·88 (0·74–1·05) | 0·16 |
Data are n (%), unless otherwise indicated. RR=risk ratio.
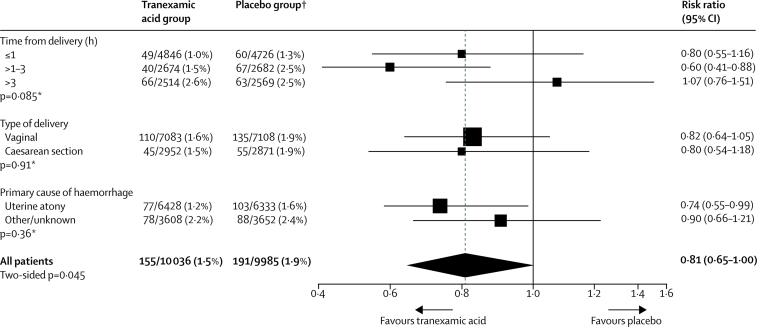
In women given tranexamic acid within 3 h of giving birth, tranexamic acid substantially reduced the risk of death due to bleeding (89 [1·2%] women died in the tranexamic acid group vs 127 [1·7%] in the placebo group, RR 0·69, 95% CI 0·52–0·91; p=0·008). There was no apparent reduction when tranexamic acid was given after 3 h (66 [2·6%] tranexamic acid group vs 63 [2·5%] placebo group, RR 1·07, 95% CI 0·76–1·51; p=0·70). There was no heterogeneity in the effect by type of birth or cause of bleeding (figure 3).
Figure 3.
Death from bleeding by subgroup
*Heterogeneity p value. †One patient excluded from subgroup analysis because of missing baseline data.
709 women had hysterectomies of which 608 (86%) were on the day of randomisation and 191 (27%) were within 1 h of randomisation (figure 2). The appendix shows the distribution of hysterectomy from hours since childbirth (appendix p 1). 578 (81%) of 709 hysterectomies were done to control bleeding. The risk of hysterectomy was not reduced with tranexamic acid (358 [3·6%] done in the tranexamic acid group vs 351 [3·5%] in the placebo group, RR 1·02, 95% CI 0·88–1·07; p=0·84). The risk of hysterectomy to control bleeding was not significantly reduced with tranexamic acid (283 [2·8%] tranexamic acid group vs 295 [3·0%] placebo group, RR 0·95, 95% CI 0·81–1·12; p=0·57).
The primary endpoint of death from all causes or hysterectomy within 42 days of giving birth occurred in 1080 women. Of these, 371 (34%) women died without undergoing a hysterectomy, 112 (10%) died after hysterectomy, and 597 (55%) survived after hysterectomy. The risk of death from all causes or hysterectomy was not reduced with tranexamic acid (534 [5·3%] tranexamic acid group vs 546 [5·6%] placebo group, RR 0·97, 95% CI 0·87–1·09; p=0·65). After adjusting for baseline risk, the risk ratio for death from all causes or hysterectomy was 0·98 (95% CI 0·87–1·10; p=0·75). There was no significant heterogeneity in the effect of tranexamic acid by time to treatment, type of birth or cause of bleeding (table 3).
Table 3.
Effect of tranexamic acid on composite primary endpoint (death or hysterectomy) by subgroup
| Tranexamic acid group | Placebo group | Risk ratio (95% CI) | ||
|---|---|---|---|---|
| Time from delivery (h) | ||||
| ≤1 | 253/4844 (5·2%) | 229/4726 (4·9%) | 1·08 (0·91–1·28) | |
| >1–≤3 | 122/2672 (4·6%) | 154/2682 (5·7%) | 0·80 (0·63–1·00) | |
| >3 | 159/2514 (6·3%) | 161/2569 (6·3%) | 1·01 (0·82–1·25) | |
| p=0·11* | ||||
| Type of delivery | ||||
| Vaginal | 255/7080 (3·6%) | 288/7108 (4·1%) | 0·89 (0·75–1·05) | |
| Caesarean section | 279/2951 (9·5%) | 257/2873 (9·0%) | 1·06 (0·90–1·24) | |
| p=0·15* | ||||
| Primary cause of haemorrhage | ||||
| Uterine atony | 249/6426 (3·9%) | 274/6333 (4·3%) | 0·90 (0·76–1·06) | |
| Other or unknown | 285/3606 (7·9%) | 272/3652 (7·5%) | 1·06 (0·90–1·24) | |
| p=0·15* | ||||
| All patients | 534/10 032 (5·3%) | 546/9985 (5·5%) | 0·97 (0·87–1·09) | |
| Two-sided p=0·65 | ||||
Data are n (%) unless otherwise indicated.
p value from likelihood ratio test.
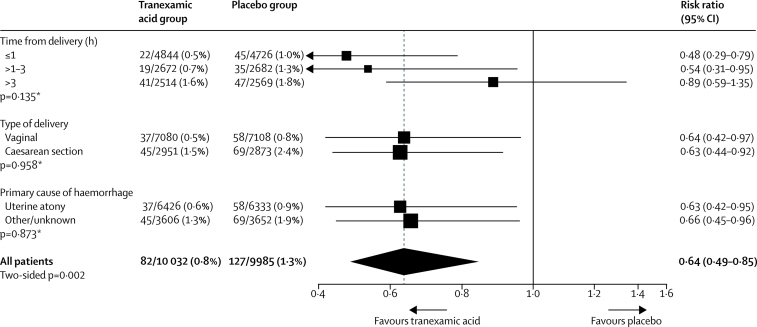
The use of intrauterine tamponade, embolisation, manual removal of the placenta, and arterial ligation did not differ significantly between the tranexamic acid and the placebo group (table 4). Brace sutures were used more often in the tranexamic group (300 [3·0%] tranexamic acid group vs 250 [2·5%] placebo group; RR 1·19, 95% CI 1·01–1·41; p=0·035). 209 laparotomies were done after randomisation to control bleeding and achieve haemostasis of which 114 (55%) followed caesarean section births and 95 (45%) followed vaginal births. There was a significant reduction in laparotomy to control bleeding with tranexamic acid (82 [0·8%] tranexamic acid group vs 127 [1·3%] placebo group; RR 0·64, 95% CI 0·49-0·85; p=0·002). We recorded no significant heterogeneity in the effect of tranexamic acid on laparotomy to control bleeding by time since giving birth, type of birth, or cause of bleeding (figure 4). Blood product transfusions were given to 5461 (54%) of 10 036 patients allocated to tranexamic acid and 5426 (54%) of 9985 women allocated to placebo. Among women who were transfused, the mean number of blood units received did not differ significantly between patients in the tranexamic acid and placebo groups. Of the women who died, 37 (7·7%) did not receive any blood products. Of these, 18 (48·7%) were in the tranexamic acid group and 19 (51·4%) were in the placebo group.
Table 4.
Effect of tranexamic acid on need for surgical intervention
|
All women |
Women who gave birth vaginally |
Women who gave birth by caesarean section |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tranexamic acid group (n=10 032) | Placebo group (n=9985) | RR (95% CI) | p value | Tranexamic acid group (n=7080) | Placebo (n=7108) | RR (95% CI) | p value | Tranexamic acid group (n=2951) | Placebo (n=2873) | RR (95% CI) | p value | |
| Intrauterine tamponade | 705 (7·0%) | 729 (7·3%) | 0·96 (0·87–1·06) | 0·45 | 519 (7·3%) | 547 (7·7%) | 0·95 (0·85–1·07) | 0·41 | 186 (6·3%) | 182 (6·3%) | 0·99 (0·82–1·21) | 0·96 |
| Manual removal of placenta | 918 (9·2%) | 961 (9·6%) | 0·95 (0·87–1·04) | 0·25 | 745 (10·5%) | 779 (11·0%) | 0·96 (0·87–1·06) | 0·40 | 173 (5·9%) | 182 (6·3%) | 0·93 (0·76–1·13) | 0·45 |
| Embolisation | 10 (0·1%) | 13 (0·1%) | 0·77 (0·34–1·75) | 0·52 | 4 (0·06%) | 7 (0·1%) | 0·57 (0·17–1·96) | 0·37 | 6 (0·2%) | 6 (0·2%) | 0·97 (0·31–3·02) | 0·96 |
| Brace sutures | 300 (3·0%) | 250 (2·5%) | 1·19 (1·01–1·41) | 0·035 | 50 (0·7%) | 50 (0·7%) | 1·00 (0·68–1·48) | 0·98 | 250 (8·5%) | 200 (7·0%) | 1·22 (1·02–1·46) | 0·031 |
| Arterial ligation | 225 (2·2%) | 254 (2·5%) | 0·88 (0·74–1·05) | 0·16 | 57 (0·8%) | 65 (0·9%) | 0·88 (0·62–1·25) | 0·48 | 168 (5·7%) | 189 (6·6%) | 0·87 (0·71–1·06) | 0·16 |
| Laparotomy for bleeding | 82 (0·8%) | 127 (1·3%) | 0·64 (0·49–0·85) | 0·002 | 37 (0·5%) | 58 (0·8%) | 0·64 (0·42–0·97) | 0·032 | 45 (1·5%) | 69 (2·4%) | 0·63 (0·44–0·92) | 0·016 |
Data are n (%), unless otherwise indicated. RR=relative risk. p values from Pearson's χ2 test.
Figure 4.
Laparotomy for bleeding by subgroup
*Heterogeneity p value.
The incidence of thromboembolic events (pulmonary embolism, deep-vein thrombosis, myocardial infarction, and stroke) did not differ significantly in the tranexamic acid versus the placebo group (table 5). The risk of organ failure (renal, cardiac, respiratory, and hepatic) and sepsis did not differ significantly between the tranexamic acid and the placebo group. 33 (0·33%) women in the tranexamic acid group had a seizure versus 43 (0·43%) in the placebo group. Eight women in the tranexamic acid group suffered the death of a breast-fed baby compared with seven women in the placebo group. No thromboembolic events were reported in breast-fed babies in either group. Of women who survived, there were no significant differences in quality of life measures. Of the women who survived, four (<1%) did not have a quality of life measure completed. 57 additional adverse events were reported (appendix p 2).
Table 5.
Effect of tranexamic acid on thromboembolic events, complications, use of uterotonics, and quality of life
| Tranexamic acid group | Placebo group | RR (95% CI) | p value | |||
|---|---|---|---|---|---|---|
| Thromboembolic events* | 10 033 | 9985 | .. | |||
| Any event | 30 (0·3%) | 34 (0·3%) | 0·88 (0·54–1·43) | 0·603 | ||
| Venous events | 20 (0·2%) | 25 (0·3%) | 0·80 (0·44–1·43) | 0·446 | ||
| Deep vein thrombosis | 3 (0·03%) | 7 (0·07%) | 0·43 (0·11–1·65) | 0·203 | ||
| Pulmonary embolism | 17 (0·2%) | 20 (0·2%) | 0·85 (0·44–1·61) | 0·611 | ||
| Arterial events | 10 (0·1%) | 9 (0·09%) | 1·11 (0·45–2·72) | 0·827 | ||
| Myocardial infarction | 2 (0·02%) | 3 (0·03%) | 0·66 (0·11–3·97) | 0·651 | ||
| Stroke | 8 (0·08%) | 6 (0·06%) | 1·33 (0·46–3·82) | 0·599 | ||
| Complications* | 10 033 | 9985 | .. | .. | ||
| Renal failure | 129 (1·3%) | 118 (1·2%) | 1·09 (0·85–1·39) | 0·505 | ||
| Cardiac failure | 110 (1·1%) | 115 (1·2%) | 0·95 (0·73–1·23) | 0·710 | ||
| Respiratory failure | 108 (1·1%) | 124 (1·2%) | 0·87 (0·67–1·12) | 0·274 | ||
| Hepatic failure | 29 (0·3%) | 30 (0·3%) | 0·96 (0·58–1·60) | 0·882 | ||
| Sepsis | 180 (1·8%) | 185 (1·9%) | 0·97 (0·79–1·19) | 0·756 | ||
| Seizure | 33 (0·3%) | 43 (0·4%) | 0·76 (0·49–1·20) | 0·242 | ||
| Use of uterotonics | 10 034 | 9984 | .. | .. | ||
| Received at least one type | 9996 (99·6%) | 9930 (99·5%) | 1·00 (1·00–1·00) | 0·090 | ||
| Oxytocin | 9940 (99·1%) | 9865 (98·8%) | 1·00 (1.00–1·01) | 0·079 | ||
| Ergometrine | 4326 (43·1%) | 4314 (43·2%) | 1·00 (0·97–1·03) | 0·891 | ||
| Misoprostol | 6707 (66·8%) | 6717 (67·3%) | 0·99 (0.97–1·01) | 0·513 | ||
| Prostaglandin | 689 (6·9%) | 722 (7·2%) | 0·95 (0·86–1·05) | 0·313 | ||
| ED-5Q+ | 9805 | 9728 | .. | .. | ||
| Mobility | 30 (0·3%) | 31 (0·3%) | 0·96 (0·58–1·58) | 0·874 | ||
| Self-care | 39 (0·4%) | 31 (0·3%) | 1·25 (0·78–2·00) | 0·355 | ||
| Usual activities | 38 (0·4%) | 44 (0·5%) | 0·86 (0·56–1·32) | 0·484 | ||
| Pain/discomfort | 13 (0·1%) | 18 (0·2%) | 0·72 (0·35–1·46) | 0·357 | ||
| Anxiety/depression | 30 (0·3%) | 29 (0·3%) | 1·03 (0·62–1·71) | 0·920 | ||
Data are n (%), unless otherwise indicated.
Fatal or non-fatal. RR=relative risk.
Discussion
The administration of tranexamic acid to women with post-partum haemorrhage reduces deaths due to bleeding and laparotomy to control bleeding with no evidence of any adverse effects or complications. When given soon after delivery, tranexamic acid reduces death due to bleeding by nearly one third.
Our study had several strengths but also some limitations. The randomisation method ensured that participating doctors had no foreknowledge of the treatment allocation. Baseline prognostic factors were well balanced and results adjusted for baseline risk were similar to the unadjusted analyses. Because almost all randomly assigned patients were followed up there is little potential for bias. We originally planned to enrol 15 000 women to assess the effect of tranexamic acid on a composite primary endpoint of death from all-causes or hysterectomy within 42 days of giving birth. However, during the trial it became apparent that the decision to conduct a hysterectomy was often made at the same time as the decision to enrol a women into the trial. Although we excluded hysterectomies done before randomisation, we could not exclude those in which the decision to conduct a hysterectomy was made at the same time as the decision to randomise or before the trial treatment had been received. We predicted that this would dilute the effect of tranexamic acid on the risk of hysterectomy. There would also be dilution from hysterectomies done several days after birth for reasons other than to prevent life-threatening bleeding. With these concerns in mind, we increased the sample size from 15 000 to 20 000 patients in the hope that the trial would have enough power to detect a reduction in post-partum haemorrhage death.17
There was a statistically significant reduction in death due to bleeding with tranexamic acid with no significant increase or decrease in any other cause of death. Because more than one quarter of deaths were not due to bleeding, the reduction in all-cause mortality with tranexamic acid, which is a weighted average of its effect on bleeding and non-bleeding deaths, was not statistically significant. Indeed, considering that one quarter of deaths after post-partum haemorrhage are not bleeding related, it would require trials many times larger than ours to show a statistically significant reduction in all-cause mortality.18 Nevertheless, because the relative contributions of bleeding and non-bleeding (eg, sepsis) deaths to all-cause mortality will vary by region or between hospitals, the effect on all-cause mortality is not generalisable. For example, tranexamic acid will have a larger effect on all-cause mortality in hospitals where sepsis death is rare than in hospitals where sepsis death is common. The effect of tranexamic acid on death due to bleeding is the generalisable measure.
Although tranexamic acid did not prevent hysterectomy, it substantially reduced the number of laparotomies to control bleeding. While hysterectomy might be a last resort to control bleeding in high-income settings, in Africa and Asia where many women are anaemic and blood supplies are limited,19, 20, 21 hysterectomy is often an early intervention to prevent death from exsanguination. Furthermore, there would probably have been a delay between randomisation and the administration of the trial treatment, so that even though the decision to randomise might have preceded the decision to do a hysterectomy, in some cases the trial treatment would not have been received when the hysterectomy decision was made. On the other hand, laparotomies which often involve re-operation to control bleeding following caesarean section, are more commonly done after other interventions including the trial treatment have been given. This might have allowed sufficient time for tranexamic acid to affect the risk of laparotomy. Randomised trials in elective surgery also show large reductions in the need for re-operation to control bleeding with tranexamic acid.22
The dilution of the effect of tranexamic acid arising from interventions that were initiated prior to receipt of the trial treatment is likely to apply to other surgical interventions and blood transfusion. Considering that there may only be a few hours from onset of primary post-partum haemorrhage to maternal death, it is not surprising that other interventions were given concurrently with the trial treatment. Given the urgency, clinicians cannot wait and see if the trial treatment has an effect before giving other treatments, not least because half of the women received placebo. The only outcome that invariably follows randomisation is death. This may explain why in this trial, and in the CRASH-2 trial of tranexamic acid in significant traumatic bleeding, there was a reduction in death due to bleeding with tranexamic acid despite no reduction in transfusion.9
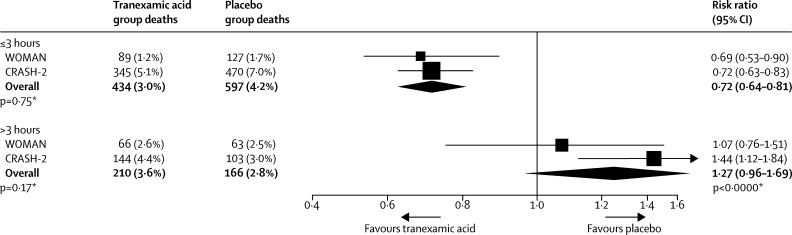
The WOMAN trial began before the results of the CRASH-2 trial of tranexamic acid in bleeding trauma patients were available. The CRASH-2 trial recruited 20 211 adults with traumatic bleeding and showed that tranexamic acid reduces death due to bleeding and all-cause mortality with no increase in vascular occlusive events. There was strong evidence of a time to treatment interaction. In patients treated within 3 h of injury, tranexamic acid reduced death due to bleeding by around one third, but when given after 3 h, it seemed to increase the risk.9, 10 Early activation of fibrinolysis is common after trauma and is associated with increased mortality.11 Because similar temporal changes in fibrinolysis have been observed after childbirth,23 we expected that early treatment with tranexamic acid would also be more effective after post-partum haemorrhage and planned to set the WOMAN trial results in the context of all available clinical data on the time to treatment interaction. Bearing in mind that even a large trial such as ours would have limited power to detect a time to treatment interaction for death due to bleeding, setting the trial results in the context of the totality of the available evidence seemed to be the most sensible approach. Although there are on-going trials of tranexamic acid in life threatening bleeding, the CRASH-2 trial is the only trial to date that provides such evidence.24, 25 Figure 5 shows the results of the WOMAN trial in the context of the CRASH-2 results. There is a strong suggestion that early treatment is most effective and late treatment is unlikely to be beneficial.
Figure 5.
Time to treatment
*Heterogeneity p value.
On the basis of clinical trials of tranexamic acid in surgery and trauma, WHO guidelines recommended tranexamic acid in post-partum haemorrhage if uterotonics fail to stop the bleeding or if it is thought that the bleeding may be due to trauma.1 Our results suggest that if tranexamic acid is used in the treatment of post-partum haemorrhage it should be given soon after the onset of post-partum haemorrhage alongside uterotonics. First, our findings show that a significant proportion of mothers die within hours of post-partum haemorrhage onset. In such circumstances, waiting to see if uterotonics fail to stop the bleeding could put some mothers' lives at risk. We found no evidence of adverse effects with tranexamic acid and it has also been shown to be safe and effective in trauma and surgery. Second, our data suggest that early administration is most effective. Treatment within 3 h of birth significantly reduced death due to bleeding and the need for laparotomy to control bleeding, an observation consistent with results of trials of tranexamic acid in traumatic bleeding. Although we did not see a monotonic decrease in the risk of death due to bleeding with decreasing time to treatment, as seen in trauma, this is more likely to reflect the imprecision of the estimates rather than the underlying biological relationship. We did observe such a monotonic decrease in the risk of laparotomy to control bleeding as time to treatment decreased. Finally, the temporal changes in fibrinolytic activation after childbirth are similar to those in trauma with an early (within one hour) increase in levels of tissue plasminogen activator.14 However, in the light of our results, further research into the timecourse of the changes in coagulation and fibrinolysis after childbirth are needed.
In the WOMAN trial, tranexamic acid was given by intravenous injection. However, in low-income and middle-income countries, many deaths from post-partum bleeding occur at home or settings where intravenous injections might not be feasible. Therefore, bioavailability of tranexamic acid after non-intravenous routes of administration needs to be assessed.
This online publication has been corrected. The corrected version first appeared at thelancet.com on May 5, 2017
Correspondence to: Clinical Trials Unit, London School of Hygiene & Tropical Medicine, London, UK thewomantrial@LSHTM.AC.UK
Acknowledgments
Acknowledgments
The run-in phase for 2000 patients' recruitment was funded by London School of Hygiene and Tropical Medicine. The funds to support the drug and placebo costs through an Investigator initiated research grant for the run-in phase was provided by Pfizer. Jack Waters who supported our funding application through Pfizer died as the trial was ongoing. The main phase was funded by the Department of Health (UK), grant number HICF-T2-0510-007 and the Wellcome Trust, grant number WT094947. The Bill & Melinda Gates Foundation (grant number OPP1095618) supported the final 5000 patients' recruitment and dissemination activities.
WOMAN Trial Collaborative Group
Writing Committee: Haleema Shakur (chair), Ian Roberts (chair), Bukola Fawole (Nigeria), Rizwana Chaudhri (Pakistan), Mohamed El-Sheikh (Sudan), Adesina Akintan (Nigeria), Zahida Qureshi (Kenya), Hussein Kidanto (Tanzania), Bellington Vwalika (Zambia), Abdulfetah Abdulkadir (Ethiopia), Saturday Etuk (Nigeria), Shehla Noor (Pakistan), Etienne Asonganyi (Cameroon), Zarko Alfirevic (UK), Danielle Beaumont (UK), Carine Ronsmans (UK), Sabaratnam Arulkumaran (UK).
Steering Committee: Sabaratnam Arulkumaran (Chair, from 2014), Adrian Grant (Chair, 2009–2014) who died as the trial was ongoing, Kaosar Afsana, Metin Gülmezoglu (2009–2015), Beverley Hunt, Oladapo Olayemi, Ian Roberts. Data Monitoring and Ethics Committee: Iain Chalmers (chair), Pisake Lumbiganon, Gilda Piaggio, Tony Brady (independent statistician).
Protocol Committee: Haleema Shakur, Ian Roberts, Zarko Alfirevic, Diana Elbourne, Metin Gülmezoglu, Carine Ronsmans. Trial coordinating team: Eni Balogun (trial manager), Tracey Pepple (data manager), Danielle Prowse (data assistant), Nigel Quashi (data manager), Lin Barneston (data manager 2010-2012), Collette Barrow (trial administrator), Danielle Beaumont (senior trial manager), Lisa Cook (assistant trial manager 2010–2011), Lauren Frimley (assistant trial manager), Daniel Gilbert (data assistant 2012–2013), Catherine Gilliam (administrator), Rob Jackson (data manager 2012–2015), Taemi Kawahara (trial manager 2010–2015), Hakim Miah (IT manager), Sergey Kostrov (systems officer), Maria Ramos (project administator 2010–2015), Ian Roberts (chief investigator), Haleema Shakur (project director), Phil Edwards (statistician), Tom Godec and Sumaya Huque (statisticial support). Nigeria coordinating team: Bukola Fawole (coordinating centre director), Olujide Okunade (assistant trial coordinator), Olusade Adetayo (assistant trial coordinator).
Pakistan coordinating team: Rizwana Chaudhri (coordinating centre director), Aasia Kayani (research coordinator), Kiran Javaid (assistant research coordinator). National Coordinators: Bukola Fawole (Nigeria), Rizwana Chaudhri (Pakistan), Chrstine Biryabarema (Uganda), Zahida Qureshi (Kenya), Robert Tchounzou (Cameroon), Mohamed El-Sheikh (Sudan), Hussein Kidanto (Tanzania), Mohan Regmi (Nepal), Bellington Vwalika (Zambia), Kastriot Dallaku (Albania), Mateus Sahani (Democratic Republic of Congo), Sayeba Akhter (Bangladesh), Abdulfetah Abdulkadir (Ethiopia), Nicolas Meda (Burkina Faso), Anthony Kwame Dah (Ghana).
Trial sites and investigators
Nigeria (5711): Mother & Child Hospital, Akure (875): Adesina Akintan, Olufemi Odekunle, Oluwabusola Monehin, Austin Ojo, Grace Akinbinu, Ifeoma Offiah; University of Calabar Teaching Hospital (555): Saturday Etuk, Ubong Akpan, Uduak Udofia, Useneno Okon, Ezukwa Omoronyia, Okpe James; University College Hospital, Ibadan (310): Oladapo Olayemi, Nike Bello, Blessed Adeyemi, Chris Aimakhu; Federal Medical Centre Owo (268): Olufemi Akinsanya, Bamidele Adeleye, Oluwaseun Adeyemi, Kayode Oluwatosin; University of Ilorin Teaching Hospital (228): Abiodun Aboyeji, Abiodun Adeniran, Adebayo Adewale, Noah Olaomo; Delta State University Teaching Hospital (198): Lawrence Omo-Aghoja, Emmanuel Okpako, Lucky Oyeye; Maitama District Hospital (188): Francis Alu, John Ogudu, Ezekiel Ladan; Federal Medical Centre Katsina (163): Ibrahim Habib, Babasola Okusanya; University of Abuja Teaching Hospital (160): Olatunde Onafowokan, David Isah, Abalaka Aye; Irrua Specialist Teaching Hospital (157): Felix Okogbo, Egbaname Aigere, Mark Ogbiti; Federal Medical Centre Lokoja (153): Temitope Onile, Olaide Salau, Yinka Amode; Federal Medical Centre Gusau (142): Kamil Shoretire, Adebola Owodunni, Kehinde Ologunde; Adeoyo Maternity Hospital (141): Akintunde Ayinde; Federal Medical Centre Abeokuta (136): Moses Alao, Olalekan Awonuga, Babatunde Awolaja; Lagos University Teaching Hospital (131): Omololu Adegbola, Fatimah Habeebu-Adeyemi, Adeyemi Okunowo; Nyanya General Hospital (124): Hadiza Idris, Ola Okike, Nneka Madueke; Jos University Teaching Hospital (112): Josiah Mutihir, Nankat Joseph, Babatunde Adebudo; Ladoke Akintola University of Technology Teaching Hospital - Osogbo Site (104): Adeniyi Fasanu, Olugbenga Akintunde, Olufemi Abidoye; Seventh Day Adventist Hospital (104): Owigho Opreh, Sophia Udonwa, Gladys Dibia; Lagos Island Maternity Hospital (103): Simeon Bazuaye, Arafat Ifemeje; University of Uyo Teaching hospital (97): Aniefiok Umoiyoho, Emmanuel Inyang-Etoh; Federal Medical Centre Birnin-Kebbi (95): Sununu Yusuf, Kayode Olayinka; Obafemi Awolowo University Teaching Hospital (92): Babalola Adeyemi, Olusegun Ajenifuja; Federal Medical Centre Azare (89): Umar Ibrahim, Yusuf Baffah Adamu; Lagos State University Teaching Hospital (71): Oluwarotimi Akinola, Grace Adekola-Oni; Braithwaite Memorial Specialist Hospital (70): Paul Kua, Roseline Iheagwam; University of Maiduguri Teaching Hospital (65): Audu Idrisa, Ado Geidam; Federal Medical Centre Makurdi (62): Andrea Jogo, Joseph Agulebe; Nnamdi Azikiwe University Teaching Hospital (57): Joseph Ikechebelu, Onyebuchi Udegbunam; Ekiti State University Teaching Hospital (56): Jacob Awoleke, Oluseyi Adelekan; Ahmadu Bello University Teaching Hospital (53): Hajaratu Sulayman, Nkeiruka Ameh; Ajeromi General Hospital (53): Nurudeen Onaolapo, Affiss Adelodun; Plateau State Specialist Hospital (53): William Golit, Dachollom Audu; Ladoke Akintola University of Technology Teaching Hospital - Ogbomoso Site (52): Adetunji Adeniji, Folasade Oyelade; Abubakar Tafawa Balewa University Teaching Hospital (51): Lamaran Dattijo, Palmer Henry; Wesley Guild Hospital (OAUTH) (49): Babalola Adeyemi, Olabisi Loto; Federal Teaching Hospital Abakaliki (38): Odidika Umeora, Abraham Onwe; Federal Medical Centre Owerri (36): Emily Nzeribe, Bartthy Okorochukwu; Federal Medical Centre Ido-Ekiti (34): Augustine Adeniyi; Kogi State Specialist Hospital (30): Emmanuel Gbejegbe, Akpojaro Ikpen; Federal Medical Centre Bida (28): Ikemefuna Nwosu, Abdulrasaq Sambo; National Hospital Abuja (23): Olubunmi Ladipo, Sola Abubakar; Karshi General Hospital (16): Ola Nene Okike; Federal Medical Centre Umuahia (14): Enyinnaya Chikwendu Nduka; University of Nigeria Teaching Hospital, Enugu (14): Eziamaka Pauline Ezenkwele; Federal Medical Center Asaba (11): Daniel Onwusulu; State Specialist Hospital Akure (11): Theresa Azonima Irinyenikan; Usmanu Danfodiyo University Teaching Hospital (11): Swati Singh; Federal Medical Centre Yenagoa (10): Amaitari Bariweni; Aminu Kano Teaching Hospital (7): Hadiza Galadanci; Federal Medical Centre Keffi (7): Peter Achara; Gwarimpa General Hospital (3): Osagie Osayande; General Hospital Minna (1): Mohammed Gana.
Pakistan(5282): Holy Family Hospital, Gyn & Obs Unit 1 (478): Rizwana Chaudhri, Kiran Jabeen, Ayesha Mobeen, Sadaf Mufti, Maliha Zafar; Ayub Teaching Hospital (420): Shehla Noor, Basharat Ahmad, Maimoona Munawar, Jeharat Gul, Naseema Usman; Holy Family Hospital, Gyn & Obs Unit 2 (303): Fehmida Shaheen, Mariam Tariq, Nadia Sadiq, Rabia Batool; Ziauddin University Kemari Campus (280): Habiba Sharaf Ali, Manahil Jaffer, Asma Baloch, Noonari Mukhtiar; Bolan Medical Complex Hospital Unit II (267): Tasneem Ashraf, Raheela Asmat, Salma Khudaidad, Ghazala Taj; Liaquat University Hospital LUMHS Gynae Unit I (230): Roshan Qazi, Saira Dars, Faryal Sardar, Sanobar Ashfaq; Rehman Medical Institute Private Limited (223): Saeeda Majeed; Lady Reading Hospital (222): Sadaqat Jabeen, Rukhsana Karim, Farzana Burki, Syeda Rabia Bukhari; Liaquat Memorial Women & Children Hospital Kohat (215): Fouzia Gul, Musarrat Jabeen, Akhtar Sherin, Qurratul Ain; Nishtar Hospital Unit III (182): Shahid Rao, Uzma Shaheen, Samina Manzoor; Fatima Bai Hospital (159): Shabeen Masood, Shabana Rizvi, Anita Ali; Lady Aitchison Hospital (143): Abida Sajid, Aisha Iftikhar, Shazia Batool; Shalamar Hospital (128): Lubna Dar, Shahenzad Sohail, Shazia Rasul; Sir Ganga Ram Hospital Lahore (111): Shamsa Humayun, Rashida Sultana, Sofia Manzoor; MCH Centre PIMS, Obs and Gynae Unit I (109): Syeda Mazhar, Afshan Batool, Asia Nazir; MCH Centre PIMS, Obs and Gynae Unit II (107): Nasira Tasnim; Nishtar Hospital Unit I (104): Hajira Masood; People's University of Medical and Health Sciences (104): Razia Khero, Neelam Surhio, Samana Aleem; Federal Government Services Hospital (FGSH) Unit III (101): Naila Israr, Saba Javed, Lubna Bashir; Sobhraj Maternity Hospital KMC (96): Samina Iqbal, Faiza Aleem; Services Hospital Lahore Unit II (91): Rubina Sohail, Saima Iqbal; Patel Hospital (89): Samina Dojki, Alia Bano; Dera Ismail Khan District Teaching Hospital (87): Naseem Saba; Sharif Medical & Dental City (85): Maimoona Hafeez, Nishat Akram; Federal Government Poly Clinic (FGPC) Hospital Unit I Islamabad (84): Naila Israr, Riffat Shaheen; Liaquat National Hospital (83): Haleema Hashmi, Sharmeen Arshad; Ziauddin University Hospital Clifton Campus (81): Rubina Hussain, Sadia Khan; CGH Cantonment General Hospital (Rawalpindi) (65): Nighat Shaheen, Safia Khalil; Isra University Hospital (61): Pushpa Sachdev, Gulfareen Arain; Jinnah Hospital Lahore (59): Amtullah Zarreen, Sara Saeed; Fatima Memorial Hospital (58): Shamayela Hanif; Shifa International Hospital (50): Nabia Tariq, Mahwish Jamil; Ziauddin University Hospital Nazimabad Campus (47): Shama Chaudhry, Hina Rajani; Services Hospital Lahore Unit III (43): Tayyiba Wasim, Summera Aslam; Combined Military Hospital (CMH) Lahore (40): Nilofar Mustafa; Nishtar Hospital Unit II (40): Huma Quddusi, Sajila Karim; Ziauddin University Hospital North Nazimabad Campus (40): Shazia Sultana, Misbah Harim; Lady Willingdon Hospital OBGYN Unit II (34): Mohd Chohan; Nescom Hospital (33): Nabila Salman; Pakistan Railway Hospital (26): Fareesa Waqar, Shamsunnisa Sadia; DHQ Hospital Rawalpindi (24): Lubna Kahloon, Shehla Manzoor; Zainab Panjwani Memorial Hospital (23): Samar Amin; Combined Military Hospital (CMH) Kharian (18): Umbreen Akram; Mian Mohammad Trust Hospital (16): Ambreen Ikram; Punjab Medical College (11): Samina Kausar; Kahota Research Laboratory (KRL) General Hospital (10): Tahira Batool; Military Hospital (MH) Rawalpindi (2): Brigadier Naila Tahir Kyani.
Uganda (2235): Mulago Hospital (1065): Christine Biryabarema, Ruth Bulime, Regina Akello, Bernadette Nakawooya Lwasa, Joselyn Ayikoru, Christine Namulwasira; Mubende Regional Referral Hospital (260): Patrick Komagum, Isabirye Rebecca, Nayiga Annet, Nakirigya Nuulu; St Francis Hospital Buluba (254): Elizabeth Nionzima, Rose Bwotya, Margret Nankya, Sarah Babirye (RIP); Mbarara Hospital (239): Joseph Ngonzi, Cesar Sanchez, Nkonwa Innocent, Kusasira Anitah; Entebbe General Hospital (143): Ayiko Jackson, Elizabeth Ndagire, Christine Nanyongo; Adjumani Hospital (103): Dominic Drametu; Angal St Luke Hospital (64): Grace Meregurwa; Church of Uganda Kisiizi Hospital (63): Francis Banya, Rita Atim; Uganda Martyrs, Ibanda Hospital (37): Emmanuel Byaruhanga; Nyakibale Hospital (7): Lema Felix.
Kenya (1031) Garissa Provincial General Hospital (317): Hussein Iman, Vincent Oyiengo, Peninah Waigi, Rose Wangui; Coast Provincial General Hospital (238): Faiza Nassir, Musimbi Soita, Rophina Msengeti, Zeinab Zubier; Moi Teaching and Referral Hospital (162): Hillary Mabeya, Antony Wanjala, Henry Mwangi; AIC Kijabe Hospital (147): Brian Liyayi, Evelyn Muthoka, Alfred Osoti; Nakuru Provincial General Hospital (85): Amos Otara, Veronicah Ongwae; Kenyatta National Hospital, University of Nairobi (53): Zahida Qureshi, Victor Wanjohi; Mwingi District Hospital (14): Bonface Musila; Bungoma District Hospital (8): Kubasu Wekesa; The Nairobi Hospital (7): Alex Nyakundi Bosire.
Cameroon (893): Kumba District Referral Hospital (271): Etienne Asonganyi, Alice Ntem, Angeline Njoache, Alice Ashu; Regional Hospital Limbe (152): André Simo, Robert Tchounzou, Dorothy Keka; Dschang District Hospital (120): Kenfack Bruno, Amadou Ndouoya, Martin Saadio; Hopital Laquintinie de Douala (120): Mesack Tchana, Odel Gwan, Pauline Assomo; St Theresa's Catholic Hospital (99): Venantius Mutsu, Nji Eric; Yaounde Gynaeco-Obstetric and Paediatric Hospital (67): Pascal Foumane, Philemon Nsem; Yaounde Central Hospital (22): Jeanne Fouedjio, Ymele Fouelifack; Centre Hospitalier et Universitaire Yaounde (20): Pierre Marie Tebeu; Sa'a District Hospital (14): Georges Nko'ayissi; Banyo District Hospital (8): Eta Ngole Mbong.
Sudan (860): Khartoum North Teaching Hospital (311): Wisal Nabag, Riham Desougi, Hadia Mustafa, Huida Eltaib; Omdurman Maternity Hospital (199): Taha Umbeli, Khalid Elfadl, Murwan Ibrahim; Kassala New Hospital (Al Saudi) (97): Abdalla Mohammed, Awadia Ali; Wad Medani Teaching Hospital of Obstetrics and Gynaecology (77): Somia Abdelrahiem, Mohammed Musa; El-Obeid Teaching Hospital (74): Khidir Awadalla, Samirra Ahmed; Kosti Hospital (34): Mahdi Bushra, Omer Babiker; Soba University Hospital (33): Hala Abdullahi, Mohamed Ahmed; Gadarif Obstetrics and Gynaecology Hospital (28): Dr. Elhassan Safa, Dr. Huida Almardi; Khartoum Teaching Hospital (6): Duria Rayis; Elmek Nimir University Hospital (1): Saeed Abdelrahman Abdelgabar.
United Kingdom (569): Liverpool Women's Hospital, Liverpool Women's NHS Foundation Trust (128): Zarko Alfirevic, Gillian Houghton, Andrew Sharpe; City Hospital Nottingham, Nottingham University Hospitals Trust (106): Jim Thornton, Nick Grace, Carys Smith; Sunderland Royal Hospital, City Hospitals Sunderland NHS Trust (96): Kim Hinshaw, Dawn Edmundson; The Royal Victoria Infirmary, Newcastle Upon Tyne Hospitals NHS Trust (92): Paul Ayuk, Alison Bates; Queen's Medical Centre, Nottingham University Hospitals Trust (91): George Bugg, Joanne Wilkins; St Mary's Hospital, Central Manchester University Hospitals NHS Foundation Trust (38): Clare Tower, Alysha Allibone; St Thomas' Hospital, Guy's and St Thomas' NHS Foundation Trust (18): Eugene Oteng-Ntim.
Tanzania (538) Muhimbili National Hospital (221): Hussein Kidanto, Ahmad Kazumari, Anna Danford, Matilda Ngarina; Temeke Municipal Hospital (118): Muzdalifat Abeid, Khadija Mayumba, Magreth Zacharia; Hospitali Teule Muheza Designated District Hospital (91): George Mtove, Leonard Madame; Bugando Medical Centre (83): Anthony Massinde, Berno Mwambe; Sekou Toure Regional Hospital (16): Rwakyendela Onesmo; Mwananyamala Municipal Hospital (9): Sebastian Kitengile Ganyaka
Nepal (533): BP Koirala Institute of Health Sciences (364): Mohan Regmi, Shyam Gupta, Rabindra Bhatt, Ajay Agrawal; Nepal Medical College Teaching Hospital (132): Pramila Pradhan, Nikita Dhakal, Punita Yadav; Birat Hospital and Research Centre (23): Gyanendra Karki; Mid Western Regional Hospital (14): Bhola Ram Shrestha.
Zambia (496): University Teaching Hospital Lusaka (362): Bellington Vwalika, Mwansa Lubeya, Jane Mumba, Willies Silwimba; Livingstone General Hospital (55): Isaiah Hansingo, Noojiri Bopili; St Francis Hospital Katete (30): Ziche Makukula; Kabwe General Hospital (20): Alexander Kawimbe; Kafue District Hospital (16): Mwansa Ketty Lubeya; Saint Paul's Mission Hospital (11): Willard Mtambo; Chipata General Hospital (2): Mathew Ng'ambi.
Albania (485) Obstetric Gynaecology University Hospital “K Gliozheni” (385): Kastriot Dallaku, Saimir Cenameri, Ilir Tasha, Aferdita Kruja; Regional Hospital Fier (72): Besnik Brahimaj; Regional Hospital Elbasan (20): Armida Tola; Lezha Regional Hospital (8): Leon Kaza.
Democratic Republic of Congo (457): Hope Medical Center (112): Mateus Sahani, Desire Tshombe, Elizabeth Buligho; Centre Medical ADEBECO (96): Roger Paluku-Hamuli, Charles Kacha; CSR Carmel (69): Kato Faida; Centre de Sante de Reference Albert Barthel (48): Badibanga Musau; Centre Medical VUHE (48): Herman Kalyana; Virunga General Hospital (40): Phanny Simisi; GESOM (Groupe d'entraide et de Solidarite Medicale) (24): Serge Mulyumba; Centre de Sante de Reference Kahembe (8): Nzanzu Kikuhe Jason; Centre Hospitalier Notre Dame d'Afrique (8): Jean Robert Lubamba; Provincial Hospital Goma (4): Willis Missumba.
Bangladesh (325): Dhaka Medical College Hospital (102): Ferdousi Islam, Nazneen Begum; Ad-din Women's Medical College & Hospital (99): Sayeba Akhter, Ferdousi Chowdhury; Chittagong Medical College Hospital (64): Rokeya Begum, Farjana Basher; Ibn Sina Medical College Hospital (30): Nazlima Nargis, Abu Kholdun; Rajshahi Medical College Hospital (30): Shahela Jesmin, Shrodha Paul.
Ethiopia (302): Jimma University Hospital (158): Hailemariam Segni, Getachew Ayana, William Haleke; St. Paul's Hospital Millennium Medical College (144): Abdulfetah Abdulkadir, Hassen Hussien, Fikre Geremew.
Burkina Faso (142): Centre Hospitalier Universitaire Souro Sanou (129): Moussa Bambara, Adolphe Somé, Amadou Ly; Centre Hospitalier Regional de Dedougou (13): Roamba Pabakba.
Jamaica (73): University Hospital of the West Indies (73): Horace Fletcher, Leslie Samuels.
Ghana (41): Komfo Anokye Teaching Hospital (39): Henry Opare-Addo, Roderick Larsen-Reindorf; Ashanti Mampong Municipal Hospital (2): Kwadwo Nyarko-Jectey.
Papua New Guinea (38): Port Moresby General Hospital (38): Glen Mola, Malts Wai.
Egypt (33): Mataria Teaching Hospital (33): Magdy El Rahman, Wafaa Basta, Hussein Khamis.
Colombia (8): Fundacion Valle del Lili (8): Maria Fernanda Escobar, Liliana Vallecilla.
Cote d'Ivoire (8): Hopital General Abobo Nord (8): Gabriel Essetchi Faye.
Contributors
Haleema Shakur and Ian Roberts conceived the study, reviewed the scientific literature, and were responsible for study design, data collection, data analysis, data interpretation, writing, and reviewing the report; they take overall responsibility for this report. Bukola Fawole, Rizwana Chaudhri, Mohamed El-Sheikh, Adesina Akintan, Zahida Qureshi, Hussein Kidanto, Bellington Vwalika, Abdulfetah Abdulkadir, Saturday Etuk, Shehla Noor, Etienne Asonganyi, and Danielle Beaumont contributed to data collection, data interpretation, and reviewing the report. Zarko Alfirevic contributed to the study design, data collection, data interpretation, and reviewing the report, Carine Ronsmans contributed to the study design, data interpretation, and reviewing the report, and Sabaratnam Arulkumaran contributed to reviewing the report.
Declaration of interests
We declare no competing interests.
Contributor Information
WOMAN Trial Collaborators:
Haleema Shakur, Ian Roberts, Bukola Fawole, Rizwana Chaudhri, Mohamed El-Sheikh, Adesina Akintan, Zahida Qureshi, Hussein Kidanto, Bellington Vwalika, Abdulfetah Abdulkadir, Saturday Etuk, Shehla Noor, Etienne Asonganyi, Zarko Alfirevic, Danielle Beaumont, Carine Ronsmans, Sabaratnam Arulkumaran, Adrian Grant, Kaosar Afsana, Metin Gülmezoglu, Beverley Hunt, Oladapo Olayemi, Ian Roberts, Iain Chalmers, Pisake Lumbiganon, Gilda Piaggio, Tony Brady, Haleema Shakur, Ian Roberts, Zarko Alfirevic, Diana Elbourne, Metin Gülmezoglu, Carine Ronsmans, Eni Balogun, Tracey Pepple, Danielle Prowse, Nigel Quashi, Lin Barneston, Collette Barrow, Danielle Beaumont, Lisa Cook, Lauren Frimley, Daniel Gilbert, Catherine Gilliam, Rob Jackson, Taemi Kawahara, Hakim Miah, Sergey Kostrov, Maria Ramos, Ian Roberts, Haleema Shakur, Phil Edwards, Tom Godec, Sumaya Huque, Bukola Fawole, Olujide Okunade, Olusade Adetayo, Rizwana Chaudhri, Aasia Kayani, Kiran Javaid, Bukola Fawole, Rizwana Chaudhri, Chrstine Biryabarema, Zahida Qureshi, Robert Tchounzou, Mohamed El-Sheikh, Hussein Kidanto, Mohan Regmi, Bellington Vwalika, Kastriot Dallaku, Mateus Sahani, Sayeba Akhter, Abdulfetah Abdulkadir, Nicolas Meda, Anthony Kwame Dah, Adesina Akintan, Olufemi Odekunle, Oluwabusola Monehin, Austin Ojo, Grace Akinbinu, Ifeoma Offiah, Saturday Etuk, Ubong Akpan, Uduak Udofia, Useneno Okon, Ezukwa Omoronyia, Okpe James, Oladapo Olayemi, Nike Bello, Blessed Adeyemi, Chris Aimakhu, Olufemi Akinsanya, Bamidele Adeleye, Oluwaseun Adeyemi, Kayode Oluwatosin, Abiodun Aboyeji, Abiodun Adeniran, Adebayo Adewale, Noah Olaomo, Lawrence Omo-Aghoja, Emmanuel Okpako, Lucky Oyeye, Francis Alu, John Ogudu, Ezekiel Ladan, Ibrahim Habib, Babasola Okusanya, Olatunde Onafowokan, David Isah, Abalaka Aye, Felix Okogbo, Egbaname Aigere, Mark Ogbiti, Temitope Onile, Olaide Salau, Yinka Amode, Kamil Shoretire, Adebola Owodunni, Kehinde Ologunde, Akintunde Ayinde, Moses Alao, Olalekan Awonuga, Babatunde Awolaja, Omololu Adegbola, Fatimah Habeebu-Adeyemi, Adeyemi Okunowo, Hadiza Idris, Ola Okike, Nneka Madueke, Josiah Mutihir, Nankat Joseph, Babatunde Adebudo, Adeniyi Fasanu, Olugbenga Akintunde, Olufemi Abidoye, Owigho Opreh, Sophia Udonwa, Gladys Dibia, Simeon Bazuaye, Arafat Ifemeje, Aniefiok Umoiyoho, Emmanuel Inyang-Etoh, Sununu Yusuf, Kayode Olayinka, Babalola Adeyemi, Olusegun Ajenifuja, Umar Ibrahim, Yusuf Baffah Adamu, Oluwarotimi Akinola, Grace Adekola-Oni, Paul Kua, Roseline Iheagwam, Audu Idrisa, Ado Geidam, Andrea Jogo, Joseph Agulebe, Joseph Ikechebelu, Onyebuchi Udegbunam, Jacob Awoleke, Oluseyi Adelekan, Hajaratu Sulayman, Nkeiruka Ameh, Nurudeen Onaolapo, Affiss Adelodun, William Golit, Dachollom Audu, Adetunji Adeniji, Folasade Oyelade, Lamaran Dattijo, Palmer Henry, Babalola Adeyemi, Olabisi Loto, Odidika Umeora, Abraham Onwe, Emily Nzeribe, Bartthy Okorochukwu, Augustine Adeniyi, Emmanuel Gbejegbe, Akpojaro Ikpen, Ikemefuna Nwosu, Abdulrasaq Sambo, Olubunmi Ladipo, Sola Abubakar, Ola Nene Okike, Enyinnaya Chikwendu Nduka, Eziamaka Pauline Ezenkwele, Daniel Onwusulu, Theresa Azonima Irinyenikan, Swati Singh, Amaitari Bariweni, Hadiza Galadanci, Peter Achara, Osagie Osayande, Mohammed Gana, Rizwana Chaudhri, Kiran Jabeen, Ayesha Mobeen, Sadaf Mufti, Maliha Zafar, Shehla Noor, Basharat Ahmad, Maimoona Munawar, Jeharat Gul, Naseema Usman, Fehmida Shaheen, Mariam Tariq, Nadia Sadiq, Rabia Batool, Habiba Sharaf Ali, Manahil Jaffer, Asma Baloch, Noonari Mukhtiar, Tasneem Ashraf, Raheela Asmat, Salma Khudaidad, Ghazala Taj, Roshan Qazi, Saira Dars, Faryal Sardar, Sanobar Ashfaq, Saeeda Majeed, Sadaqat Jabeen, Rukhsana Karim, Farzana Burki, Syeda Rabia Bukhari, Fouzia Gul, Musarrat Jabeen, Akhtar Sherin, Qurratul Ain, Shahid Rao, Uzma Shaheen, Samina Manzoor, Shabween Masood, Shabana Rizvi, Anita Ali, Abida Sajid, Aisha Iftikhar, Shazia Batool, Lubna Dar, Shahenzad Sohail, Shazia Rasul, Shamsa Humayun, Rashida Sultana, Sofia Manzoor, Syeda Mazhar, Afshan Batool, Asia Nazir, Nasira Tasnim, Hajira Masood, Razia Khero, Neelam Surhio, Samana Aleem, Naila Israr, Saba Javed, Lubna Bashir, Samina Iqbal, Faiza Aleem, Rubina Sohail, Saima Iqbal, Samina Dojki, Alia Bano, Naseem Saba, Maimoona Hafeez, Nishat Akram, Naila Israr, Riffat Shaheen, Haleema Hashmi, Sharmeen Arshad, Rubina Hussain, Sadia Khan, Nighat Shaheen, Safia Khalil, Pushpa Sachdev, Gulfareen Arain, Amtullah Zarreen, Sara Saeed, Shamayela Hanif, Nabia Tariq, Mahwish Jamil, Shama Chaudhry, Hina Rajani, Tayyiba Wasim, Summera Aslam, Nilofar Mustafa, Huma Quddusi, Sajila Karim, Shazia Sultana, Misbah Harim, Mohd Chohan, Nabila Salman, Fareesa Waqar, Shamsunnisa Sadia, Lubna Kahloon, Shehla Manzoor, Samar Amin, Umbreen Akram, Ambreen Ikram, Samina Kausar, Tahira Batool, Brigadier Naila, Tahir Kyani, Christine Biryabarema, Ruth Bulime, Regina Akello, Bernadette Nakawooya Lwasa, Joselyn Ayikoru, Christine Namulwasira, Patrick Komagum, Isabirye Rebecca, Nayiga Annet, Nakirigya Nuulu, Elizabeth Nionzima, Rose Bwotya, Margret Nankya, Sarah Babirye, (RIP), Joseph Ngonzi, Cesar Sanchez, Nkonwa Innocent, Kusasira Anitah, Ayiko Jackson, Elizabeth Ndagire, Christine Nanyongo, Dominic Drametu, Grace Meregurwa, Francis Banya, Rita Atim, Emmanuel Byaruhanga, Lema Felix, Hussein Iman, Vincent Oyiengo, Peninah Waigi, Rose Wangui, Faiza Nassir, Musimbi Soita, Rophina Msengeti, Zeinab Zubier, Hillary Mabeya, Antony Wanjala, Henry Mwangi, Brian Liyayi, Evelyn Muthoka, Alfred Osoti, Amos Otara, Veronicah Ongwae, Zahida Qureshi, Victor Wanjohi, Bonface Musila, Kubasu Wekesa, Alex Nyakundi Bosire, Etienne Asonganyi, Alice Ntem, Angeline Njoache, Alice Ashu, André Simo, Robert Tchounzou, Dorothy Keka, Kenfack Bruno, Amadou Ndouoya, Martin Saadio, Mesack Tchana, Odel Gwan, Pauline Assomo, Venantius Mutsu, Nji Eric, Pascal Foumane, Philemon Nsem, Jeanne Fouedjio, Ymele Fouelifack, Pierre Marie Tebeu, Georges Nko'ayissi, Eta Ngole Mbong, Wisal Nabag, Riham Desougi, Hadia Mustafa, Huida Eltaib, Taha Umbeli, Khalid Elfadl, Murwan Ibrahim, Abdalla Mohammed, Awadia Ali, Somia Abdelrahiem, Mohammed Musa, Khidir Awadalla, Samirra Ahmed, Mahdi Bushra, Omer Babiker, Hala Abdullahi, Mohamed Ahmed, Elhassan Safa, Huida Almardi, Duria Rayis, Saeed Abdelrahman Abdelgabar, Zarko Alfirevic, Gillian Houghton, Andrew Sharpe, Jim Thornton, Nick Grace, Carys Smith, Kim Hinshaw, Dawn Edmundson, Paul Ayuk, Alison Bates, George Bugg, Joanne Wilkins, Clare Tower, Alysha Allibone, Eugene Oteng-Ntim, Hussein Kidanto, Ahmad Kazumari, Anna Danford, Matilda Ngarina, Muzdalifat Abeid, Khadija Mayumba, Magreth Zacharia, George Mtove, Leonard Madame, Anthony Massinde, Berno Mwambe, Rwakyendela Onesmo, Sebastian Kitengile Ganyaka, Mohan Regmi, Shyam Gupta, Rabindra Bhatt, Ajay Agrawal, Pramila Pradhan, Nikita Dhakal, Punita Yadav, Gyanendra Karki, Bhola Ram Shrestha, Bellington Vwalika, Mwansa Lubeya, Jane Mumba, Willies Silwimba, Isaiah Hansingo, Noojiri Bopili, Ziche Makukula, Alexander Kawimbe, Mwansa Ketty Lubeya, Willard Mtambo, Mathew Ng'ambi, Kastriot Dallaku, Saimir Cenameri, Ilir Tasha, Aferdita Kruja, Besnik Brahimaj, Armida Tola, Leon Kaza, Mateus Sahani, Desire Tshombe, Elizabeth Buligho, Roger Paluku-Hamuli, Charles Kacha, Kato Faida, Badibanga Musau, Herman Kalyana, Phanny Simisi, Serge Mulyumba, Nzanzu Kikuhe Jason, Jean Robert Lubamba, Willis Missumba, Ferdousi Islam, Nazneen Begum, Sayeba Akhter, Ferdousi Chowdhury, Rokeya Begum, Farjana Basher, Nazlima Nargis, Abu Kholdun, Shahela Jesmin, Shrodha Paul, Hailemariam Segni, Getachew Ayana, William Haleke, Abdulfetah Abdulkadir, Hassen Hussien, Fikre Geremew, Moussa Bambara, Adolphe Somé, Amadou Ly, Roamba Pabakba, Horace Fletcher, Leslie Samuels, Henry Opare-Addo, Roderick Larsen-Reindorf, Kwadwo Nyarko-Jectey, Glen Mola, Malts Wai, Magdy El Rahman, Wafaa Basta, Hussein Khamis, Maria Fernanda Escobar, Liliana Vallecilla, and Gabriel Essetchi Faye
Supplementary Material
References
- 1.WHO . WHO recommendations for the prevention and treatment of postpartum haemorrhage. World Health Organisation; Geneva: 2012. [PubMed] [Google Scholar]
- 2.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.WHO, UNFPA and The World Bank . Trends in maternal mortality: 1990 to 2010 - WHO. UNICEF, UNFPA and The World Bank estimates; 2012. [Google Scholar]
- 4.Ronsmans C, Graham WJ, LMSSS Group Maternal mortality: who, when, where, and why. Lancet. 2006;368:1189–1200. doi: 10.1016/S0140-6736(06)69380-X. [DOI] [PubMed] [Google Scholar]
- 5.Say L, Chou D, Gemmill A. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 6.Electronic Medicines Compendium, Summary of Product Characteristics: Tranexamic acid. http://www.medicines.org.uk/emc/medicine/1489 (accessed March 3, 2017).
- 7.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ker K, Prieto-Merino D, Roberts I. Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br J Surg. 2013;100:1271–1279. doi: 10.1002/bjs.9193. [DOI] [PubMed] [Google Scholar]
- 9.CRASH-2 Collaborators Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 10.CRASH-2 Collaborators The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 11.Sawamura A, Hayakawa M, Gando S. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res. 2009;124:608–613. doi: 10.1016/j.thromres.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Chapman MP, Moore EE, Moore HB. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80:16–23. doi: 10.1097/TA.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X, Darlington DN, Cap AP. Procoagulant and fibrinolytic activity after polytrauma in rat. Am J Physiol Regul Integr Comp Physiol. 2016;310:R323–R329. doi: 10.1152/ajpregu.00401.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruithof EK, Tran-Thang C, Gudinchet A. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–466. [PubMed] [Google Scholar]
- 15.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice E6(R1), 1996 June 10. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4.pdf (accessed March 3, 2017).
- 16.Shakur H, Elbourne D, Gulmezoglu M. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials. 2010;11:40. doi: 10.1186/1745-6215-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shakur H, Roberts I, Edwards P, Elbourne D, Alfirevic Z, Ronsmans C. The effect of tranexamic acid on the risk of death and hysterectomy in women with post-partum haemorrhage: statistical analysis plan for the WOMAN trial. Trials. 2016;17:249. doi: 10.1186/s13063-016-1332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prieto-Merino D, Smeeth L, Staa TP, Roberts I. Dangers of non-specific composite outcome measures in clinical trials. BMJ. 2013;347:f6782. doi: 10.1136/bmj.f6782. [DOI] [PubMed] [Google Scholar]
- 19.Tort J, Rozenberg P, Traore M, Fournier P, Dumont A. Factors associated with postpartum hemorrhage maternal death in referral hospitals in Senegal and Mali: a cross-sectional epidemiological survey. BMC Pregnancy Childbirth. 2015;15:235. doi: 10.1186/s12884-015-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oladapo OT, Adetoro OO, Ekele BA. When getting there is not enough: a nationwide cross-sectional study of 998 maternal deaths and 1451 near-misses in public tertiary hospitals in a low-income country. BJOG. 2016;123:928–938. doi: 10.1111/1471-0528.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah N, Hossain N, Shoaib R, Hussain A, Gillani R, Khan NH. Socio-demographic characteristics and the three delays of maternal mortality. J Coll Physicians Surg Pak. 2009;19:95–98. [PubMed] [Google Scholar]
- 22.Myles PS, Smith JA, Forbes A. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376:136–148. doi: 10.1056/NEJMoa1606424. [DOI] [PubMed] [Google Scholar]
- 23.Ducloy-Bouthors AS, Duhamel A, Kipnis E. Postpartum haemorrhage related early increase in D-dimers is inhibited by tranexamic acid: haemostasis parameters of a randomized controlled open labelled trial. Br J Anaesth. 2016;116:641–648. doi: 10.1093/bja/aew021. [DOI] [PubMed] [Google Scholar]
- 24.Dewan Y, Komolafe EO, Mejia-Mantilla JH, Perel P, Roberts I, Shakur H. CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012;13:87. doi: 10.1186/1745-6215-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts I, Coats T, Edwards P. HALT-IT--tranexamic acid for the treatment of gastrointestinal bleeding: study protocol for a randomised controlled trial. Trials. 2014;15:450. doi: 10.1186/1745-6215-15-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.