Abstract
Although third-line antiretroviral therapy (ART) is available in South Africa's public sector, its cost is substantially higher than first and second line. Identifying risk factors for failure on second-line treatment remains crucial to reduce the need for third-line drugs. We conducted a case–control study including 194 adult patients (≥18 years; 70 cases and 124 controls) who initiated second-line ART in Johannesburg, South Africa. Unconditional logistic regression was used to assess predictors of virologic failure (defined as 2 consecutive viral load measures ≥1000 copies/mL, ≥3 months after switching to second line). Variables included a social instability index, ART adherence, self-reported as well as diagnosed adverse drug reactions (ADRs), HIV disclosure, depression, and factors affecting access to HIV clinics. Overall 60.0% of cases and 54.0% of controls were female. Mean ages of cases and controls were 41.8 ± 9.6 and 43.3 ± 8.0, respectively. Virologic failure was predicted by ART adherence <90% [odds ratio (OR) 4.7; 95% confidence interval (95% CI): 2.1–10.5], younger age (<40 years of age; OR 0.6; 95% CI: 0.3–1.1), high social instability (OR 3.8; 95% CI: 1.30–11.5), self-reported ADR (OR 1.9; 95% CI: 1.0–3.5), disclosure to friends/colleagues rather than partner/relatives (OR 3.4; 95% CI: 1.3–9.1), and medium/high depression compared to low/no depression (OR 4.4; 95% CI: 1.5–13.4). Our results suggest complex socioeconomic factors contributing to risk of virologic failure, possibly by impacting ART adherence, among patients on second-line therapy in South Africa. Identifying patients with possible indicators of nonadherence could facilitate targeted interventions to reduce the risk of second-line treatment failure and mitigate the demand for third-line regimens.
Keywords: : HIV treatment, second-line regimen, South Africa, antiretroviral therapy, virologic failure
Introduction
Globally, the scale-up of antiretroviral therapy (ART) in the mid-2000s has dramatically reduced HIV-related morbidity and mortality and improved life expectancy.1–3 Despite high ART coverage in sub-Saharan Africa, recent evidence has shown that between 8% and 23% of patients will fail first-line ART by 5 years after initiation and will require transition to more expensive protease inhibitor (PI) based second-line ART regimens.4–6
Unlike in many resource-limited settings, second-line ART is readily available in South Africa, although at over double the cost of first-line ART.7 Hence, the rate of switching to second-line ART in South Africa is substantially higher than in other resource-limited settings.8–11 In 19 studies reporting outcomes of second-line ART in resource-limited settings, the proportion of adult patients who experienced virologic failure varied widely from 8.3% to 41.2% at 24 months.12 In South Africa, 23–35.6% of patients will experience treatment failure by 12 months on second-line ART.13–15 These proportions may be underestimated due to limited routine viral load (VL) monitoring in these settings or challenges with missing VL data.16 If these rates are sustained, the total number of patients on second-line regimens requiring very expensive third-line regimens will increase, stretching already constrained budgets. At current prices, third-line treatment costs about $1,300 per patient year in South Africa, about five times as much as first-line treatment.17
Clinical predictors of virologic failure among patients on second-line treatment include younger age and lower VL at the initiation of second-line ART, while shorter duration of viremia before the switch and higher CD4 count have been associated with virologic suppression.15 Virologic failure among patients on second-line ART is not driven by resistance to PIs. Among patients failing second-line ART (RNA >400 copies/mL) in the Themba Lethu clinical cohort, only 18% were found to have clinically meaningful resistance to lopinavir and 25% to atazanavir.18 Poor adherence is the most common behavioral predictor of treatment failure,13 yet the contextual factors and societal factors that present barriers to ART adherence are poorly understood, particularly among treatment-experienced patients on second-line ART.
In addition to clinical indicators of poor ART outcomes, understanding psychosocial and contextual predictors is necessary to inform interventions to improve ART adherence and outcomes, particularly among patients on second-line ART. The aim of this article is to determine predictors of virologic failure among HIV-positive patients on second-line ART in Johannesburg, South Africa, to identify intervention approaches to mitigate the need for third-line treatment.
Methods
Study design and population
We conducted a case–control study among HIV-positive adult patients who had been switched to second-line ART at the Themba Lethu HIV Clinic (TLC) in Johannesburg from April 2004 to January 2016.19 The Themba Lethu clinic is a large public sector HIV treatment site with nearly 2400 patients currently receiving second-line ART.19 The clinic receives technical support from Right to Care through the President's Emergency Plan for AIDS Relief program. Themba Lethu follows South Africa's National HIV Treatment Guidelines that recommend the use of triple therapy, including a non-nucleoside reverse transcriptase inhibitor (NNRTI) and two NRTIs for first-line therapy. Patients who fail first-line therapy (2 consecutive VL measures ≥1000 copies/mL, ≥3 months after switching to second-line therapy and 3 months apart) are switched to a second-line ART regimen, including two NRTIs and a PI, typically ritonavir-boosted lopinavir (LPV/r).20
Cases
We defined cases as patients who experienced a first virologic failure while on second-line ART from December 2013 onward. We defined virologic failure on second-line ART as having 2 consecutive VL measures above 1000 copies/mL at least 3 months apart and at least 3 months after switching to second-line therapy.
Controls
Controls were sampled using risk-set sampling from all subjects meeting the inclusion criteria who were in care on the date that a case was identified.21 Risk-set sampling samples controls from all subjects who are still at risk for the outcome (i.e., are in the risk set) at the time that a case becomes a case. We defined controls as patients who had not yet experienced a virologic failure at the date a case arose. Two controls who were still in care were enrolled for each case. We used, as controls, the next two eligible consenting patients who presented to the clinic after a case was enrolled. Both cases and controls provided informed consent.
Data collection
Consenting patients were enrolled in the study through referral from the attending physician and were interviewed on the day of enrolment using a structured interview. Enrolled participants consented to being interviewed and for researchers to access their medical records at the TLC. Study participants were asked to complete a questionnaire about events occurring in the month before the date of failure (date of first failing VL) for cases, and 1 month before the date of the last VL test for controls. All interviews were conducted in English.
The questionnaire was designed to collect demographic data (age, sex, education, and employment), factors affecting access to the clinic (distance from clinic, work hours, mobility, and transport methods), as well as experiences with HIV treatment, alcohol use, and HIV status disclosure. Participants were asked to indicate the proportion of antiretroviral (ARV) doses that they missed in the period of interest. Patients reporting 10% or more missed doses were considered to be nonadherent.22,23 Participants were also asked to indicate whether they had experienced a side effect while taking ART and if they did, to specify the side effect that they felt was the most serious (based on their symptoms) and indicate how well they coped with it. These self-reported adverse drug reactions (ADRs) were then categorized as follows: body changes, central nervous system (CNS)-related conditions (headache, sleeplessness, and dizziness), gastrointestinal problems (diarrhea and bloating), muscle/joint pains, and skin conditions.
Coping with ADR was assessed using a 7-item scale (Cronbach α: 0.73) developed by Johnson and Neilands.24 Depression was measured using the shortened CES-D scale (Cronbach α: 0.94).25 A social instability index was developed based on participants' living arrangements, their employment, food insecurity, and sexual partnership status and their dwelling type.26 A dichotomous variable was generated based on the above characteristics with one set to represent the negative responses (e.g., unemployed = 1 and employed = 0). A score was calculated by summing all the negative responses of the instability index, and categorized as low (0, 1), medium (2, 3), and high (4, 5) instability.
Data on clinical characteristics (both clinical conditions and laboratory tests) on second-line ART were collected from patients' medical records and were reported up to 6 months before the date of second-line failure for cases or the last VL date for controls. The following diagnosed conditions were considered to be possible ADRs: lipodystrophy, dermatitis, muscle/joint pain, neuropathy, gastroenteritis, gynaecomastia, hepatitis, or lactic acidosis. Anemia was defined as any hemoglobin result <11.5 g/dL for females and <13.0 g/dL for males. Creatinine clearance was measured in mL/min and was considered to be low if <88 mL/min among females and <97 mL/min among males. CD4 count was categorized as CD4 < 350, 350–500, and CD4 > 500 cells/μL. Advanced World Health Organization stage was defined as any stage III or IV condition, while low body mass index (BMI) was defined as any BMI result <18.5 kg/m2 and normal/high as ≥18.5 kg/m2.
Ethics approval for this study was obtained from the Wits Human Research Ethics Committee (HREC No. M140915) and the Boston University Independent Review Board (IRB No. H-33399).
Statistical analysis
Data analysis was conducted using STATA version 14 (StataCorp, College Station, TX). Continuous variables were described using medians and interquartile ranges. Categorical variables were described using percentages. Unconditional logistic regression was used to estimate both odds ratios (OR) for experiencing a virologic failure on second-line ART and the associated 95% confidence intervals (95% CIs). Owing to the relatively small sample size, only crude models are presented.
Results
Between December 2014 and January 2016, we enrolled 70 cases (consecutive HIV-positive patients who had a virologic failure on second-line treatment) and 124 controls. Among both cases and controls, the majority of participants were female (60.0% for cases and 54.0% for controls). Overall, 76.8% of participants were between 31 and 50 years, and over 80% had at least some secondary school level of education. Most (63.5%) study participants lived at least 11 km from the clinic and (86.1%) accessed the clinic on foot or by public transport.
Demographic and contextual predictors of virologic failure
Table 1 shows demographic and contextual characteristics of study participants. The odds of virologic failure among patients on second-line ART did not differ by sex or level of education. Patients aged 40 years or younger were at higher odds of virologic failure than participants over 41 (OR 0.6; 95% CI: 0.3–1.1). Compared to the unemployed, participants who worked part-time or all day were less likely to experience virologic failure (OR 0.6; 95% CI: 0.3–1.1). Patients who were dependent on public transportation or went to the clinic on foot were also at increased odds of failure (OR 5.4; 95% CI: 1.6–18.5) compared to patients who used a private car (a likely marker of high socioeconomic status), although use of private cars was rare overall. Further, there was no association between the distance travelled and the mobility methods. We also found that the odds of virologic failure was higher among patients who experienced high social instability (OR 3.8; 95% CI: 1.3–11.5) than those with low instability. Moreover, participants who had been on second-line ART for at least 2 years were 65% less likely to fail compared to those who were recently switched (<12 months) (OR 0.4; 95% CI: 0.2–0.9).
Table 1.
Crude Demographic and Contextual Predictors of Missed Doses and Virologic Failure Among Patients on Second-Line Antiretroviral Therapy in Johannesburg, South Africa
| Cases (N = 70) n (col%) | Control (N = 124) n (col%) | OR virologic failure (95% CI) | |
|---|---|---|---|
| Sex | |||
| Female | 42 (60.0) | 67 (54.0) | 1 |
| Male | 28 (40.0) | 57 (46.0) | 0.8 (0.4–1.4) |
| Age category, years | |||
| ≤40 | 31 (44.3) | 45 (36.3) | 1 |
| 41–50 | 25 (35.7) | 61 (49.2) | 0.6 (0.3–1.1) |
| >50 | 14 (20.0) | 18 (14.5) | 1.1 (0.5–2.6) |
| Education level | |||
| Unknown | 6 (8.6) | 17 (13.7) | 1 |
| Primary school | 3 (4.3) | 7 (5.7) | 1.3 (0.2–6.6) |
| High school or higher | 61 (87.1) | 100 (80.1) | 1.7 (0.6–4.5) |
| Employment and work hours | |||
| Unemployed | 31 (44.3) | 41 (33.1) | 1 |
| Employed | 39 (55.7) | 83 (66.9) | 0.6 (0.3–1.1) |
| Mobility method | |||
| By private car | 3 (4.3) | 24 (19.4) | 1 |
| On foot/public transport | 67 (95.7) | 100 (80.7) | 5.4 (1.6–18.5) |
| How far did you live from the nearest health clinic or hospital? | |||
| ≤10 km | 20 (29.9) | 44 (36.1) | 1 |
| ≥11 km | 45 (67.2) | 75 (61.5) | 1.3 (0.7–2.5) |
| Don't know | 2 (3.0) | 3 (2.5) | 1.5 (0.2–9.5) |
| Social instability | |||
| Low | 18 (26.1) | 48 (39.7) | 1 |
| Medium | 41 (59.4) | 66 (54.6) | 1.7 (0.8–3.2) |
| High | 10 (14.5) | 7 (5.8) | 3.8 (1.3–11.5) |
| Duration on second-line ART, months | |||
| ≥12 | 30 (42.9) | 38 (30.7) | 1 |
| 13–24 | 17 (24.3) | 21 (16.9) | 1.0 (0.5–2.3) |
| ≥25 | 23 (32.9) | 65 (52.4) | 0.4 (0.2–0.9) |
95% CI, 95% confidence interval; ART, antiretroviral therapy; OR, odds ratio.
Adherence-related predictors of virologic failure
As expected, poor adherence was a strong predictor of second-line ART failure. Overall 17.0% of participants reported having missed at least 10% of their ARV doses, but cases were more likely to miss ARV doses than controls (OR 4.7; 95% CI: 2.1–10.5) (Table 2). The majority of patients relied on a wall chart or alarm clock to remember to take their drugs (57.2%), and those who remembered to take their ART doses from memory had lower odds of failure than those who relied on wall charts or alarm clocks (OR 0.5; 95% CI: 0.2–1.0).
Table 2.
Crude Adherence-Related Predictors of Missed Doses and Virologic Failure in the 30 Days Before Failure/Last Viral Load Test Among Second-Line Antiretroviral Therapy Patients in Johannesburg, South Africa
| Cases (N = 70) n (col%) | Control (N = 130) n (col%) | OR virologic failure (95% CI) | |
|---|---|---|---|
| Missed doses | |||
| <10% | 48 (68.6) | 113 (91.1) | 1 |
| ≥10% | 22 (31.4) | 11 (8.9) | 4.7 (2.1–10.5) |
| ARV reminders | |||
| Wall chart or electronic alarm | 45 (64.3) | 66 (52.2) | 1 |
| Family or friends | 6 (8.6) | 16 (12.9) | 0.5 (0.2–1.5) |
| From memory | 11 (15.7) | 34 (27.4) | 0.5 (0.2–1.0) |
| TV or radio program | 8 (11.4) | 8 (6.5) | 1.5 (0.5–4.2) |
| Self-reported ADR | |||
| No | 31 (44.3) | 75 (60.5) | 1 |
| Yes | 39 (55.7) | 49 (39.5) | 1.9 (1.1–3.5) |
| Coping with self-reported ADR | |||
| No ADR reported | 31 (44.3) | 74 (59.7) | 1 |
| Low/medium coping | 28 (40.0) | 40 (32.3) | 1.7 (0.9–3.2) |
| High coping | 11 (15.7) | 10 (8.1) | 2.6 (1.0–6.8) |
| Disclosure | |||
| Current partner/close relatives | 58 (82.9) | 115 (92.7) | 1 |
| Friends or colleagues | 12 (17.1) | 7 (5.7) | 3.4 (1.3–9.1) |
| Not disclosed | 0 | 2 (1.6) | — |
| Depression | |||
| None/low | 59 (84.3) | 119 (96.0) | 1 |
| Medium/high | 11 (15.7) | 65 (4.0) | 4.4 (1.5–13.4) |
| Alcohol use | |||
| No alcohol | 44 (62.9) | 95 (77.2) | 1 |
| Any alcohol | 26 (37.1) | 28 (22.7) | 2.0 (1.1–3.8) |
95% CI, 95% confidence interval; ADR, adverse drug reaction; ARV, antiretroviral; OR, odds ratio.
Disclosure, a factor that likely influences adherence, was common in the study sample, only two participants had not disclosed their HIV status to anyone. Those who disclosed only to friends or work colleagues were more likely to experience virologic failure compared to those who disclosed to a partner or close relatives (OR 3.4; 95% CI: 1.3–9.1). Depression, another factor likely to influence adherence, was also common and 39.2% of participants reported medium to high levels of depression. Participants reporting medium to high depression had considerably higher odds of virologic failure than those with low or no depression (OR 4.4; 95% CI: 1.5–13.4). Further, compared to patients who did not consume alcohol, those who did had higher odds of virologic failure (OR 2.0; 95% CI: 1.1–3.8).
Clinical predictors of virologic failure
Table 3 highlights the clinical predictors of virologic failure on second-line ART. The majority of participants initiated second-line ART on a regimen of either of the following: zidovudine (AZT), lamivudine (3TC), or LPVr (45.9%), or a regimen of tenofovir (TDF), 3TC or emtricitabine (FTC), and LPVr (33.0%). Only 9.8% (n = 19) were started on AZT, didanosine (ddI), and LPVr, a regimen allowed under the 2004 guidelines. However, at the time of the first failure event (cases) or last VL test (controls), most participants were receiving either AZT +3TC+LPVr (58.2%) or TDF +3TC/FTC+LPVr (34.0%). Neither the initial second-line regimen nor the regimen at the time of the interview/failure event was predictive of failure. Having a low BMI (OR 2.0; 95% CI: 0.9–4.4) was an imprecise predictor of virologic failure.
Table 3.
Crude Clinical Predictors of Virologic Failure Up To 6 Months Before Switch to Second-Line Antiretroviral Therapy Among Patients in Johannesburg, South Africa
| Variables | Cases (N = 70) n (col%) | Control (N = 130) n (col%) | OR virologic failure (95% CI) |
|---|---|---|---|
| Regimen at switch to second-line ART | |||
| AZT+ddI+LPVr | 5 (7.1) | 14 (11.3) | 1 |
| AZT +3TC+LPVr | 34 (48.6) | 55 (44.4) | 1.7 (0.6–5.2) |
| TDF +3TC/FTC+LPVr | 25 (35.7) | 39 (31.5) | 1.8 (0.5–5.6) |
| d4T+3TC+LPVr | 3 (4.3) | 3 (2.4) | 2.8 (0.4–18.7) |
| Other | 3 (4.3) | 13 (10.5) | 0.6 (0.1–3.3) |
| Regimen at interview or failure | |||
| TDF +3TC/FTC+LPVr | 24 (34.3) | 42 (33.9) | 1 |
| AZT +3TC+LPVr | 38 (54.3) | 75 (60.5) | 0.9 (0.5–1.7) |
| Other | 8 (11.4) | 7 (5.7) | 2.0 (0.6–6.2) |
| Diagnosed ADR in medical records | |||
| No | 62 (88.6) | 115 (92.7) | 1 |
| Yes | 8 (11.4) | 9 (7.3) | 1.6 (0.6–4.5) |
| Anemia | |||
| No | 49 (70.0) | 96 (77.4) | 1 |
| Yes | 21 (30.0) | 28 (22.6) | 1.5 (0.8–2.8) |
| Creatinine clearance | |||
| Normal | 45 (64.3) | 73 (58.9) | 1 |
| Low | 25 (35.7) | 51 (41.1) | 0.8 (0.4–1.5) |
| CD4 count | |||
| ≤350 cell/μL | 34 (48.6) | 28 (21.5) | 1 |
| 350–500 cells/μL | 7 (10.0) | 4 (3.1) | 1.4 (0.4–5.4) |
| >500 cells/μL | 10 (14.3) | 11 (8.5) | 0.7 (0.3–2.0) |
| Missing | 19 (27.1) | 87 (66.9) | |
| WHO stage | |||
| I/II | 52 (74.3) | 97 (78.2) | 1 |
| III/IV | 18 (25.7) | 27 (21.8) | 1.2 (0.6–2.5) |
| BMI | |||
| Normal/high (≥18.5 kg) | 56 (80.0) | 110 (88.7) | 1 |
| Low (<18.5 kg) | 14 (20.0) | 14 (11.3) | 2.0 (0.9–4.4) |
3TC, lamivudine; 95% CI, 95% confidence interval; ADR, adverse drug reaction; ART, antiretroviral therapy; AZT, zidovudine; BMI, body mass index; ddI, didanosine; FTC, emtricitabine; LPV/r, ritonavir-boosted lopinavir; OR, odds ratio; TDF, tenofovir; WHO, World Health Organization.
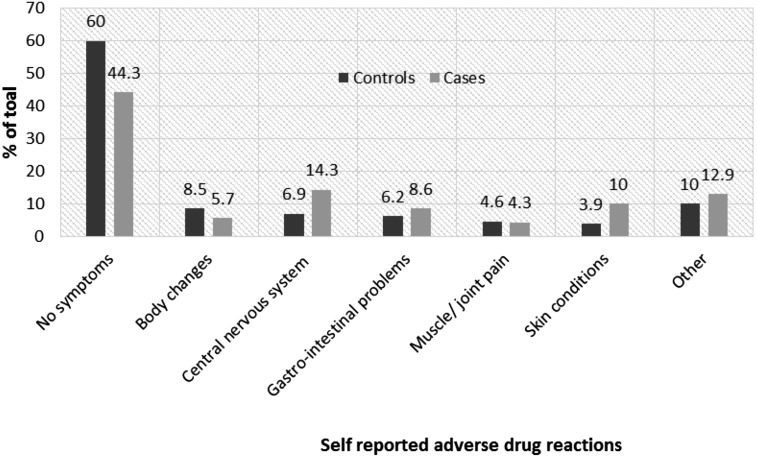
Only 8.8% of patients had a confirmed diagnosis of an ADR before the last VL test or failure, yet 45.4% patients self-reported experiencing an ADR-related symptom. Although a slightly higher proportion of cases (11.4%) had a diagnosed ADR compared to controls (7.3%), having a confirmed ADR diagnosis was not a strong predictor of second-line virologic failure (OR 1.6; 95% CI: 0.6–4.5). Overall 55.7% of cases and 39.5% of controls self-reported an ADR. Figure 1 shows the proportions of cases and controls reporting various ADRs. ADRs reported included body changes (5.7% of cases and 8.5% of controls), CNS-related conditions (14% of cases and 6.9% of controls), gastrointestinal problems (8.6% of cases and 6.2% of controls), muscle or joint pains (4.3% of cases and 4.6% of controls), and skin conditions (10.0% of cases and 3.9% of controls). Participants who self-reported at least one ADR were more likely to fail treatment (OR 1.9; 95% CI: 1.0–3.5) compared to those with no ADR symptoms.
FIG. 1.
Self-reported adverse drug reactions in the 30 days before virologic failure/last viral load test among patients receiving second-line antiretroviral therapy in Johannesburg, South Africa.
Discussion
In South Africa, third-line therapy, although much more expensive than second-line therapy, is available in the public health sector. Accordingly, the high rates of virologic failure among HIV-positive patients who are receiving second-line ART warrant further efforts to understand predictors of virologic failure to inform interventions to improve patient outcomes. In this study, we set out to examine demographic, contextual, and adherence-related predictors of virologic failure on second-line ART. Similar to previous studies, our results show that poor treatment adherence remains an important predictor of virologic failure.12,13,27–29 We found that patients with self-reported adherence lower than 90% in the month before virologic failure/last VL test were 4.6 times more likely to fail second-line ART. A multi-center analysis found that patients with an adherence index of <80% reported twice higher treatment failure rates compared with those with an adherence index of at least 95%30,31 on second-line therapy. In addition, a study from Malawi also found that patients on second-line therapy who had “ever missed a dose” were less likely to achieve virologic suppression (<400 copies/mL) compared to patients who had never missed a dose.27
In addition to nonadherence, virologic failure was associated with younger age, social instability, self-reported ADRs, HIV status disclosure to people other than close family members and sexual partner, alcohol consumption, and depression. Younger age, low social support, and internalized HIV stigma have been previously cited as important predictors of ART failure.32 HIV-related stigma could also be a predictor of social instability, nondisclosure, and depression by affecting patients' abilities to access social support (sexual partnerships and supportive home) as well as economic well-being (employability and food security).33,34 While interventions focusing on patients' personal abilities to overcome barriers to adherence have had promising results; continued efforts to normalize HIV are needed and the involvement of social services may be required to support socially unstable patients.13,35,36
The risk of virologic failure among those who were employed was slightly lower than among unemployed patients. While transportation cost could be a contributing factor among unemployed patients, among patients who worked all day, inability to secure paid leave for clinic visits and unfavorable clinic operating hours may also hinder regular attendance.
We also found that self-reported ADRs, but not clinically confirmed ADRs were associated with higher risk of virologic failure. The recording of ADRs is generally poor, with even less data on ADR among patients on second-line ART.37 This could partly explain the disparity between the proportions of patients with clinically diagnosed ADR compared to self-reported data. However, patient complaints of ADR, including low-grade ADRs, could be regarded as important early warning indicators of nonadherence.
Our findings should be considered within the following limitations. The small sample size and weak statistical power limited our ability to adjust for potential confounders and, therefore, make stronger inferences based on the results, and further studies with larger sample sizes are needed. In addition, unlike controls, cases were asked to recall events that occurred up to 12 months before the interview. Although the date of virologic failure was provided to assist cases with recall, differential recall between cases and controls may have affected our estimates. In addition, patients were aware of their study assignment and health condition that could have affected their responses. Moreover, the study sample was restricted to patients from one large HIV clinic in Johannesburg who spoke and understood English, which may not be representative of patients from other settings. Finally, our cases were not confirmed to have had resistance to second-line therapy, but rather used the definition of failure used in practice based on repeated elevated VL measures.
Nevertheless, our results highlight the complex interplay between socioeconomic and drug-related factors contributing to ART adherence and subsequent risk of virologic failure among patients on second-line ART in South Africa. Adherence counseling should take into consideration patients' self-reported experiences of ADRs as well as their social circumstances and, whenever possible, include material support and linking to social services. Also, interventions that support easy access to clinics and expedient service among stable patients alongside continued social support programs are needed to improve adherence to second-line ART and curb the need for third-line regimens.
Acknowledgments
The authors gratefully acknowledge the directors and staff of the Temba Lethu HIV clinic as well as Right to Care, the Non-Governmental Organization supporting the study site through a partnership of the South African National and Gauteng provincial Department of Health with the United States Agency for International Development (USAID). Most of all, we thank the patients attending the clinic for their continued trust in the treatment provided at the clinic. Funding was provided by USAID under the terms of Cooperative Agreement 674-A-12-00020 to Right to Care and INROADS USAID-674-A-12-00029 to the Health Economics and Epidemiology Research Unit and Cooperative Agreement 674-A-00-09-00018-00 to Boston University. The contents of this article are the responsibility of the authors and do not necessarily reflect the views of the funding agencies, Temba Lethu clinic, or patients.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: Collaborative analysis of cohort studies. PLoS Med 2013;10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: A cohort analysis from Uganda. Ann Intern Med 2011;155:209–216 [DOI] [PubMed] [Google Scholar]
- 3.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: Valuing the scale-up of HIV treatment. Science (New York) 2013;339:961–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 2010;24:563–572 [DOI] [PubMed] [Google Scholar]
- 5.Fox MP, Cutsem GV, Giddy J, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012;60:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nglazi MD, Lawn SD, Kaplan R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr 2011;56:e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS 2010;24:915–919 [DOI] [PubMed] [Google Scholar]
- 8.ART-LINC of IeDEA Study Group, Keiser O, Tweya H, et al. Switching to second-line antiretroviral therapy in resource-limited settings: Comparison of programmes with and without viral load monitoring. AIDS 2009;23:1867–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pujades-Rodriguez M, O'Brien D, Humblet P, Calmy A. Second-line antiretroviral therapy in resource-limited settings: The experience of Medecins Sans Frontieres. AIDS 2008;22:1305–1312 [DOI] [PubMed] [Google Scholar]
- 10.Palombi L, Marazzi MC, Guidotti G, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis 2009;48:115–122 [DOI] [PubMed] [Google Scholar]
- 11.Madec Y, Leroy S, Rey-Cuille MA, Huber F, Calmy A. Persistent difficulties in switching to second-line ART in sub-saharan Africa—A systematic review and meta-analysis. PLoS One 2013;8:e82724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: A systematic review and meta-analysis. AIDS 2012;26:929–938 [DOI] [PubMed] [Google Scholar]
- 13.Fox MP, Ive P, Long L, Maskew M, Sanne I. High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010;53:500–506 [DOI] [PubMed] [Google Scholar]
- 14.Murphy RA, Sunpath H, Castilla C, et al. Second-line antiretroviral therapy: Long-term outcomes in South Africa. J Acquir Immune Defic Syndr 2012;61:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston V, Fielding K, Charalambous S, et al. Second-line antiretroviral therapy in a workplace and community-based treatment programme in South Africa: Determinants of virological outcome. PLoS One 2012;7:e36997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecher S, Ellenberger D, Kim AA, et al. Scale-up of HIV viral load monitoring—seven sub-Saharan African countries. MMWR 2015;64:1287–1290 [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Rath G, Pillay Y, Blecher M, et al. Total cost and potential cost savings of the national antiretroviral treatment (ART) programmein South Africa 2010 to 2017. 9th International AIDS Economics Network (IAEN) pre-conference, 2016, Durban [Google Scholar]
- 18.Berhanu Rea. Second line failure and protease inhibitor resistance in a clinic in Johannesburg, South Africa. [CROI abstract 1049]. In Special Issue: Abstracts From the 2014 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med 2014
- 19.Fox MP, Maskew M, MacPhail AP, et al. Cohort profile: The Themba Lethu Clinical Cohort, Johannesburg, South Africa. Int J Epidemiol 2013;42:430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NDoH. National Consolidated Guidelines for the Prevention of Mother-to-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents and Adults. Pretoria: South African National Department of Health, 2015 [Google Scholar]
- 21.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Philadelphia: Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 22.Turner BJ. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. J Infect Dis 2002;185(Suppl 2):S143–S151 [DOI] [PubMed] [Google Scholar]
- 23.Peltzer K, Friend-du Preez N, Ramlagan S, Anderson J. Antiretroviral treatment adherence among HIV patients in KwaZulu-Natal, South Africa. BMC Public Health 2010;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson MO, Neilands TB. Coping with HIV treatment side effects: Conceptualization, measurement, and linkages. AIDS Behav 2007;11:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, O'Brien N, Forrest JI, et al. Validating a shortened depression scale (10 item CES-D) among HIV-positive people in British Columbia, Canada. PLoS One 2012;7:e40793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.German D, Latkin CA. Social stability and health: Exploring multidimensional social disadvantage. J Urban Health 2012;89:19–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosseinipour MC, Kumwenda JJ, Weigel R, et al. Second-line treatment in the Malawi antiretroviral programme: High early mortality, but good outcomes in survivors, despite extensive drug resistance at baseline. HIV Med 2010;11:510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Win MM, Maek ANW, Phonrat B, Kiertiburanakul S, Sungkanuparph S. Virologic and immunologic outcomes of the second-line regimens of antiretroviral therapy among HIV-infected patients in Thailand. J Int Assoc Physicians AIDS Care 2011;10:57–63 [DOI] [PubMed] [Google Scholar]
- 29.Marconi VC, Wu B, Hampton J, et al. Early warning indicators for first-line virologic failure independent of adherence measures in a South African Urban Clinic. AIDS Patient Care STDs 2013;27:657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pujades-Rodriguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A, AIDS Working Group of MSF. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA 2010;304:303–312 [DOI] [PubMed] [Google Scholar]
- 31.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis JAMA 2006;296:679–690 [DOI] [PubMed] [Google Scholar]
- 32.Bleijenberg MP, Bierman WFW, Jaglall C, et al. Prediction of virological failure in HIV-infected individuals treated with cART in Suriname. J Int AIDS Soc 2010;13(Suppl. 4):P56 [Google Scholar]
- 33.Brashers DE, Neidig JL, Goldsmith DJ. Social support and the management of uncertainty for people living with HIV or AIDS. Health Communication 2004;16:305–331 [DOI] [PubMed] [Google Scholar]
- 34.van Loggerenberg F, Gray D, Grant A, et al. A qualitative study of patient motivation to adhere to combination antiretroviral therapy in South Africa. AIDS Patient Care STDS 2015;29:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garone DB, Conradie K, Patten G, et al. High rate of virological re-suppression among patients failing second-line antiretroviral therapy following enhanced adherence support: A model of care in Khayelitsha, South Africa. SAJ HIV Med 2013;14:166–169 [Google Scholar]
- 36.Johnston V, Cohen K, Wiesner L, et al. Viral suppression following switch to second-line antiretroviral therapy: Associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch. J Infect Dis 2014;209:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: Key determinants. BMJ Open 2014;4:e005869. [DOI] [PMC free article] [PubMed] [Google Scholar]