Abstract
Background: Oxidative stress may play an important role in both initiation and progression of breast cancer. We conducted the first systematic epidemiologic review to summarize the published literature on oxidative stress biomarkers and breast cancer.
Materials and Methods: We implemented systematic search strategies to identify published studies of oxidative stress biomarkers and (1) risk of developing breast cancer and (2) breast cancer prognosis using the PRISMA statement guidelines.
Results: We identified eleven case–control studies of oxidative stress biomarkers and breast cancer. Biomarkers utilized varied and menopausal status was a key modifying factor. Across three nested case–control studies with biomarkers measured before diagnosis, one reported increased risk of postmenopausal breast cancer in association with 8-oxodG (DNA damage biomarker), while two (one of F2-isoprostanes and one of fluorescent oxidation products) reported inverse associations for premenopausal breast cancer only. We identified eight prognostic studies. Two reported associations for lipid peroxidation and breast cancer prognosis; results for other studies were null.
Conclusions: DNA damage may increase risk of breast cancer among postmenopausal women, while lipid peroxidation may be inversely associated with premenopausal breast cancer. Lipid peroxidation may be associated with survival after breast cancer diagnosis; however, results require evaluation in large, prospective cohort studies.
Keywords: : breast cancer, oxidative stress biomarkers, epidemiology, systematic review, cohort studies, case–control studies
Introduction
The generation of reactive oxygen and nitrogen species unrestrained, and subsequent oxidative stress, has been implicated in the pathogenesis of many chronic diseases, including cancer, diabetes, and cardiovascular disease, as well as aging in general.1–4 Oxidative stress can be broadly defined as an imbalance between oxidants and antioxidants in favor of the oxidants, potentially leading to damage.5,6 If the level of reactive species is high and overcomes the antioxidant defense mechanisms of the human body, oxidative damage can occur to lipids, proteins, or directly to DNA.7 DNA damage is hypothesized to play an important role in the initiation of carcinogenesis.
Oxidative stress mechanisms are also involved in the activation of cell signaling pathways, including tumor cell proliferation, increased tumor cell migration, and increased tumor cell proangiogenic factors, and play a key role in apoptosis, mechanisms that can impact both cancer progression and metastasis.2,8–10 Increased reactive oxygen species (ROS) and the resulting high oxidative stress are key characteristics of malignant tumors.11 Many cancer treatments, such as radiotherapy and certain chemotherapy agents, act through oxidative stress pathways via the production of ROS to kill tumor cells.12–14
Several biomarkers of oxidative stress have been identified for use in epidemiologic studies and can be measured in various biological samples, including both blood and urine.7,15–21 Commonly used biomarkers include DNA damage biomarkers, such as 8-hydroxy-2′-deoxyguanosine (8-oxodG),22 protein carbonyl groups as a marker of protein oxidation,23 and malondialdehyde (MDA) and F2-isoprostanes as markers of lipid peroxidation.21,24–27 Studies have also evaluated the role of oxidative stress in cancer and other chronic diseases using plasma fluorescent oxidation products (FlOPs), which are considered a global biomarker of oxidative stress.20,28
Biomarkers of oxidative stress have been investigated for their association with the development and progression of several cancer types, and in particular breast cancer, as oxidative stress mechanisms may be involved in several known breast cancer risk factors, including obesity and daily alcohol intake, and circulating estrogen levels.29–34 Breast cancer cells have been shown to be susceptible to oxidative damage and have high levels of oxidative stress, including protein damage, DNA damage, and lipid peroxidation.9,35 Furthermore, several breast cancer risk factors may alter levels of endogenous oxidative stress.36,37 To our knowledge, no review has synthesized the published epidemiologic literature on oxidative stress biomarkers and breast cancer risk and prognosis. Therefore, we conducted a systematic literature review to identify and summarize all published epidemiologic studies of oxidative stress biomarkers in association with (1) risk of developing breast cancer and (2) prognosis after breast cancer and identify areas for future research.
Materials and Methods
Search strategy and study selection
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines for the present review.38 We conducted two separate systematic searches to identify peer-reviewed original articles that evaluated one or more oxidative stress biomarkers and breast cancer in epidemiologic studies of case–control, nested case–control, or cohort study design. The first systematic search was to identify studies of oxidative stress biomarkers and risk of developing breast cancer and the second was for studies of oxidative stress biomarkers and breast cancer prognosis. To our knowledge, no other systematic reviews on biomarkers of oxidative stress and cancer have been conducted, with the exception of one study focused on prostate cancer.39
The PubMed interface of the electronic database Medline was searched systematically for all articles published in peer-reviewed journals through August 2015. The search was updated in February 2016. The search strategy and algorithms were devised by two authors (J.D.L., S.J.N.). Both search strategies were limited to studies conducted in female humans and published in English.
The search algorithm for breast cancer risk studies (Search A, Fig. 1) included (“breast cancer” OR “breast neoplasms”) AND (risk or odds) AND (“oxidative stress” OR “DNA damage” OR “Lipid peroxidation”) NOT review[Publication Type] NOT comment[Publication Type] NOT editorial[Publication Type] NOT “breast cancer cells”[Title] NOT “breast epithelial cells”[Title] NOT “cell line”[Title]. For prognosis studies (Search B, Fig. 1), we replaced risk or odds in the search terms with the following terms in the search algorithm: (prognosis OR prognostic OR death OR mortality OR survival OR recurrence OR relapse OR “disease-free” OR progression OR survivorship OR survivor).
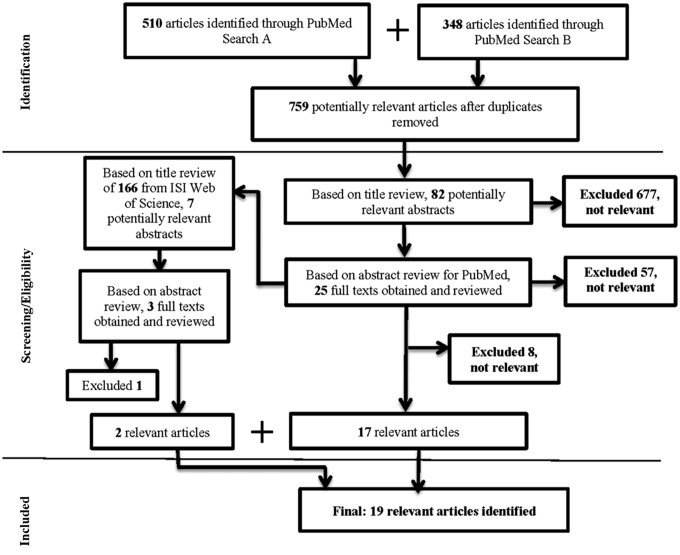
FIG. 1.
PRISMA study flow diagram.
The search strategy first included a title review followed by abstract review by one coauthor (S.J.N.). Potentially relevant full texts were reviewed by both coauthors (S.J.N. and J.D.L.). In addition to the PubMed search, (1) the reference lists for each study and relevant review articles were manually reviewed and (2) citation lists from Web of Science for each relevant article were manually reviewed to identify any additional relevant studies by two authors (J.D.L. and S.J.N.). Based on our study objective, relevant articles were those that evaluated one or more oxidative stress biomarkers and odds/risk of developing breast cancer or breast cancer prognosis in epidemiologic studies of case–control, nested case–control, or cohort study design. We did not exclude articles based on publication date or sample size.
Data synthesis
Data were extracted independently using duplicate Excel spreadsheets by two separate abstracters and reviewed by a third independent reviewer (S.J.N.) in the case of any discrepancies for each individual study. For the risk studies, major data items extracted (if available) included first author and study year, study location, study design, study period, study population (sample size, invasive/in situ status, data source), biomarkers of oxidative stress (type of biological sample, assay, units, timing of measurement in relation to diagnosis), results (odds ratios/relative risk (RRs)/hazard ratios and 95% confidence intervals [CIs] ([or other relevant effect estimate] for the association(s) of the biomarker and risk of breast cancer), and covariates. As menopausal status was a key effect modifier in several studies, if available, we show results by menopausal status. For the prognosis studies, similar data items were abstracted with additional information collected on age at diagnosis, stage of diagnosis (or grade if stage was not available), years of diagnosis, prognostic outcomes and data source, and length of follow-up. For prognosis studies, if a multivariable analysis was not performed, mean survival time was abstracted.
Results
As shown in Figure 1, 759 nonduplicate potentially relevant studies were identified based on the systematic PubMed searches using the search algorithms described above. After title review, 82 abstracts were identified as potentially relevant. After abstract review, 25 full texts were obtained as potentially relevant studies, with a total of 17 relevant articles that evaluated one or more biomarkers of oxidative stress and breast cancer identified. We identified two additional relevant studies via manual searching of citation reports from Web of Science for a total of 19 relevant articles identified via all search methods.
Oxidative stress biomarkers and risk of developing breast cancer
Study design and study population details are displayed in Table 1 and results are summarized in Table 2. A total of 12 case–control studies, including five nested case–control studies, were identified. Oxidative stress biomarkers varied across studies, with measures of damage/oxidation to lipids, DNA, and protein, as well as a global biomarker of oxidative stress, FlOP levels.
Table 1.
Observational Studies of Oxidative Stress Biomarkers and Risk of Developing Breast Cancer: Study Design and Study Population
| Refs. | Study design | Recruitment period | Location | Cases/controls | Study population characteristics (stage and data source) |
|---|---|---|---|---|---|
| Smith et al.44 | Population-based case–control | 1995–1996 | U.S. | 70/70 | Stage NR Cases and controls from Georgetown University Medical Center |
| Rossner et al. 40 | Population-based case–control | 1996–1997 | U.S. | 400/401 | Invasive and in situ Controls from RDD (<65 years) and Health Care Financing Administration rosters (≥65 years of age); data source for cases NR |
| Rossner et al.31 | Population-based case–control | 1996–1997 | U.S. | 1050/1107 | Invasive and in situ Controls from RDD (<65 years) and Health Care Financing Administration rosters (≥65 years of age); data source for cases NR |
| Sharhar et al.43 | Case–control study | 2005–2006 | Malaysia | 57/139 | Invasive Cases were from multiple health centers and controls were recruited from various institutions and residential areas. |
| Zipprich et al.42 | Case–control study | 1995–2006 | U.S. | 268 sister sets, n = 645 | Invasive or in situ: NR Cases and controls were recruited from hospitals, clinics, and community organizations in the New York metropolitan area. |
| Dai et al.45 | Prospective nested case–control study | 1997–2006 | Shanghai, China | 436/852 | Invasive Participants were from the Shanghai Women's Health Study, cases were identified via active follow-up and via linkage to the Shanghai Cancer Registry. |
| Shen et al.41 | Population-based case–control | 1996–1997 | U.S. | 1061/1108 | Invasive and in situ Controls from RDD (<65 years) and Health Care Financing Administration rosters (≥65 years of age); data source for cases NR |
| Lee et al.46 | Prospective nested case–control | 1997–2004 | Shanghai, China | 354/654 | Invasive or in situ: NR Participants were from the Shanghai Women's Health Study, cases were identified via active follow-up and the Shanghai Cancer Registry |
| Fortner et al.28 | Prospective nested case–control | 1st blood collection 1989–1990; 2nd Blood collection 2000–2002 Follow-up 2000–2006 |
U.S. | 377/377 | Invasive and in situ Participants were from with the Nurses' Health Study with cancer diagnoses self-reported with confirmation by medical records (99%) |
| Loft et al.30 | Prospective nested case–control | 1993–2000, 3–7 years of follow-up | Denmark | 336/336 | Invasive Participants were from a prospective Danish cohort study, cases were diagnosed in the Danish Cancer Registry. |
| Sisti et al.47 | Prospective nested case–control | Blood collection 1989–1990 (NHS I) Blood collection 1996–1999 (NHS II) |
U.S. | 392/392 | Invasive and in situ Participants were from the Nurses' Health Study with cancer diagnoses self-reported with confirmation by medical records (99%) |
NR, not reported; RDD, random digit dialing.
Table 2.
Observational Studies of Oxidative Stress Biomarkers and Risk of Developing Breast Cancer
| Refs. | Oxidative stress biomarker measurement and timing | Results overall and by menopausal status (OR/RR/IRR and 95% CIs) | Commentsa | |
|---|---|---|---|---|
| 44 | DNA damage measured as comet tail moments using single-cell gel electrophoresis in peripheral lymphocytes. Timing: two groups (1) cases diagnosed with breast cancer within 5 years and free of cancer/treatments for at least 6 months before study entry and (2) cases before treatment. Sample type: blood |
Overallb | Adjustment factors: age | |
| High DNA damage at baseline | 13.44 (5.97–30.24) | Additional subgroup analysis: Results were stratified by median BMI; associations were stronger among women with higher BMI (P for interaction NR). | ||
| High DNA damage after ionizing radiation | 13.65 (6.07–30.71) | |||
| High DNA damage after repair | 6.54 (3.11–13.79) | |||
| Results by menopausal status NR | ||||
| 40 | Urinary 15-F2t-IsoP (nmol/mmol creatinine) levels were analyzed using immunoassay kit. Urinary 8-oxodG (nmol/mmol creatinine) levels were analyzed by competitive ELISA. Timing: samples were collected, on average, within 3 months after diagnosis (most before chemotherapy, all after surgery) Sample type: spot urine |
Overall |
Adjustment factors: for 15-F2t-IsoP, the model was adjusted for age, average physical activity level, and BMI; for 8-oxodG, the model was adjusted for age, average physical activity level, fruit and vegetable intake, average lifetime alcohol intake, active smoking status, menopausal status, and BMI. Additional subgroup analysis: age, average physical activity level, fruit and vegetable intake, average lifetime alcohol intake, cigarette smoking status, and BMI not shown to modify associations, statistical tests for interaction NR. |
|
| 15-F2t-IsoP | ||||
| <0.45 | 1.00 (Reference) | |||
| 0.45–0.64 | 1.25 (0.81–1.94) | |||
| 0.64–0.99 | 1.53 (0.99–2.35) | |||
| >0.99 | 1.88 (1.23–2.88) | |||
| 8-oxodG | ||||
| <18.85 | 1.00 (Reference) | |||
| 18.85–27.92 | 0.99 (0.64–1.51) | |||
| 27.92–38.28 | 0.75 (0.48–1.16) | |||
| >38.28 | 0.79 (0.51–1.22) | |||
| Premenopausal | ||||
| 15-F2t-IsoP | ||||
| <0.45 | 1.00 (Reference) | |||
| 0.45–0.64 | 1.13 (0.57–2.20) | |||
| 0.64–0.99 | 1.16 (0.56–2.41) | |||
| >0.99 | 1.85 (0.91–3.76) | |||
| Postmenopausal: | ||||
| 15-F2t-IsoP | ||||
| <0.45 | 1.00 (Reference) | |||
| 0.45–0.64 | 1.40 (0.75–2.60) | |||
| 0.64–0.99 | 1.93 (1.08–3.45) | |||
| >0.99 | 2.06 (1.15–3.69) | |||
| 8-oxodG by menopausal status NR | ||||
| 31 | Plasma protein carbonyl groups (nmol/mL) were assessed using noncompetitive ELISA. Timing: samples were collected, on average, within 3 months after diagnosis (most before chemotherapy, all after surgery) Sample type: blood |
Overall | Adjustment factors: age, physical activity, fruit and vegetable intake, alcohol intake, active smoking status, menopausal status, BMI, ever use HRT, ever use OC, age at menarche, parity, and age at first birth. | |
| <11.5 | 1.00 (Reference) | |||
| 11.5–14.8 | 1.18 (0.90–1.55) | |||
| >14.8–19.2 | 1.51 (1.16–1.97) | |||
| >19.2 | 1.60 (1.23–2.09) | |||
| Premenopausal | ||||
| <11.5 | 1.00 (Reference) | |||
| 11.5–14.8 | 1.21 (0.76–1.93) | Additional subgroup analysis: age, physical activity, fruit and vegetable intake, alcohol intake, smoking status, BMI, HRT and OC use, age of menarche, age at first birth, and parity. Authors noted results were stronger among women with higher levels of physical activity, alcohol intake, and HRT users (p values for interaction were statistically significant for exercise (0.02) and HRT (0.04)). | ||
| >14.8–19.2 | 1.74 (1.09–2.75) | |||
| >19.2 | 1.87 (1.17–3.00) | |||
| Postmenopausal | ||||
| <11.5 | 1.00 (Reference) | |||
| 11.5–14.8 | 1.14 (0.81–1.61) | |||
| >14.8–19.2 | 1.39 (0.99–1.94) | |||
| >19.2 | 1.49 (1.07–2.07) | |||
| 43 | Plasma MDA (mmol/g protein) was assessed using thiobarbituric acid-reactive substances. Timing: NR, cases were not receiving cancer treatment, except surgery. Sample type: blood |
Overall | Adjustment factors: education, occupation, family history of breast cancer, lactation, and waist circumference. | |
| ≥4.8 (vs. <4.8) | 6.82 (1.95–23.9) | |||
| Results by menopausal status NR | Additional subgroup analysis: NR | |||
| 42 | Levels of plasma protein carbonyls (nmol/mg) were assessed from blood sample using noncompetitive ELISA. Timing: months to years after diagnosis Sample type: blood |
Overall | Adjustment factors: menopausal status, age at menarche. | |
| ≤11.0 | 1.00 (Reference) | |||
| 11.1–13.0 | 1.43 (0.77–2.66) | Additional subgroup analysis: NR | ||
| 13.1–15.3 | 2.36 (1.14–4.89) | |||
| >15.3 | 1.87 (0.83–4.18) | |||
| Continuous | 1.99 (0.74–5.36) | |||
| Authors noted that the trends in risk were generally similar by menopausal status (data not shown). | ||||
| 45 | Levels of urinary excretion of isoprostanes (ng/mg of creatinine) measured by GC/NICI MS from spot urine samples. Timing: samples were collected at baseline or during first follow-up approximately 2 years later (prediagnosis). Sample type: spot urine |
Overall | Adjustment factors: age, education, age at menarche, age at first live birth, months of breastfeeding, history of BBD, first-degree family cancer history, ever smoker, total intake of red meat and isoflavones, and use of HRT. | |
| 15-F2t-IsoP | ||||
| <1.32 | 1.00 (Reference) | |||
| 1.32–1.99 | 1.00 (0.72–1.38) | |||
| >1.99 | 0.91 (0.64–1.28) | |||
| 15-F2t-IsoPM | Additional subgroup analysis: Results varied by BMI for both biomarkers, with a stronger positive association seen in women with higher BMI (p-values for interaction were statistically significant depending on BMI category). | |||
| <0.44 | 1.00 (Reference) | |||
| 0.44–0.66 | 1.10 (0.80–1.52) | |||
| >0.66 | 0.98 (0.68–1.41) | |||
| Premenopausal (tertile 3 vs. tertile 1) | ||||
| 15-F2t-IsoP | 0.58 (0.35–0.98) | |||
| 15-F2t-IsoPM | 0.68 (0.41–1.14) | |||
| Postmenopausal (tertile 3 vs. tertile 1): | ||||
| 15-F2t-IsoP | 1.33 (0.83–2.13) | |||
| 15-F2t-IsoPM | 1.47 (0.86–2.53) | |||
| 41 | Urinary 15-F2t-IsoP (nmol/mmol creatinine) levels were analyzed using immunoassay kit. Urinary 8-oxodG (nmol/mmol creatinine) levels were analyzed by competitive ELISA. Timing: samples were collected, on average, within 3 months after diagnosis (most before chemotherapy, all after surgery) Sample type: spot urine |
Overall | Adjustment factors: age at reference and the other two biomarkers (includes telomere length). | |
| 15-F2t-IsoP | ||||
| <0.71 | 1.00 (Reference) | Additional subgroup analysis: NR | ||
| ≥0.71 | 1.15 (0.96–1.37) | |||
| <0.49 | 1.00 (Reference) | |||
| 0.49–0.70 | 1.09 (0.84–1.40) | |||
| 0.71–1.06 | 1.22 (0.95–1.57) | |||
| ≥1.07 | 1.17 (0.91–1.50) | |||
| 8-oxodG | ||||
| <22.17 | 1.00 (Reference) | |||
| ≥22.17 | 0.96 (0.80–1.14) | |||
| <15.27 | 1.00 (Reference) | |||
| 15.27–22.16 | 0.95 (0.74–1.21) | |||
| 22.17–31.67 | 0.93 (0.73–1.19) | |||
| >31.68 | 0.94 (0.74–1.21) | |||
| Results by menopausal status NR | ||||
| 46 | Urinary MDA (mg/g creatinine) was measured based on the reaction with 2-thiobarbituric acid. Urinary 8-oxodG (μg/g creatinine) levels were determined by a competitive ELISA kit. Timing: Prediagnosis Sample type: spot urine |
Overall | Adjustment factors: age at baseline ±2 years, sample collection date and time, antibiotic use in the past week, previous cancer history, and menopausal status. | |
| MDA | ||||
| ≤0.481 | 1.00 (Reference) | |||
| >0.481 to ≤0.677 | 0.96 (0.60–0.39) | Additional subgroup analysis: NR | ||
| >0.677 to ≤1.011 | 0.96 (0.66–1.40) | |||
| >1.011 | 0.98 (0.67–1.42) | |||
| 8-oxodG | ||||
| ≤10.364 | 1.00 (Reference) | |||
| >10.364 to ≤14.497 | 1.28 (0.80–1.86) | |||
| >14.497 to ≤20.209 | 1.13 (0.78–1.65) | |||
| >20.209 | 1.11 (0.7–1.62) | |||
| No modification by menopausal status observed (results not shown) | ||||
| 28 | Levels of plasma FlOP, measured as relative fluorescent intensity per milliliter of plasma (FI/mL) via spectrofluorometric readings. Timing: cases had one sample ≤6 years before diagnosis (proximate) and one sample at ≥10 years before diagnosis (distant) Sample type: blood |
FlOP_360 | Adjustment factors: age at blood draw, fasting status, menopausal status and HRT use at blood draw, time of blood draw, family history of breast cancer, history of BBD, BMI at blood draw, age at menarche, age at first birth/parity, alcohol use, and physical activity. | |
| Proximate | ||||
| <174 | 1.0 (Reference) | |||
| ≥174 to <207 | 0.8 (0.5–1.3) | |||
| ≥207 to <251 | 0.6 (0.4–0.9) | |||
| ≥251 | 0.8 (0.5–1.3) | |||
| Distant | Additional subgroup analysis: associations were evaluated by BMI, HRT use, and total plasma carotenoid levels. A positive association for FlOP_320 was found only among women with low BMI (P for interaction was not statistically significant). | |||
| <174 | 1.0 (Reference) | |||
| ≥174 to <207 | 1.0 (0.7–1.6) | |||
| ≥207 to <251 | 1.2 (0.8–1.9) | |||
| ≥251 | 1.0 (0.7–1.6) | |||
| FlOP_320 | ||||
| Proximate | ||||
| <296 | 1.0 (Reference) | |||
| ≥296 to <388 | 1.0 (0.6–1.6) | |||
| ≥388 to <577 | 0.8 (0.5–1.2) | |||
| ≥577 | 1.1 (0.7–1.7) | |||
| Distant | ||||
| <296 | 1.0 (reference) | |||
| ≥296 to <388 | 0.8 (0.5–1.2) | |||
| ≥388 to <577 | 1.1 (0.7–1.7) | |||
| ≥577 | 1.3 (0.9–2.0) | |||
| FlOP_400 | ||||
| Proximate | 1.0 (Reference) | |||
| <47.8 | 1.6 (1.0–2.5) | |||
| ≥47.8 to <57.8 | 1.3 (0.8–2.1) | |||
| ≥57.8 to <73.0 | 1.3 (0.8–2.0) | |||
| ≥73.0 | ||||
| Distant | ||||
| <47.8 | 1.0 (Reference) | |||
| ≥47.8 to <57.8 | 0.9 (0.6–1.3) | |||
| ≥57.8 to <73.0 | 1.0 (0.6–1.6) | |||
| ≥73.0 | 1.0 (0.7–1.5) | |||
| Results by menopausal status NR | ||||
| 30 | 8-OxodG in nmol/mmol creatinine excretion in urine samples was determined by column-switching HPLC with electrochemical detection. Timing: samples collected at entry ≤5 years before diagnosis Sample type: spot urine |
Postmenopausal women only in the study | Adjustment factors: smoking, parity (age at first birth and number of births), education, intake of alcohol, BMI, and duration of HRT use. | |
| Per unit increase in 8-oxodG | 1.08 (1.00–1.17) | |||
| 8-oxodG | Additional subgroup analysis: when stratified by ER status, significant association was limited to women with ER+ breast cancer. Associations were shown by HRT use, smoking status, intake of fruit, vegetables, and iron, and iron supplement use. A statistically significant interaction was found for iron use, with the positive association limited to women who consumed lower iron levels (0.02). | |||
| 0.12–1.15 | 1.00 (Reference) | |||
| 1.15–1.78 | 0.78 (0.50–1.22) | |||
| 1.78–2.84 | 0.88 (0.54–1.43) | |||
| 2.84–25.42 | 1.42 (0.86–2.34) | |||
| 47 | Levels of plasma FlOP, measured as relative fluorescent intensity per milliliter of plasma (FI/mL) via spectrofluorometric readings. Timing: before diagnosis of breast cancer Sample type: blood |
FlOP_360 | Adjustment factors: BMI at blood draw, age at menarche, alcohol intake, parity/age at first birth, family history of breast cancer, and history of BBD. | |
| <176 | 1.00 (Reference) | |||
| 176 to <209 | 0.71 (0.52–0.96) | |||
| 209 to <252 | 0.85 (0.63–1.16) | Additional subgroup analysis: When stratified by ER status, results for FlOP_400 were suggestively inversely associated with ER- breast cancer only (p-value for heterogeneity = 0.04). | ||
| ≥252 | 0.68 (0.50–0.95) | |||
| FlOP_320 | ||||
| <271 | 1.00 (Reference) | |||
| 271 to <339 | 0.97 (0.71–1.32) | |||
| 339 to <453 | 0.83 (0.59–1.16) | |||
| ≥453 | 0.76 (0.55–1.06) | |||
| FlOP_400 | ||||
| <53 | 1.00 (Reference) | |||
| 53 to <64 | 1.14 (0.83–1.56) | |||
| 64 to <79 | 1.14 (0.83–1.57) | |||
| ≥79 | 1.03 (0.74–1.44) | |||
Adjustment factors for overall models.
Reference group is low damage.
15-F2t-IsoP, 15-F2t-isoprostane; 15-F2t-IsoPM, 2,3-dinor-5,6-dihydro-15-F2t-IsoP; 8-oxodG, 8-oxo-7,8-dihyrdo-2′-deoxyguanosine; BBD, benign breast disease; BMI, body–mass index; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; FlOP, fluorescent oxidation products; GC/NICI MS, gas chromatography/negative ion chemical ionization mass spectrometry; HPLC, high-performance liquid chromatography; HRT, hormone replacement therapy; IRR, incidence rate ratio; MDA, malondialdehyde; NHS, Nurses' Health Study; NR, not reported; OC, oral contraceptive; OR, odds ratio; RR, relative risk.
Case–control studies with biomarkers measured after diagnosis
In a population-based case–control study of 400 cases and 401 controls from the Long Island Breast Cancer Study Project, a positive association was reported for urinary 15-F2t-IsoP measured via immunoassay and risk of breast cancer, but not urinary 8-oxodG measured by competitive enzyme-linked immunosorbent assay (ELISA).40 All samples for the cases were collected within on average 3 months after diagnosis of breast cancer, with most before the initiation of chemotherapy. The positive association between 15-F2t-IsoP and increased risk of breast cancer was found among both pre- and postmenopausal women. Subsequently, investigators from the Long Island Breast Cancer Study Project conducted a larger study with 1061 breast cancer cases and 1108 controls (including the original cases/controls from the first study) and found no statistically significant associations between 15-F2t-IsoP or 8-oxodG and breast cancer.41
In a separate report, investigators from this same study evaluated the association between plasma protein carbonyl levels and breast cancer among 1050 cases and 1107 controls.31 They reported a trend for increasing odds of breast cancer risk in relation to increasing quartiles of plasma protein carbonyl levels measured via noncompetitive ELISA. The association was not modified by menopausal status.
In a population-based case–control study of 268 sister sets from the Breast Cancer Family Registry, where one sister was a case and the other was a control, plasma protein carbonyls measured via noncompetitive ELISA were associated with increased odds of breast cancer, adjusting for known breast cancer risk factors. All samples were taken months to years after diagnosis for the cases.42 A small case–control study with 57 cases and 139 controls conducted in Malaysia reported that higher levels of plasma MDA were associated with increased odds of breast cancer, adjusting for type of education, type of occupation, relatives, lactation, and waist circumference.43 A very small case–control study among 70 cases and 70 controls reported that higher DNA damage in lymphocytes measured via the comet assay was associated with increased odds of breast cancer.44 None of these three small studies evaluated associations by menopausal status.
Nested case–control studies with biomarkers measured before diagnosis
Five prospective nested case–control studies were identified, all with samples for cases collected before diagnosis. In a nested case–control study in the Shanghai Women's Health Study with 436 cases/852 controls, urinary 15-F2t-IsoP and 2,3-dinor-5,6-dihydro-15-F2t-IsoP (15-F2t-IsoPM) measured via gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI MS) were not associated with breast cancer risk overall, adjusting for known breast cancer risk factors and lifestyle factors.45 However, when stratified by menopausal status, an inverse association was observed for both biomarkers among premenopausal women only (e.g., 15-F2t-IsoP in the highest tertile [reference was the lowest] was associated with 42% reduced risk of premenopausal breast cancer).
In a second nested case–control study in the same cohort with a smaller sample size (354 breast cancer cases/654 controls), breast cancer was not associated with urinary levels of MDA or 8-oxodG,46 with authors reporting that associations were not modified by menopausal status. A nested case–control study in the Nurses' Health Study of 377 cases/377 controls evaluated the associations of FlOPs and breast cancer risk. Biomarkers were classified as proximate exposure (≤6 years before diagnosis) and distant exposure (≥10 years before diagnosis).28 For FlOP_360, proximate levels for the second quartile (207–251 FI/mL) only (RRs [95% CI]: 0.6 [0.4–0.9]) were inversely associated with breast cancer risk, associations were not observed for the second or third quartile, and associations for this biomarker measured distantly were null. For FlOP_320 and FlOP_400, no clear pattern of association with breast cancer overall was observed.
A fourth prospective nested case–control study of 336 cases/336 controls in Denmark evaluated urinary 8-oxodG levels measured via column-switching high-performance liquid chromatography in association with risk of postmenopausal breast cancer. Samples from cases were collected at cohort entry within 5 years of diagnosis. Overall, a positive association between 8-oxodG and risk of breast cancer was observed,30 and when stratified by ER status, the positive association was limited to women with ER+ breast cancer. In 2015, a large nested case–control study focusing on premenopausal breast cancer in the Nurses' Health Study I and II studies reported some evidence for an inverse association for FlOPs and premenopausal breast cancer (e.g., FlOP_360 highest quartile levels [compared with lowest quartile levels] were associated with a 32% decreased risk of premenopausal breast cancer in adjusted models).47
Subgroup analyses for lifestyle factors
Lifestyle factors may be associated with oxidative damage, and several studies considered body–mass index (BMI) and other lifestyle factors as potential effect modifiers/interaction variables, with results summarized (if available) in the comments section of Table 2. Briefly, three studies reported that results differed by BMI. In an early small case–control study, Smith et al. reported that the association between DNA damage and breast cancer was stronger among women with higher BMI, although no statistical test for interaction was provided.44 Dai et al. reported a positive association for 15-F2t-IsoPM among women with higher BMI and inverse association among women with lower BMI.45 In the Nurses' Health Study, one of the FlOP biomarkers was found to have a significant positive association only among women with low BMI; however, the p-value for interaction was not statistically significant (0.13).28 One study reported that the positive association of 8-oxodG was only present in women with low dietary iron intake; p-value for interaction = 0.02.30
Oxidative stress biomarkers and breast cancer prognosis
Study design and study population details are displayed in Table 3 and results are summarized in Table 4. We identified a total of eight studies that investigated biomarkers of oxidative stress in association with breast cancer prognosis with various oxidative stress biomarkers in blood, urine, and tissue utilized. The largest and earliest study identified, a prospective cohort of 363 breast cancer patients, reported that lipoperoxides measured in plasma before surgery were statistically significantly associated with increased risk of recurrence (>0.5 μmol/L = 2.13 [1.13–4.01]). However, lipoperoxides were not significantly associated with breast cancer-specific mortality (>0.5 μmol/L = 1.60 [0.73–3.51]).48
Table 3.
Observational Studies of Oxidative Stress Biomarkers and Breast Cancer Prognosis: Study Design and Study Population
| Refs. | Study design | Location | Years of diagnosis | Study population characteristics | Prognostic outcomes and follow-up |
|---|---|---|---|---|---|
| Saintot et al.48 | Prospective cohort | France | 1989–1992 | 363 Cancer cases (353 invasive, 10 in situ) Mean age at diagnosis: 59 years, range: 28–91 Scarff Blum and Richardson grade I–III |
Outcome(s): mortality and recurrence Data source: Cancer Center in France Follow-up: minimum of 8 years |
| Sova et al.49 | Retrospective cohort | Finland | 2003–2005 | 173 Cancer cases (invasive only) Mean age of patients in study: 51.7, age/range at diagnosis NR Grade I–III |
Outcome(s): BCSS Data source: hospital records Follow-up: mean of 40.5 months |
| Vera-Ramirez et al.52 | Prospective cohort | 2005–2007 | Spain | 70 Cancer cases (invasive only) Median age: 53 years, range 29–74 (Neoadjuvant) and 51 years, range 28–73 (adjuvant) AJCC Stage: I–III |
Outcome(s): DFS and OS Data source: hospital records Follow-up: mean of 50.1 months, range 9–58 months |
| Karihtala et al.50 | Prospective cohort | 2000–2008 | Finland | 79 Invasive cases Age NR Grade I–II |
Outcome(s): BCSS Data source: hospital records Follow-up: mean of 96.6 months |
| Roszkowski et al.64 | Prospective cohort | NR | Poland | 32 Breast cancer cases (invasive only), 99 total cancer cases Median age at diagnosis: NR years, range: NR Stage: III |
Outcome(s): OS Data source: NR Follow-up: up to 60 months |
| Karihtala et al.51 | Prospective cohort | 2003–2006 | Finland | 116 Cancer cases (96 invasive and 20 in situ) Age/range at diagnosis NR Stage: I–III |
Outcome(s): BCSS Data source: hospital records Follow-up: NR |
| Vera-Ramirez et al.53 | Prospective cohort | NR | Spain | 30 Invasive cases Median age at diagnosis: 51 years Range: 29–70 Stage: all metastatic |
Outcome(s): DFS and OS Source: oncology department registry Follow-up: mean of 21.86 months, range 2–45 months |
| Nechuta et al.54 | Prospective nested case–control | 2002–2005 | Shanghai, China | 160 Cases Controls (survived): median age 51.7; Cases (died): median age 51.6 Stage: I–III |
Outcome(s): total mortality Source: Shanghai vital statistics registry Follow-up: NR |
AJCC, American Joint Committee on Cancer; BCSS, breast cancer-specific survival; DFS, disease-free survival; OS, overall survival.
Table 4.
Observational Studies of Oxidative Stress Biomarkers and Breast Cancer Prognosis
| Refs. | Oxidative stress biomarker measurement and timing | Results | Covariates |
|---|---|---|---|
| Saintot et al.48 | Plasma MDA in μmol/L (lipoperoxides) via HPLC Timing: after diagnosis and before surgery Sample type: blood |
Breast cancer death, HRs (95% CI) | Age at diagnosis, menopausal status, ER status, and PR status. |
| <0.3, 1.00 (reference) | |||
| 0.3–0.5, 1.49 (0.68–3.26) | |||
| >0.5, 1.60 (0.73–3.51) | |||
| Recurrence, HRs (95% CI) | |||
| <0.3, 1.00 (reference) | |||
| 0.3–0.5, 1.69 (0.89–3.21) | |||
| >0.5, 2.13 (1.13–4.01) | |||
| Sova et al.49 | 8-OxodG (ng/mL) in serum samples using the ELISA and tumor tissue using IHC (4 groups of staining categories from negative to very positive) Timing: preoperative Sample types: blood, tumor tissue |
Serum 8-oxodG and BCSS Authors noted that this biomarker was not statistically significantly associated with BCSS (data not shown). Tumor tissue 8-oxodG and BCSS Negative 8-oxodG was associated with lower BCSS in univariate analysis, mean survival in months was 66.9 for positive and 49.5 for negative, p-value <0.01 Negative 8-oxodG was significantly associated with reduced BCSS in multivariable analysis (data not shown) |
Tumor tissue marker only: tumor size, node status, grade, Ki-67, HER2, p53, and ER/PR status |
| Vera-Ramirez et al52 | DNA strand breaks were detected using the alkaline comet assay Levels of plasma protein carbonyl groups (nmol/mg) were assessed using Protein Carbonyl Kit Timing: both before and after chemotherapy Sample type: blood |
Univariate, DFS (HRs and 95% CIs) | NR |
| DNA strand breaks, 1.009 (0.963–1.058) | |||
| Protein carbonyl level, 1.001 (0.99–1.013) | |||
| Univariate, OS HRs and (95% CIs) | |||
| DNA strand breaks, 1 (0.948–1.054) | |||
| Protein carbonyl level, 1.003 (0.991–1.016) | |||
| Multivariable results: NR | |||
| Karihtala et al.50 | 8-OxodG in tumor tissues using IHC Timing: NR Sample type: tumor tissue |
8-OxodG-positive immunostaining was associated with higher BCSS in univariate analysis (p = 0.011).> Authors note no association found in multivariable analysis (data not shown). |
NR |
| Roszkowski et al.64 | Urinary 8-oxodG (nmol/mmol creatinine) and 8-oxo-Gua (nmol/mmol creatinine) using HPLC with electrochemical detection or HPLC and GC-MS. Timing: before/after radiation treatment Sample type: spot urine |
No results reported specifically for breast cancer patients in the study. | NA |
| Karihtala et al.51 | 8-OxodG measured via IHC hOGG1a measured via IHC (4 groups of staining categories from negative to very positive) Sample type: tumor tissue |
Univariate, Mean survival time (95% CI)a | Traditional clincopathological factors (NR) |
| 8-oxodG and/or hOGG1 positive | |||
| 67.5 months (65.3–69.7) | |||
| 8-oxodG and hOGG1 negative | |||
| 34.5 months (21.8–47.2) | |||
| Multivariable: results for 8-oxodG alone or combined with hOGG1 NR | |||
| Vera-Ramirez et al.53 | DNA strand breaks detected using the alkaline comet assay. Lipid peroxidation was evaluated by measuring the concentration of TBARS (nmol/mL). Levels of plasma protein carbonyl (nmol/mg) groups were assessed using Protein Carbonyl Kit. Timing: before and after chemotherapy Sample type: blood |
Univariate, DFS, HRs (95% CIs) | NR |
| DNA strand breaks, 0.994 (0.969–1.02) | |||
| TBARS, 1.101 (0.91–1.33) | |||
| Protein carbonyl level, 1.026 (0.958–1.099) | |||
| Univariate, OS, HRs (95% CIs) | |||
| DNA strand breaks, 0.982 (0.951–1.015) | |||
| TBARS, 0.953 (0.74–1.225) | |||
| Protein carbonyl level, 1.063 (0.975–1.158) | |||
| Multivariable: NR | |||
| Nechuta et al.54 | Urinary levels of 15-F2t-IsoPs (ng/mg creatinine) and 15-F2t-IsoPM (ng/mg creatinine) were quantified by GC/NICI MS Timing: after primary treatment Sample type: spot urine |
15-F2t-IsoP, ORs (95% CIs) | Age, stage, year of diagnosis, clinical factors, weeks between diagnosis and urine collection, education income, BMI, menopausal status, vitamin supplement use |
| <1.725, 1.00 (reference) | |||
| ≥1.725, 0.36 (0.14–0.96) | |||
| <1.48, 1.00 (reference) | |||
| 1.48–2.07, 0.58 (0.20–1.67) | |||
| ≥2.07, 0.35 (0.11–1.13) | |||
| 15-F2t-IsoPM, ORs (95% CIs) | |||
| <0.908, 1.00 (reference) | |||
| ≥0.908, 1.39 (0.62–3.09) | |||
| <0.745, 1.00 (reference) | |||
| 0.745–1.07, 0.97 (0.38–2.50) | |||
| ≥1.07, 1.89 (0.67–5.32) |
This study only reported results that included both an oxidative stress biomarker and DNA repair biomarker combined, which is why the DNA repair biomarker is included in the study description.
DFS, disease-free survival; ER, estrogen receptor; HER-2, human epidermal growth factor receptor; hOGG1, human 8-oxoguanine glycosylase; IHC, immunohistochemistry; PR, progesterone receptor; TBARS, thiobarbituric acid-reactive substances.
In 2010, Sova et al. reported that negative 8-oxodG tumor status based on immunohistochemistry was associated with lower breast cancer-specific survival (BCSS).49 This study also reported that serum 8-oxodG, from blood collected before surgery, was not associated with BCSS, but data were not shown. Two small Finnish cohort studies evaluated 8-oxodG tumor status in association with BCSS, with the first evaluating 8-oxodG alone50 and the second evaluating 8-oxodG in combination with 8-oxoguanine glycosylase.51 The authors reported that no statistically significant associations were observed in multivariable analyses (effect estimates were not reported; Table 4).
Vera-Ramirez et al. conducted two studies, the first among 70 breast cancer patients52 and the second among 30 metastatic only patients.53 In both studies, they investigated biomarkers of DNA damage, lipid peroxidation, and protein damage in association with disease-free survival and overall survival both before and after chemotherapy and found no evidence for an association between any of the studied biomarkers and prognosis. In a small nested case–control study of 160 breast cancer cases from the Shanghai Breast Cancer Survival Study, GC/NICI MS was used to measure 15-F2t-IsoP and 15-F2t-IsoPM in urine samples collected after completion of cancer treatment.54 Adjusting for clinical and lifestyle factors, higher urinary levels of 15-F2t-IsoP were statistically significantly inversely associated with total mortality, while levels of 15-F2t-IsoPM were not significantly associated with survival.
Discussion
Biomarkers of oxidative stress and risk of developing breast cancer
Associations between oxidative stress biomarkers and breast cancer risk were inconsistent across studies, with evidence for both positive and inverse associations depending on the biomarkers evaluated and/or menopausal status. Three studies evaluated 8-oxodG, a valid marker of DNA damage, with two case–control studies reporting null results and one prospective cohort study reporting increased risk of postmenopausal breast cancer in association with higher levels of DNA damage. Two prospective cohort studies with measurement of oxidative stress biomarkers before breast cancer diagnosis reported inverse associations with breast cancer risk among premenopausal women only. Two case–control studies reported increased risk of breast cancer in association with protein oxidation measured after breast cancer diagnosis.
While some studies measured two oxidative stress biomarkers; none evaluated markers of DNA, lipid, and protein damage together and only one study measured biomarkers at more than one time point. Beyond menopausal status, results from subgroups analyses defined by BMI, dietary factors, and/or ER status suggested potential effect modification of the associations of oxidative stress and breast cancer risk by BMI and ER status in particular, which may contribute to differences across studies.
Some studies have reported that higher levels of oxidative stress are associated with obesity and adipose tissue.37,55–57 The finding of higher levels of oxidative stress and increased risk of postmenopausal breast cancer could reflect the known obesity and postmenopausal breast cancer association.58 In fact, Dai et al. conducted analyses stratified by BMI and found a positive association among women with higher BMI, but not among women with lower BMI.45
One key limitation that may contribute to the inconsistent results observed across studies is the timing of sample collection. Several studies collected samples after diagnosis of breast cancer, with some after surgery and/or during cancer treatment (such as chemotherapy). Levels of oxidative stress may change based on the presence and progression of the tumor itself and due to cancer treatments, including surgery, radiotherapy, and chemotherapy.9,13 While nested case–control studies can help minimize this limitation, the concern that oxidative stress may be generated by the preclinical tumor remains.32 This potential bias can be evaluated by stratifying associations by length of follow-up time, which was rarely done in the identified reports.
Another limitation is that breast cancer has been shown to be an etiologically heterogeneous disease, with studies showing modification of known associations by tumor subtype.58,59 However, to date, only two studies examined associations of biomarkers of oxidative stress with breast cancer risk by tumor ER status,30,47 and no studies have evaluated associations by molecular subtypes. Finally, differences in how menopausal status was defined across studies, a known methodological limitation in epidemiological studies considering menopausal status,60 or lack of stratifying results by menopausal status could have contributed to inconsistencies in findings.
Many reviews have described the limitations and strengths of various noninvasive biomarkers of oxidative stress used in epidemiological studies, including measurement issues (e.g., see Refs.7,15–19,21,24,61). Herein, we highlight some key issues to demonstrate how measurement assays and type of biomarker can also contribute to inconsistencies and lack of replication of findings. MDA, a biomarker of lipid peroxidation, was used in several studies summarized in this review, but is known to have the key issue of nonspecificity.
The gold standard in lipid peroxidation measurement includes 15-F2t-IsoP and 15-F2t-IsoPM, valid biomarkers of systematic in vivo oxidative stress. Highly sensitive and specific assays have been developed to measure F2-isoprostanes using GC/NICI MS.62 In our review, some studies used GC/NICI MS and others used immunoassays to measure F2-isoprostanes, which are less accurate.26,62 8-OxodG is one of the most commonly used markers for assessing oxidative DNA damage as it is one of the most abundant DNA lesions caused by ROS. However, 8-oxodG is a function not only of oxidation of DNA but also of excision repair,51 and studies that measure both DNA repair and oxidative DNA damage are needed to improve interpretation of findings. In summary, inconsistent results may be attributed to using unreliable assays and markers for the detection of systematic oxidative stress, lack of assaying biomarkers at more than one time point before diagnosis, and lack of careful consideration of the impact of preclinical cancer on oxidative stress biomarkers.
Biomarkers of oxidative stress and breast cancer prognosis
Oxidative stress plays a critical role in cancer treatment, with cytotoxic therapies increasing oxidative damage to potentially kill tumor cells. Beyond cancer treatment, oxidative stress from endogenous (e.g., metabolism, immune response) and exogenous sources (e.g., ionizing radiation, smoking, chemicals) may result in changes in the metabolic pathways in tumor cells, tumor vascular networks, and tumor macrophage infiltration.8,33 These alterations can impact not only tumor progression but also cancer cell adaption to oxidative stress, potentially leading to increased resistance to therapy, angiogenesis, and increased risk of metastasis.8,9 Due to the critical role of oxidative stress mechanisms in both cancer treatment and potentially cancer metastasis, it has been suggested that oxidative stress may be particularly important in cancer prognosis; however, as we found in this review, the epidemiologic literature in this area is very limited to date.
Overall, studies of oxidative stress biomarkers and breast cancer prognosis have been limited by small sample sizes (ranged from 30 to 363 cases) and/or lack of standardized analytic approaches (e.g., multivariable survival analyses) with consideration of key covariates/potential confounding factors. In addition, most were single-hospital studies and not population-based studies. Various biomarkers were used, with some studies measuring DNA damage, lipid peroxidation, and/or protein damage in various biological samples (blood, tissue, urine). Furthermore, the timing of biomarkers varied, with some studies measuring markers before surgery, after surgery and before/during chemotherapy, and after any cancer treatments. The largest and earliest study reported a positive association between a marker of lipid damage and prognosis48; however, this study used a marker of lipid peroxidation known to be nonspecific. Other studies generally reported limited evidence for an association. In summary, the lack of sufficient statistical power, use of various biomarkers, variation in time at biomarker assessments, and inadequate adjustment for confounding factors across studies hinder our ability to make a clear conclusion regarding the role of the biomarkers of oxidative stress in breast cancer prognosis at this time.
Research needs summary and conclusions
A key limitation of the literature to date is lack of studies in minority populations, including African American and Hispanic women, who may have different risk factor profiles for breast cancer and altered levels of oxidative stress biomarkers.63 Another key limitation is that no studies evaluated associations by breast cancer molecular subtype, which could be particularly informative for understanding mechanisms of oxidative stress both in the etiology and prognosis of breast cancer. Studies were further limited as most did not evaluate multiple valid biomarkers in multiple biological samples (e.g., urine and blood or blood and tumor tissue), which could help overcome limitations found in using a single biomarker. Finally, there were insufficient data to conduct a meta-analysis at this time; however, this may be possible after future research.
In conclusion, prospective studies provide inconsistent evidence that oxidative stress may increase risk of postmenopausal breast cancer (specifically DNA damage), but may be inversely associated with risk of premenopausal breast cancer (specifically F2-ispoprostanes and FlOPs). This association may be modified by ER status and/or BMI, but future studies with a larger sample size, applying standardized and multiple markers for measuring oxidative damage in prediagnostic biological samples, are needed to confirm these findings. Lipid peroxidation may play a role in breast cancer prognosis based on two small studies to date. Future large prospective studies that include multiple sample types (tissue, urine) using valid biomarkers measured at multiple time points after diagnosis (before and after cancer treatments) may be particularly informative and help to overcome methodological limitations of previous prognostic studies.
Acknowledgments and Funding
The authors thank Min Gao for helping with data abstraction. This work was partly supported by the National Cancer Institute at the National Institutes of Health (NIH) (grant number K07CA184257 to S.J.N.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Maritim AC, Sanders RA, Watkins JB., III. Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol 2003;17:24–38 [DOI] [PubMed] [Google Scholar]
- 2.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 2004;44:239–267 [DOI] [PubMed] [Google Scholar]
- 3.Basu S. F2-isoprostanes in human health and diseases: From molecular mechanisms to clinical implications. Antioxid Redox Signal 2008;10:1405–1434 [DOI] [PubMed] [Google Scholar]
- 4.Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev 2009;2:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch Biochem Biophys 1986;246:501–514 [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol 1997;82:291–295 [DOI] [PubMed] [Google Scholar]
- 7.Mayne ST. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr 2003;133:933s–940s [DOI] [PubMed] [Google Scholar]
- 8.Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer—Oxidative stress: Its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 2001;3:323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, et al. . Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells. Biological bases to develop oxidative-based therapies. Crit Rev Oncol Hematol 2011;80:347–368 [DOI] [PubMed] [Google Scholar]
- 10.Echiburu-Chau C, Roy D, Calaf GM. Metastatic suppressor CD44 is related with oxidative stress in breast cancer cell lines. Int J Oncol 2011;39:1481–1489 [DOI] [PubMed] [Google Scholar]
- 11.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013;12:931–947 [DOI] [PubMed] [Google Scholar]
- 12.Lawenda BD, Kelly KM, Ladas EJ, et al. . Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst 2008;100:773–783 [DOI] [PubMed] [Google Scholar]
- 13.Sangeetha P, Das UN, Koratkar R, Suryaprabha P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Rad Biol Med 1990;8:15–19 [DOI] [PubMed] [Google Scholar]
- 14.Weijl NI, Cleton FJ, Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev 1997;23:209–240 [DOI] [PubMed] [Google Scholar]
- 15.Niki E. Lipid peroxidation products as oxidative stress biomarkers. BioFactors 2008;34:171–180 [DOI] [PubMed] [Google Scholar]
- 16.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem 2006;52:601–623 [DOI] [PubMed] [Google Scholar]
- 17.Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci 2009;46:241–281 [DOI] [PubMed] [Google Scholar]
- 18.Ogino K, Wang DH. Biomarkers of oxidative/nitrosative stress: An approach to disease prevention. Acta Med Okayama 2007;61:181–189 [DOI] [PubMed] [Google Scholar]
- 19.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:120–139 [DOI] [PubMed] [Google Scholar]
- 20.Wu TY, Willett WC, Rifai N, Rimm EB. Plasma fluorescent oxidation products as potential markers of oxidative stress for epidemiologic studies. Am J Epidemiol 2007;166:552–560 [DOI] [PubMed] [Google Scholar]
- 21.Roberts LJ, Morrow JD. Measurement of F-2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 2000;28:505–513 [DOI] [PubMed] [Google Scholar]
- 22.Olinski R, Gackowski D, Rozalski R, Foksinski M, Bialkowski K. Oxidative DNA damage in cancer patients: A cause or a consequence of the disease development? Mutation Res 2003;531:177–190 [DOI] [PubMed] [Google Scholar]
- 23.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329:23–38 [DOI] [PubMed] [Google Scholar]
- 24.Kadiiska MB, Gladen BC, Baird DD, et al. . Biomarkers of oxidative stress study II. Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 2005;38:698–710 [DOI] [PubMed] [Google Scholar]
- 25.Knasmuller S, Nersesyan A, Misik M, et al. . Use of conventional and -omics based methods for health claims of dietary antioxidants: A critical overview. Br J Nutr 2008;99 E Suppl 1:ES3–ES52 [DOI] [PubMed] [Google Scholar]
- 26.Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. J Biol Chem 2008;283:15533–15537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halliwell B, Lee CYJ. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid Redox Signal 2010;13:145–156 [DOI] [PubMed] [Google Scholar]
- 28.Fortner RT, Tworoger SS, Wu T, Eliassen AH. Plasma florescent oxidation products and breast cancer risk: Repeated measures in the Nurses' Health Study. Breast Cancer Res Treat 2013;141:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakhi AK, Russnes KM, Thoresen M, et al. . Pre-radiotherapy plasma carotenoids and markers of oxidative stress are associated with survival in head and neck squamous cell carcinoma patients: A prospective study. BMC Cancer 2009;9:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loft S, Olsen A, Moller P, Poulsen HE, Tjonneland A. Association between 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and risk of postmenopausal breast cancer: Nested case-control study. Cancer Epidemiol Biomarkers Prev 2013;22:1289–1296 [DOI] [PubMed] [Google Scholar]
- 31.Rossner P, Jr, Terry MB, Gammon MD, et al. . Plasma protein carbonyl levels and breast cancer risk. J Cell Mol Med 2007;11:1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leufkens AM, van Duijnhoven FJB, Woudt SHS, et al. . Biomarkers of oxidative stress and risk of developing colorectal cancer: A cohort-nested case-control study in the european prospective investigation into cancer and nutrition. Am J Epidemiol 2012;175:653–663 [DOI] [PubMed] [Google Scholar]
- 33.Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutation Res 2011;711:193–201 [DOI] [PubMed] [Google Scholar]
- 34.Ambrosone CB. Oxidants and antioxidants in breast cancer. Antioxidants Redox Signal 2000;2:903–917 [DOI] [PubMed] [Google Scholar]
- 35.Jezierska-Drutel A, Rosenzweig SA, Neumann CA. Role of oxidative stress and the microenvironment in breast cancer development and progression. Adv Cancer Res 2013;119:107–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorjgochoo T, Gao YT, Chow WH, et al. . Obesity, age, and oxidative stress in middle-aged and older women. Antioxidants Redox Signal 2011;14:2453–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keaney JF, Larson MG, Vasan RS, et al. . Obesity and systemic oxidative stress—Clinical correlates of oxidative stress in the Framingham Study. Arterioscl Thromb Vasc 2003;23:434–439 [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh B, Figtree G, Costa D, et al. . Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int 2016;4:71–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossner P, Jr., Gammon MD, Terry MB, et al. . Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2006;15:639–644 [DOI] [PubMed] [Google Scholar]
- 41.Shen J, Gammon MD, Terry MB, et al. . Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer 2009;124:1637–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zipprich J, Terry MB, Liao Y, et al. . Plasma protein carbonyls and breast cancer risk in sisters discordant for breast cancer from the New York site of the Breast Cancer Family Registry. Cancer Res 2009;69:2966–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharhar S, Normah H, Fatimah A, et al. . Antioxidant intake and status, and oxidative stress in relation to breast cancer risk: A case-control study. Asian Pac J Cancer Prev 2008;9:343–349 [PubMed] [Google Scholar]
- 44.Smith TR, Miller MS, Lohman KK, Case LD, Hu JJ. DNA damage and breast cancer risk. Carcinogenesis 2003;24:883–889 [DOI] [PubMed] [Google Scholar]
- 45.Dai Q, Gao YT, Shu XO, et al. . Oxidative stress, obesity, and breast cancer risk: Results from the Shanghai Women's Health Study. J Clin Oncol 2009;27:2482–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KH, Shu XO, Gao YT, et al. . Breast cancer and urinary biomarkers of polycyclic aromatic hydrocarbon and oxidative stress in the Shanghai Women's Health Study. Cancer Epidemiol Biomarkers Prev 2010;19:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sisti JS, Lindstrom S, Kraft P, et al. . Premenopausal plasma carotenoids, fluorescent oxidation products, and subsequent breast cancer risk in the nurses' health studies. Breast Cancer Res Treat 2015;151:415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saintot M, Mathieu-Daude H, Astre C, et al. . Oxidant-antioxidant status in relation to survival among breast cancer patients. Int J Cancer 2002;97:574–579 [DOI] [PubMed] [Google Scholar]
- 49.Sova H, Jukkola-Vuorinen A, Puistola U, Kauppila S, Karihtala P. 8-Hydroxydeoxyguanosine: A new potential independent prognostic factor in breast cancer. Br J Cancer 2010;102:1018–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karihtala P, Kauppila S, Soini Y, Arja Jukkola V. Oxidative stress and counteracting mechanisms in hormone receptor positive, triple-negative and basal-like breast carcinomas. BMC Cancer 2011;11:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karihtala P, Kauppila S, Puistola U, Jukkola-Vuorinen A. Absence of the DNA repair enzyme human 8-oxoguanine glycosylase is associated with an aggressive breast cancer phenotype. Br J Cancer 2012;106:344–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, et al. . Does chemotherapy-induced oxidative stress improve the survival rates of breast cancer patients? Antioxid Redox Signal 2011;15:903–909 [DOI] [PubMed] [Google Scholar]
- 53.Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortosa MC, et al. . Oxidative stress status in metastatic breast cancer patients receiving palliative chemotherapy and its impact on survival rates. Free Radic Res 2012;46:2–10 [DOI] [PubMed] [Google Scholar]
- 54.Nechuta S, Cai Q, Zheng Y, et al. . Urinary biomarkers of oxidative stress and breast cancer survival. Cancer Causes Cont 2014;25:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomson CA, Giuliano AR, Shaw JW, et al. . Diet and biomarkers of oxidative damage in women previously treated for breast cancer. Nutr Cancer 2005;51:146–154 [DOI] [PubMed] [Google Scholar]
- 56.Block G, Dietrich M, Norkus EP, et al. . Factors associated with oxidative stress in human populations. Am J Epidemiol 2002;156:274–285 [DOI] [PubMed] [Google Scholar]
- 57.Crujeiras AB, Diaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: A potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res 2013;47:243–256 [DOI] [PubMed] [Google Scholar]
- 58.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 2014;36:114–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambertini M, Santoro L, Del Mastro L, et al. . Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev 2016;49:65–76 [DOI] [PubMed] [Google Scholar]
- 60.Phipps AI, Ichikawa L, Bowles EJ, et al. . Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas 2010;67:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weimann A, Broedbaek K, Henriksen T, Stovgaard ES, Poulsen HE. Assays for urinary biomarkers of oxidatively damaged nucleic acids. Free Radic Res 2012;46:531–540 [DOI] [PubMed] [Google Scholar]
- 62.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F-2-isoprostanes as a biomarker of oxidative stress. Nat Protoc 2007;2:221–226 [DOI] [PubMed] [Google Scholar]
- 63.Watters JL, Satia JA, Kupper LL. Correlates of antioxidant nutrients and oxidative DNA damage differ by race in a cross-sectional study of healthy African American and white adults. Nutr Res 2008;28:565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roszkowski K, Olinski R. Urinary 8-oxoguanine as a predictor of survival in patients undergoing radiotherapy. Cancer Epidemiol Biomarkers Prev 2012;21:629–634 [DOI] [PubMed] [Google Scholar]