Abstract
Background
Corynebacterium striatum is a member of the non-diphtherial corynebacteria, which are ubiquitous in nature and generally colonize the skin and mucous membranes of humans. Rarely, it causes infective endocarditis (IE). We report a case of rare left atrial bacterial vegetative mass due to C. striatum masquerading as a myxoma identified through a tortuous diagnostic process, and present a brief review of the relevant literature.
Case presentation
We present a case of 63-year-old man who presented with progressively worsening dyspnea on exertion and lower leg edema, and was diagnosed with heart failure. Transesophageal echocardiography (TEE) revealed that the left atrium was filled with a 2.7 cm × 2.6 cm mass. The patient, who had no signs of infection or related risk factors, was suspected of having a left atrial myxoma clinically. After excising the mass, the histopathology suggested thrombus with no myxocytes. Postoperatively, a fever appeared and C. striatum was isolated from the blood cultures. Although antibiotics were used, the symptoms of heart failure worsened gradually and echocardiography revealed valve vegetation. The patient underwent a second operation because of IE. Surprisingly, the mass was confirmed to be a bacterial vegetation due to C. striatum based on Gram staining at a 1000× magnification, although this was not noted on routine pathological examination of the two surgical specimens.
Conclusions
Physicians should be aware of Corynebacterium in blood cultures, which cannot simply be assumed to be a contaminant. A diagnosis of IE should be suspected, particularly in high-risk patients or those with an unexplained fever. Our patient had IE due to C. striatum with no risk factors. This case supports the diagnosis of IE using a combination of pathology and etiology.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2468-8) contains supplementary material, which is available to authorized users.
Keywords: Infective endocarditis, Corynebacterium Striatum, Atrial myxoma, Bacterial vegetation, Case report
Background
Infective endocarditis (IE) is a lethal disease that has undergone major changes in both host and pathogens over the past 20 years [1]. The clinical presentation includes a fever, new or changing heart murmur, embolic phenomena [2], heart failure, dyspnea, splinter hemorrhages, Roth spots, and glomerulonephritis. However, atypical clinical presentations of IE are common in the elderly or immunocompromised patients [3]. Staphylococcus aureus is the most common cause of IE in most of the industrialized world, whereas Corynebacterium striatum is a very rare cause.
Here, we present a patient with a left atrial bacterial vegetative mass due to C. striatum mimicking a myxoma, who was treated successfully with combined medical and surgical methods after a tortuous diagnostic process.
Case presentation
A 63-year-old man with a history of hypertension and atrial fibrillation presented with progressively worsening dyspnea on exertion and aggravated lower leg edema for 1 month without a fever, coughing, or sore throat. He had no history of surgery. The symptoms of heart failure markedly improved after a 2-week treatment with diuretics in a local hospital. However, echocardiography showed a mobile mass in the left atrium; therefore, the patient was transferred to our hospital for further treatment. On admission, his vital signs were blood pressure of 145/90 mmHg, atrial fibrillation with a ventricular rate of 81 beats per min, and a body temperature of 36.3 °C. Laboratory tests showed a white blood cell (WBC) count of 3.8 × 103/μL (reference range 4.0–10.0 × 103/μL) with 44.7% neutrophils, and a serum hypersensitive C-reactive protein (hs-CRP) level of 8 mg/L (reference range 0.8–8 mg/L). His urine and feces examinations were normal. Transthoracic echocardiography (TTE) revealed a 2.7 cm × 2.6 cm left atrial mass that was suspected of being a myxoma (Fig. 1a). TEE also revealed a 2.7 cm left atrial mass attached by a thin stalk (0.15 cm) to the base of the atrial apex, swinging freely within the left atrial cavity (Fig. 1b and Additional file 1). The patient was managed surgically. The mass was confirmed to originate from the atrial apex and appeared grossly myxoid and fragile. Surprisingly, however, the histopathology of the specimen showed no characteristic stellate mesenchymal cells to support the diagnosis of myxoma. Instead, it revealed a fibrinous exudate and chronic inflammation with a few mononuclear cells and a macrophage reaction (Fig. 2a). Therefore, the mass was considered to be a left atrial thrombus.
Fig. 1.
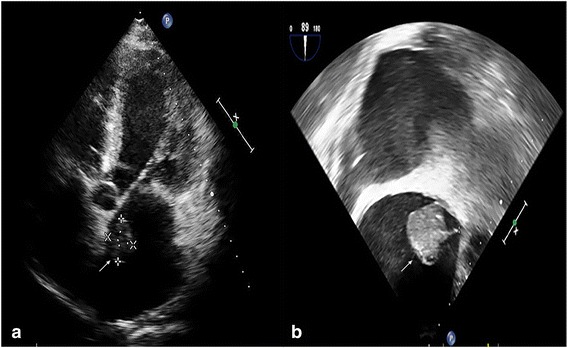
a Transthoracic echocardiography and (b)transesophageal echocardiography showing a 2.7 cm × 2.6 cm hypoechoic mass (arrow) attached by a thin stalk (measuring 0.15 cm in size) to the base of the atrial apex, freely swing within the left atrial cavity
Fig. 2.
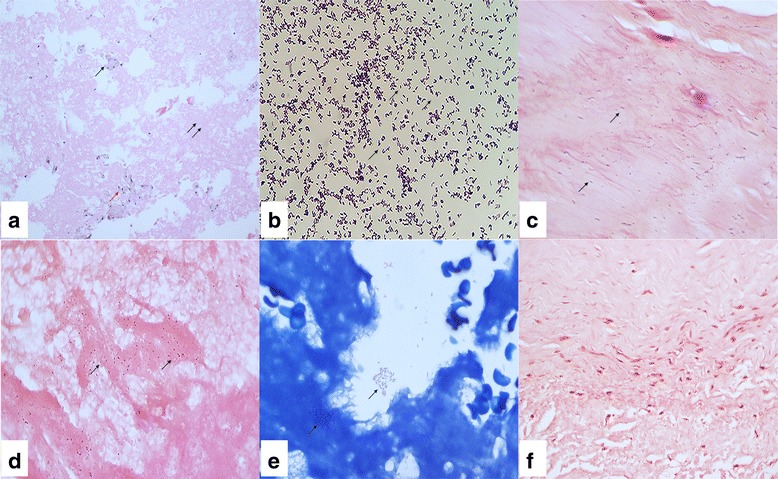
Pathological findings and staining of bacteria. a Hematoxylin and eosin (H&E) stain of the first excised mass showing large numbers of fibrinous exudate (double →) and chronic inflammation with a few mononuclear cells (→) and macrophage (→) (×200). b Gram’s staining of blood cultures showing large numbers of C. striatum (×1000). c Gram’s staining of the second excised vegetation showing Corynebacterium (×1000). d Gram’s staining and (e)Wright’s staining of the first excised mass for Corynebacterium (×1000). f Corynebacterium can not be found in second excised vegetation by Gram’s staining (×400)
Additional file 1: Video that demonstrates a 2.7 cm left atrial mass attached by a thin stalk to the base of the atrial apex, which was extremely similar to the myxoma, freely swing within the left atrial cavity, 7 s, 6.8 MB. (MP4 6644 kb)
Despite the preventive use of cefuroxime sodium (1500 mg every 12 h), his temperature increased to 39.5 °C on the fourth day. The WBC count increased to 9.5 × 10 [3]/μL with 85.9% neutrophils and his hs-CRP level rose to 183.9 mg/L. Four sets of blood cultures were drawn. Urgent computed tomography (CT) of the chest showed bilateral pulmonary infiltrates with sputum culture positive for Acinetobacter baumannii. Consequently, cefoperazone, sulbactam, and fosfomycin were started. Nevertheless, his fever recurred for 2 weeks. The blood cultures yielded C. striatum (Fig. 2b) that was resistant to ceftriaxone, penicillin, meropenem, and clindamycin and sensitive to vancomycin. Instead of vancomycin, linezolid was chosen to treat the C. striatum bacteremia after considering the elevated creatinine level of 117 μmol/L (reference range 59–104 μmol/L). However, the symptoms of heart failure worsened gradually and echocardiography revealed valve excrescence and severe aortic insufficiency. Therefore, the patient underwent surgical management and treatment with 500 mg daptomycin daily. A double valve replacement was performed. At surgery, a 1 cm × 1 cm vegetation was seen at the left coronary cusp of the aortic valve, with perforation of the right coronary cusp of the aortic valve and moderate mitral regurgitation. The fever subsided rapidly and six sets of blood cultures obtained postoperatively were negative. Histological examination of the new vegetation indicated large numbers of Corynebacterium on Gram staining (Fig. 2c) and vegetation cultures confirmed that the bacterium was C. striatum. We also found the same Corynebacterium on the first surgical specimen using Gram and Wright’s staining (Fig. 2e). We speculate that the left atrial mass was an occult bacterial embolus and large amounts of bacteria entered the blood stream and induced the valve damage after the first operation. Subsequently, the patient received a 4-week course of intravenous daptomycin and was switched to oral linezolid for 3 weeks. The patient’s general condition markedly improved and he was discharged from our hospital.
Discussion
IE is a potentially lethal disease with a low incidence (1.7–7.9 cases/100,000 inhabitants). C. striatum is a rare cause of IE, causing about 0.33% of all cases of IE [4]. As a member of the corynebacteria, C. striatum is a Gram-positive, aerobic, non-sporulating bacillus that grows slowly in cultures and is distributed in the skin and mucous membranes of normal hosts and hospitalized patients. It is one of the more commonly isolated coryneform bacteria in the clinical microbiology laboratory and is usually considered a contaminant because of its low virulence. However, C. striatum can cause not only IE but also a variety of different infections such as pneumonia, empyema, peritonitis, arthritis, keratitis, intrauterine infections, wound infection, breast abscess, and osteomyelitis [5]. Risk factors for Corynebacterium endocarditis include pre-existing cardiac disease, a history of bacterial endocarditis, and the presence of prosthetic devices [6]. Rufael et al. [7] reported the first case of native valve endocarditis due to C. striatum, which required a combination of medical and surgical treatments [7]. Of the 24 cases of C. striatum endocarditis found in PubMed (Table 1) [6–30], most showed a predilection for heart valves. In our case, however, C. striatum colonized the left atrial apex masquerading as a left atrial myxoma instead of attaching to the heart valves. Looking back on our data, there were three main potential causes of misdiagnosis. First, the patient had no classic symptoms of IE on admission. Second, both TEE and the surgical findings supported the diagnosis of left atrial myxoma. Third, the initial histopathology was misleading.
Table 1.
Summarizing previously reported cases of C. striatum endocarditis
| Reference | Age | Sex | Associated illness | Valve | Intervention | Outcome |
|---|---|---|---|---|---|---|
| 8 | 76 | M | None | Aortic | Medical | Died |
| 7 | 54 | M | Hypertension | Aortic | Medical and surgical | Survived |
| 9 | 73 | M | Pacemaker | Tricuspid | Medical and surgical | Survived |
| 10 | 24 | M | Ventricular shunt | Pulmonary | Medical | Survived |
| 11 | 68 | M | Hypertension | Mitral | Medical | Survived |
| 12 | 72 | F | Prosthetic valve | Aortic | Medical | Died |
| 13 | 62 | F | Prosthetic valve | Aortic | Medical | Survived |
| 14 | 50 | M | Mycotic aneurysm | Aortic | Medical and surgical | Survived |
| 15 | 61 | F | Rheumatic fever | Mitral | Medical | Survived |
| 15 | 72 | F | Prosthetic valve | Mitral | Medical | Survived |
| 16 | 46 | F | Hemodialysis | Tricuspid | Medical | Survived |
| 17 | 68 | M | Prosthetic valve | Mitral | Medical | Survived |
| 18 | 69 | F | Endometrial cancer | Mitral | Medical and surgical | Survived |
| 19 | 77 | F | None | Mitral | Medical | Survived |
| 6 | 62 | M | Hypertension | Aortic | Medical and surgical | Survived |
| 20 | 73 | F | Hypertension, chronic kidney disease, and diabetes mellitus | Mitral | Medical | Survived |
| 21 | 83 | M | Metastatic prostate cancer | Mitral | Medical | Died |
| 22 | 71 | M | Diabetes mellitus | Mitral | Medical and surgical | Died |
| 23 | 71 | F | Pacemaker prosthetic valve |
Mitral | Medical and surgical | Survived |
| 24 | 62 | M | Cardiomyopathy, diabetes mellitus and osteomyelitis | Aortic | Medical and surgical | Survived |
| 25 | 69 | F | ANCA+ vasculitis | Mitral | Medical and surgical | Died |
| 26 | 51 | M | Pacemaker | Not described | Medical and surgical | Survived |
| 27 | 56 | M | Diabetes mellitus, chronic kidney disease, and osteomyelitis | Mitral | Medical and surgical | Died |
| 28 | 78 | M | Chronic kidney disease, diabetes mellitus and pacemaker | Tricuspid and right ventricular wall | Medical and surgical | Survived |
| 29 | 53 | F | None | Quadricuspid aortic | Medical and surgical | Survived |
The category “definite IE based on clinical criteria” involves with at least two major criteria, or one major criterion and three minor criteria, or five minor criteria. Major criteria include blood culture positive for IE, evidence of endocardial involvement, echocardiogram positive for IE, and new valvular regurgitation. According to the clinical, echocardiographic and biological findings, as well as the results of serologies, the patient did not meet the modified Duke’s criteria for diagnosis of endocarditis when he was transferred to our hospital. However, two of the major Duke criteria were met after the first operation―the positive blood culture and echocardiographic findings―enabling a definitive diagnosis [31]. Looking back, Corynebacterium was present in the first surgical specimen in our case. Therefore, the left atrial mass should have been considered an occult bacterial vegetation. The C. striatum was likely completely surrounded by fibrous tissue, so the patient had no signs of infection until bacteria were released by the first surgery. Ori Elkayam et al. [22] pointed out that IE is the most common manifestation of C. striatum, particularly in patients with nosocomial risk factors. But it is a potential pathogen even in normal hosts with no risk factors, such as our patient. Therefore, in addition to the routine pathological examination, special stains such as Gram staining or Wright’s staining should be performed if a few macrophages are seen. When a patient with suspected myxoma develops an unmanageable fever postoperatively, physicians should be alert to the progress of IE and evaluate the possibility with a combined pathological examination and blood cultures. The pathological examination of resected tissue or embolic fragments remains the gold standard for the diagnosis of IE. Nevertheless, we fell into a trap in this case because no pathogens were found in the first or second surgical specimens on examination with a medical microscope at a 400× magnification (Fig. 2f). We confirmed that C. striatum in positive blood cultures was responsible for the IE and that this diagnosis was supported by the results of Gram staining and Wright’s staining of tissue specimens viewed at a 1000× magnification with an oil immersion lens. Daptomycin is an effective drug for C. striatum and has been used in some patients with endocarditis caused by this organism [28]. Combined antibiotic treatment and surgery were performed because of the uncontrolled infection and severe aortic regurgitation based on the ESC guidelines [32]. The patient had a slow, uneventful recovery.
Conclusions
Corynebacterium in positive blood cultures cannot simply be assumed to be a contaminant. The diagnosis of IE should be suspected, particularly in high-risk patients or those with an unexplained fever. Gram staining can provide additional support for a diagnosis of IE, particularly when the pathological examination implies an inflammatory response, and we should consider the size of the pathogenic bacteria and select the appropriate magnification when examining slides. This can guide postoperative antibiotic use and reduce the risk of a second surgery. In addition, it is necessary to make a suitable standard analysis protocol of cardiac masses to include thorough microbiological analysis, which can reduce misdiagnosis and improve the standard of care.
Acknowledgements
None.
Funding
Not applicable.
Availability of data and materials
All data contained within the manuscript.
Authors’ contributions
Jun Xu was responsible for the acquisition of data and drafted the manuscript; Xia Zheng revised it critically for important intellectual content and gave final approval to the version to be published. Qing Yang and Jun Li were responsible for Lab test and histologic examination. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- C. striatum
Corynebacterium striatum
- CT
Computed tomography
- hs-CRP
Hypersensitive C-reactive protein
- IE
Infective endocarditis
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
- WBC
White blood cell counts
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-017-2468-8) contains supplementary material, which is available to authorized users.
Contributor Information
Jun Xu, Email: xujun5156@zju.edu.cn.
Qing Yang, Email: yq720226@163.com.
Jun Li, Email: lijunfee@163.com.
Xia Zheng, Phone: 86-571-87236838, Phone: 86-13958174689, Email: zxicu@zju.edu.cn.
References
- 1.Duval X, Delahaye F, Alla F, et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol. 2012;59(22):1968–1976. doi: 10.1016/j.jacc.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Thuny F, Di Salvo G, Disalvo G, et al. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112(1):69–75. doi: 10.1161/CIRCULATIONAHA.104.493155. [DOI] [PubMed] [Google Scholar]
- 3.Pérez de Isla L, Zamorano J, Lennie V, Vázquez J, Ribera JM, Macaya C. Negative blood culture infective endocarditis in the elderly: long-term follow-up. Gerontology. 2007;53(5):245–249. doi: 10.1159/000101691. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz P, Kestler M, De Alarcon A, et al. Current epidemiology and outcome of infective Endocarditis: a multicenter, prospective, cohort study. Medicine (Baltimore) 2015;94(43):e1816. doi: 10.1097/MD.0000000000001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Severo CB, Guazzelli LS, Barra MB, Hochhegger B, Severo LC. Multiple pulmonary nodules caused by Corynebacterium Striatum in an immunocompetent patient. Rev Inst Med Trop Sao Paulo. 2014;56(1):89–91. doi: 10.1590/S0036-46652014000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belmares J, Detterline S, Pak JB, Parada JP. Corynebacterium endocarditis species-specific risk factors and outcomes. BMC Infect Dis. 2007;7(1):4. doi: 10.1186/1471-2334-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rufael DW, Cohn SE. Native valve endocarditis due to Corynebacterium Striatum: case report and review. Clin Infect Dis. 1994;19(6):1054–1061. doi: 10.1093/clinids/19.6.1054. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz SM, Coudron PE. Native valve endocarditis caused by an organism resembling Corynebacterium Striatum. J Clin Microbiol. 1990;28(1):8–10. doi: 10.1128/jcm.28.1.8-10.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melero-Bascones M, Muñoz P, Rodríguez-Créixems M, Bouza E. Corynebacterium Striatum: an undescribed agent of pacemaker-related endocarditis. Clin Infect Dis. 1996;22(3):576–577. doi: 10.1093/clinids/22.3.576. [DOI] [PubMed] [Google Scholar]
- 10.Tattevin P, Cremieux AC, Muller-Serieys C, Carbon C. Native valve endocarditis due to Corynebacterium Striatum: first reported case of medical treatment alone. Clin Infect Dis. 1996;23(6):1330–1331. doi: 10.1093/clinids/23.6.1330. [DOI] [PubMed] [Google Scholar]
- 11.Juurlink DN, Borczyk A, Simor AE. Native valve endocarditis due to Corynebacterium Striatum. Eur J Clin Microbiol Infect Dis. 1996;15(12):963–965. doi: 10.1007/BF01690520. [DOI] [PubMed] [Google Scholar]
- 12.de Arriba JJ, Blanch JJ, Mateos F, Martínez-Alfaro E, Solera J. Corynebacterium Striatum first reported case of prosthetic valve endocarditis. J Inf Secur. 2002;44(3):193. doi: 10.1053/jinf.2001.0927. [DOI] [PubMed] [Google Scholar]
- 13.Houghton T, Kaye GC, Meigh RE. An unusual case of infective endocarditis. Postgrad Med J. 2002;78(919):290–291. doi: 10.1136/pmj.78.919.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocazeybek B, Ozder A, Kucukoglu S, Kucukates E, Yuksel H, Olga R. Report of a case with polymicrobial endocarditis related to multiresistant strains. Chemotherapy. 2002;48(6):316–319. doi: 10.1159/000069710. [DOI] [PubMed] [Google Scholar]
- 15.Stoddart B, Sandoe JAT, Denton M. Corynebacterium Striatum endocarditis masquerading as connective tissue disorders. Rheumatology (Oxford) 2005;44(4):557–558. doi: 10.1093/rheumatology/keh519. [DOI] [PubMed] [Google Scholar]
- 16.Shah M, Murillo JL. Successful treatment of Corynebacterium Striatum endocarditis with daptomycin plus rifampin. Ann Pharmacother. 2005;39(10):1741–1744. doi: 10.1345/aph.1G242. [DOI] [PubMed] [Google Scholar]
- 17.Mashavi M, Soifer E, Harpaz D, Beigel Y. First report of prosthetic mitral valve endocarditis due to Corynebacterium Striatum: successful medical treatment. Case report and literature review. J Inf Secur. 2006;52(5):e139–e141. doi: 10.1016/j.jinf.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Tibrewala AV, Woods CJ, Pyrgos VJ, Ruiz ME. Native valve endocarditis caused by C. striatum. Scand J Infect Dis. 2006;38(9):805–807. doi: 10.1080/00365540600606598. [DOI] [PubMed] [Google Scholar]
- 19.Elshibly S, Xu J, Millar BC, Armstrong C, Moore JE. Molecular diagnosis of native mitral valve endocarditis due to Corynebacterium Striatum. Br J Biomed Sci. 2006;63(4):181–184. doi: 10.1080/09674845.2006.11978096. [DOI] [PubMed] [Google Scholar]
- 20.Marull J, Casares PA. Nosocomial valve endocarditis due to corynebacterium striatum: a case report. Cases J. 2008;1(1):388. doi: 10.1186/1757-1626-1-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat Y, Bal AM, Rochow S, Gould IM. An unusual case of Corynebacterium Striatum endocarditis and a review of the literature. Int J Infect Dis. 2008;12(6):672–674. doi: 10.1016/j.ijid.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Boltin D, Katzir M, Bugoslavsky V, et al. Corynebacterium Striatum--a classic pathogen eluding diagnosis. Eur J Intern Med. 2009;20(3):e49–e52. doi: 10.1016/j.ejim.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Oliva A, Belvisi V, Iannetta M, et al. Pacemaker lead endocarditis due to multidrug-resistant Corynebacterium Striatum detected with sonication of the device. J Clin Microbiol. 2010;48(12):4669–4671. doi: 10.1128/JCM.01532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batalla AS, de La Blanchardière A, Vergnaud M, Dargère S, Verdon R. Recurrent Corynebacterium Striatum endocarditis, secondary to osteomyelitis. Med Mal Infect. 2011;41(3):160–163. doi: 10.1016/j.medmal.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Deligeoroglou E, Fotaki P, Kokkalis D, Creatsas G. Description of 8 cases with gonadal dysgenesis syndrome type 46XY. Akush Ginekol (Sofiia) 2001;42(2):9–12. doi: 10.3201/eid0801.010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knox KL, Holmes AH. Nosocomial endocarditis caused by Corynebacterium amycolatum and other nondiphtheriae corynebacteria. Emerging Infect Dis. 2002;8(1):97–99. doi: 10.3201/eid0801.010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran TT, Jaijakul S, Lewis CT, et al. Native valve endocarditis caused by Corynebacterium Striatum with heterogeneous high-level daptomycin resistance: collateral damage from daptomycin therapy? Antimicrob Agents Chemother. 2012;56(6):3461–3464. doi: 10.1128/AAC.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernández Guerrero ML, Molins A, Rey M, Romero J, Gadea I. Multidrug-resistant Corynebacterium Striatum endocarditis successfully treated with daptomycin. Int J Antimicrob Agents. 2012;40(4):373–374. doi: 10.1016/j.ijantimicag.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Fernández Guerrero ML, Robles I, Nogales MDC, Nuevo D. Corynebacterium Striatum: an emerging nosocomial drug-resistant endocardial pathogen. J Heart Valve Dis. 2013;22(3):428–430. [PubMed] [Google Scholar]
- 30.Mizoguchi H, Sakaki M, Inoue K, et al. Quadricuspid aortic valve complicated with infective endocarditis: report of a case. Surg Today. 2014;44(12):2388–2391. doi: 10.1007/s00595-014-0844-1. [DOI] [PubMed] [Google Scholar]
- 31.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 32.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the Management of Infective Endocarditis: the task force for the management of infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data contained within the manuscript.


