Abstract
Microglia are the resident immune cells of the CNS and constitute a self‐sustaining population of CNS‐adapted tissue macrophages. As mononuclear phagocytic cells, they express high levels of superoxide‐producing NADPH oxidases (NOX). The sole function of the members of the NOX family is to generate reactive oxygen species (ROS) that are believed to be important in CNS host defence and in the redox signalling circuits that shape the different activation phenotypes of microglia. NOX are also important in pathological conditions, where over‐generation of ROS contributes to neuronal loss via direct oxidative tissue damage or disruption of redox signalling circuits. In this review, we assess the evidence for involvement of NOX in CNS physiopathology, with particular emphasis on the most important surface receptors that lead to generation of NOX‐derived ROS. We evaluate the potential significance of the subcellular distribution of NOX isoforms for redox signalling or release of ROS to the extracellular medium. Inhibitory mechanisms that have been reported to restrain NOX activity in microglia and macrophages in vivo are also discussed. We provide a critical appraisal of frequently used and recently developed NOX inhibitors. Finally, we review the recent literature on NOX and other sources of ROS that are involved in activation of the inflammasome and discuss the potential influence of microglia‐derived oxidants on neurogenesis, neural differentiation and culling of surplus progenitor cells. The degree to which excessive, badly timed or misplaced NOX activation in microglia may affect neuronal homeostasis in physiological or pathological conditions certainly merits further investigation.
Linked Articles
This article is part of a themed section on Redox Biology and Oxidative Stress in Health and Disease. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.12/issuetoc
Abbreviations
- CR3
complement receptor 3
- DPI
diphenylene iodonium
- HMGB1
high mobility group box 1
- JAK
Janus kinase
- NAC
N‐acetylcysteine
- NLRP3
NOD‐like receptor family, pyrin domain containing 3
- SVZ
subventricular zone
- TLR
toll‐like receptor
Tables of Links
| TARGETS | |
| Catalytic receptors a | Ligand‐gated ion channels c |
| CD11b (integrin αM) | NMDA receptor |
| CD18 (integrin β2) | P2X7 receptor |
| NLRP3, NOD‐like receptor family, pyrin domain containing 3 | Enzymes d |
| TLR2 | Akt |
| TLR4 | ERK1/2 |
| GPCRs b | Haem oxygenase 1 |
| mGlu3 receptor | Inducible NO synthase |
| mGlu5 receptor | Lyn |
| P2Y2 receptor | p21‐activated kinase 1 (PAK1) |
| P2Y4 receptor | p38 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d Alexander et al., 2015a, 2015b, 2015c, 2015d).
Introduction
Microglia are the tissue‐specific macrophages of the CNS, and unlike other brain cells, they derive from yolk sac haematopoietic stem cells, which populate the mouse brain around embryonic day 9 (Alliot et al., 1999; Ginhoux et al., 2010; Kierdorf et al., 2013). Once established in the brain parenchyma, the population of CNS microglia is maintained throughout life by proliferation when needed, which occurs independently of bone marrow‐derived precursors (Prinz and Priller, 2014; Ajami et al., 2007; Elmore et al., 2014; Bruttger et al., 2015).
Since they were first identified by Pio del Rio‐Hortega in 1920, most research has concentrated on the neuropathological associations of microglia (Kettenmann and Verkhratsky, 2011). Many effector functions of microglia are potentially cytotoxic, and a substantial body of evidence links excessive activation of microglia to the neuroinflammation that accompanies many forms of acute or chronic neuropathology. Release of pro‐inflammatory cytokines, arachidonic acid derivatives, excitatory neurotransmitters, proteinases and ROS may all contribute to neurodegenerative disease, if unchecked. In particular, ROS production by microglia is considered to be a major cause of neuronal dysfunction, damage and death (Block et al., 2007; Gao et al., 2012) through direct oxidative damage to neuronal macromolecules (Wu et al., 2006; Rojo et al., 2014) or derangement of neuronal redox signalling circuits.
On the other hand, microglia have a vital role in cell survival. In common with other tissue‐specific macrophages, such as skin Langerhans cells and spleen red pulp macrophages (which microglia most resemble) (Butovsky et al., 2014), they have the same basic functions as immune surveillance cells, namely, clearance capabilities and maintenance of local homeostasis (Casano and Peri, 2015). Indeed, microglia are now recognized as being extremely plastic, versatile and multifunctional cells that, importantly, play key roles in the healthy brain (Hanisch and Kettenmann, 2007; Saijo and Glass, 2011; Prinz and Priller, 2014; Casano and Peri, 2015). Microglial processes constantly survey the parenchyma for pathogens and debris from damaged or dying cells and remodel and maintain the local environment (Davalos et al., 2005; Nimmerjahn et al., 2005). In the developing brain, microglia are responsible for clearance of apoptotic cells, neurogenesis and axonal growth, synaptic pruning (synaptic refinement) and vessel patterning (Casano and Peri, 2015). It is also increasingly recognized that regulated oxidant generation by the family of NADPH oxidases (NOX; Sorce and Krause, 2009; Lambeth and Neish, 2014; Nayernia et al., 2014) contributes to cell homeostasis through the regulation of key redox‐dependent pathways (Holmstrom and Finkel, 2014). For example, H2O2 modulates transcription factor activity, cytoskeleton dynamics, ion channel activity, receptor activation and tyrosine kinase cascades through reversible oxidation of cysteines with low pKa values in target proteins (Go et al., 2015; Holmstrom and Finkel, 2014). Regulated H2O2 release to the surroundings is conceivably also involved in the paracrine modulation of redox sensitive targets in neighboring neurons or glia cells.
In this review, we discuss the state of the art of oxidant production in microglia, emphasizing the critical role of NOX and the sensory receptors that regulate NOX activity, or are subjected to NOX‐generated redox signalling, in the healthy or diseased brain. Because of the (still) limited literature on the subject in microglia, we rely in part on lessons learned from macrophages and, to a lesser extent, from mesenchymal cell types.
Oxidant production in microglia
With the exception of H2O2, which is not a free radical, ROS are small molecules or ions characterized by the presence of unpaired electrons (radicals). In biological settings, they are generated either in a regulated manner from specific enzymes, such as NOX, or as by‐products or end‐products of oxidative metabolism.
The discovery of NOX family members as enzymes with the sole purpose of generating superoxide (O2 −) and H2O2, together with the recognition of widespread regulatory redox modification of target proteins (Herrmann and Dick, 2012; Go et al., 2015), has led to the concept that ROS form part of a highly complex and sophisticated regulatory system, referred to as the Redox Code (Jones and Sies, 2015). Both O2 − and H2O2 play important roles in cell signalling as second messengers (see Holmstrom and Finkel, 2014). Antioxidants act as a counterbalance to ROS, and in microglia, they both regulate redox signalling and oppose the toxicity associated with free radical production. The accompanying review (Vilhardt et al., this issue) provides a detailed analysis of the role of antioxidant systems in microglia.
NADPH oxidase (NOX)
NOX is by far the most important source of oxidants in cells, but mitochondria may also produce oxidants under certain conditions. Discussion of other cellular sources of oxidants is beyond the scope of this review, but we refer to a recent review for details (Casas et al., 2015).
The NOX family consists of seven enzymes, NOX1–5 and DUOX1–2, all of which generate O2 −, or in the case of NOX4, H2O2 (Lambeth, 2004; Bedard and Krause, 2007). NOX2 (also known as gp91phox) is highly expressed in microglia of both humans and rodents (Sorce et al., 2014) and, to a differing degree, in other CNS cell types including neurons and neuronal stem cells (Nayernia et al., 2014). Expression of NOX1 and NOX4 has been documented in microglia, but the results are less clear‐cut, because of a lack of specific antibodies. NOX1, NOX2 and NOX4 are similar in size and domain structure (Lambeth, 2004). They consist of a short cytosolic N‐terminal domain followed by six transmembrane domains, where two haem prosthetic groups are contained, and a long C‐terminus that has flavin adenine dinucleotide (FAD) and NADPH binding sites. A membrane‐bound flavocytochrome b558 complex containing subunits p22phox and NOX2 (gp91phox) together with cytosolic subunits constitutes the phagocyte NADPH oxidase.
When activated, gp91phox abstracts electrons from cytosolic NADPH and shuttles them through the membrane for the one‐electron reduction of molecular oxygen on the other (luminal or extracellular) side of the membrane to generate O2 −. The NOX2 complex consists of additional cytosolic regulatory proteins p40phox, p47phox and p67phox and the small GTPase Rac1 (Figure 1). Translocation of these subunits to flavocytochrome b558 in the membrane is necessary for ROS‐generating NOX activation, as p67phox and Rac1 in tandem regulate the electron flow from NADPH to FAD (Diebold and Bokoch, 2001). Rac1/2 is mobilized separately from the cytosolic phox proteins but is available at the membrane following release from Rho‐GDI (DerMardirossian et al., 2004) and interaction with GDP/GTP exchange factors such as VAV1 (Roepstorff et al., 2008). In microglia, the rate‐limiting step in NOX2 activation is the critical serine (and threonine) phosphorylation of p47phox (Roepstorff et al., 2008). This is catalysed by kinases that are activated downstream of cell surface receptor stimulation and include PKC isoforms, p21‐activated kinase 1, Akt, p38MAP kinase and ERK1/2 (El‐Benna et al., 2009).
Figure 1.
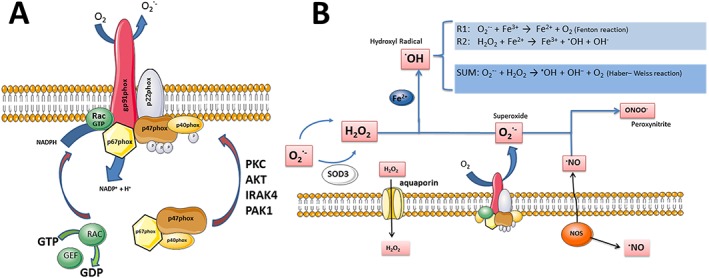
The phagocyte NADPH oxidase (NOX2). (A) Assembly of the phagocyte NADPH oxidase consisting of the integral flavocytochrome b558 complex [cyt b558; composed of gp91phox (NOX2) and p22phox subunits] and cytosolic subunits p40, p47 and p67phox and the small GTPase Rac1. Of the cytosolic subunits, p67phox and Rac1 are catalytic, while p40phox and p47phox serve to guide, position and retain p67phox interactions with cyt b558 in the membrane. In resting cells, binding motifs contained within p47phox (and p40phox) for interaction with PIP3 in the membrane and p22phox are shielded by the so‐called auto‐inhibitory region. However, upon multiple serine and threonine phosphorylations by activating kinases, the auto‐inhibition is released, and either p47phox or p40phox in a ligand/receptor‐specific manner transports p67phox to cyt b558 in the membrane. Rac1 is activated by release from RhoGDI and subsequent nucleotide exchange by a GTP/GDP exchange factor (GEF). Rac1 in concert with p67phox mediates electron transfer from NADPH to the redox centers of gp91phox (FAD and haem) and finally to molecular oxygen on the extracellular side of the membrane to form O2 −. (B) NOX2‐derived oxidant reactions. The membrane permeability of O2 − is low, and release of oxidants to the extracellular environment therefore requires that NOX2 is localized to the cell surface. Released O2 − quickly dismutates to H2O2 either spontaneously or through the action of extracellular SOD (SOD3). In the presence of free iron, H2O2 and O2 − can react to form the highly reactive hydroxyl radical .OH, while O2 − in the presence of NO can form the similarly neurotoxic peroxynitrite (ONOO−). A small fraction of extracellularly produced H2O2 can diffuse into the cytosol either directly through the membrane or via aquaporin channels, to alter the activity of redox targets.
In general, mitochondrial production of ROS (complexes I and III of the electron transport chain are the main sites of O2 − production) is orders of magnitude smaller than the oxidant output generated by NOX enzymes and is not associated with the extracellular release of ROS (Brown and Borutaite, 2012). Nonetheless, it has been hypothesized that mitochondrial and NOX‐mediated ROS production may be functionally linked, particularly in the stimulation of microglial activation by pro‐inflammatory mediators. A discussion of the association is the subject of an excellent critical review by Bordt and Polster (2014).
Subcellular localization of NOX in microglia
The site of oxidant production is important because of the differing membrane permeability of the various ROS, coupled with their varying susceptibility to neutralization by the cytosolic reducing environment. Thus, the precise subcellular localization, timing and nature of the ROS generated are essential for biological function. The chemical reactivity of the different ROS also has an important bearing on their potency. For example, in the presence of transition state metals such as iron, O2 − and H2O2 form the highly reactive and destructive hydroxyl radical (.OH) in the well‐known Haber–Weiss reaction (Figure 1B). By this mechanism, O2 − reduces free iron to the Fe2 + form (Fenton reaction) that, in turn, oxidises H2O2 to .OH. This chain reaction may have marked effects in specific brain regions with high concentrations of iron, such as the substantia nigra (Youdim et al., 1991), and may explain the particular vulnerability of dopaminergic neurons to oxidative stress (Qin et al., 2004). The reaction product of O2 − and NO, peroxynitrite anion (ONOO−) is also highly reactive, and in many instances, the oxidative toxicity experienced by neurons and glia cells depends on the combined production of NO and O2 − (Wang et al., 2004; Li et al., 2005).
It is generally assumed that H2O2 and ONOO− generated in the cytosol can cross the plasma membrane by diffusion through aquaporins (Bienert and Chaumont, 2014) or anion channels. The membrane permeability of O2 − and .OH is low, and a substantial release to the surroundings requires that NOX is localized to the cell surface.
In resting macrophages and microglia, NOX2 is present on the plasma membrane, but following cell activation, it is redistributed (partly or fully depending on species) by clathrin‐mediated endocytosis to an intracellular, agonist‐regulated storage compartment consisting of numerous small (<100 nm) vesicles (Figure 2) (Ejlerskov et al., 2012). When resident on the cell surface, NOX2 associates with lipid rafts (glycosphingolipid and cholesterol‐enriched microdomains) in microglia and other cell types (Vilhardt and van Deurs, 2004). These domains may form a platform for NOX2 mobility and signalling. In contrast, NOX1 in microglia is contained in intracellular vacuoles of undefined, but perhaps lysosomal, nature (Cheret et al., 2008). There are very few reports of NOX4 expression in microglia, and all refer to identification of mRNA, rather than the protein (Harrigan et al., 2008; Li et al., 2009a; Mead et al., 2012). However, in monocyte‐derived macrophages, NOX4 has been detected at the protein level and appears to occupy an intracellular compartment of small vesicles (Lee et al., 2013b). NOX4 does not seem to be required for microglia activation in vivo (Kallenborn‐Gerhardt et al., 2012) or for expression of most pro‐inflammatory genes in vitro (Li et al., 2009a).
Figure 2.
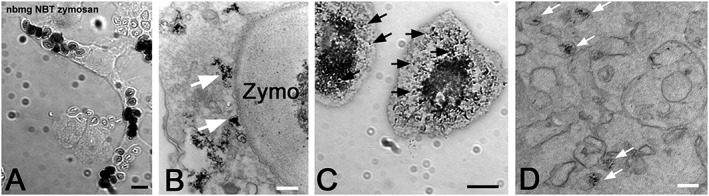
Microglia oxidant production in culture. Cytochemical reactions of primary rat microglia exposed to zymosan (A and B) or phorbol 12‐myristate 13‐acetate (C and D) in the presence of either NBT to measure O2 − production (A and C) for light microscopy or the H2O2‐sensitive CeCl3 (B and D) for electron microscopy. Notice that oxidant production is mainly intracellular and resides with a population of small vesicles (arrows in C and D), which become mobilized for fusion with the phagosome containing zymosan (arrows in B). Bars A and C, 10 μm; B and D, 100 nm (unpublished results; F. Vilhardt).
Knowledge of the cellular sorting machinery that governs localization and agonist‐regulated distribution of the NOX is fragmentary. A hierarchy of undefined sorting signals is presumed to regulate NOX trafficking (Helmcke et al., 2009; von Lohneysen et al., 2010). In microglia, together with neutrophils, dendritic cells and macrophages, the small GTPase Rab27A/B determines trafficking of NOX2‐associated organelles to phagosomes containing IgG‐opsonized targets (Jancic et al., 2007; Anderson et al., 2011; Ejlerskov et al., 2012). Additionally, in microglia, Rab27 is required to sequester the NOX2 complex in intracellular vesicles away from the cell surface (Ejlerskov et al., 2012). In other cell types, Ras GTPase‐activating‐like protein (IQGAP1) is a tethering factor for NOX2 at the plasma membrane (Ikeda et al., 2005). In many instances, stimulation of phagocytes does not lead to overt NOX activation, but rather primes NOX for enhanced oxidant production following stimuli that directly activate the oxidase. A part of this priming derives from the shuttling of NOX2 from internal stores to the cell surface (Ward et al., 2000; Uriarte et al., 2011; Ejlerskov et al., 2012).
Receptor‐mediated NOX activation in microglia
Microglia are endowed with an enormous array of sensing receptors (Hanisch and Kettenmann, 2007; Hickman et al., 2013) that guide each cell to assume an activation status that is finely tuned to the environment. In this section, selected examples of microglial surface receptors are described, in which NOX expression and ROS generation have been implicated in signalling and/or disease. For more detailed information on signalling pathways in microglia, the reader is referred to a recent review on immune cell receptor expression (Hu et al., 2014).
Toll‐like receptors (TLR)
The TLR family of pattern recognition receptors binds a variety of endogenous ligands that are relevant in innate immune responses. The TLRs mediate their effect through two arms of signal transduction, depending on the requirement for myeloid differentiation factor 88 (MyD88), leading to activation of MAPK and NF‐kB and transcription of IFNβ genes. Microglia express most TLRs, and their expression levels are altered by microglia activation (Olson and Miller, 2004; Jack et al., 2005; McKimmie and Fazakerley, 2005). TLRs contribute to neuropathology, because of association with disease‐related molecules that include amyloid β (Aβ; Jana et al., 2008), α‐synuclein (Fellner et al., 2012; Codolo et al., 2013; Kim et al., 2013), mutant SOD1 (Liu et al., 2009; Zhao et al., 2010), oxidized high‐mobility group box protein 1 (Agalave et al., 2014), oxidized phospholipids (Imai et al., 2008), galectin‐3 (Burguillos et al., 2015) and gangliosides (Jou et al., 2006). In many cases, NOX expression and oxidant production are up‐regulated by these ligands (Imai et al., 2008; Liu et al., 2009; Zhao et al., 2010; Fellner et al., 2012; Codolo et al., 2013). However, in some neuropathological conditions where genetic evidence points to a role for TLRs and oxidant production, the endogenous ligands remain unknown, for example, in cerebral ischaemia–reperfusion injury (Abe et al., 2010; Suzuki et al., 2012).
While TLR signalling is believed to set the level of oxidant production as a component of a general phagocyte activation programme, TLR activation induces a small, acute oxidant burst. It has been found that TLRs rely on NOX activity to initiate signalling. For instance, NOX‐derived ROS directly regulate the partitioning of TLRs to lipid rafts in the membrane (Nakahira et al., 2006; Wong et al., 2009) or promote the assembly of signalling complexes (Matsuzawa et al., 2005; Yang et al., 2008), which are required for efficient signalling. In this respect, the direct physical interaction between TLR2 and either NOX1 or NOX2, and TLR4 and NOX4 in phagocyte and mesenchymal cell types may be important (Park et al., 2004; Yang et al., 2009; Suzuki et al., 2012; Lee et al., 2013a). Additionally, signalling through TLRs is important for priming (hyperresponsiveness) of NOX2. Thus, bacterial LPS induce the recruitment of cytosolic phox proteins to the membrane (DeLeo et al., 1998) by the phosphorylation of Ser345 in p47phox (Dang et al., 2006). Priming of NOX2 downstream of other cell surface receptors is often dependent on p38MAPK and ERK (El Benna et al., 1996; Forsberg et al., 2001); however, TLR signalling also activates IL‐1 receptor‐associated kinase 4 (IRAK4) in the MyD88‐dependent TLR signalling axis. IRAK4 not only primes NOX but also phosphorylates p47phox on several residues to activate the oxidase directly (Pacquelet et al., 2007), which correlates with the inability of IRAK4‐deficient neutrophils to prime and activate NOX2 (Picard et al., 2003), and the dependency of TLR‐mediated NOX activation on the MyD88 signalling arm of TLRs (Laroux et al., 2005). TLR engagement also leads to activation of GTP/GDP exchange factor VAV, which mediates nucleotide exchange on Rac1, a catalytic subunit of the NOX complex (Miletic et al., 2007). In addition, Rac1 activated downstream of TLRs also activates p38MAPK, which may participate in mobilization of p47phox (Miletic et al., 2007).
Complement receptor 3 (CR3; CD11b/CD18)
LPS‐mediated activation of microglia has been used as a model in numerous studies of neuroinflammation. Infusion of LPS into the mouse brain causes wide‐spread microglia activation, increased oxidant production and development of a Parkinsonian‐like brain disease with loss of (selectively vulnerable) dopaminergic neurons (Gao et al., 2002; Gao et al., 2003a). Similarly, microglial activation and ROS production are features of in vitro models of Parkinson's disease (Gao et al., 2003a; Kim et al., 2007b; Rodriguez‐Pallares et al., 2008). Significantly, disease progression is inhibited in NOX2‐deficient animals (Gao et al., 2003b; Wu et al., 2003; Hernandes et al., 2013), which underlines the importance of ROS as a contributing factor in Parkinson's disease.
LPS is mostly a ligand for TLR4, acting in concert with CD14 and LPS binding protein. However, LPS stimulation of NOX2 activity in microglia mainly occurs through binding of LPS to complement receptor 3 (CR3) (Qin et al., 2005b; Pei et al., 2007). CR3 is composed of CD11b (integrin αM) and CD18 (integrin β2) subunits. CD11b is a frequently used histopathological marker for microglia activation, and its expression is partly regulated by NOX2 and inducible NO synthase (Roy et al., 2008). CR3 also acts as a phagocytic receptor for C3b/iC3b‐opsonized targets including endogenous targets such as synapses (Schafer et al., 2012) and neurites (Linnartz et al., 2012). The correlation between CR3 ligation and NOX2 activation in neutrophils and other phagocytes, even in the absence of phagocytosis, is well established (Lofgren et al., 1999; Serrander et al., 1999). Integrins can signal on their own, but leukocyte integrins (β2 integrins) pair up with receptors such as DAP12 or FcγRs (Zhou and Brown, 1994) containing an immunoreceptor tyrosine‐based activation motif, which direct Src kinase‐mediated signal transduction (Linnartz and Neumann, 2013). Proximal signal transduction arms involve Syk, Vav and PLC, the latter supporting PKC activation through inositol trisphosphate and DAG release.
Furthermore, as in the case of TLR signalling, NOX2‐derived oxidants are implicated in the redox regulation of signalling pathways downstream of CR3 ligation in macrophages, for example, MAPK and NF‐kB activation (Zhou et al., 2013).
In terms of pathology, an interaction between CR3 and fibrinogen is important in axonal damage and demyelination (Adams et al., 2007). Part of this pathology is likely to derive from the CR3‐induced and fibrinogen‐induced activation of microglial ROS production (as measured by fluorescent probes by two‐photon microscopy) (Davalos et al., 2012). The high‐mobility group box 1 protein (HMGB1) is normally not present in the CNS extracellular space but acts as a damage‐associated molecular pattern protein in neuropathological states and binds to CR3 to elicit oxidant production from microglia (Gao et al., 2011), leading to death of neurons. Binding of aggregated α‐synuclein to CR3 also induces NOX‐mediated oxidant release from microglia (Zhang et al., 2005) (Figure 3), leading to a redox‐mediated regulation of the non‐receptor tyrosine kinase Lyn to evoke chemotaxis (Wang et al., 2015c). It is worth noting that zebrafish phagocytes chemotactically respond to gradients of H2O2 released from transformed or damaged cells in living tissues (Niethammer et al., 2009; Feng et al., 2010). Interestingly, Lyn is an important redox sensor in this context and may be activated by both exogenous (Yoo et al., 2011) or endogenously produced ROS (Wang et al., 2015c) to effect chemotaxis. As yet, no direct correlate for this phenomenon has been discovered in the mammalian CNS.
Figure 3.
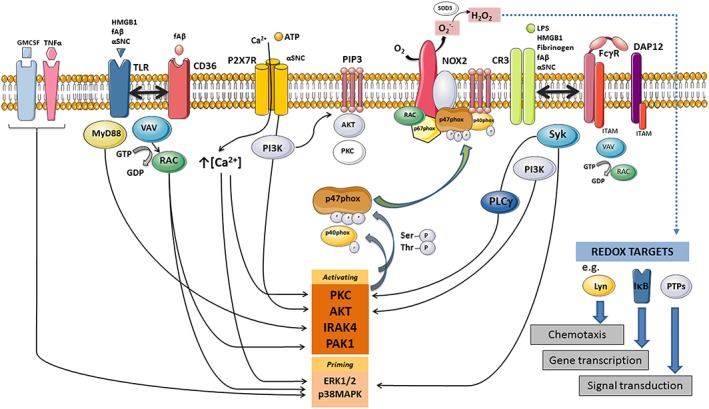
Activating microglia cell surface receptors, proximal signalling proteins and NOX2 activation by p47phox phosphorylation and Rac1 nucleotide exchange. Note that the figure is meant to organize activating surface receptors, their ligands and second messengers discussed in the review and that several important regulators of NOX activity have been omitted for clarity. The rate‐limiting step for NOX2 activation in microglia is activation of cytosolic subunit p47phox by phosphorylation of a number or serine and threonine residues in an auto‐inhibitory region of p47phox. Depending on the specific residues, phosphorylated p47phox can become ‘primed’ by different kinases including IRAK4, p38MAPK and ERK1/2, while full activation requires a number of residues to become phosphorylated by kinases such as PKC, Akt, IRAK4 or p21‐activated kinase 1 (PAK1). In some instances, for example, following FcγR signalling, p40phox rather than p47phox phosphorylation is required for mobilization of p67phox to the membrane. PI3K phosphorylates PI lipids in the membrane to produce PIP3, which serves as a recruitment factor of both regulatory proteins such as AKT and PKC isoforms, and certain GTP/GDP exchange factors, in addition to retaining mobilized p40phox and p47phox at the membrane through interactions with their PX domains. PLC contributes to activation by producing inositol trisphosphate, which increases cytosolic calcium levels, and DAG, which activates several PKC isoforms. In macrophages, GTP/GDP nucleotide exchange on Rac1 is performed by VAV isoforms. Released superoxide dismutates to H2O2 either spontaneously or through the catalytic activity of extracellular superoxide dismutase (SOD3) but can diffuse into the cytosol through the membrane either directly or through aquaporin channels (Hara‐Chikuma et al., 2015) to alter activity of cytosolic redox targets. In this kind of autocrine redox modification, extracellular catalase, but not SOD, deactivates the response (Pawate et al., 2004; Mander et al., 2006; Wang et al., 2015c).
Ionotropic and metabotropic purinergic receptors
Ionotropic P2X and metabotropic P2Y purinergic receptors are important for regulation of the microglial actin cytoskeleton, which controls various cellular functions, such as process motility, migration, pinocytosis and phagocytosis (Madry and Attwell, 2015). In microglia, the expression pattern of purinergic receptors differs significantly from that of peripheral macrophages (Hickman et al., 2013). This suggests that microglia have adapted for specific perception of cell damage or messenger molecules secreted by neurons or astrocytes. There is firm evidence that stimulation of the ionotropic P2X7 receptor by ATP induces ROS production and release in microglia. However, formal proof of an association between P2X7 and NOX activity has yet to be provided (Parvathenani et al., 2003; Mead et al., 2012).
Calcium entry is required for signalling and ROS production to occur following ATP stimulation in microglia and macrophages (Kim et al., 2007a; Martel‐Gallegos et al., 2013). An increase in intracellular calcium also increases NOX activity through ERK1/2‐dependent (Apolloni et al., 2013; Martel‐Gallegos et al., 2013), p38MAPK‐dependent and PI3K‐dependent (Parvathenani et al., 2003) pathways. Signalling through these kinases in macrophages is reinforced by NOX‐derived H2O2 (Cruz et al., 2007). In vivo, the source of ATP that stimulates microglial activation and ROS production could be derived from neurons or other glia but may also be provided in an autocrine fashion, because it is known that microglia secrete ATP in response to certain stimuli (Kim et al., 2007a; Higashi et al., 2011; Pascual et al., 2012).
Activation of microglial purinergic receptors has also been implicated in neurodegenerative disease (Parvathenani et al., 2003). Indeed, Aβ‐mediated and α‐synuclein‐mediated activation of microglia is reduced in P2X7 (Parvathenani et al., 2003; Kim et al., 2007a; Jiang et al., 2015) or P2Y receptor‐deficient cells (Kim et al., 2012). A role for NOX in this activation pattern has been proposed in several cases (Parvathenani et al., 2003; Kim et al., 2007a; Jiang et al., 2015). For example, a direct association between α‐synuclein and P2X7 receptor stimulation is thought to involve downstream signalling through PI3K, leading to NOX activation (Jiang et al., 2015).
Activating neurotransmitter receptors
Microglia express a large number of neurotransmitter receptors in their surveying and activated states (Pocock and Kettenmann, 2007; Kettenmann et al., 2013). However, the first studies that established NOX2 as the primary source of NMDA receptor‐induced O2 − production were performed on cultured neurons and mouse hippocampus and neocortex (Girouard et al., 2009; Brennan et al., 2009). Later, it was observed that NOX was activated by agonists of glutamate metabotropic (mGlu3 and group III), GABAA and purinergic P2X7 or mGlu5 receptors in the rodent BV2 microglial cell line (Mead et al., 2012). A neuroprotective microglial phenotype was promoted by GABA and purinergic receptor stimulation, whereas activation of NOX by glutamate receptor agonists had the opposite effect. The results from a complementary study revealed that activation of microglia in vitro, following NMDA receptor stimulation, was accompanied by secretion of ROS (and pro‐inflammatory cytokines) that was toxic to neurons (Kaindl et al., 2012). It has also been shown that, in mesencephalic mixed cultures, substance P exerts neurotoxicity, mediated via microglial NOX activation (Block et al., 2006). Moreover, dynorphin‐derived peptides inhibit NOX2‐mediated intracellular and extracellular ROS production following LPS stimulation of microglia (Qin et al., 2005a). Overall, it is evident that disease‐associated disruptions to neurotransmitter systems have a significant effect on the activation state of microglia and, consequently, neuronal integrity. Information on receptors that couple negatively to NOX is provided in the following section on NOX inhibition.
CD36
CD36 is a class B scavenger receptor that is essential for NOX‐mediated oxidant production in microglia activated with Aβ (Bianca et al., 1999; Coraci et al., 2002). Further work revealed that CD36 on microglia engages Aβ in conjunction with cell surface receptors CD47 and integrin α6β1, which are all required for binding, signalling and oxidant production (Bamberger et al., 2003). Downstream signalling involves tyrosine kinase activation (Bamberger et al., 2003; Wilkinson et al., 2006) of Rac1 GDP/GTP exchange factor VAV1 (Wilkinson et al., 2006) to elicit NOX activation. However, CD36 also induces VAV–Rac1 signalling (and MAPK38 activation) and oxidant production following stimulation with Aβ in concert with TLR2 and TLR4 (Reed‐Geaghan et al., 2009). CD36 also acts as a co‐receptor for TLR ligands in the setting of CNS ischaemic insults (Abe et al., 2010; Park et al., 2011) where CD36 is required for development of vascular oxidative stress (Cho et al., 2005) although endogenous TLR ligands have not been defined.
NOX inhibition
Endogenous mechanisms
There are a number of physiological mechanisms that decrease NOX activity. TGFβ signalling opposes expression of inflammatory mediators in microglia (Paglinawan et al., 2003) and induces a quiescent phenotype in microglia or recruited myeloid cells in organotypic hippocampal cultures (Abutbul et al., 2012). However, only recently has the importance of TGFβ as a major determinant of both mouse and human microglial gene expression and physiology been appreciated (Butovsky et al., 2014). To date, the correlation between the anti‐inflammatory activity of TGFβ and NOX activity in microglia (Qian et al., 2008) has not been explored. TGFβ mediates NOX2 inhibition through inhibition of Ser345 phosphorylation of p47phox (Qian et al., 2008), a phosphorylation event, which is known to prime the NOX2 system and greatly increase ROS release to subsequent stronger stimuli (Dang et al., 2006). IL‐10, another anti‐inflammatory compound, affords neuroprotection and reduces NOX2 activity by impeding JAK activation and mobilization of p47phox to the membrane following LPS challenge (Qian et al., 2006).
An important antioxidative protein that is induced by alternative (IL‐4) activation of microglia and macrophages is haem oxygenase 1, which degrades haem to free iron, biliverdin and CO. Carbon monoxide thus produced in turn inhibits NOX2 in macrophages, maybe by binding to gp91phox, and blunts LPS‐induced inflammatory responses and other TLR signalling events, by preventing lipid raft recruitment of TLRs required for signalling (Nakahira et al., 2006). Bilirubin formed from biliverdin in an additional step also inhibits NOX2 activity required for induction of inducible NO synthase expression in macrophages (Lanone et al., 2005), while the haem degradation in itself is limiting for the incorporation into NOX (Taille et al., 2004).
Recently, the membrane‐bound factor Slamf8 was found to negatively regulate NOX2 activity in response to a large range of stimuli in murine macrophages (Wang et al., 2012b). Slamf8 could be identical to the NOX2 repressive membrane factor previously described in dendritic cells (Elsen et al., 2004). In Slamf8(−/−) macrophages, excessive phosphorylation of cytosolic subunit p40phox was observed, which correlates with an enhanced O2 − production (Wang et al., 2012b). On the other hand, Slamf1, through interaction with Beclin and PI3K, may enhance NOX2 activity in response to certain stimuli (Ma et al., 2012). Reciprocal signalling through these two receptors to differentially regulate NOX activity was found to affect migration of myeloid cells (monocytes, macrophages and dendritic cells) in vivo. Moreover, in Slamf8 (−/−) cells, the accelerated migration rate was abolished in diphenyleneiodonium (DPI)‐treated animals (Wang et al., 2015a).
A number of studies highlight the association between glutamate stimulation of microglia and decreased NOX activity (Loane et al., 2009). In microglia, mGlu3 and mGlu5 are the dominant metabotropic glutamate receptors expressed (Pocock and Kettenmann, 2007; Berger et al., 2012), and of these, the mGlu5 receptor is emerging as a potential target to modify microglial activation and prevent neuronal apoptosis in a number of experimental models of microglial cell activation. Studies of traumatic brain injury in mice or subarachnoid haemorrhage in rats have provided evidence that pharmacological activation of mGlu5 receptors reduces microglial activation and promotes neuronal survival (Loane et al., 2013; Zhang et al., 2015). The results have been replicated using brain‐permeable, positive allosteric modulators of mGlu5 receptors with greater potency (Loane et al., 2014; Xue et al., 2014). Data suggest that the benefits of mGlu receptor activation derive from NOX inhibition (Loane et al., 2013), because the selective mGlu receptor agonist, (RS)‐2‐chloro‐5‐hydroxyphenylglycine, reduced NF‐κB activity and nitrite production in LPS‐stimulated microglia but was ineffective in NOX2 deficient (gp91(phox−/−)) cultures. In complementary work, Chantong and colleagues conversely showed that blockade of mGlu5 receptors using 2‐methyl‐6‐(phenylethynyl)‐pyridine in BV‐2 microglial cells induces a stress response characterized by increased intracellular ROS, mitochondrial ROS production and enhanced expression of inducible NOS and IL‐6 (Chantong et al., 2014).
Pharmacological inhibitors
Because of the involvement of NOX activity in a large array of diseases, there is considerable interest in developing suitable NOX inhibitors, in particular, for microglial NOX2 activity. A large number of potentially therapeutic molecules have been reported to act through NOX inhibition in microglia, including ligands that bind μ‐opioid receptor (Liu et al., 2000; Qin et al., 2005a; Qian et al., 2007b; Qian et al., 2007c; Wang et al., 2012c; Yang et al., 2014), neurotransmitter receptors (Moon et al., 2008; Hu et al., 2012), other receptors (Liu et al., 2003; Zhou et al., 2008; Chechneva et al., 2011; Chung et al., 2012), ion channels (Li et al., 2009b; Liu et al., 2011) and general anti‐inflammatory drugs (Colton and Chernyshev, 1996; Choi et al., 2005; Huo et al., 2011; Wang et al., 2012a).
However, careful evaluation through a stringent flow chart involving the use of ROS‐measuring probes, NOX‐associated oxygen consumption and semi‐recombinant assays (Jaquet et al., 2009; Zielonka et al., 2014; Hirano et al., 2015) has so far validated only a handful of molecules with unequivocal NOX inhibitory activity. DPI inhibits all forms of NOX in vitro in the low micromolar range (Jaquet et al., 2011), but its use in vivo is limited by high toxicity (Cooper et al., 1988). DPI is a potent flavoprotein inhibitor, which non‐specifically and irreversibly inhibits FAD‐mediated electron transfer. It therefore inhibits other flavin‐containing enzymes, such as NO synthase and mitochondrial cytochromes. However, recent studies indicate that almost homeopathic doses (subpicomolar concentrations) of DPI can exert neuroprotective effects in mixed neuron‐glia cultures (Qian et al., 2007a; Wang et al., 2014) or in animal models of Parkinson's disease (Wang et al., 2015b). DPI at these concentrations did not affect several cytosolic or mitochondrial flavoproteins (Wang et al., 2014; Wang et al., 2015b) but did reduce phorbol 12‐myristate 13‐acetate‐induced and LPS‐induced NOX2 activity and oxidative stress, as assessed by 4‐hydroxynonenal staining. However, no stringent proof of a direct effect of DPI on NOX at these concentrations was provided.
Celastrol inhibits NOX activity in vitro (Jaquet et al., 2011) but appeared inactive on NOX activity following in vivo administration (Hirano et al., 2015). N‐substituted phenothiazines show NOX inhibitory activity in the low micromolar range in vitro (Seredenina et al., 2015) and cross the blood–brain barrier; however, similarly to celastrol, they contain highly promiscuous chemical structures and thus interfere with many pharmacological targets, making it difficult to distinguish NOX inhibitory activity from other pharmacological effects, such as dopamine receptor antagonism. The thioxo‐dihydroquinazolin‐one compound 43 has been forwarded as a validated potent NOX2 inhibitor (Zielonka et al., 2014) but is more likely a myeloperoxidase inhibitor (Li et al., 2015). Recently, a novel specific NOX2 inhibitor, GSK2795039, has been described (Hirano et al., 2015). It shows efficacy in vivo and can penetrate into the brain. It remains to be determined whether GSK2795039 or other newly discovered NOX inhibitors have therapeutic utility in CNS disease through microglial NOX2 inhibition.
Apocynin is often referred to as an NOX inhibitor. It penetrates the CNS and has shown therapeutic benefit in numerous CNS disorders, possibly by inhibiting microglia activation (Sorce and Krause, 2009). However, although it may prove useful as a therapeutic agent, evidence that it acts as a bona fide NOX inhibitor is lacking (Gatto et al., 2013). Apocynin more likely acts as an anti‐inflammatory drug or an antioxidant (Heumuller et al., 2008). Although antioxidant supplements have not reached therapeutic expectations (Schmidt et al., 2015), novel therapeutic antioxidant molecules are emerging for CNS disease, with a mode of action possibly involving dampening of microglial oxidant generation. Treatment with the potent antioxidant edaravone in patients with stroke and cerebral infarction is used in some countries (Isahaya et al., 2012; Kikuchi et al., 2013) and may be useful in treatment of Parkinson's disease as well (Yuan et al., 2008). With edaravone's effect attributed to its antioxidant properties, it seems strange that the effects on microglia NOX have not been directly investigated. N‐acetylcysteine (NAC) is another antioxidant with documented experimental neuroprotective effects but is currently only in clinical use for acetaminophen intoxication and as a mucolytic agent. N‐acetylcysteine amide is a modified compound with higher bioavailability, being able to cross the blood–brain barrier and the mitochondrial membrane and with a higher radical scavenging ability than NAC itself, that shows promising results and decreased neuronal degeneration and apoptosis following experimental penetrating traumatic brain injury (Gunther et al., 2015), but again, this compound has not been tested directly on NOX activation in microglia.
NOX in inflammasome activation and neurogenesis
The involvement of NOX activity in microglia in chronic neurodegenerative diseases or acute brain disorders such as ischaemia–reperfusion syndrome has recently been carefully reviewed (Saijo and Glass, 2011; Gao et al., 2012; Nayernia et al., 2014). Here, we discuss the recent implication of NOX‐derived oxidants in mechanisms relevant to neurological disease, namely, inflammasome activation and suppression of neurogenesis in the inflamed brain.
Inflammasome activation
The inflammasome is a multimeric protein conglomerate consisting of NOD‐like receptor family, pyrin domain containing 3 (NLRP3), ASC/PYCARD, and caspase‐1, which, when activated by diverse insults of both foreign and endogenous nature (Cruz et al., 2007; Tschopp and Schroder, 2010; Mead et al., 2012), cleaves the pro‐form of IL‐1β and IL‐18 to their active form for release. Regardless of stimulus, inflammasome activation requires ROS generation (Tschopp and Schroder, 2010), calcium mobilization (Murakami et al., 2012) and a decreased cytosolic K+ concentration. α‐synuclein aggregates trigger NLRP3‐dependent activation and release of IL‐1β in a ROS‐dependent manner in microglia (Codolo et al., 2013). Also extracellular challenge of microglia with amyotrophic lateral sclerosis mutants of 43 kDa TAR DNA binding protein up‐regulates the expression of gp91phox concurrent with inflammasome induction and IL‐1β secretion, but no mechanistic coupling was attempted (Zhao et al., 2015). The source of ROS for inflammasome activation has been debated for some time after the initial report of p22phox dependency (Dostert et al., 2008). However, later and more thorough studies making use of macrophages derived from chronic granulomatous disease patients with different forms of NOX deficiency (including p22phox required for NOX1–NOX4) found IL‐1β secretion to be undisturbed, or even enhanced (Meissner et al., 2010; van Bruggen et al., 2009; van de Veerdonk et al., 2010). None of the calcium‐activated NOX ‐ NOX5 and DUOX1 and 2 ‐ play a role in inflammasome activation (Rada et al., 2014). Therefore, mitochondria, or other systems of oxidant generation, are likely to be the source of ROS for inflammasome activation (Zhou et al., 2011). The exact role of ROS in the signalling cascade leading to inflammasome activation is unclear (Bauernfeind et al., 2011), although the recent identification of specific ROS targets is an important step forward in this respect (Zhou et al., 2010). Thioredoxin‐interacting protein normally associates with thioredoxin, but following oxidative modification, it is released and binds to and activates NLRP3, thereby providing a link between ROS and inflammasome activation (Zhou et al., 2010).
Neurogenesis
Microglia are present in the subventricular zone (SVZ) and the subgranular zone of the dentate gyrus of the hippocampus (Sato, 2015) where new neurons are born in the postnatal brain. Indeed, the specific recruitment of microglia to these zones by neuronal factors (Lelli et al., 2013; Arno et al., 2014) seems to indicate that microglia fulfil important roles (Ziv et al., 2006; Ueno et al., 2013; Shigemoto‐Mogami et al., 2014; Kizil et al., 2015). Here, NOX2 activity is important in mediating the microglial chemotactic response downstream of colony stimulating factor‐1 and VEGF receptor stimulation and in ensuring correct migration of microglia from lateral ventricles to SVZ (Lelli et al., 2013).
Alluringly, although not uncontested (Forsberg et al., 2013), it is assumed that neural progenitor cells maintain their potential for self‐renewal and neurogenesis by oxidant production. This occurs in part by signalling through the PI3K/Akt pathway that is sustained by NOX2‐mediated oxidant production (Le Belle et al., 2011). This takes place potentially downstream of vascular cell adhesion molecule (VCAM) ligation (necessary to maintain the neurogenic niche in the SVZ), which increases NOX2 expression and oxidant production (Kokovay et al., 2012).
Forebrain neurons that are still developing and maturing (relative to terminally differentiated forms) have a very low antioxidant defence, because of epigenetic down‐regulation of neuronal Nrf2 (Bell et al., 2015). In some cases, exogenous addition of H2O2 is sufficient to bring about alterations in self‐renewal and differentiation of progenitors (Le Belle et al., 2011). This naturally opens the question of whether activated microglia, via release of oxidants, could modulate either the maintenance of the neural stem cell population (Le Belle et al., 2011) or the differentiation of progenitors (Forsberg et al., 2013). In the MPTP+‐induced model of Parkinson's disease, reduced proliferation of neural progenitor cells correlates with the phase of maximal microglial activation. Moreover, in vitro experiments using co‐cultures of progenitors with microglia suggested that (apocynin‐sensitive) NOX‐derived ROS is important in the inhibition of progenitor proliferation through activation of neuronal glycogen synthase kinase β3, which affects the wnt/β‐catenin signalling pathway that is integral to neurogenesis (L'Episcopo et al., 2012). However, the correlation between neurogenesis and microglial activation is far from clear‐cut. Studies have shown that neurogenesis can be depressed (Ekdahl et al., 2003; Monje et al., 2003; Butovsky et al., 2005; Su et al., 2014; Kizil et al., 2015), enhanced (Cacci et al., 2008; Deierborg et al., 2010; Kizil et al., 2015) or unaltered (Heldmann et al., 2011; Ng et al., 2012) by microglia activation and neuroinflammation. Therefore, at present, the best defined role for microglia‐derived ROS in neurogenesis is the physiological culling of brain Purkinje cells (followed by phagocytosis) (Marin‐Teva et al., 2004) or surplus neurons in the hippocampus (Wakselman et al., 2008) during development, both processes being dependent on microglial NOX activity.
Open questions and conclusions
In the current literature, there are few attempts to separate the microglial NOX from that of neurons. There is no doubt that NOX2 is expressed at much higher levels in microglia than in nerve cells, with a correspondingly higher oxidant production when activated. However, the expression of NOX 2 has also been described in different neuronal populations, and its activation may cause cellular dysfunction and pathology that does not involve microglia (Nayernia et al., 2014). Neuronal NOX should thus be given serious consideration as a source of oxidative stress. Because of potential overlapping expression patterns of NOX1, NOX2 and NOX4 in microglia, other glia cells and different neuronal populations (Nayernia et al., 2014), it has not been possible by the use of currently existing global NOX knock‐out mouse strains to precisely identify the cellular sources of oxidants, their exact physiological role and involvement in pathology. Moreover, in neuropathological states, it is impossible to know, from the current evidence, whether a ‘surge’ of microglial oxidants would be more detrimental to a nerve cell than a smaller, but misplaced or badly timed, production of oxidants from within the nerve cell itself. Generation of mouse models with either inducible expression of NOX2 on a p22phox(−/−) background or conditional knock out of NOX2 specifically in microglia could aid in the elucidation of these questions. Further, the use of biologically encoded ROS sensors specifically expressed in neurons or microglia by transgenics or viral transduction would allow (i) separation of the cellular sources of ROS, (ii) separation of microglia‐mediated oxidative stress from other pro‐inflammatory functions of microglia on neuronal function and survival (to the extent that ROS generation in the absence of other activating signals would not induce an M1 phenotype) and (iii) analysis of the potential of microglia‐derived ROS to affect redox targets in the neuronal cytosol. Finally, as discussed at length in the accompanying review (Vilhardt et al., this issue), it will be important to resolve microglia‐fuelled oxidative stress into its components of direct oxidative damage to crucial neuronal proteins or deranged redox signalling (Jones and Sies, 2015). Perturbation of redox signalling circuits could either take place in microglia themselves to forward a pro‐inflammatory state or work in a paracrine mode to affect neuronal or cerebrovascular signalling networks either globally or locally.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The present work was supported by the European Cooperation in Science and Research (COST Action BM1203/EU‐ROS) and the European Community's Framework Programme (FP7/2007–2013) under grant 278611 (Neurinox).
Haslund‐Vinding, J. , McBean, G. , Jaquet, V. , and Vilhardt, F. (2017) NADPH oxidases in oxidant production by microglia: activating receptors, pharmacology and association with disease. British Journal of Pharmacology, 174: 1733–1749. doi: 10.1111/bph.13425.
References
- Abe T, Shimamura M, Jackman K, Kurinami H, Anrather J, Zhou P, et al. (2010). Key role of CD36 in toll‐like receptor 2 signaling in cerebral ischemia. Stroke 41: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutbul S, Shapiro J, Szaingurten‐Solodkin I, Levy N, Carmy Y, Baron R, et al. (2012). TGF‐beta signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia 60: 1160–1171. [DOI] [PubMed] [Google Scholar]
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. (2007). The fibrin‐derived gamma377‐395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 204: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, et al. (2014). Spinal HMGB1 induces TLR4‐mediated long‐lasting hypersensitivity and glial activation and regulates pain‐like behavior in experimental arthritis. Pain 155: 1802–1813. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM (2007). Local self‐renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10: 1538–1543. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Catalytic receptors. Br J Pharmacol 172: 5979–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G Protein‐Coupled Receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E, et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B (1999). Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 117: 145–152. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Chessa TA, Davidson K, Henderson RB, Walker S, Tolmachova T, et al. (2011). PtdIns3P and Rac direct the assembly of the NADPH oxidase on a novel, pre‐phagosomal compartment during FcR‐mediated phagocytosis in primary mouse neutrophils. Blood 116: 4978–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carri MT, Cozzolino M, et al. (2013). The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1‐G93A microglia model of amyotrophic lateral sclerosis. J Immunol 190: 5187–5195. [DOI] [PubMed] [Google Scholar]
- Arno B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, et al. (2014). Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun 5: 5611. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE (2003). A cell surface receptor complex for fibrillar beta‐amyloid mediates microglial activation. J Neurosci 23: 2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V (2011). Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 187: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K, Krause KH (2007). The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313. [DOI] [PubMed] [Google Scholar]
- Bell KF, Al‐Mubarak B, Martel MA, McKay S, Wheelan N, Hasel P, et al. (2015). Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat Commun 6: 7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JV, Dumont AO, Focant MC, Vergouts M, Sternotte A, Calas AG, et al. (2012). Opposite regulation of metabotropic glutamate receptor 3 and metabotropic glutamate receptor 5 by inflammatory stimuli in cultured microglia and astrocytes. Neuroscience 205: 29–38. [DOI] [PubMed] [Google Scholar]
- Bianca VD, Dusi S, Bianchini E, Dal Pra I, Rossi F (1999). beta‐amyloid activates the O‐2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer's disease. J Biol Chem 274: 15493–15499. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Chaumont F (2014). Aquaporin‐facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840: 1596–1604. [DOI] [PubMed] [Google Scholar]
- Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, et al. (2006). Potent regulation of microglia‐derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J. 20: 251–258. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS (2007). Microglia‐mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8: 57–69. [DOI] [PubMed] [Google Scholar]
- Bordt EA, Polster BM (2014). NADPH oxidase‐ and mitochondria‐derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radic Biol Med 76: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, et al. (2009). NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nature Neurosci 12: 857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Borutaite V (2012). There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion 12: 1–4. [DOI] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, et al. (2015). Genetic cell ablation reveals clusters of local self‐renewing microglia in the mammalian central nervous system. Immunity 43: 92–106. [DOI] [PubMed] [Google Scholar]
- Burguillos MA, Svensson M, Schulte T, Boza‐Serrano A, Garcia‐Quintanilla A, Kavanagh E, et al. (2015). Microglia‐secreted galectin‐3 acts as a Toll‐like receptor 4 ligand and contributes to microglial activation. Cell Rep . doi:10.1016/j.celrep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. (2014). Identification of a unique TGF‐beta‐dependent molecular and functional signature in microglia. Nat Neurosci 17: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. (2005). Microglia activated by IL‐4 or IFN‐gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31: 149–160. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone‐Cat MA, Anelli T, Biagioni S, Minghetti L (2008). In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia 56: 412–425. [DOI] [PubMed] [Google Scholar]
- Casano AM, Peri F (2015). Microglia: multitasking specialists of the brain. Dev Cell 32: 469–477. [DOI] [PubMed] [Google Scholar]
- Casas AI, Dao VT, Daiber A, Maghzal GJ, di Lisa F, Kaludercic N, et al. (2015). Reactive oxygen‐related diseases: therapeutic targets and emerging clinical indications. Antioxid Redox Signal 23: 1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantong B, Kratschmar DV, Lister A, Odermatt A (2014). Inhibition of metabotropic glutamate receptor 5 induces cellular stress through pertussis toxin‐sensitive Gi proteins in murine BV‐2 microglia cells. J Neuroinflamm 11: 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechneva OV, Mayrhofer F, Daugherty DJ, Pleasure DE, Hong JS, Deng W (2011). Low dose dextromethorphan attenuates moderate experimental autoimmune encephalomyelitis by inhibiting NOX2 and reducing peripheral immune cells infiltration in the spinal cord. Neurobiol Dis 44: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, et al. (2008). Neurotoxic activation of microglia is promoted by a nox1‐dependent NADPH oxidase. J Neurosci 28: 12039–12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, et al. (2005). The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci 25: 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Chung ES, Hong YB, Kim SU, Jin BK (2005). Inhibition of thrombin‐induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. J Neurochem 95: 1755–1765. [DOI] [PubMed] [Google Scholar]
- Chung ES, Bok E, Chung YC, Baik HH, Jin BK (2012). Cannabinoids prevent lipopolysaccharide‐induced neurodegeneration in the rat substantia nigra in vivo through inhibition of microglial activation and NADPH oxidase. Brain Res 1451: 110–116. [DOI] [PubMed] [Google Scholar]
- Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, et al. (2013). Triggering of inflammasome by aggregated alpha‐synuclein, an inflammatory response in synucleinopathies. PLoS One 8 e55375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Chernyshev ON (1996). Inhibition of microglial superoxide anion production by isoproterenol and dexamethasone. Neurochem Int 29: 43–53. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Petty RK, Hayes DJ, Morgan‐Hughes JA, Clark JB (1988). Chronic administration of the oral hypoglycemic agent diphenyleneiodonium to rats. An animal model of impaired oxidative phosphorylation (mitochondrial myopathy). Biochem Pharmacol 37: 687–694. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, et al. (2002). CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta‐amyloid fibrils. Am J Pathol 160: 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM (2007). ATP activates a reactive oxygen species‐dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282: 2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, et al. (2006). A specific p47phox ‐serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest 116: 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752–758. [DOI] [PubMed] [Google Scholar]
- Davalos D, Ryu JK, Merlini M, Baeten KM, le Moan N, Petersen MA, et al. (2012). Fibrinogen‐induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierborg T, Roybon L, Inacio AR, Pesic J, Brundin P (2010). Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere‐derived cells into neurons and oligodendrocytes. Neuroscience 171: 1386–1396. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, et al. (1998). Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest 101: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerMardirossian C, Schnelzer A, Bokoch GM (2004). Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol Cell 15: 117–127. [DOI] [PubMed] [Google Scholar]
- Diebold BA, Bokoch GM (2001). Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol 2: 211–215. [DOI] [PubMed] [Google Scholar]
- Dostert C, Petrilli V, van Bruggen R, Steele C, Mossman BT, Tschopp J (2008). Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejlerskov P, Christensen DP, Beyaie D, Burritt JB, Paclet MH, Gorlach A, et al. (2012). NADPH oxidase is internalized by clathrin‐coated pits and localizes to a Rab27A/B GTPase‐regulated secretory compartment in activated macrophages. J Biol Chem 287: 4835–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 100: 13632–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Benna J, Dang PM, Gougerot‐Pocidalo MA, Marie JC, Braut‐Boucher F (2009). p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med 41: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Benna J, Han J, Park JW, Schmid E, Ulevitch RJ, Babior BM (1996). Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch Biochem Biophys 334: 395–400. [DOI] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. (2014). Colony‐stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82: 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen S, Doussiere J, Villiers CL, Faure M, Berthier R, Papaioannou A, et al. (2004). Cryptic O2—generating NADPH oxidase in dendritic cells. J Cell Sci 117: 2215–2226. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, et al. (2012). Toll‐like receptor 4 is required for alpha‐synuclein dependent activation of microglia and astroglia. Glia 2012: 22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P (2010). Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol 8: e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K, Wuttke A, Quadrato G, Chumakov PM, Wizenmann A, di Giovanni S (2013). The tumor suppressor p53 fine‐tunes reactive oxygen species levels and neurogenesis via PI3 kinase signaling. J Neurosci 33: 14318–14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg M, Lofgren R, Zheng L, Stendahl O (2001). Tumour necrosis factor‐alpha potentiates CR3‐induced respiratory burst by activating p38 MAP kinase in human neutrophils. Immunology 103: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B (2003a). Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci 23: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002). Microglial activation‐mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem 81: 1285–1297. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS (2003b). Critical role of microglial NADPH oxidase‐derived free radicals in the in vitro MPTP model of Parkinson's disease. FASEB J 17: 1954–1956. [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Hong JS (2012). NADPH oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci 33: 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS (2011). HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31: 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ Jr, Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, et al. (2013). NADPH oxidase‐dependent and ‐independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem 28: 95–104. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, et al. (2009). NMDA receptor activation increases free radical production through nitric oxide and NOX2. Neurosci 29: 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Chandler JD, Jones DP (2015). The cysteine proteome. Free Radic Biol Med 84: 227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther M, Davidsson J, Plantman S, Norgren S, Mathiesen T, Risling M (2015). Neuroprotective effects of N‐acetylcysteine amide on experimental focal penetrating brain injury in rats. J Clin Neurosci 22: 1477–1483. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10: 1387–1394. [DOI] [PubMed] [Google Scholar]
- Hara‐Chikuma M, Satooka H, Watanabe S, Honda T, Miyachi Y, Watanabe T, et al. (2015). Aquaporin‐3‐mediated hydrogen peroxide transport is required for NF‐kappaB signalling in keratinocytes and development of psoriasis. Nat Commun 6: 7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourd'heuil D, Mongon AA (2008). Activation of microglia with zymosan promotes excitatory amino acid release via volume‐regulated ion channels: the role of NADPH oxidases. J Neurochem 106: 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldmann U, Mine Y, Kokaia Z, Ekdahl CT, Lindvall O (2011). Selective depletion of Mac‐1‐expressing microglia in rat subventricular zone does not alter neurogenic response early after stroke. Exp Neurol 229: 391–398. [DOI] [PubMed] [Google Scholar]
- Helmcke I, Heumüller S, Tikkanen R, Schröder K, Brandes RP (2009). Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 11: 1279–1287. [DOI] [PubMed] [Google Scholar]
- Hernandes MS, Santos GD, Cafe‐Mendes CC, Lima LS, Scavone C, Munhoz CD, et al. (2013). Microglial cells are involved in the susceptibility of NADPH oxidase knockout mice to 6‐hydroxy‐dopamine‐induced neurodegeneration. PLoS One 8 e75532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann JM, Dick TP (2012). Redox biology on the rise. Biol Chem 393: 999–1004. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa‐Sicard E, Schmidt HH, Busse R, Schroder K, et al. (2008). Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, et al. (2013). The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 16: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Segawa S, Matsuo T, Nakamura S, Kikkawa Y, Nishida K, et al. (2011). Microglial zinc uptake via zinc transporters induces ATP release and the activation of microglia. Glia 59: 1933–1945. [DOI] [PubMed] [Google Scholar]
- Hirano K, Chen WS, Chueng AL, Dunne AA, Seredenina T, Filippova A, et al. (2015). Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxid Redox Signal 23: 358–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom KM, Finkel T (2014). Cellular mechanisms and physiological consequences of redox‐dependent signalling. Nat Rev Mol Cell Biol 15: 411–421. [DOI] [PubMed] [Google Scholar]
- Hu X, Liou AK, Leak RK, Xu M, An C, Suenaga J, et al. (2014). Neurobiology of microglial action in CNS injuries: receptor‐mediated signaling mechanisms and functional roles. Prog Neurobiol 119‐120: 60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhou H, Zhang D, Yang S, Qian L, Wu HM, et al. (2012). Clozapine protects dopaminergic neurons from inflammation‐induced damage by inhibiting microglial overactivation. J Neuroimmune Pharmacol 7: 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Rangarajan P, Ling EA, Dheen ST (2011). Dexamethasone inhibits the Nox‐dependent ROS production via suppression of MKP‐1‐dependent MAPK pathways in activated microglia. BMC Neurosci 12: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Yamaoka‐Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, et al. (2005). IQGAP1 regulates reactive oxygen species‐dependent endothelial cell migration through interacting with Nox2. Arterioscler Thromb Vasc Biol 25: 2295–2300. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kuba K, Neely GG, Yaghubian‐Malhami R, Perkmann T, van Loo G, et al. (2008). Identification of oxidative stress and Toll‐like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isahaya K, Yamada K, Yamatoku M, Sakurai K, Takaishi S, Kato B, et al. (2012). Effects of edaravone, a free radical scavenger, on serum levels of inflammatory biomarkers in acute brain infarction. J Stroke Cerebrovasc Dis 21: 102–107. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, et al. (2005). TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175: 4320–4330. [DOI] [PubMed] [Google Scholar]
- Jana M, Palencia CA, Pahan K (2008). Fibrillar amyloid‐beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol 181: 7254–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancic C, Savina A, Wasmeier C, Tolmachova T, El‐Benna J, Dang PM, et al. (2007). Rab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomes. Nat Cell Biol 9: 367–378. [DOI] [PubMed] [Google Scholar]
- Jaquet V, Marcoux J, Forest E, Leidal KG, McCormick S, Westermaier Y, et al. (2011). NADPH oxidase (NOX) isoforms are inhibited by celastrol with a dual mode of action. Br J Pharmacol 164: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD (2009). Small‐molecule NOX inhibitors: ROS‐generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11: 2535–2552. [DOI] [PubMed] [Google Scholar]
- Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, et al. (2015). P2X7 receptor is critical in alpha‐synuclein–mediated microglial NADPH oxidase activation. Neurobiol Aging 36: 2304–2318. [DOI] [PubMed] [Google Scholar]
- Jones DP, Sies H (2015). The redox code. Antioxid Redox Signal 23: 734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou I, Lee JH, Park SY, Yoon HJ, Joe EH, Park EJ (2006). Gangliosides trigger inflammatory responses via TLR4 in brain glia. Am J Pathol 168: 1619–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, et al. (2012). Activation of microglial N‐methyl‐D‐aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol 72: 536–549. [DOI] [PubMed] [Google Scholar]
- Kallenborn‐Gerhardt W, Schroder K, del Turco D, Lu R, Kynast K, Kosowski J, et al. (2012). NADPH oxidase‐4 maintains neuropathic pain after peripheral nerve injury. J Neurosci 32: 10136–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Kirchhoff F, Verkhratsky A (2013). Microglia: new roles for the synaptic stripper. Neuron 77: 10–18. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Verkhratsky A (2011). Neuroglia–living nerve glue. Fortschr Neurol Psychiatr 79: 588–597. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. (2013). Microglia emerge from erythromyeloid precursors via Pu.1‐ and Irf8‐dependent pathways. Nat Neurosci 16: 273–280. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Miura N, Kawahara KI, Murai Y, Morioka M, Lapchak PA, et al. (2013). Edaravone (Radicut), a free radical scavenger, is a potentially useful addition to thrombolytic therapy in patients with acute ischemic stroke. Biomed Rep 1: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, et al. (2013). Neuron‐released oligomeric alpha‐synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4: 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Gibson Wood W, et al. (2012). Nucleotides released from Abeta(1)(‐)(4)(2) ‐treated microglial cells increase cell migration and Abeta(1)(‐)(4)(2) uptake through P2Y(2) receptor activation. J Neurochem 121: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Moon JH, Lee HG, Kim SU, Lee YB (2007a). ATP released from beta‐amyloid‐stimulated microglia induces reactive oxygen species production in an autocrine fashion. Exp Mol Med 39: 820–827. [DOI] [PubMed] [Google Scholar]
- Kim YS, Choi DH, Block ML, Lorenzl S, Yang L, Kim YJ, et al. (2007b). A pivotal role of matrix metalloproteinase‐3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J 21: 179–187. [DOI] [PubMed] [Google Scholar]
- Kizil C, Kyritsis N, Brand M (2015). Effects of inflammation on stem cells: together they strive? EMBO Rep 16: 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, et al. (2012). VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11: 220–230. [DOI] [PubMed] [Google Scholar]
- L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Deleidi M, et al. (2012). Plasticity of subventricular zone neuroprogenitors in MPTP (1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine) mouse model of Parkinson's disease involves cross talk between inflammatory and Wnt/beta‐catenin signaling pathways: functional consequences for neuroprotection and repair. J Neurosci 32: 2062–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD (2004). NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Neish AS (2014). Nox enzymes and new thinking on reactive oxygen: a double‐edged sword revisited. Annu Rev Pathol 9: 119–145. [DOI] [PubMed] [Google Scholar]
- Lanone S, Bloc S, Foresti R, Almolki A, Taille C, Callebert J, et al. (2005). Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J 19: 1890–1892. [DOI] [PubMed] [Google Scholar]
- Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C (2005). Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram‐negative bacteria. J Immunol 175: 5596–5600. [DOI] [PubMed] [Google Scholar]
- le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, et al. (2011). Proliferative neural stem cells have high endogenous ROS levels that regulate self‐renewal and neurogenesis in a PI3K/Akt‐dependant manner. Cell Stem Cell 8: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Joo JH, Kim J, Lim HJ, Kim S, Curtiss L, et al. (2013a). Interaction of NADPH oxidase 1 with Toll‐like receptor 2 induces migration of smooth muscle cells. Cardiovasc Res 99: 483–493. [DOI] [PubMed] [Google Scholar]
- Lee SF, Ullevig S, Kim HS, Asmis R (2013b). Regulation of monocyte adhesion and migration by Nox4. PLoS One 8 e66964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli A, Gervais A, Colin C, Cheret C, Ruiz de Almodovar C, Carmeliet P, et al. (2013). The NADPH oxidase Nox2 regulates VEGFR1/CSF‐1R‐mediated microglial chemotaxis and promotes early postnatal infiltration of phagocytes in the subventricular zone of the mouse cerebral cortex. Glia 61: 1542–1555. [DOI] [PubMed] [Google Scholar]
- Li B, Bedard K, Sorce S, Hinz B, Dubois‐Dauphin M, Krause KH (2009a). NOX4 expression in human microglia leads to constitutive generation of reactive oxygen species and to constitutive IL‐6 expression. J Innate Immun 1: 570–581. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA (2005). Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A 102: 9936–9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ganesh T, Diebold BA, Zhu Y, McCoy JW, Smith SM, et al. (2015). Thioxo‐dihydroquinazolin‐one compounds as novel inhibitors of myeloperoxidase. ACS Med Chem Lett 6: 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu X, Liu Y, Bao Y, An L (2009b). Nimodipine protects dopaminergic neurons against inflammation‐mediated degeneration through inhibition of microglial activation. Neuropharmacol 56: 580–589. [DOI] [PubMed] [Google Scholar]
- Linnartz B, Kopatz J, Tenner AJ, Neumann H (2012). Sialic acid on the neuronal glycocalyx prevents complement C1 binding and complement receptor‐3‐mediated removal by microglia. J Neurosci 32: 946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnartz B, Neumann H (2013). Microglial activatory (immunoreceptor tyrosine‐based activation motif)‐ and inhibitory (immunoreceptor tyrosine‐based inhibition motif)‐signaling receptors for recognition of the neuronal glycocalyx. Glia 61: 37–46. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Hong JS (2000). Naloxone protects rat dopaminergic neurons against inflammatory damage through inhibition of microglia activation and superoxide generation. J Pharmacol Exp Ther 293: 607–617. [PubMed] [Google Scholar]
- Liu Y, Hao W, Dawson A, Liu S, Fassbender K (2009). Expression of amyotrophic lateral sclerosis‐linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J Biol Chem 284: 3691–3699. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lo YC, Qian L, Crews FT, Wilson B, Chen HL, et al. (2011). Verapamil protects dopaminergic neuron damage through a novel anti‐inflammatory mechanism by inhibition of microglial activation. Neuropharmacol 60: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Qin L, Li G, Zhang W, An L, Liu B, et al. (2003). Dextromethorphan protects dopaminergic neurons against inflammation‐mediated degeneration through inhibition of microglial activation. J Pharmacol Exp Ther 305: 212–218. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Byrnes KR, Jeong W, Faden AI (2013). Activation of mGluR5 and inhibition of NADPH oxidase improves function recovery after traumatic brain injury. J Neurotrauma 30: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Pajoohesh‐Ganji A, Byrnes KR, Faden AI (2009). Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem 284: 15629–15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Stoica BA, Tchantchou F, Kumar A, Barrett JP, Akintola T, et al. (2014). Novel mGluR5 positive allosteric modulator improves functional recovery, attenuates neurodegeneration and alters microglial polarization after experimental traumatic brain injury. Neurotherapeutics 11: 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren R, Serrander L, Forsberg M, Wilsson A, Wasteson A, Stendahl O (1999). CR3, FcgammaRIIA and FcgammaRIIIB induce activation of the respiratory burst in human neutrophils: the role of intracellular Ca(2+), phospholipase D and tyrosine phosphorylation. Biochim Biophys Acta 1452: 46–59. [DOI] [PubMed] [Google Scholar]
- Ma C, Wang N, Detre C, Wang G, O'Keeffe M, Terhorst C (2012). Receptor signaling lymphocyte‐activation molecule family 1 (Slamf1) regulates membrane fusion and NADPH oxidase 2 (NOX2) activity by recruiting a Beclin‐1/Vps34/ultraviolet radiation resistance‐associated gene (UVRAG) complex. J Biol Chem 287: 18359–18365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madry C, Attwell D (2015). Receptors, ion channels, and signaling mechanisms underlying microglial dynamics. J Biol Chem 290: 12443–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander PK, Jekabsone A, Brown GC (2006). Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol 176: 1046–1052. [DOI] [PubMed] [Google Scholar]
- Marin‐Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M (2004). Microglia promote the death of developing Purkinje cells. Neuron 41: 535–547. [DOI] [PubMed] [Google Scholar]
- Martel‐Gallegos G, Casas‐Pruneda G, Ortega‐Ortega F, Sanchez‐Armass S, Olivares‐Reyes JA, Diebold B, et al. (2013). Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c‐Src/Pyk2 and ERK1/2. Biochim Biophys Acta 1830: 4650–4659. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, et al. (2005). ROS‐dependent activation of the TRAF6‐ASK1‐p38 pathway is selectively required for TLR4‐mediated innate immunity. Nat Immunol 6: 587–592. [DOI] [PubMed] [Google Scholar]
- McKimmie CS, Fazakerley JK (2005). In response to pathogens, glial cells dynamically and differentially regulate toll‐like receptor gene expression. J Neuroimmunol 169: 116–125. [DOI] [PubMed] [Google Scholar]
- Mead EL, Mosley A, Eaton S, Dobson L, Heales SJ, Pocock JM (2012). Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial‐neuronal interactions. J Neurochem 121: 287–301. [DOI] [PubMed] [Google Scholar]
- Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A (2010). Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116: 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic AV, Graham DB, Montgrain V, Fujikawa K, Kloeppel T, Brim K, et al. (2007). Vav proteins control MyD88‐dependent oxidative burst. Blood 109: 3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302: 1760–1765. [DOI] [PubMed] [Google Scholar]
- Moon JH, Kim SY, Lee HG, Kim SU, Lee YB (2008). Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1‐42)‐stimulated microglia. Exp Mol Med 40: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. (2012). Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109: 11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, et al. (2006). Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS‐induced trafficking of TLRs to lipid rafts. J Exp Med 203: 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayernia Z, Jaquet V, Krause KH (2014). New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal 20: 2815–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SY, Semple BD, Morganti‐Kossmann MC, Bye N (2012). Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. J Neurotrauma 29: 1410–1425. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ (2009). A tissue‐scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD (2004). Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173: 3916–3924. [DOI] [PubMed] [Google Scholar]
- Pacquelet S, Johnson JL, Ellis BA, Brzezinska AA, Lane WS, Munafo DB, et al. (2007). Cross‐talk between IRAK‐4 and the NADPH oxidase. Biochem J 403: 451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglinawan R, Malipiero U, Schlapbach R, Frei K, Reith W, Fontana A (2003). TGFbeta directs gene expression of activated microglia to an anti‐inflammatory phenotype strongly focusing on chemokine genes and cell migratory genes. Glia 44: 219–231. [DOI] [PubMed] [Google Scholar]
- Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS (2004). Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide‐induced production of reactive oxygen species and activation of NF‐kappa B. J Immunol 173: 3589–3593. [DOI] [PubMed] [Google Scholar]
- Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, et al. (2011). Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid‐beta. Proc Natl Acad Sci U S A 108: 5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R (2003). P2X7 mediates superoxide production in primary microglia and is up‐regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem 278: 13309–13317. [DOI] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A (2012). Microglia activation triggers astrocyte‐mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 109: E197–E205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR (2004). Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77: 540–551. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SPH, Buneman OP, et al. , NC‐IUPHAR(2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl Acids Res 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Pang H, Qian L, Yang S, Wang T, Zhang W, et al. (2007). MAC1 mediates LPS‐induced production of superoxide by microglia: the role of pattern recognition receptors in dopaminergic neurotoxicity. Glia 55: 1362–1373. [DOI] [PubMed] [Google Scholar]
- Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, et al. (2003). Pyogenic bacterial infections in humans with IRAK‐4 deficiency. Science 299: 2076–2079. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H (2007). Neurotransmitter receptors on microglia. Trends in Neurosci. 30: 527–535. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J (2014). Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15: 300–312. [DOI] [PubMed] [Google Scholar]
- Qian L, Block M, Wei SJ, Lin CF, Reece J, Pang H, et al. (2006). Interleukin‐10 protects lipopolysaccharide‐induced neurotoxicity in primary midbrain cultures by inhibiting the function of NADPH oxidase. J Pharmacol Exp Ther 319: 44–52. [DOI] [PubMed] [Google Scholar]
- Qian L, Gao X, Pei Z, Wu X, Block M, Wilson B, et al. (2007a). NADPH oxidase inhibitor DPI is neuroprotective at femtomolar concentrations through inhibition of microglia over‐activation. Parkinsonism Relat Disord 13 (Suppl 3): S316–S320. [DOI] [PubMed] [Google Scholar]
- Qian L, Tan KS, Wei SJ, Wu HM, Xu Z, Wilson B, et al. (2007b). Microglia‐mediated neurotoxicity is inhibited by morphine through an opioid receptor‐independent reduction of NADPH oxidase activity. J Immunol 179: 1198–1209. [DOI] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, et al. (2008). Potent anti‐inflammatory and neuroprotective effects of TGF‐beta1 are mediated through the inhibition of ERK and p47phox‐Ser345 phosphorylation and translocation in microglia. J Immunol 181: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Xu Z, Zhang W, Wilson B, Hong JS, Flood PM (2007c). Sinomenine, a natural dextrorotatory morphinan analog, is anti‐inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflamm 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, et al. (2005a). Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress. FASEB J 19: 550–557. [DOI] [PubMed] [Google Scholar]
- Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, et al. (2005b). Interactive role of the Toll‐like receptor 4 and reactive oxygen species in LPS‐induced microglia activation. Glia 52: 78–84. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, et al. (2004). NADPH oxidase mediates lipopolysaccharide‐induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279: 1415–1421. [DOI] [PubMed] [Google Scholar]
- Rada B, Park JJ, Sil P, Geiszt M, Leto TL (2014). NLRP3 inflammasome activation and interleukin‐1beta release in macrophages require calcium but are independent of calcium‐activated NADPH oxidases. Inflamm Res 63: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed‐Geaghan EG, Savage JC, Hise AG, Landreth GE (2009). CD14 and Toll‐like receptors 2 and 4 are required for fibrillar A{beta}‐stimulated microglial activation. J Neurosci 29: 11982–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira‐Garcia JL (2008). Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH‐derived ROS. Neurobiol Dis 31: 58–73. [DOI] [PubMed] [Google Scholar]
- Roepstorff K, Rasmussen I, Sawada M, Cudre‐Maroux C, Salmon P, Bokoch G, et al. (2008). Stimulus‐dependent regulation of the phagocyte NADPH oxidase by a VAV1, Rac1, and PAK1 signaling axis. J Biol Chem 283: 7983–7993. [DOI] [PubMed] [Google Scholar]
- Rojo AI, McBean G, Cindric M, Egea J, Lopez MG, Rada P, et al. (2014). Redox control of microglial function: molecular mechanisms and functional significance. Antioxid Redox Signal 21: 1766–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Jana A, Yatish K, Freidt MB, Fung YK, Martinson JA, et al. (2008). Reactive oxygen species up‐regulate CD11b in microglia via nitric oxide: implications for neurodegenerative diseases. Free Radic Biol Med 45: 686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK (2011). Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787. [DOI] [PubMed] [Google Scholar]
- Sato K (2015). Effects of microglia on neurogenesis. Glia 63: 1394–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron 74: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Stocker R, Vollbracht C, Paulsen G, Riley D, Daiber A, et al. (2015). Antioxidants in translational medicine. Antioxid Redox Signal 23: 1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredenina T, Chiriano G, Filippova A, Nayernia Z, Mahiout Z, Fioraso‐Cartier L, et al. (2015). A subset of N‐substituted phenothiazines inhibits NADPH oxidases. Free Radic Biol Med 86: 239–249. [DOI] [PubMed] [Google Scholar]
- Serrander L, Larsson J, Lundqvist H, Lindmark M, Fallman M, Dahlgren C, et al. (1999). Particles binding beta(2)‐integrins mediate intracellular production of oxidative metabolites in human neutrophils independently of phagocytosis. Biochim Biophys Acta 1452: 133–144. [DOI] [PubMed] [Google Scholar]
- Shigemoto‐Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K (2014). Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34: 2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Krause KH (2009). NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11: 2481–2504. [DOI] [PubMed] [Google Scholar]
- Sorce S, Nuvolone M, Keller A, Falsig J, Varol A, Schwarz P, et al. (2014). The role of the NADPH oxidase NOX2 in prion pathogenesis. PLoS Pathog 10 e1004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P, Zhang J, Zhao F, Aschner M, Chen J, Luo W (2014). The interaction between microglia and neural stem/precursor cells. Brain Res Bull 109: 32–38. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Hattori K, Hamanaka J, Murase T, Egashira Y, Mishiro K, et al. (2012). Pharmacological inhibition of TLR4‐NOX4 signal protects against neuronal death in transient focal ischemia. Sci Rep 2: 896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taille C, El‐Benna J, Lanone S, Dang MC, Ogier‐Denis E, Aubier M, et al. (2004). Induction of heme oxygenase‐1 inhibits NAD(P)H oxidase activity by down‐regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem 279: 28681–28688. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K (2010). NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, et al. (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16: 543–551. [DOI] [PubMed] [Google Scholar]
- Uriarte SM, Rane MJ, Luerman GC, Barati MT, Ward RA, Nauseef WM, et al. (2011). Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J Immunol 187: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, et al. (2009). Human NLRP3 inflammasome activation is Nox1‐4 independent. Blood 115: 5398–5400. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, et al. (2010). Reactive oxygen species‐independent activation of the IL‐1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A 107: 3030–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhardt F, van Deurs B (2004). The phagocyte NADPH oxidase depends on cholesterol‐enriched membrane microdomains for assembly. EMBO J 23: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lohneysen K, Noack D, Wood MR, Friedman JS, Knaus UG (2010). Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol 30: 961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A (2008). Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci 28: 8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhai H, Huang L, Li H, Xu Y, Qiao X, et al. (2012a). Aspirin protects dopaminergic neurons against lipopolysaccharide‐induced neurotoxicity in primary midbrain cultures. J Mol Neurosci 46: 153–161. [DOI] [PubMed] [Google Scholar]
- Wang G, Abadia‐Molina AC, Berger SB, Romero X, O'Keeffe MS, Rojas‐Barros DI, et al. (2012b). Cutting edge: Slamf8 is a negative regulator of Nox2 activity in macrophages. J Immunol 188: 5829–5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, van Driel BJ, Liao G, O'Keeffe MS, Halibozek PJ, Flipse J, et al. (2015a). Migration of myeloid cells during inflammation is differentially regulated by the cell surface receptors Slamf1 and Slamf8. PLoS One 10 e0121968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Chu CH, Oyarzabal E, Jiang L, Chen SH, Wilson B, et al. (2014). Subpicomolar diphenyleneiodonium inhibits microglial NADPH oxidase with high specificity and shows great potential as a therapeutic agent for neurodegenerative diseases. Glia 62: 2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Qian L, Chen SH, Chu CH, Wilson B, Oyarzabal E, et al. (2015b). Post‐treatment with an ultra‐low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson's disease models. Brain 138: 1247–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Rowan MJ, Anwyl R (2004). Beta‐amyloid‐mediated inhibition of NMDA receptor‐dependent long‐term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci 24: 6049–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhou H, Gao H, Chen SH, Chu CH, Wilson B, et al. (2012c). Naloxone inhibits immune cell function by suppressing superoxide production through a direct interaction with gp91phox subunit of NADPH oxidase. J Neuroinflamm 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chu CH, Stewart T, Ginghina C, Wang Y, Nie H, et al. (2015c). alpha‐Synuclein, a chemoattractant, directs microglial migration via H2O2‐dependent Lyn phosphorylation. Proc Natl Acad Sci U S A 112: E1926–E1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RA, Nakamura M, McLeish KR (2000). Priming of the neutrophil respiratory burst involves p38 mitogen‐activated protein kinase‐dependent exocytosis of flavocytochrome b558‐containing granules. J Biol Chem 275: 36713–36719. [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Koenigsknecht‐Talboo J, Grommes C, Lee CY, Landreth G (2006). Fibrillar beta‐amyloid‐stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J Biol Chem 281: 20842–20850. [DOI] [PubMed] [Google Scholar]
- Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH (2009). Fatty acids modulate Toll‐like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species‐dependent manner. J Biol Chem 284: 27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S (2006). The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A 103: 12132–12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson‐Lewis V, Ischiropoulos H, et al. (2003). NADPH oxidase mediates oxidative stress in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine model of Parkinson's disease. Proc Natl Acad Sci U S A 100: 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Stoica BA, Hanscom M, Kabadi SV, Ai f (2014). Positive allosteric modulators (PAMs) of metabotropic glutamate receptor 5 (mGLuR5) attenuate microglial activation. CNS Neurol Disord Drug Targets 13: 558–566. [DOI] [PubMed] [Google Scholar]
- Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, et al. (2009). NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol 182: 3696–3705. [DOI] [PubMed] [Google Scholar]
- Yang CS, Shin DM, Lee HM, Son JW, Lee SJ, Akira S, et al. (2008). ASK1‐p38 MAPK‐p47phox activation is essential for inflammatory responses during tuberculosis via TLR2‐ROS signalling. Cell Microbiol 10: 741–754. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu Y, Yuan F, Li Z, Huang S, Shen H, et al. (2014). Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol Immunol 60: 109–114. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Starnes TW, Deng Q, Huttenlocher A (2011). Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Ben‐Shachar D, Riederer P (1991). Iron in brain function and dysfunction with emphasis on Parkinson's disease. Eur Neurol 31 (Suppl 1): 34–40. [DOI] [PubMed] [Google Scholar]
- Yuan WJ, Yasuhara T, Shingo T, Muraoka K, Agari T, Kameda M, et al. (2008). Neuroprotective effects of edaravone‐administration on 6‐OHDA‐treated dopaminergic neurons. BMC Neurosci 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. (2005). Aggregated alpha‐synuclein activates microglia: a process leading to disease progression in Parkinson's disease. FASEB J 19: 533–542. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Sun BL, Liu JK, Yang MF, Li DW, Fang J, et al. (2015). Activation of mGluR5 attenuates microglial activation and neuronal apoptosis in early brain injury after experimental subarachnoid hemorrhage in rats. Neurochem Res 40: 1121–1132. [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Bell S, Wang J, Wen S, Baloh RH, et al. (2015). TDP‐43 activates microglia through NF‐kappaB and NLRP3 inflammasome. Exp Neurol 273: 24–35. [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, et al. (2010). Extracellular mutant SOD1 induces microglial‐mediated motoneuron injury. Glia 58: 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Liao J, Aloor J, Nie H, Wilson BC, Fessler MB, et al. (2013). CD11b/CD18 (Mac‐1) is a novel surface receptor for extracellular double‐stranded RNA to mediate cellular inflammatory responses. J Immunol 190: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang S, Zhao X, Wei T (2008). Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid‐beta1‐42. J Pineal Res 45: 157–165. [DOI] [PubMed] [Google Scholar]
- Zhou MJ, Brown EJ (1994). CR3 (Mac‐1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc gamma RIII and tyrosine phosphorylation. J Cell Biol 125: 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010). Thioredoxin‐interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225. [DOI] [PubMed] [Google Scholar]
- Zielonka J, Cheng G, Zielonka M, Ganesh T, Sun A, Joseph J, et al. (2014). High‐throughput assays for superoxide and hydrogen peroxide: design of a screening workflow to identify inhibitors of NADPH oxidases. J Biol Chem 289: 16176–16189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, et al. (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9: 268–275. [DOI] [PubMed] [Google Scholar]


