ABSTRACT
The alternative sigma factor σE is a key component of the Escherichia coli response to cell envelope stress and is required for viability even in the absence of stress. The activity of σE increases during entry into stationary phase, suggesting an important role for σE when nutrients are limiting. Elevated σE activity has been proposed to activate a pathway leading to the lysis of nonculturable cells that accumulate during early stationary phase. To better understand σE-directed cell lysis and the role of σE in stationary phase, we investigated the effects of elevated σE activity in cultures grown for 10 days. We demonstrate that high σE activity is lethal for all cells in stationary phase, not only those that are nonculturable. Spontaneous mutants with reduced σE activity, due primarily to point mutations in the region of σE that binds the −35 promoter motif, arise and take over cultures within 5 to 6 days after entry into stationary phase. High σE activity leads to large reductions in the levels of outer membrane porins and increased membrane permeability, indicating membrane defects. These defects can be counteracted and stationary-phase lethality delayed significantly by stabilizing membranes with Mg2+ and buffering the growth medium or by deleting the σE-dependent small RNAs (sRNAs) MicA, RybB, and MicL, which inhibit the expression of porins and Lpp. Expression of these sRNAs also reverses the loss of viability following depletion of σE activity. Our results demonstrate that appropriate regulation of σE activity, ensuring that it is neither too high nor too low, is critical for envelope integrity and cell viability.
IMPORTANCE The Gram-negative cell envelope and cytoplasm differ significantly, and separate responses have evolved to combat stress in each compartment. An array of cell envelope stress responses exist, each of which is focused on different parts of the envelope. The σE response is conserved in many enterobacteria and is tuned to monitor pathways for the maturation and delivery of outer membrane porins, lipoproteins, and lipopolysaccharide to the outer membrane. The activity of σE is tightly regulated to match the production of σE regulon members to the needs of the cell. In E. coli, loss of σE results in lethality. Here we demonstrate that excessive σE activity is also lethal and results in decreased membrane integrity, the very phenotype the system is designed to prevent.
KEYWORDS: cell envelope, stress response, transcriptional regulation
INTRODUCTION
Stress responses allow cells to rapidly adapt their gene expression to cope with changing conditions. Signal transduction pathways sense an inducing stress and transduce that information to a transcription factor, which, in turn, regulates the expression of specialized sets of genes required to combat the stress. Once the stress is removed, the response is downregulated and gene expression returns to the basal state. In bacteria, major cellular stress responses are mediated by alternative sigma factors, which rapidly reprogram gene expression by replacing the housekeeping sigma factor and directing RNA polymerase to the genes in their regulons (1–3). Gram-negative bacteria have compartmentalized responses to address stress in their two cellular compartments, the cytoplasm and the cell envelope. The alternative sigma factors σE and σ32 mediate the envelope and cytoplasmic stress responses, respectively (4–6). Although mutants lacking σ32 are very temperature sensitive, Escherichia coli can grow without σ32 at temperatures below 20°C (7). In contrast, rpoE, the gene encoding σE, is essential for viability (8). When σE activity is depleted, cells develop envelope defects and lyse, indicating that σE is required to maintain cell envelope integrity and that combating envelope stress via σE is critical for survival (5).
σE directs the transcription of a regulon that has a significant impact on the cell envelope (9). There are two arms of the response, one mediated by proteins and the other by small RNAs (sRNAs) (9, 10). σE transcribes genes encoding proteases, which degrade misfolded envelope proteins, and chaperones and assembly factors, which escort outer membrane proteins and lipopolysaccharide (LPS) as they transit from the inner membrane across the periplasm to the outer membrane (9, 11, 12). σE also transcribes genes encoding several sRNAs that block the expression of a series of mRNAs, including those encoding every major outer membrane porin and several lipoproteins (10, 13). As a result, when conditions in the cell envelope are unfavorable, σE serves to increase the capacity of the pathways that deliver LPS and porins to the outer membrane, while decreasing the load on the system by reducing de novo synthesis of outer membrane constituents. The sRNA arm of the response is of particular importance because expression of the RybB, MicA, or MicL σE-dependent sRNAs can restore viability when σE activity is severely reduced (10, 13).
The activity of σE is tuned to the state of outer membrane protein folding through a regulatory pathway that is activated by unfolded outer membrane proteins (14). The activity of σE is directly regulated by the anti-sigma factor RseA. RseA is an inner membrane protein whose cytoplasmic domain binds to σE and prevents σE from associating with RNA polymerase (15–17). Unfolded outer membrane proteins trigger a proteolytic cascade that results in the complete degradation of RseA, freeing σE to direct transcription (18, 19). In addition to the pathway that controls the proteolysis of RseA, transcription by the σE-RNA polymerase holoenzyme is activated by the global stress alarmone guanosine-3′,5′-bisdiphosphate (ppGpp) (20, 21). ppGpp is a potent signal of starvation, and its levels increase during entry into stationary phase in rich medium or when growth slows due to depletion of specific nutrients (22, 23). The activity of σE increases at such times in a ppGpp-dependent manner, independent of RseA and the envelope stress signaling pathway, suggesting that σE might facilitate the survival of E. coli after the exponential phase of growth (20, 24).
The role of σE in stationary-phase survival remains relatively unexplored. σE has been proposed to direct a pathway that leads to the lysis of viable but nonculturable cells (VBNC) in early stationary phase. This proposal is based on observations that cultures with elevated σE activity due to σE overexpression or deletion of the rseA gene have lower optical densities (ODs) in early stationary phase than do isogenic wild-type cells, although the CFU per milliliter for the two strains are comparable. Cultures with elevated σE activity also have higher levels of proteins released into the growth medium than wild-type cultures, suggesting that cells that are not able to form colonies have lysed (25–27).
To better understand σE-directed cell lysis and its contribution to the role of σE in stationary-phase survival, we investigated the effects of elevated σE activity in cultures grown for 10 days, into stationary phase, in rich medium. Importantly we found that σE-directed cell lysis is likely to be due to membrane defects in cells with elevated σE activity, rather than a purposeful regulated process. In fact, elevated σE activity is toxic in stationary phase for all cells, and mutants with reduced σE activity take over cultures within 4 to 5 days. The toxicity is due almost entirely to the RybB, MicA, and MicL sRNAs, indicating that proper regulation of their expression is a critical role of the σE response.
RESULTS
High σE activity causes a lethal phenotype in stationary phase.
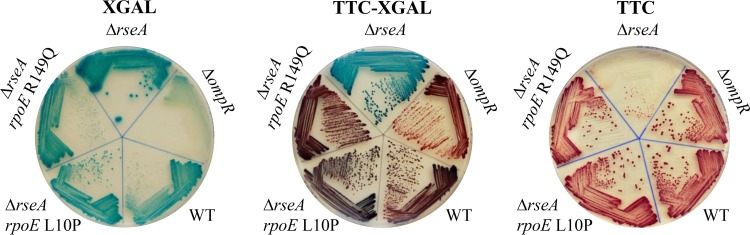
In previous studies of σE-directed cell lysis with a ΔrseA mutant in early stationary phase, the optical densities, CFU per milliliter, and release of protein into culture supernatants were examined, but σE activity was not investigated (25–27). Because we had observed that mutants with reduced σE activity occasionally appeared when overnight cultures of a ΔrseA strain were plated, we decided to monitor σE activity using the σE-dependent rpoHp3-lacZ fusion, in addition to measuring the optical density and CFU per milliliter. To better distinguish colonies with a wide range of σE activities, we developed a method that combined two indicators of β-galactosidase activity, with different dynamic ranges, i.e., 2,3,5-triphenyltetrazolium chloride (TTC) and 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (X-Gal). Cells with high levels of lacZ expression, such as the ΔrseA strain, form dark blue colonies on X-Gal agar, white to light pink colonies on lactose-TTC agar, and blue colonies on lactose–TTC–X-Gal agar (Fig. 1). Cells with intermediate levels of σE activity, such as the wild-type strain, form medium blue colonies with X-Gal, red colonies with TTC, and purple colonies with TTC and X-Gal (Fig. 1). Cells with low levels of σE activity, such as a ΔompR strain (6), form light blue colonies with X-Gal, deep red colonies with TTC, and red colonies with the combination (Fig. 1).
FIG 1.
Cells with a wide range of σE activities can be distinguished on lactose–TTC–X-Gal agar. The activity of σE from the σE-dependent rpoHp3-lacZ reporter is shown on LB–X-Gal (left), lactose–TTC–X-Gal (center), and lactose-TTC (right) plates for the ΔompR strain with very low levels of σE activity, the wild-type (WT) strain with moderate levels of σE activity, and the ΔrseA strain with high levels of σE activity. Also shown are two mutants, isolated from stationary-phase ΔrseA cultures, that have mutations in rpoE that reduce its activity to different extents (8-fold decrease for the L10P mutation and 33-fold decrease for the R149Q mutation) (Table 1).
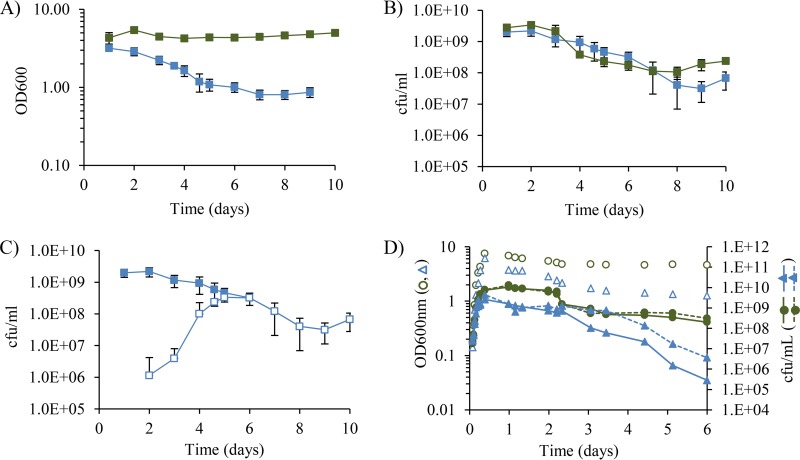
To gain a more complete view of the role of σE-directed cell lysis during stationary phase, we grew cultures of wild-type and ΔrseA strains in Luria-Bertani (LB) broth for 10 days, into the start of long-term stationary phase. Previous studies focused only on early-stationary-phase cultures grown for 72 h or less (25–27). The optical density at 600 nm (OD600) of the ΔrseA cultures decreased approximately 4-fold during the first 5 days in stationary phase and then remained at the same level for the following 5 days (Fig. 2A, blue squares). In contrast, the OD600 of the wild-type cultures did not change significantly for the entire 10 days (Fig. 2A, green squares). Despite the observation that the OD600 of the ΔrseA cultures was less than that of the wild-type cultures, the CFU per milliliter of the two strains were similar (Fig. 2B). The CFU per milliliter began to decrease after 2 to 3 days and dropped by 90 to 99% before leveling off by day 7 (Fig. 2B), as expected for cultures grown in rich medium that are transitioning from early stationary phase through death phase and into long-term stationary phase (28). When examined by phase-contrast microscopy, the ΔrseA cultures had numerous small translucent cells indicative of lysis, which were likely responsible for the lower optical density. The remaining phase-dense cells looked similar to those in the wild-type cultures and had no obvious morphological defects. Our results from the first 72 h in stationary phase for wild-type and ΔrseA cultures were similar to those reported previously (25–27).
FIG 2.
Mutants with reduced σE activity outcompete the parental ΔrseA strain in stationary-phase cultures. (A and B) OD600 (A) and CFU per milliliter (B) of ΔrseA (blue squares) and wild-type (green squares) cultures grown for 10 days are shown. (C) Total CFU per milliliter of ΔrseA cultures (closed squares) and CFU per milliliter of mutants with reduced σE activity that arose in ΔrseA cultures (open squares) are shown. Data presented are averages with standard deviations from 4 independent cultures. (D) σE overexpression from the plasmid pLC245 also reduces survival in stationary phase. The OD600 values for a wild-type strain carrying pLC245 (blue triangles) or the control plasmid pTrc99a (green circles) grown under inducing conditions in the absence of selective pressure for the plasmids are shown as open symbols. The total CFU per milliliter (closed symbols, dashed lines) and the CFU per milliliter of cells still carrying the plasmid, as measured by ampicillin resistance (closed symbols, solid lines), are shown for each strain.
A critical difference between the wild-type and ΔrseA strains was observed when σE activity was monitored. The colonies from wild-type cultures were a purple color throughout the experiment on TTC–X-Gal agar, indicating that the levels of σE activity remained relatively constant. Within 2 to 3 days, however, the population of cells from the ΔrseA cultures with high σE activity that formed blue colonies in the presence of lactose, TTC, and X-Gal started to decrease and purple colonies with lower levels of σE activity began to appear (Fig. 2C, open squares). By day 5 or 6 of stationary phase, all colonies were purple (Fig. 2C), indicating that bacteria with high levels of σE activity did not survive more than few days in stationary phase and were replaced by a population of cells with lower levels of σE activity. Colonies of two mutants with reduced σE activity, the ΔrseA rpoE L10P and ΔrseA rpoE R149Q strains, are shown in Fig. 1. The OD600 of the ΔrseA cultures also stopped decreasing around day 6 (Fig. 2A), consistent with replacement of the dying ΔrseA strain by cells with lower levels of σE activity. Similar results were obtained with two ΔrseA strains, each carrying a different tightly linked marker, nadB::Tn10 or yfiC::kan.
The stationary-phase defects are due to elevated σE activity and not loss of RseA.
The decrease in the number of cells with high levels of σE activity could be caused by toxic effects associated with high σE activity or by a σE-independent defect caused by deletion of the rseA gene. To distinguish between these possibilities, we compared the growth of a wild-type strain containing a plasmid with the rpoE gene under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter with the growth of a strain with the empty vector plasmid. The strains were grown into early exponential phase in the presence of ampicillin to ensure that all cells had the plasmid. Cells were then transferred to LB medium without ampicillin to allow plasmid loss, if it proved to be toxic, and with IPTG to induce overexpression of rpoE. The OD600 of the strain overexpressing rpoE was lower in stationary phase than was that of the control strain (Fig. 2D), similar to findings observed with the ΔrseA strain. Overexpression of rpoE also reduced survival (measured as the total CFU per milliliter) in stationary phase, compared to the control strain without the plasmid or the overexpression strain without induction of rpoE (Fig. 2D). Ninety percent of the cells overexpressing rpoE were sensitive to ampicillin after 3 days in stationary phase, indicating that the rpoE overexpression plasmid had been lost and/or cells with the plasmid were not able to survive (Fig. 2D). In contrast, the pTrc99a control plasmid was stably maintained even in the absence of selective pressure (Fig. 2D). These data indicate that failure to survive in stationary phase is a property of elevated σE activity and is not due to a function of RseA that is independent of σE.
Spontaneous mutations with lower σE activity map primarily to the rpoE gene.
Several of the strains isolated from the stationary-phase cultures were analyzed to identify the mutations causing lower σE activity. In cultures of the ΔrseA nadB::Tn10 strain, many mutants also lost tetracycline resistance. PCR amplification using primers for the promoter region failed, suggesting that the rpoE promoter was disrupted by recombination events due to excision of Tn10 from nadB, which is directly upstream of rpoE. In mutants isolated from cultures of the ΔrseA ΔyfiC::kan strain, the kanamycin resistance marker was stable and rpoE could be successfully amplified by PCR. Fifteen mutants isolated from stationary-phase cultures of the ΔrseA ΔyfiC::kan strain and the ΔrseA nadB::Tn10 strain that retained tetracycline resistance were analyzed. Thirteen mutants had point mutations in rpoE and two had mutations mapping outside rpoE, one to the rpoBC region encoding the β and β′ subunits of RNA polymerase and one to nsrR, a nitrite-sensitive transcriptional repressor. All mutations were moved by P1 transduction into a clean ΔrseA background, to verify that the mutations were responsible for the phenotypes. σE activity and protein levels were measured for the isolates with mutations in rpoE. We found that all of the mutations decreased σE activity to levels similar to or below those found in the wild-type strain with a functional rseA gene.
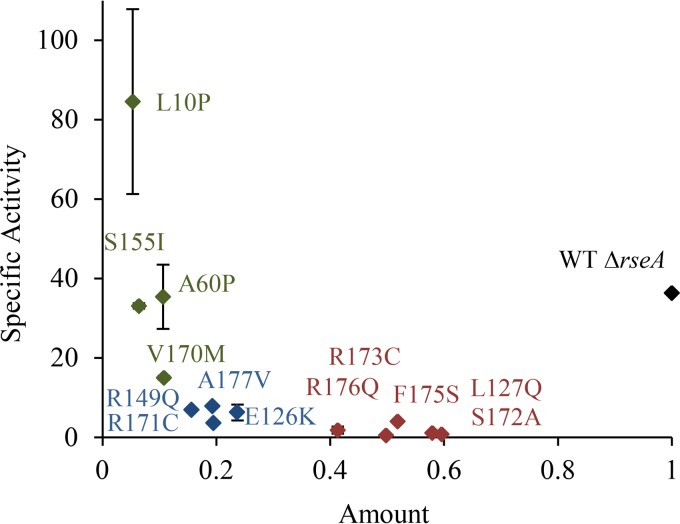
The mutations could be roughly divided into three classes: those that significantly decreased the specific activity and had only minor effects on the amount of σE in the cell, those that had moderate effects on both the specific activity and the amount of σE, and those that had stronger effects on the amount than on the specific activity of σE (Table 1 and Fig. 3). The L127Q, S172A, R173C, F175S, and R176Q mutations belong to the first class (Fig. 3). The mutations decreased the specific activity of σE 9- to 72-fold, with only small effects on the amount of σE. These residues have been shown to form direct contacts with the −35 region of the promoter DNA, with the exception of L127, which is buried in the hydrophobic core of σE domain 4 (15, 29). The rpoE gene is transcribed from both σ70- and σE-dependent promoters, and the slightly reduced amounts of σE in these mutants could be due to reduced transcription from the σE-dependent promoter or reduced stability of the protein.
TABLE 1.
Effects of rpoE mutations on σE amounts and activity in the ΔrseA strain background
| rpoE variant | Relative amount of σEa | Activity of σE (arbitrary units) | Specific activity of σEb |
|---|---|---|---|
| Wild type | 1.00 | 36.37 ± 1.04 | 36.37 ± 1.04 |
| R173C | 0.50 ± 0.12 | 0.25 ± 0.00 | 0.50 ± 0.00 |
| S172A | 0.60 ± 0.20 | 0.47 ± 0.03 | 0.79 ± 0.05 |
| L127Q | 0.58 ± 0.14 | 0.65 ± 0.02 | 1.11 ± 0.04 |
| R176Q | 0.41 ± 0.15 | 0.75 ± 0.38 | 1.82 ± 0.92 |
| F175S | 0.52 ± 0.17 | 2.08 ± 0.08 | 4.01 ± 0.16 |
| R171C | 0.20 ± 0.11 | 0.69 ± 0.01 | 3.53 ± 0.07 |
| E126K | 0.24 ± 0.12 | 1.48 ± 0.47 | 6.22 ± 2.00 |
| R149Q | 0.16 ± 0.05 | 1.08 ± 0.01 | 6.87 ± 0.09 |
| A177V | 0.19 ± 0.02 | 1.51 ± 0.03 | 7.81 ± 0.15 |
| V170M | 0.11 ± 0.05 | 1.62 ± 0.05 | 15.01 ± 0.46 |
| A60P | 0.06 ± 0.03 | 2.11 ± 0.04 | 33.09 ± 0.67 |
| S155I | 0.11 ± 0.05 | 3.76 ± 0.86 | 35.41 ± 8.07 |
| L10P | 0.05 ± 0.00 | 4.47 ± 1.23 | 84.57 ± 23.28 |
Amount of the σE variant relative to the amount of wild-type σE in the parental ΔrseA strain.
Activity of σE normalized to the relative amount of σE.
FIG 3.
Mutants with reduced σE activity that have been recovered from stationary-phase cultures of the ΔrseA strain have mutations in the rpoE gene that reduce the amount and/or specific activity of σE. The amount of σE in each strain was measured by Western blotting and normalized to the level of wild-type (WT) σE in the parental ΔrseA strain. The specific activity was determined by measuring σE activity from the rpoHp3-lacZ reporter gene in liquid cultures and normalizing the activity to the amount of σE in that particular strain. Mutations that primarily reduce the amount of σE but not the specific activity are shown in green, those that reduce the amount and specific activity of σE are shown in blue, and those that reduce the specific activity but not the amount of σE are shown in red.
The E126K, R149Q, R171C, and A177V mutations affected both the amount of σE (4- to 6-fold reductions) and the specific activity (5- to 10-fold reductions) (Fig. 3). E126 is exposed on the surface and potentially at the interface between core RNA polymerase and σE, R149 and R171 make DNA contacts in the −35 promoter region, and A177 forms part of the hydrophobic core (15, 29).
The remaining mutations, L10P, A60P, S155I, and V170M, reduced the amount of σE in the cell by ≥10-fold, with little to no reduction in specific activity (Fig. 3). These mutations are likely to destabilize the folded structure of the protein, leading to increased degradation by proteases. The L10P and A60P mutations introduce prolines in the first position of a turn and in the middle of an α helix, respectively (30, 31). An isoleucine substitution at position 155 likely exposes a hydrophobic residue on the surface of the protein, and V170 is buried in the hydrophobic core (15, 29).
Reduced σE activity is sufficient to eliminate the stationary-phase defects.
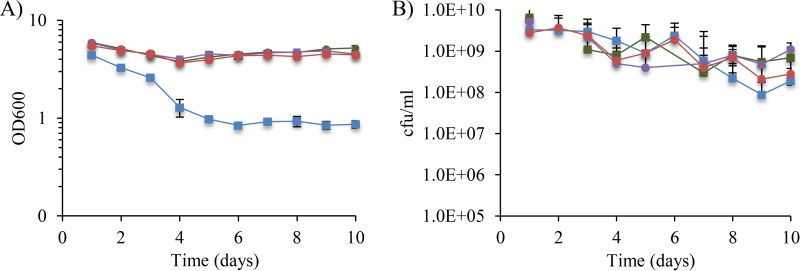
In addition to having elevated σE activity, cells lacking RseA cannot modulate σE in response to envelope stress, either of which could lead to the stationary-phase defects. To distinguish between these possibilities, we asked whether ΔrseA mutants with reduced σE activity exhibited the same phenotype as the parent ΔrseA strain in stationary phase. Two mutants belonging to the first two classes described above, the ΔrseA rpoE S172A and ΔrseA rpoE R171C strains, were selected for further examination. When cultures of these strains were grown for 10 days, the OD600 did not decrease (Fig. 4A), the CFU per milliliter were comparable to those of the wild-type strain (Fig. 4B), and the colonies remained the same color (purple) on lactose–TTC–X-Gal plates, indicating that decreasing σE activity reversed the survival defect of ΔrseA strains.
FIG 4.
Mutations that decrease σE activity reverse the stationary-phase defects of the ΔrseA strain. The OD600 (A) and total CFU per milliliter (B) of ΔrseA (blue squares), wild-type (green squares), ΔrseA rpoE S172A (red circles), and ΔrseA rpoE R171C (purple circles) cultures are shown. Mutants with lower levels of σE activity did not appear for the wild-type, ΔrseA rpoE S172A, and ΔrseA rpoE R171C strains. Data shown are averages with standard deviations from 2 independent cultures.
High σE activity reduces outer membrane integrity.
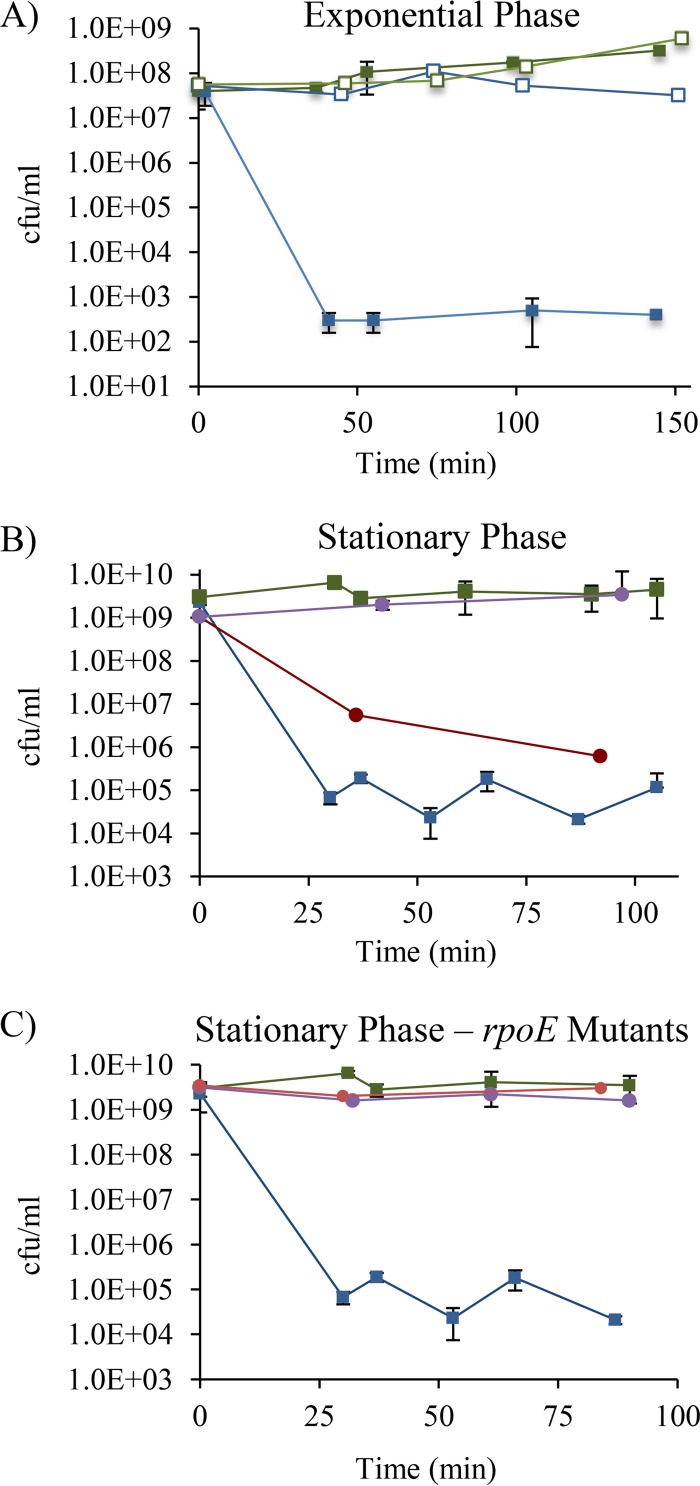
Given the impact of σE regulon members on the outer membrane (9, 10, 13, 32, 33), we reasoned that high σE activity might lead to an imbalance in the synthesis of outer membrane components, altering the permeability of the cell envelope and potentially making the ΔrseA cells less fit in stationary phase. To test this idea, we examined the sensitivity of the ΔrseA strain to the detergent SDS and rifampin, a large hydrophobic antibiotic that normally is not able to cross the outer membrane of E. coli but can enter cells with increased permeability. Exponential-phase ΔrseA cultures rapidly lost viability (a decrease in CFU per milliliter of >5 orders of magnitude) in the presence of 5% SDS and stopped growing within 90 min after the addition of rifampin to a final concentration of 8 μg/ml (Fig. 5A). In contrast, growth of the wild-type strain was not affected at these concentrations of SDS and rifampin over the same time frame (Fig. 5A). Approximately one-half of the ΔrseA cells that survived SDS treatment had reduced σE activity, as indicated by colony color on lactose–TTC–X-Gal agar, whereas no colonies with reduced σE activity were seen for untreated ΔrseA cultures. These data indicated that a small population of cells with low σE activity were already present in exponential-phase cultures and were enriched by SDS treatment.
FIG 5.
The ΔrseA strain exhibits membrane permeability defects that are stabilized by Mg2+ and by mutations that reduce σE activity. (A) CFU per milliliter of the ΔrseA (blue squares) and wild-type (green squares) strains from exponentially growing cultures in LB medium treated with 5% SDS (closed symbols) or 8 μg/ml rifampin (open symbols) is shown. (B) CFU per milliliter of 1-day-old stationary-phase cultures treated with 5% SDS is shown for ΔrseA cultures in LB medium (blue squares), LB-MOPS (red circles), and LB-Mg (purple circles) and wild-type cultures in LB medium (green squares). (C) CFU per milliliter of ΔrseA (blue squares), ΔrseA rpoE S172A (red circles), ΔrseA rpoE R171C (purple circles), and wild-type (green squares) stationary-phase cultures in LB medium treated with 5% SDS is shown. Data presented are averages with standard deviations from at least 2 independent cultures. In all panels, time indicates minutes after treatment.
To determine whether the membrane defects persisted into stationary phase, 28- to 30-h cultures (before mutants took over the population) were treated with 5% SDS. The CFU per milliliter of cultures of the ΔrseA strain decreased by approximately 4 orders of magnitude within 30 min after SDS treatment, while the wild-type strain was not affected (Fig. 5B). Of the cells that survived SDS treatment, 99% exhibited reduced σE activity on lactose–TTC–X-Gal agar, suggesting that ΔrseA mutants with low σE activity were less sensitive to SDS and were present in the cultures after 28 h of growth at a frequency of about 0.5 × 10−4 to 1 × 10−4.
To determine whether elevated σE activity was responsible for the SDS sensitivity of the ΔrseA strain, stationary-phase cultures of the ΔrseA rpoE S172A and ΔrseA rpoE R171C mutants were treated with SDS. Both mutations reversed the SDS sensitivity of the ΔrseA strain (Fig. 5C), indicating that high σE activity is responsible for reduced outer membrane integrity as well as the stationary-phase lethality phenotypes.
Stabilization of the outer membrane reduces stationary-phase lethality and reverses membrane permeability defects.
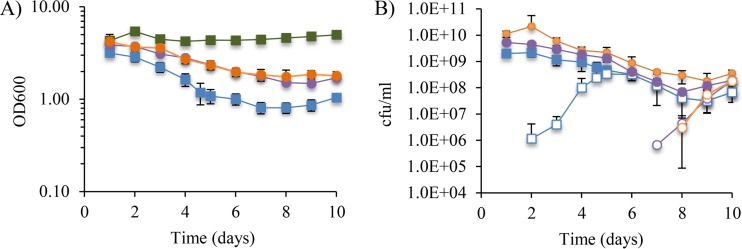
Mg2+ is known to stabilize the outer membrane, most likely by stabilizing interactions between adjacent LPS molecules (34). If the increased membrane permeability of the ΔrseA strain is related to its instability in stationary phase and is due to a problem with the LPS component of the outer membrane, then supplementing the growth medium with Mg2+ should decrease both defects associated with the ΔrseA strain. Indeed, we found that stationary-phase ΔrseA cultures grown in LB medium with 20 mM MgCl2 (LB-Mg) were no longer sensitive to 5% SDS (Fig. 5B). In addition, the decrease in optical density during the first 5 days of stationary phase was significantly reduced when the ΔrseA strain was grown in LB-Mg, compared to growth without Mg2+ supplementation (Fig. 6A), the appearance of mutants with low σE activity was delayed an average of 48 h in the LB-Mg cultures (Fig. 6B), and mutants with low σE activity did not take over even after 10 days in stationary phase (Fig. 6B). The number of mutants stopped increasing by day 8, and the mutants constituted approximately 30% of the total CFU per milliliter for the last 3 days of culture growth (Fig. 6B).
FIG 6.
Supplementation of LB medium with Mg2+ and buffering with MOPS stabilize the ΔrseA strain in stationary phase. The ΔrseA strain was grown into stationary phase for 10 days in LB medium (blue squares), LB-Mg (purple circles), LB-MOPS (red circles), or LB-MOPS-Mg (orange circles). The wild-type strain grown in LB medium (green squares) is also shown. (A) OD600 of the cultures is shown. (B to D) CFU per milliliter is shown as total CFU per milliliter (closed symbols) and CFU per milliliter of mutants with reduced σE activity (open symbols) for cultures grown in LB-Mg (B), LB-MOPS (C), or LB-MOPS-Mg (D). Data shown are averages with standard deviations from at least 4 independent cultures.
Growth in buffered medium partially reverses SDS sensitivity.
The pH of the growth medium increased in the stationary-phase cultures over time, as is known to occur for E. coli grown in LB medium, which could contribute to the reduced survival of ΔrseA cells. To relieve the alkali stress, cultures were grown in LB medium buffered to pH 7.3 with 40 mM 3-(N-morpholino)propanesulfonic acid (MOPS) (LB-MOPS). The pH remained constant for the 10 days of culture growth. Mutants with lower σE activity appeared at approximately the same time as they did in cultures grown in unbuffered LB medium (Fig. 6C), and the decrease in OD600 was not reversed (Fig. 6A). However, survival after the addition of SDS increased by 1 order of magnitude for stationary-phase cultures of the ΔrseA strain grown in LB-MOPS compared to the same strain grown in unbuffered LB medium (Fig. 5B), although the sensitivity was not fully reversed. A strong effect of growth medium buffering on stationary-phase survival was not observed; however, buffering potentiated the stabilization seen with Mg2+. Mutants with reduced σE activity appeared in MOPS-buffered LB medium supplemented with 20 mM MgCl2 24 h later than in cultures grown in LB-Mg and 72 h later than in cultures grown in LB medium (Fig. 6B and D). The percentage of mutants with reduced σE activity stopped increasing by day 8, and mutants constituted approximately 14% of the total CFU per milliliter for the last 3 days of the experiment (Fig. 6D).
Buffering of the growth medium did not have a large effect on survival of the ΔrseA strain. However, mutants that arose in ΔrseA cultures grown in LB-MOPS exhibited a wider range of σE activity than did those obtained from cultures grown in unbuffered LB medium or in LB-Mg. Pale purple colonies with about 2-fold lower σE activity than the parent ΔrseA strain were seen frequently on lactose–TTC–X-Gal agar, while only deep red colonies with 6- to 70-fold lower σE activity were isolated from cultures in unbuffered LB medium or LB-Mg. A similar high level of diversity of mutants with various amounts of σE activity was found when the ΔrseA strain was grown in the MOPS-based EZ rich defined medium. The phenotype of a pale purple mutant isolated from a culture grown in LB-MOPS was stable when the mutant was grown in MOPS-buffered LB medium for 10 days. When the same mutant was grown in unbuffered LB medium for 10 days, deep red mutants with further decreased σE activity accumulated, although their accumulation was delayed by 6 days, compared to a ΔrseA strain. Thus, 2-fold decreased σE activity was sufficient to allow survival for 10 days in MOPS-buffered medium, but lower σE activity was required for survival beyond day 6 in unbuffered LB medium. This result indicates that buffering of the medium partially relieves the lethal phenotype associated with elevated σE activity.
Overexpression of σE-dependent sRNAs in the ΔrseA mutant makes a significant contribution to SDS sensitivity and stationary-phase lethality.
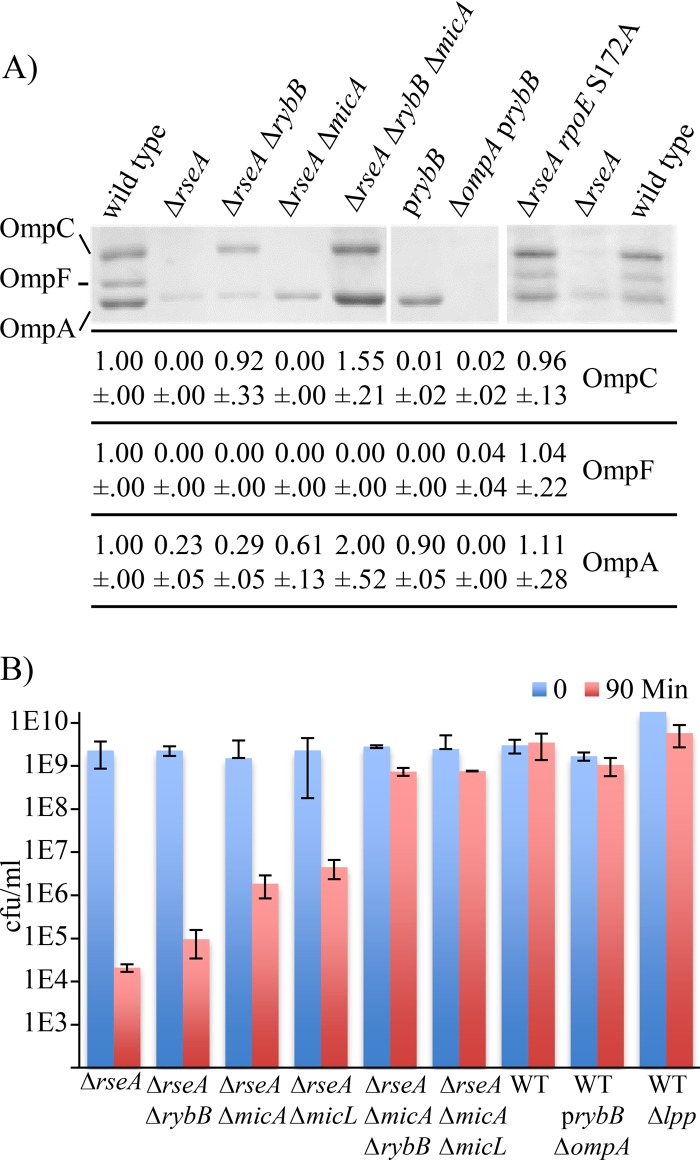
During envelope stress, σE reduces the load on the outer membrane biogenesis systems by transcribing the RybB and MicA sRNAs, which inhibit the expression of every major porin as well as several other genes that affect the envelope (10). These sRNAs are highly expressed in the ΔrseA mutant, resulting in clear reductions in the levels of OmpC, OmpF, and OmpA in the outer membrane (Fig. 7A). To examine whether sRNA overexpression contributed to the phenotype of the ΔrseA strain, double and triple mutants lacking one or both of the sRNAs were made in the ΔrseA background. OmpC amounts were restored in the ΔrseA ΔrybB mutant (Fig. 7A), but SDS sensitivity was only partially reversed (Fig. 7B). OmpA amounts in the ΔrseA ΔmicA mutant were about one-half of those found in the wild-type strain (Fig. 7A), and a moderate reduction in SDS sensitivity was observed (Fig. 7B). Deletion of both the rybB and micA genes in the ΔrseA strain restored OmpC and OmpA but not OmpF to levels found in wild-type cells (Fig. 7A) and reversed the SDS sensitivity of the strain (Fig. 7B). We do not understand why OmpF levels were not restored in the ΔrseA ΔrybB mutant, despite the fact that RybB has been demonstrated to negatively regulate ompF expression (10). Regulation of ompF expression is known to be complex and is inhibited by other regulators such as CpxR and OmpR, either of which could keep expression levels low in the ΔrseA ΔrybB background (35, 36). Levels of all of the porins, as well as SDS tolerance, were restored in the ΔrseA rpoE S172A mutant (Fig. 5C and 7A). Interestingly, low porin levels alone are not sufficient to explain the SDS sensitivity of the ΔrseA mutant. When the ompA gene was deleted and the rybB gene was overexpressed from a plasmid in a wild-type strain, OmpC, OmpF, and OmpA were nearly undetectable but the SDS sensitivity was similar to that of wild-type cells (Fig. 7).
FIG 7.
Levels of the major outer membrane porins are severely reduced in the ΔrseA strain and are completely or partially restored by deletion of sRNAs or mutations that reduce σE activity. (A) A representative gel with outer membrane preparations separated by 6 M urea-SDS-PAGE is shown. Quantification of the levels of OmpC, OmpF, and OmpA relative to those in the wild-type (WT) strain (averages and standard deviations from multiple biological repeats) are shown in the table below. (B) CFU per milliliter of the indicated strains before (0) and 90 min after treatment of stationary-phase cultures with 5% SDS is shown. Data presented are averages with standard deviations from at least 2 independent cultures.
To determine whether high levels of RybB and MicA play a role in stationary-phase lethality in addition to SDS sensitivity, the ΔrseA ΔrybB ΔmicA strain was grown into stationary phase. The OD600 did not decrease to the same extent as that of the ΔrseA strain, although it did decrease more than that of the wild-type culture (Fig. 8A). The appearance of mutants with low σE activity was delayed by 5 days for the triple mutant, compared to the ΔrseA strain, and approximately 5 to 10% of the culture still had high levels of σE activity by day 10, suggesting that aberrant expression of MicA and RybB made a significant contribution to the reduced viability of the ΔrseA mutant in stationary phase (Fig. 8B). These effects were not due to a reduction in σE activity in the triple mutant, because the σE activity of the ΔrseA ΔrybB ΔmicA strain was similar to that of the ΔrseA single mutant.
FIG 8.
MicA, RybB, and MicL sRNAs are responsible for the instability of the ΔrseA strain during the first part of stationary phase. The OD600 (A) and CFU per milliliter, as total CFU per milliliter (closed symbols) and CFU per milliliter of mutants with reduced σE activity (open symbols) (B), of ΔrseA (blue squares), ΔrseA ΔmicA ΔrybB (purple circles), ΔrseA ΔmicA ΔrybB ΔmicL (orange circles), and wild-type (green squares) cultures grown in LB medium, measured at the indicated times, are shown. Data are averages with standard deviations from at least 2 independent cultures.
In addition to the sRNAs that regulate porin expression, σE transcribes the MicL sRNA, which blocks translation of the mRNA encoding Lpp (13). Lpp is the most abundant protein in E. coli and connects the outer membrane to the peptidoglycan layer, enhancing the structural integrity of the cell envelope (37). Deleting MicL in the ΔrseA background increased the survival of stationary-phase cells after SDS treatment to a similar extent as deleting MicA alone (Fig. 7B). Deleting both the micL and micA genes in the ΔrseA strain prevented lysis (Fig. 7B), indicating that the ΔrseA strain can be stabilized by alleviating the repression of target gene expression by either MicA and RybB or MicA and MicL. As we observed with porin levels, reduced amounts of Lpp alone were not responsible for the SDS sensitivity, because stationary-phase cultures of a Δlpp mutant in an otherwise wild-type strain were not sensitive to SDS (Fig. 7B).
To determine whether high levels of MicL were responsible for the remaining stationary-phase instability of the ΔrseA ΔmicA ΔrybB strain, we constructed the ΔrseA ΔmicA ΔrybB ΔmicL quadruple mutant. Stationary-phase survival was marginally improved (Fig. 8). Colonies with reduced σE activity did not appear until after 8 days of culture growth, and approximately 50% of the colonies still had elevated σE activity by day 10, as opposed to 5 to 10% for the ΔrseA ΔmicA ΔrybB strain.
DISCUSSION
Tuning σE activity to the proper level is necessary for outer membrane homeostasis.
The cell envelope of Gram-negative bacteria is critical for maintaining cell integrity. Its composition can be altered to meet challenges imposed by the environment, and σE plays an essential role in maintaining envelope homeostasis (5). Here we demonstrate not only that loss of σE activity is toxic to E. coli but also that elevated levels of σE activity are deleterious. Both the ΔrseA strain with excess σE activity and ΔrpoE strains with no σE activity (containing suppressor mutations to support viability) exhibit increased membrane permeability (38). It is only when σE activity is within the appropriate range, neither too low nor too high, that proper envelope homeostasis is maintained. Interestingly, expression of the RybB, MicA, and MicL sRNAs plays a central role in cellular defects arising from both limited and excessive σE activity. Cell viability following depletion of σE activity could be restored by expressing the micL, rybB, or micA gene from the σ70-dependent promoter (10, 13). Conversely, deleting the micL, rybB, and micA genes from the ΔrseA mutant restored membrane integrity and significantly delayed the onset of stationary-phase defects. Taken together, these data demonstrate that the sRNAs are a critical part of the σE response and their expression must be appropriately fine-tuned to match the needs of the cells.
Recent work with the Cpx envelope stress response parallels our finding that σE activity must be properly controlled (39). The Cpx response is activated by disruption of peptidoglycan homeostasis and helps E. coli survive treatment with β-lactam antibiotics that block peptidoglycan synthesis. Similar to σE, the level of Cpx activity must be appropriately modulated; both too little activity and too much are deleterious. Mutants lacking the Cpx response are more sensitive to β-lactam antibiotics, while constitutive activation causes peptidoglycan-associated problems, including morphological changes, cell division defects, and increased sensitivity to β-lactams (39).
What is the cause of stationary-phase lethality due to elevated σE activity?
A large part of the stationary-phase lethality of the ΔrseA strain can be attributed to increased membrane permeability. Stabilizing the membranes of ΔrseA cells by supplementing the growth medium with Mg2+, which bridges negative charges on the LPS and strengthens the permeability barrier, eliminated the SDS sensitivity, nearly reversed the decrease in optical density following entry into stationary phase, and delayed the appearance of mutants with reduced σE activity. Increased pH of the growth medium in stationary phase appeared to exacerbate the membrane defects of the ΔrseA strain because buffering to neutral pH decreased the SDS sensitivity, although not to the same extent as supplementation with Mg2+. Growth in medium that was both buffered and supplemented with Mg2+ had the largest effect on survival of the ΔrseA strain in stationary phase. Mutants with low levels of σE activity arose but did not take over the cultures during the time frame of the experiment. The appearance of these mutants with low levels of σE activity suggests that ΔrseA cells are sensitive to stresses present later in stationary phase that are different, in nature and/or severity, from those encountered during early stationary phase and the deleterious effects of these stresses cannot be fully mitigated by the extent of membrane stabilization provided by Mg2+ supplementation and pH neutralization.
The membrane permeability defects and stationary-phase lethality are caused by elevated σE activity and are not due to an unrelated effect of the rseA deletion. Both defects were reversed by mutations in the ΔrseA strains that decreased σE activity. Since the only known role of σE in cells is to direct transcription, aberrant expression of σE-dependent genes is responsible for the phenotypes. Our data show that high levels of the RybB and MicA sRNAs are major contributing factors. Deleting RybB and MicA from a ΔrseA strain reversed the permeability defects and significantly delayed the appearance of mutants with low σE activity. MicA and RybB regulate the expression of all major porin genes, such that ΔrseA cells exhibit dramatic reductions in outer membrane porins. Low porin levels are known to result in the appearance of phospholipids in the outer leaflet of the outer membrane, which destabilizes the structure of the outer membrane and increases the sensitivity of the bacteria to SDS and hydrophobic antibiotics (40, 41). However, the membrane permeability defects of the ΔrseA strain are not due solely to low levels of porins. When porin levels were reduced by deleting the ompA gene and overexpressing rybB in a wild-type background, the cells were still resistant to 5% SDS. MicA has additional targets beyond porin mRNAs, and reductions in their expression in addition to ompA may contribute to the phenotype (10). For example, the MicA regulon includes pal, lpxT, and ycfS, all of which encode proteins that have the potential to affect outer membrane integrity (10). LpxT is involved in lipid A biosynthesis, Pal plays a role in maintaining outer membrane integrity in complex with the Tol protein, and YcfS is a l,d-transpeptidase involved in peptidoglycan maturation (42–44).
In addition to MicA and RybB, the MicL sRNA contributes to phenotypes associated with the ΔrseA strain. Lpp, the only known target of MicL, stabilizes the outer membrane, and complete deletion of lpp results in leakage of periplasmic contents and increased outer membrane vesiculation (13, 45). Deletion of MicL from the ΔrseA strain increased survival following SDS treatment, completely reversed SDS sensitivity when combined with the ΔmicA allele, and increased stationary-phase survival when combined with deletions of the rybB and micA genes. The observations that the stationary-phase instability of the ΔrseA strain was not completely eliminated in the quadruple mutant and was achieved only by decreasing σE activity indicate either that aberrant overexpression of members of the protein arm of the σE regulon is deleterious when the cultures persist longer into stationary phase or that expression of σE-dependent sRNAs is required for survival later in stationary phase.
The membrane permeability defects of the ΔrseA strain are also apparent in exponential phase, indicating that these defects are inherent to elevated σE activity and are not due to stationary-phase-specific changes in physiology. We do not currently know whether the ΔrseA cells would fail to survive if the cultures were maintained in exponential phase for sufficient time to allow selection of mutants with reduced σE activity or whether stationary phase imposes additional stresses that make membrane integrity important for survival.
Mutations in the rpoE gene reduce σE activity and appear at many residues shown to contact the −35 region of promoter DNA.
In the course of these experiments, we isolated a series of mutations in rpoE that reduced its activity. Sigma factors bind to promoter DNA with two conserved domains (3). Domain 4 binds to the −35 promoter motif and helps position RNA polymerase properly on the DNA. Domain 2 binds to the −10 promoter motif and helps to separate the DNA strands so that the polymerase can access the template strand and initiate RNA synthesis. Most of the mutations we recovered in rpoE map to domain 4, and many were shown to form contacts with the −35 motif in the cocrystal structure of isolated domain 4 bound to DNA (29). Interestingly, only one mutation in domain 2 was recovered, and the mutation reduced the stability of σE in the cells, while having little effect on specific activity. The failure to recover mutations in domain 2 residues that contact the DNA suggests that domain 2 of σE is less tolerant to mutation than domain 4. The importance of domain 2-promoter interactions has been clearly demonstrated for the housekeeping sigma factor σ70, which can direct transcription from promoters with an extended −10 region and no −35 region (2). However, alternative sigma factors have more stringent promoter recognition properties, leading to the idea that interactions with both regions of the promoter are critical (2). The appearance of mutations in many of the residues that contact the DNA in the −35 region demonstrates that the interaction between domain 4 of σE and the −35 promoter motif can be weakened while still supporting transcription initiation.
Does σE direct lysis of viable but nonculturable cells during early stationary phase?
E. coli cultures are known to accumulate a population of nonculturable cells following entry into stationary phase (28, 46). The discrepancies between the optical densities and CFU per milliliter for wild-type and ΔrseA strains have led to the hypothesis that elevated σE activity activates a σE-dependent cell death pathway that results in lysis of this subpopulation of nonculturable cells and concomitant reduction in the optical density (25–27). The work presented here suggests that lysis of this nonculturable cell population is due to the physical consequences of the cellular defects associated with high σE activity. Early-stationary-phase E. coli cultures have been shown to contain relatively large subpopulations of cells that do not form colonies and exhibit extensive protein oxidation (46). Both the Cpx and σE envelope stress responses are activated to a greater extent in these cells than in the culturable cells, indicating that cell envelope homeostasis is disrupted (46). Therefore, it is likely that the membrane perturbations and other defects associated with the ΔrseA mutation further destabilize the nonculturable population, resulting in lysis, whereas these cells remain intact but do not form colonies in a wild-type background. Furthermore, our findings show that the deleterious effects of high σE activity are not limited to the nonculturable population present during the first few days in stationary phase but ultimately lead to lethality for all cells with elevated σE activity.
What is the role of σE during entry into stationary phase?
The activity of σE does increase during entry into stationary phase, although not to the same high levels seen in strains lacking rseA or overexpressing the rpoE gene (20, 21). Entry into stationary phase is accompanied by a large change in cell physiology that involves remodeling of the outer membrane, including decreases in outer membrane protein contents and increases in LPS contents (47). Rather than directing cell lysis, a more likely role for σE in stationary phase is to help orchestrate these changes through transcription of its regulon to the appropriate extent at the appropriate time.
Concluding thoughts.
Stress responses provide extra protection for cells in the face of harm. Therefore, it might be predicted that having a response on at an elevated level all the time would render the cells more stress resistant. For example, the Rcs phosphorelay is activated by damage to the peptidoglycan layer, and constitutively activating it increases survival in the presence of β-lactam antibiotics (48). However, other stress responses are tuned to combat particular sets of importune conditions, and expression of the genes required to cope with such conditions can actually be deleterious in the absence of stress. As shown for the σE and Cpx responses, it is the precise regulation of the stress responses, both activation to the appropriate extent in response to specific signals and downregulation as stress is alleviated, that ensures cell survival.
MATERIALS AND METHODS
Strains, plasmids, media, and growth conditions.
Strains and plasmids used in this work are described in Table 2. Cultures were grown in LB medium adjusted to pH 7.3 and supplemented when indicated with 20 mM MgCl2 or 40 mM MOPS adjusted to pH 7.3. The defined rich MOPS medium (EZ rich medium) was purchased from Teknova. Lactose–TTC–X-Gal agar contained 1% lactose, 50 μg/ml TTC, 50 μg/ml X-Gal, 2 g/liter yeast extract, 10 g/liter tryptone, 5 g/liter NaCl, and 1.5% agar. Selection for the plasmids was performed using 100 μg/ml ampicillin. Because studies of σE can involve mutants with thermosensitivity at elevated temperatures, all cultures were grown at 30°C, with aeration. Mutant alleles were moved into the indicated strains using P1 transduction.
TABLE 2.
Strains and plasmids used
| Strain or plasmid | Genotype or characteristicsa | Source or reference, P1vir donor strain, and/or description |
|---|---|---|
| Strains | ||
| CAG45113 | MG1655 ΔlacX74 | |
| SEA001 | MG1655 ϕλ[rpoHp3::lacZ] ΔlacX74 | 20 |
| SEA2000 | SEA001 ΔrseA nadB::Tn10 | 20 |
| SEA6462 | SEA001 ΔrseA ΔyfiC::kan | This work; P1 donor JW2559 (51), ΔyfiC::kan transduced into SEA2000 |
| SEA6647 | CAG45113(pJV300) | This work; CAG45113 transformed with plasmid pJV300 |
| SEA6649 | CAG45113(pKP109-6) | This work; CAG45113 transformed with plasmid pKP109-6 |
| SEA6713 | SEA001 ΔrseA ΔyfiC | This work; kan marker excised from SEA6462 by Flp recombinase |
| SEA6718 | SEA001 ΔrseA ΔyfiC ΔrybB::kan | This work; P1 donor KMT197 (32), ΔrybB::kan transduced into SEA6713 |
| SEA6759 | SEA001 ΔrseA ΔyfiC ΔrybB::kan ΔmicA::cam | This work; P1 donor G897 (52), ΔmicA::cam transduced into SEA6718 |
| SEA6767 | SEA001 ΔrseA ΔyfiC ΔmicA::cam | This work; P1 donor G897 (52), ΔmicA::cam transduced into SEA6713 |
| SEA6771 | CAG45113 ΔompA::kan(pKP109-6) | This work; P1 donor JW0940 (51), ΔompA::kan transduced into SEA6649 |
| SEA278 | SEA001 ΔrseA ΔyfiC ΔmicL::kan | This work; P1 donor JW1863 (51), ΔmicL::kan transduced into SEA6713 |
| SEA276 | SEA001 ΔrseA ΔyfiC ΔmicA::cam ΔmicL::kan | This work; P1 donor G897 (52), ΔmicA::cam transduced into SEA278 |
| SEA281 | SEA001 ΔrseA ΔyfiC ΔmicL ΔmicA::cam ΔrybB::kan | This work; P1 donor KMT197 (32), ΔrybB::kan transduced into SEA276 after excision of kan marker from micL by Flp recombinase |
| SEA272 | SEA001 Δlpp::kan | This work; P1 donor JW1667 (51), Δlpp::kan transduced into SEA001 |
| Spontaneous mutants | ||
| SEA6525 | SEA001 ΔrseA yfiC::kan rpoE R171C | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6526 | SEA001 ΔrseA yfiC::kan rpoE L10P | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6527 | SEA001 ΔrseA yfiC::kan rpoE S172A | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6542 | SEA001 ΔrseA yfiC::kan rpoE L127Q | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6543 | SEA001 ΔrseA yfiC::kan rpoE V170 M | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6544 | SEA001 ΔrseA yfiC::kan rpoE R149Q | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6663 | SEA001 ΔrseA yfiC::kan rpoE R173C | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA6666 | SEA001 ΔrseA yfiC::kan rpoE A177V | This work; spontaneous mutant of SEA6462 isolated after growth in LB broth |
| SEA7008 | SEA001 ΔrseA nadB::Tn10 rpoE E126K | This work; spontaneous mutant of SEA2000 isolated after growth in LB broth |
| SEA7012 | SEA001 ΔrseA nadB::Tn10 rpoE R176Q | This work; spontaneous mutant of SEA2000 isolated after growth in LB broth |
| SEA7035 | SEA001 ΔrseA nadB::Tn10 rpoE A60P | This work; spontaneous mutant of SEA6462 isolated after growth in EZ rich medium |
| SEA7036 | SEA001 ΔrseA nadB::Tn10 rpoE S155I | This work; spontaneous mutant of SEA6462 isolated after growth in EZ rich medium |
| SEA7037 | SEA001 ΔrseA nadB::Tn10 rpoE F175S | This work; spontaneous mutant of SEA6462 isolated after growth in EZ rich medium |
| Plasmids | ||
| pJV300 | Vector, ColE1 ori rep, IPTG inducible, Apr | 53 |
| pKP109-6 | rybB in pJV300, Apr | Gift from J. Vogel; rybB under control of Plac promoter |
| pLC245 | rpoE in pTrc99a, Apr | 9 |
| pTrc99a | Vector, pBR322 ori, IPTG inducible, Apr |
Apr, ampicillin resistant.
Stationary-phase lethality studies.
For the stationary-phase growth studies, strains were first streaked onto lactose–TTC–X-Gal agar to verify the expected levels of σE activity. One isolated colony for each strain was grown at 30°C in 1 ml LB broth until an OD600 of ∼1.0 was reached. Cultures were diluted by a factor of 10−5 in fresh LB medium, and 5 μl of the diluted cells was used to inoculate 4 ml LB broth, supplemented with MOPS and/or MgCl2 as indicated. The low cell count in the inoculates (<50 bacteria) reduced the probability that a mutant with low σE activity would be present at the beginning of the growth of ΔrseA strains. Cultures were incubated for 10 days at 30°C, with aeration. At the indicated times, the absorbance of the cultures was measured, and the CFU were determined on LB agar or lactose–TTC–X-Gal agar. The pH of MOPS-buffered cultures was monitored and was shown to remain the same, whereas the pH of unbuffered cultures increased over the 10-day period.
Plasmid-induced lethality and plasmid stability studies.
Overnight cultures in LB medium with ampicillin of the wild-type strain SEA6003 carrying the plasmid pLC245 (for overexpression of σE) or the empty vector control plasmid pTrc99a were diluted into fresh LB medium with ampicillin to an OD600 of 0.025 and were incubated at 30°C, with aeration. When the cultures reached an OD600 of ∼0.15, the cells were pelleted by centrifugation and the pellets were resuspended in fresh LB medium with 25 μM IPTG and without ampicillin. The cultures were then incubated for 6 days at 30°C, with aeration. At the indicated times, the OD600 was measured and the CFU per milliliter were determined on LB plates (total CFU per milliliter) and LB plates supplemented with ampicillin (CFU per milliliter of bacteria still carrying the plasmid).
Membrane sensitivity tests.
To assess membrane sensitivity in stationary phase, strains were first streaked onto lactose–TTC–X-Gal agar to verify the expected levels of σE activity. Cultures were then inoculated with isolated colonies and grown for ∼28 h at 30°C, with aeration. Aliquots of the cultures were mixed with SDS to a final concentration of 5% or with an equal volume of water as a control and were incubated at 30°C, with aeration. At the indicated times, the OD600 of the cultures was measured and the CFU per milliliter were determined. For membrane sensitivity in exponential phase, overnight cultures were diluted to an OD600 of 0.025 and incubated at 30°C, with aeration. When the cultures reached an OD600 of 0.1 to 0.2, SDS was added to a final concentration of 5%, rifampin was added to a final concentration of 8 μg/ml, or an equal volume of water was added. The OD600 was measured and the CFU per milliliter were determined at the indicated times after the addition of SDS or rifampin.
β-Galactosidase assays.
β-Galactosidase assays were performed as described previously (19).
Western blot hybridization.
Cells were grown in LB medium to an OD600 of 0.8, and whole-cell extracts were prepared as described previously (49). Proteins extracted from 0.16 OD units of cells were separated by SDS-PAGE and transferred to Amersham Hybond-P polyvinylidene difluoride (PVDF) transfer membranes. Blots were probed with anti-σE and anti-RpoB polyclonal antibodies, and bound complexes were detected with horseradish peroxidase-coupled donkey anti-rabbit IgG. The secondary antibody was visualized with the Amersham ECL reagent according to the manufacturer's recommendations, using HyBlot CL autoradiography film (Denville Scientific Inc., Metuchen, NJ). Films were scanned using an Epson Perfection 3170 photo scanner, and the protein bands were quantified using ImageQuant 5.2 software. The intensity of each σE band was normalized to that of RpoB to control for loading differences.
Outer membrane preparations and electrophoresis.
Outer membranes were separated from inner membranes on the basis of their insolubility in 0.5% sarcosyl, as described previously (6). Proteins in the resulting membrane extracts were separated by 6 M urea-SDS-PAGE (10% polyacrylamide) and visualized using Coomassie blue. Gels were scanned, and the amounts of proteins were quantified using ImageQuant 5.2 software.
Mapping of spontaneous mutations.
Because the mutants had reduced σE activity, we first sequenced the rpoE gene to determine whether the mutations were located in that region. The rpoE gene and its promoter were amplified with the primers rpoEnadBmid (5′-CCGCTACCGATAATCAACACG-3′) and rseB1R (5′-GCTGATGAATGACAGCTCG-3′), using Taq polymerase. Amplified fragments were purified using QIAquick spin columns and were sequenced using the primers rpoE1 (5′-ACTGGTAGTGCGCTATCAGC-3′) and rpoE1R (5′-TTTACAGCAATCCGATACAGCC-3′). All rpoE mutations were moved by P1 transduction to a clean ΔrseA strain, to ensure that the phenotypes were linked to the mutations.
To map mutations not located in rpoE, a library of mini-Tn10::kan insertions was made in the wild-type E. coli strain. The resulting transposon library was moved into the mutant strains by P1 transduction, and colonies reversing the phenotype of interest were isolated. The mini-Tn10::kan insertion site was determined using a PCR-based protocol, with primers hybridizing to the transposon and a random primer (32, 50). The resulting PCR fragments were sequenced to identify the region of the chromosome linked to the mutation, although the precise mutation was not identified.
Structural analysis.
In silico analysis of the σE point mutations was performed using the σE structures with Protein Data Bank (PDB) accession numbers 1OR7 (15), 4LUP (30), and 2H27 (29).
ACKNOWLEDGMENTS
We are thankful to Carol A. Gross for the anti-σE and anti-RpoB antibodies and to Susan Gottesman, Gerhart Wagner, and Jörg Vogel for strains and plasmids. We thank Beth Luff for helping with some of the experiments.
This work was supported by grant MCB-0347302 from the National Science Foundation and grant R01 GM097365 from the National Institutes of Health to S.E.A.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Paget MSB, Helmann JD. 2003. The σ70 family of sigma factors. Genome Biol 4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feklístov A, Sharon BD, Darst SA, Gross CA. 2014. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol 68:357–376. doi: 10.1146/annurev-micro-092412-155737. [DOI] [PubMed] [Google Scholar]
- 3.Gruber TM, Gross CA. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol 57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 4.Guisbert E, Yura T, Rhodius VA, Gross CA. 2008. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol Mol Biol Rev 72:545–554. doi: 10.1128/MMBR.00007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayden JD, Ades SE. 2008. The extracytoplasmic stress factor, σE, is required to maintain cell envelope integrity in Escherichia coli. PLoS One 3:e1573. doi: 10.1371/journal.pone.0001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mecsas J, Rouviere PE, Erickson JW, Donohue TJ, Gross CA. 1993. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev 7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YN, Kusukawa N, Erickson JW, Gross CA, Yura T. 1988. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor sigma 32. J Bacteriol 170:3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Las Peñas A, Connolly L, Gross CA. 1997. SigmaE is an essential sigma factor in Escherichia coli. J Bacteriol 179:6862–6864. doi: 10.1128/jb.179.21.6862-6864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. 2006. Conserved and variable functions of the σE stress response in related genomes. PLoS Biol 4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gogol EB, Rhodius VA, Papenfort K, Vogel J, Gross CA. 2011. Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci U S A 108:12875–12880. doi: 10.1073/pnas.1109379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperandeo P, Dehò G, Polissi A. 2009. The lipopolysaccharide transport system of Gram-negative bacteria. Biochim Biophys Acta 1791:594–602. doi: 10.1016/j.bbalip.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Rigel NW, Silhavy TJ. 2012. Making a beta-barrel: assembly of outer membrane proteins in Gram-negative bacteria. Curr Opin Microbiol 15:189–193. doi: 10.1016/j.mib.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G. 2014. MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein. Genes Dev 28:1620–1634. doi: 10.1101/gad.243485.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh NP, Alba BM, Bose B, Gross CA, Sauer RT. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61–71. doi: 10.1016/S0092-8674(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 15.Campbell EA, Tupy JL, Gruber TM, Wang S, Sharp MM, Gross CA, Darst SA. 2003. Crystal structure of Escherichia coli σE with the cytoplasmic domain of its anti-σ RseA. Mol Cell 11:1067–1078. doi: 10.1016/S1097-2765(03)00148-5. [DOI] [PubMed] [Google Scholar]
- 16.De Las Peñas A, Connolly L, Gross CA. 1997. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol Microbiol 24:373–385. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- 17.Missiakas D, Mayer MP, Lemaire M, Georgopoulos C, Raina S. 1997. Modulation of the Escherichia coli σE (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol Microbiol 24:355–371. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- 18.Ades SE, Connolly LE, Alba BM, Gross CA. 1999. The Escherichia coli σE-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-σ factor. Genes Dev 13:2449–2461. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barchinger SE, Ades SE. 2013. Regulated proteolysis: control of the Escherichia coli σE-dependent cell envelope stress response. Subcell Biochem 66:129–160. doi: 10.1007/978-94-007-5940-4_6. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo A, Ades SE. 2006. Growth phase-dependent regulation of the extracytoplasmic stress factor, σE, by guanosine 3′,5′-bispyrophosphate (ppGpp). J Bacteriol 188:4627–4634. doi: 10.1128/JB.01981-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costanzo A, Nicoloff H, Barchinger SE, Banta AB, Gourse RL, Ades SE. 2008. ppGpp and DksA likely regulate the activity of the extracytoplasmic stress factor σE in Escherichia coli by both direct and indirect mechanisms. Mol Microbiol 67:619–632. doi: 10.1111/j.1365-2958.2007.06072.x. [DOI] [PubMed] [Google Scholar]
- 22.Dennis PP, Ehrenberg M, Bremer H. 2004. Control of rRNA synthesis in Escherichia coli: a systems biology approach. Microbiol Mol Biol Rev 68:639–668. doi: 10.1128/MMBR.68.4.639-668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 24.Gopalkrishnan S, Nicoloff H, Ades SE. 2014. Co-ordinated regulation of the extracytoplasmic stress factor, sigmaE, with other Escherichia coli sigma factors by (p)ppGpp and DksA may be achieved by specific regulation of individual holoenzymes. Mol Microbiol 93:479–493. doi: 10.1111/mmi.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabir MS, Yamashita D, Koyama S, Oshima T, Kurokawa K, Maeda M, Tsunedomi R, Murata M, Wada C, Mori H, Yamada M. 2005. Cell lysis directed by σE in early stationary phase and effect of induction of the rpoE gene on global gene expression in Escherichia coli. Microbiology 151:2721–2735. doi: 10.1099/mic.0.28004-0. [DOI] [PubMed] [Google Scholar]
- 26.Noor R, Murata M, Nagamitsu H, Klein G, Raina S, Yamada M. 2009. Dissection of σE-dependent cell lysis in Escherichia coli: roles of RpoE regulators RseA, RseB and periplasmic folding catalyst PpiD. Genes Cells 14:885–899. doi: 10.1111/j.1365-2443.2009.01318.x. [DOI] [PubMed] [Google Scholar]
- 27.Nitta T, Nagamitsu H, Murata M, Izu H, Yamada M. 2000. Function of the σE regulon in dead-cell lysis in stationary-phase Escherichia coli. J Bacteriol 182:5231–5237. doi: 10.1128/JB.182.18.5231-5237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zambrano MM, Siegele DA, Almirón M, Tormo A, Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]
- 29.Lane WJ, Darst SA. 2006. The structural basis for promoter −35 element recognition by the group IV sigma factors. PLoS Biol 4:e269. doi: 10.1371/journal.pbio.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campagne S, Marsh ME, Capitani G, Vorholt JA, Allain FH. 2014. Structural basis for −10 promoter element melting by environmentally induced sigma factors. Nat Struct Mol Biol 21:269–276. doi: 10.1038/nsmb.2777. [DOI] [PubMed] [Google Scholar]
- 31.Campbell EA, Greenwell R, Anthony JR, Wang S, Lim L, Das K, Sofia HJ, Donohue TJ, Darst SA. 2007. A conserved structural module regulates transcriptional responses to diverse stress signals in bacteria. Mol Cell 27:793–805. doi: 10.1016/j.molcel.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson KM, Rhodius VA, Gottesman S. 2007. σE regulates and is regulated by a small RNA in Escherichia coli. J Bacteriol 189:4243–4256. doi: 10.1128/JB.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P. 2006. Conserved small non-coding RNAs that belong to the σE regulon: role in down-regulation of outer membrane proteins. J Mol Biol 364:1–8. doi: 10.1016/j.jmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev 67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt LA, Hsing W, Gibson KE, Silhavy TJ. 1996. From acids to osmZ: multiple factors influence synthesis of the OmpF and OmpC porins in Escherichia coli. Mol Microbiol 20:911–917. doi: 10.1111/j.1365-2958.1996.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 36.Batchelor E, Walthers D, Kenney LJ, Goulian M. 2005. The Escherichia coli CpxA-CpxR envelope stress response system regulates expression of the porins OmpF and OmpC. J Bacteriol 187:5723–5731. doi: 10.1128/JB.187.16.5723-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowles CE, Li Y, Semmelhack MF, Cristea IM, Silhavy TJ. 2011. The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Mol Microbiol 79:1168–1181. doi: 10.1111/j.1365-2958.2011.07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Button JE, Silhavy TJ, Ruiz N. 2007. A suppressor of cell death caused by the loss of σE downregulates extracytoplasmic stress responses and outer membrane vesicle production in Escherichia coli. J Bacteriol 189:1523–1530. doi: 10.1128/JB.01534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delhaye A, Collet JF, Laloux G. 2016. Fine-tuning of the Cpx envelope stress response is required for cell wall homeostasis in Escherichia coli. mBio 7:e00047-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y. 2002. The function of OmpA in Escherichia coli. Biochem Biophys Res Commun 292:396–401. doi: 10.1006/bbrc.2002.6657. [DOI] [PubMed] [Google Scholar]
- 41.Schweizer M, Schwarz H, Sonntag I, Henning U. 1976. Mutational change of membrane architecture: mutants of Escherichia coli K12 missing major proteins of the outer cell envelope membrane. Biochim Biophys Acta 448:474–491. doi: 10.1016/0005-2736(76)90301-1. [DOI] [PubMed] [Google Scholar]
- 42.Touzé T, Tran AX, Hankins JV, Mengin-Lecreulx D, Trent MS. 2008. Periplasmic phosphorylation of lipid A is linked to the synthesis of undecaprenyl phosphate. Mol Microbiol 67:264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cascales E, Bernadac A, Gavioli M, Lazzaroni J-C, Lloubes R. 2002. Pal lipoprotein of Escherichia coli plays a major role in outer membrane integrity. J Bacteriol 184:754–759. doi: 10.1128/JB.184.3.754-759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanders AN, Pavelka MS. 2013. Phenotypic analysis of Escherichia mutants lacking l,d-transpeptidases. Microbiology 159:1842–1852. doi: 10.1099/mic.0.069211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwechheimer C, Kulp A, Kuehn MJ. 2014. Modulation of bacterial outer membrane vesicle production by envelope structure and content. BMC Microbiol 14:324. doi: 10.1186/s12866-014-0324-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desnues B, Cuny C, Grégori G, Dukan S, Aguilaniu H, Nyström T. 2003. Differential oxidative damage and expression of stress defense regulons in culturable and non-culturable Escherichia coli cells. EMBO Rep 4:400–404. doi: 10.1038/sj.embor.embor799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro Llorens JM, Tormo A, Martínez-García E. 2010. Stationary phase in Gram-negative bacteria. FEMS Microbiol Rev 34:476–495. doi: 10.1111/j.1574-6976.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 48.Laubacher ME, Ades SE. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J Bacteriol 190:2065–2074. doi: 10.1128/JB.01740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentry DR, Hernandez VJ, Nguyen LH, Jensen DB, Cashel M. 1993. Synthesis of the stationary-phase sigma factor sigma s is positively regulated by ppGpp. J Bacteriol 175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YG, Huang N. 1998. Efficient amplification of insert end sequences from bacterial artificial chromosome clones by thermal asymmetric interlaced PCR. Plant Mol Biol Rep 16:175. doi: 10.1023/A:1007420918645. [DOI] [Google Scholar]
- 51.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio Collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Udekwu KI, Darfeuille F, Vogel J, Reimegård J, Holmqvist E, Wagner EG. 2005. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev 19:2355–2366. doi: 10.1101/gad.354405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sittka A, Pfeiffer V, Tedin K, Vogel J. 2007. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol 63:193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]