Abstract
The function of cortical GABAergic interneurons is largely determined by their integration into specific neural circuits, but the mechanisms controlling the wiring of these cells remain largely unknown. This is particularly true for a major population of basket cells that express the neuropeptide cholecystokinin (CCK). Here we found that the tyrosine kinase receptor ErbB4 is required for the normal integration of CCK+/VGlut3+ basket cells into cortical circuits. The number of inhibitory synapses made by CCK+/VGlut3+ basket cells and the inhibitory drive they exert on pyramidal cells are severely reduced in conditional mice lacking ErbB4. Developmental disruption of the connectivity of these cells diminishes the power of theta oscillations during exploratory behavior, disrupts spatial coding by place cells, and causes selective alterations in spatial learning and memory in adult mice. These results suggest that normal integration of CCK+ basket cells in cortical networks is key to support spatial coding information in hippocampus.
The function of cortical networks relies on the precise interaction between pyramidal cells and interneurons. Interneurons are uniquely placed to orchestrate cortical activity in manifold ways due their great diversity, defined by a unique set of neurochemical, electrophysiological and morphological features1. Accordingly, interneurons have adopted a division of labor within cortical circuits, through which distinct classes of interneurons specialize in innervating particular classes of neurons and, within them, targeting different subcellular compartments at precise time windows in reference to specific behavioral events or brain states2, 3. Parsing the function of different classes of interneurons is therefore crucial for unveiling how the cerebral cortex encodes information.
Basket cells comprise a heterogeneous collection of interneurons that confine their synapses around the soma and primary dendrites of target neurons, thereby exerting a powerful control over their output. There are two main classes of basket cells, with distinct developmental origins and singular neurochemical and electrophysiological properties, parvalbumin-expressing (PV+) fast-spiking basket cells and cholecystokinin-expressing (CCK+) regular spiking basket cells4–6. CCK+ basket cells are further subdivided in two main subtypes based on the mutually exclusive expression of vesicular glutamate transporter 3 (VGlut3+) or vasoactive intestinal peptide (VIP+) (Ref. 7).
Several lines of evidence suggest that PV+ and CCK+ basket cells contribute differently to cortical operations. In the hippocampus, PV+ and CCK+ basket cells modulate different microcircuits by preferentially targeting pyramidal cells located in deep or superficial layers of the stratum pyramidale8. The fast dynamics and reliable release of PV+ basket cells make them suitable to operate as clockworks by actively generating fast network oscillations9, 10. In contrast, the relatively unreliable and asynchronous release of GABA by CCK+ basket cells suggests a weaker contribution to gamma rhythms11, 12. Indeed, the precise contribution of CCK+ basket cells to cortical rhythms remains unknown. Current views suggest that they may contribute to place cell firing during theta oscillations3–5, but this hypothesis remains to be experimentally tested.
The molecular mechanisms controlling the wiring of basket cells in specific neural circuits are poorly understood13–18. It has been previously shown that loss of the neuregulin receptor ErbB4 decreases the number of excitatory synapses received by PV+ basket cells14, 16–18. Here, we have generated CCK-Cre;Erbb4F/F mutants to investigate the function of this receptor in the connectivity of CCK+ basket cells. Because ErbB4 expression is restricted to some GABAergic interneurons in the cerebral cortex14, 19, this strategy also offered a unique opportunity to investigate the consequences of disrupting the development of CCK+ basket cells. In the past, the absence of unique markers for CCK+ basket cells has limited the functional interrogation of this population of interneurons to single cell analyses12, 20–22. Our results reveal that ErbB4 is required both for the normal wiring of CCK+/VGlut3+ basket cells. These developmental defects impair the normal inhibitory function of CCK+/VGlut3+ basket cells in the adult brain, decreases the power of theta oscillations during exploratory behavior, disrupts spatial coding by place cells, and causes selective alterations in spatial learning and memory deficits.
Results
Basket CCK+ interneurons containing VGlut3 also express ErbB4
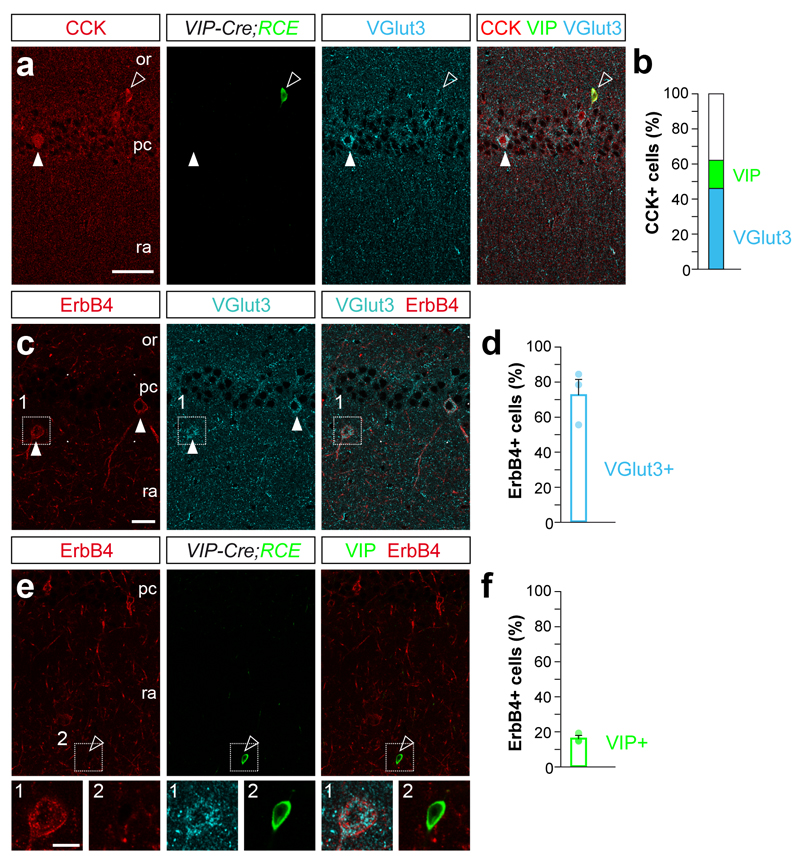
We observed that nearly half of the CCK+ interneurons in the mouse CA1 hippocampal area express VGlut3 (46%), whereas only a small fraction of them contain VIP (16%, Fig. 1a,b). Previous studies have shown that the tyrosine kinase receptor ErbB4 is abundantly expressed among CCK+ interneurons15 (Supplementary Fig. 1a,b), but it is unclear whether only a particular subset of these cells expresses ErbB4. We found that ErbB4 was prominently expressed by CCK+/VGlut3+ interneurons (72%), but only marginally expressed by CCK+/VIP+ (16%, Fig. 1c–f and data not shown). These observations therefore revealed that ErbB4 is enriched in the largest population of CCK+ basket cells, which also contain VGlut3.
Figure 1. Subsets of CCK+ interneurons express ErbB4.
(a) Triple immunohistochemistry for CCK (red, arrowheads), GFP (green, open arrowheads) and VGlut3 (cyan, arrowheads) in the hippocampus of Vip-Cre;RCE mice. (b) Percentage of VGlut3+ (cyan) and VIP+ (green) among CCK+ interneurons. (c) Triple immunohistochemistry for ErbB4 (red, arrows), GFP (cyan, open arrowheads) and VGlut3 (arrows) in the hippocampus of Vip-Cre;RCE mice; n = 166 cells from three brains. (d) Percentage of ErbB4+ among VGlut3+ cells (cyan, n = 112 VGlut3 cells from three brains). (e) Immunolabeling showing Erbb4+ (red) and GFP+ (VIP, green) interneurons in Vip-Cre;RCE mice. (f) Percentage of ErbB4+ among VIP+ cells (green, n = 202 VIP cells from three brains). Lower panel 1, High magnification images of c. Lower panel 2, High magnification images of e. or, stratum oriens; pc, stratum pyramidale; ra, stratum radiatum. Data are expressed as mean ± s.e.m. Scale bars, 50 μm (a, f, j). Scale bar inset 20 μm.
Wiring defects in CCK+/VGlut3+ basket cells lacking ErbB4
Others and we have previously shown that ErbB4 plays a prominent role in the development of the connectivity of fast spiking interneurons in the cerebral cortex14, 16, 17. To examine the function of ErbB4 in the wiring of CCK+/VGlut3+ interneurons, we generated CCK-specific conditional Erbb4 mutants by breeding CCK-Cre mice 23 with mice carrying floxed Erbb4 alleles 24. As described before23, CCK-Cre mice triggered recombination in the majority of CCK+/VGlut3+ cells (Supplementary Fig. 1c,d). Consistently, we observed a prominent decrease in the number of CCK+ and VGlut3+ interneurons containing ErbB4 in the mutants compared to controls. As expected, expression of ErbB4 was maintained in interneurons lacking VGlut3 (putative PV+ interneurons) (Supplementary Fig.2). We found that ErbB4 is dispensable for the generation and migration of cortical CCK+ interneurons, since the density and laminar organization of CCK+ cells in the hippocampus is similar between both genotypes (Supplementary Fig. 3).
ErbB4 is required for the normal development of excitatory synapses onto fast spiking interneurons14, 16, 17. In the hippocampus, ErbB4 is also prominently clustered in the somatic proximal area of CCK+/VGlut3+ interneurons (Fig. 1c). We quantified the density of excitatory presynaptic inputs (VGlut1+/PSD95+) impinging into the soma of CCK+ cells at the end of synaptogenesis (P30). We found a significant decrease in the number of VGlut1+/PSD95+ structures around the soma of these cells, but similar total density in CCK-Cre;Erbb4F/F mutants compared to control mice (Supplementary Fig. 4). These results indicate that CCK+/VGlut3+ interneurons receive a reduced number of excitatory inputs in the absence of ErbB4.
We next examined whether ErbB4 plays a role in the establishment of the inhibitory synapses made by CCK+/VGlut3+ interneurons. ErbB4 is present in about a quarter of the inhibitory synapses contacting the soma of pyramidal cells14, but PV+ basket cells do not require ErbB4 to make a normal complement of synapses16, 18. One possible explanation is that ErbB4 is only present in synapses made by CCK+/VGlut3+ interneurons. To test this hypothesis, we used antibodies for both VGlut3 and the cannabinoid receptor 1 (CB1), which identifies somatic CCK+ axon terminals25, and quantified the number of VGlut3+/CB1+ boutons contacting the soma of CA1 pyramidal cells (Fig. 2a,b). We found that the density of VGlut3+/CB1+ boutons targeting the soma of pyramidal cells was significantly reduced in CCK-Cre;Erbb4F/F mutants compared to controls (Fig. 2c–e). Similar reduction was observed in the CA3 region (data not shown). This analysis revealed that hippocampal CCK+/VGlut3+ basket cells make fewer inhibitory synapses onto pyramidal cells in the absence of ErbB4.
Figure 2. Deletion of Erbb4 from CCK+ interneurons decreases the number of synapses made on hippocampal pyramidal and parvalbumin cells at P30 in CA1 hippocampal region.
(a) Schematic drawing of Erbb4 deletion from CCK+ interneurons. The circle indicates the synapses analyzed in this experiment. (b) Triple immunohistochemistry showing VGlut3 (red), CB1 (green) and NeuN staining (grey). (c,d) Confocal images showing high magnification of double positive VGlut3+/CB1+ boutons apposed to NeuN+ somas in the pyramidal cell layer (top, arrows). (c’–d’) Binary images used for quantification; co-localization of VGlut3/CB1 in yellow (bottom). (e) Density of VGlut3+/CB1+ boutons contacting the soma of pyramidal cells in control and mutant mice. t test, t (6) = 2.931 P = 0.026, n= 366 and 280 neurons in CA1 from four controls and four mutants respectively. Distribution of densities of VGlut3+/CB1+ boutons on the soma of pyramidal cells in control and mutant mice at P30. χ2 = 36.172, d.f. = 4, P < 0.001, n = 366 and 280 neurons from 4 controls and 4 mutants, respectively. (f) Scheme of Erbb4 deletion from CCK+ interneurons. The circle indicates the synapses analyzed in this experiment. (g, h) Top panels, representative confocal images showing VGlut3+ boutons (green, arrows) contacting a PV+ interneuron (red) in control (g) and mutant mice (h). Bottom panels, confocal images showing VGlut3+ boutons (green, arrows) contacting an ErbB4+ interneuron (red) in control (g) and mutant mice (h). (i) Density of VGlut3+ boutons contacting the soma of PV+ interneurons. t test, t (4) = 4.048, P = 0.016, n = 35 and 29 neurons from 3 controls and 3 mutants, respectively. (j) Density of VGlut3+ boutons contacting the soma of ErbB4+ interneurons. P = 0.033, t test, t (4) = 3.211, n = 67 and 57 neurons from 3 controls and 3 mutants, respectively. or, stratum oriens; pc, stratum pyramidale; ra, stratum radiatum. Scale bars, 20 μm (b) and 3 μm (c,d,g,h). Data are expressed as mean ± s.e.m.
Since VGlut3+/CB1+ terminals are also very abundant around the soma of PV+ interneurons in the hippocampus (Supplementary Fig. 1e,f), we hypothesized that ErbB4 might also be required for the development of these synapses. We found that PV+ fast spiking interneurons received significantly fewer VGlut3+ boutons in CCK-Cre;Erbb4F/F mutants than in control mice (Fig. 2g–j). In addition, we observed a decrease in total protein levels for GAD65, but not GAD67, in CCK-Cre;Erbb4F/F mutants compared to control mice (Supplementary Fig. 5). Since GAD65 is particularly enriched in CCK+ interneurons26, these observations reinforced the notion that CCK+/VGlut3+ basket cells have important synaptic deficits in the absence of ErbB4.
We next explored whether the synaptic deficits observed at P30 were maintained in adult mice. At P60, we found a consistent reduction in the density of CCK+/VGlut3+ basket cell boutons impinging onto both pyramidal (Supplementary Fig. 6a–d) and PV+ cells (Supplementary Fig. 6e–h) in CCK-Cre;Erbb4F/F mutants. To further strengthen our conclusions, we filled VGlut3+ interneurons with neurobiotin in control and CCK-Cre;Erbb4F/F mutant mice and analyzed the density of varicosities per axon. We found a significant decrease in the density of neurobiotin-filled varicosities in CCK-Cre;Erbb4F/F mutants compared to controls (Supplementary Fig. 6i,j). Altogether, our results revealed that in CCK-Cre;Erbb4F/F mutants CCK+/VGlut3+ basket cells have prominent wiring defects that begin during postnatal development and persist into adulthood.
Decreased inhibitory drive to pyramidal cells in CCK-specific Erbb4 mutants
We investigated the functional consequences of the abnormal connectivity of CCK+/VGlut3+ basket cells lacking ErbB4. To this end, we first recorded spontaneous inhibitory postsynaptic currents (sIPSC) from individual CA1 pyramidal cells in acute slices from P60-70 control and CCK-Cre;Erbb4F/F mutant mice (Fig. 3a). We found that pyramidal cells of CCK-Cre;Erbb4F/F mutant mice exhibit a modest but significant decrease in sIPSC amplitude, with no significant changes in sIPSC frequency (Fig. 3a–d). Moreover, we observed no apparent changes in the intrinsic properties of CCK+ interneurons (Supplementary 7a). These findings indicated that basal inhibitory synaptic transmission is compromised in CCK-Cre;Erbb4F/F mutants.
Figure 3. Functional impairment of inhibition in CA1 pyramidal cells of CCK-Cre;ErbB4F/F mice at P60-70.
(a) Scheme of Erbb4 deletion from CCK+ interneurons. The circle indicates the recording site. sIPSC sample traces (b), mean sIPSC amplitude (c) and mean sIPSC frequency (d) in pyramidal neurons. t test, t (25) = 2.646, P = 0.015, t (25) = 1.03, P = 0.313, n = 10 and 17 neurons from 3 control and 6 mutant mice, respectively. (e) Schematic of experiment configuration. (f) CCK interneurons in CCK-Cre and CCK-Cre;ErbB4F/F mice fire multiple action potentials in response to full-field optogenetic stimulation (1 ms 473nm LED flash). (g) Spikes fired per stimulus, irradiance effect. Two-way ANOVA, F (8, 72) = 14.65, P < 0.001, the two groups do not differ significantly in their response to photostimulation, Two-way ANOVA genotype effect, F (8, 72) = 14.65, P = 0.150, interaction effect, F (8, 72) = 0.357, P = 0.939, n = 5 cells in each group. (h) Schematic of experiment configuration. (i) Effects for photostimulation irradiances on the peak amplitude of evoked IPSCs, Two-way ANOVA, genotype effect, F(1, 189) = 102.3, P < 0.001, Two-way ANOVA, interaction effect, (F(8, 182) = 1.532, P = 0.149, n = 11 and 12 neurons from 5 control and 5 mutant mice, respectively. Multiple comparison testing (Sidak’s test) revealed significant differences at 2 mW/mm2 irradiance and above. (j) Minimal optogenetic stimulation to evoke IPSCs, Mann-Whitney test, U=12, P = 0.034, n = 11 and 6 neurons from 5 control and 4 mutant mice, respectively. Data are expressed as mean ± s.e.m.
We reasoned that compensatory mechanisms and/or polysynaptic effects could mask a more vigorous synaptic transmission phenotype. To circumvent this problem, we directly assessed the function of CCK+ interneurons in CCK-Cre;Erbb4F/F mutants using optogenetics. We injected the CA1 region of control and conditional mutants with adeno-associated viruses (AAV) carrying a conditional construct expressing Channelrhodopsin-2 (ChR2) fused to EYFP (Supplementary Fig. 8a–d). Since CCK drives the expression of Cre in pyramidal cells as well as CCK+ interneurons23, AAV injections led to ChR2 expression in both cell types, which precluded the exclusive activation of CCK+ interneurons. To overcome this limitation, we developed a strategy through which we could specifically examine the inhibition arising from infected CCK+ interneurons. First, we recorded from control and mutant ChR2+ pyramidal cells in voltage-clamp under GABAergic and glutamatergic blockade, obtaining I-V curves in response to 473nm photostimulation (Fig. 3e,h and Supplementary Fig. 8e–g). At a holding potential of +10 mV, direct ChR2 photocurrents were negative and modest for all pyramidal cells recorded (Supplementary Fig. 8e, n = 21 cells, -59.1 ± 10.2 pA), which enable us to unequivocally distinguish them from CCK-light evoked positive synaptic GABAergic currents (Supplementary Fig. 8f). This observation held when splitting results by genotype (n = 15 control cells, 6 mutant cells), as there were no significant differences in photocurrent amplitude at +10 mV (Mann-Whitney U = 32, P > 0.05, Control median = -45 pA, CCK-Cre;Erbb4F/F median = -60 pA). All subsequent optogenetic experiments were thus performed under glutamatergic receptor blockade, at a holding potential of +10 mV.
We then characterized light-evoked spiking in CCK+ interneurons to verify that photostimulation produced similar responses in both genotypes. Recordings were targeted to CCK+ cells in CA1 stratum radiatum, where the VGlut3+ population is enriched (Supplementary Fig. 3f-h), and revealed no effect of genotype on the number of spikes fired per stimulus (Fig. 3e–g). We then recorded optogenetically-evoked IPSCs in CA1 pyramidal neurons (Fig. 3h). The peak amplitude of evoked IPSCs was significantly reduced in CCK-Cre;Erbb4F/F mutants compared to controls, with multiple-comparisons testing revealing statistically-significant effects for photostimulation irradiances above and including 2 mW/mm2 (Fig. 3i). GABAzine completely abolished synaptic currents, confirming them to be GABAergic (Supplementary Fig. 8g). These results confirmed that the inhibitory input of CCK+ interneurons onto pyramidal cells is functionally impaired in CCK-Cre;Erbb4F/F mutants. Under optogenetic stimulation at low illumination intensities, a mixture of successes and failures in spiking can be observed (Fig. 3g, 0.23 mW/mm2 irradiance), similar to minimal stimulation using electrodes. For a subset of pyramidal neurons, we were able to lower illumination intensity to a point where we correspondingly observed a mixture of successes and failures in IPSCs in an “all-or-none” pattern (Supplementary Fig. 8h inset, 8i, j). Successfully evoked synaptic currents under these conditions are interpreted as arising from a single action potential from one pre-synaptic neuron27. In the subset of pyramidal cells where minimal intensity IPSCs could be distinguished from the direct ChR2 photocurrent, we observed that the average peak amplitude of IPSCs was significantly lower in CCK-Cre;ErbB4F/F mutants than in controls (Fig. 3j). The frequency distribution of all such events also suggested a shift towards lower IPSC amplitudes in the mutants (Supplementary Fig. 8h–j). Altogether, these results demonstrated that the inhibition of pyramidal cells by CCK+ interneurons is functionally impaired in CCK-Cre;Erbb4F/F mutants.
To assess the impact of these defects on the activity of pyramidal cells, we next recorded spontaneous excitatory postsynaptic currents (sEPSC) in CA1 pyramidal cells from acute slices at P60-70. We observed that the activity of pyramidal cells is moderately disrupted in CCK-Cre;Erbb4F/F mutants (Supplementary Fig. 7). Altogether, our findings demonstrate that synaptic deficits in the wiring of CCK+/VGlut3+ interneurons caused by the loss of ErbB4 impair the inhibition of pyramidal cells.
Reduced hippocampal theta activity in CCK-specific Erbb4 mutants
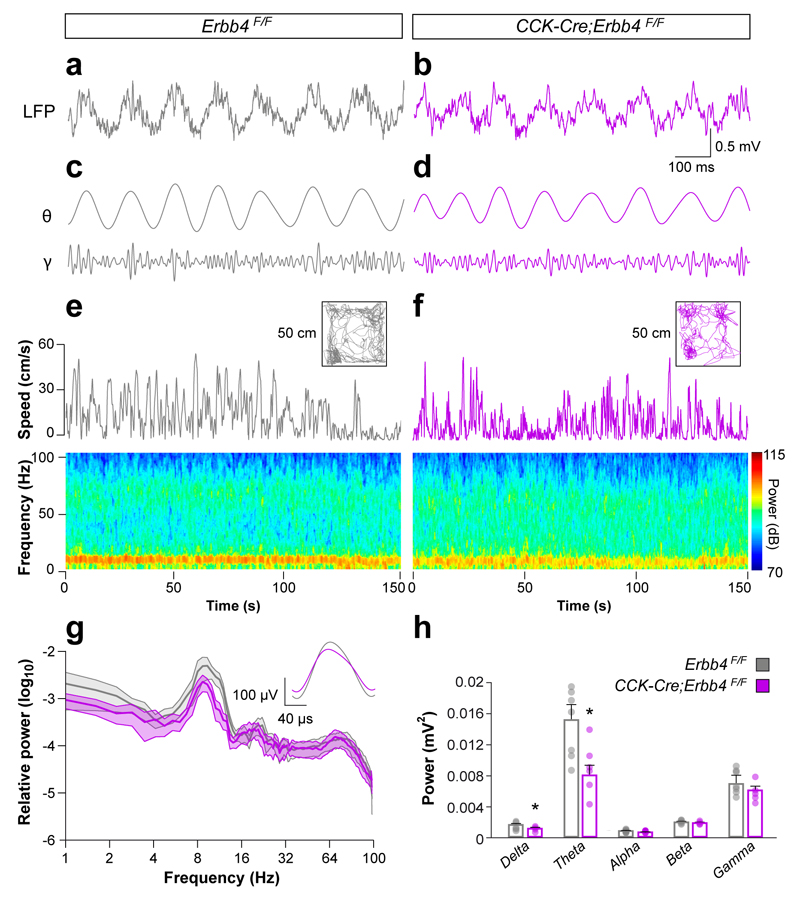
We next sought to investigate the impact of the observed deficits in vivo by recording hippocampal local field potential (LFP) in freely moving control and CCK-Cre;Erbb4F/F mutant mice. We implanted tetrodes in the dorsal hippocampus and recorded LFP during spontaneous exploration in a square open field arena. We found that CCK-Cre;Erbb4F/F mutants have a significant decrease in the power of delta and, most prominently, theta oscillations, with no significant changes in the theta/delta ratio, theta frequency, or power of high frequency oscillations (Fig. 4a–h). Since CCK-Cre mice do not drive recombination in the septum or brainstem pontine nuclei (Supplementary Fig. 9), two structures that influence theta activity in the hippocampus28, 29, the prominent decrease in the power of theta frequency observed in CCK-Cre;Erbb4F/F mutants is likely caused by defects in cortical CCK+ interneurons. These observations revealed that developmental disruption of the wiring of CCK+/VGlut3+ basket cells alters hippocampal rhythms, most notably in the theta frequency.
Figure 4. Disturbed hippocampal oscillatory activity in Erbb4 conditional mutant mice in CA1 hippocampal region.
(a, b) Spontaneous local field potentials (LFP) from control (a) and mutant (b) mice. Epochs in which animals were moving above 5 cm/s were selected to obtain LFP epochs during similar behaviors. (c and d) Filtered LFP signal in the theta and gamma bands (top and bottom traces, respectively). (e and f) Spontaneous exploration, instantaneous speed for depicted open fields (50 x 50 cm) and bottom spectrograms for control (e) and mutant (f) mice. (g) Power spectrum of CA1 LFP from 0.5–100 Hz in control and mutant mice. The inset depicts the mean theta cycle in control and mutant mice. (h) CA1 LFP band-power in the delta (0.5–4 Hz), theta (4–12 Hz), alpha (13–15 Hz), beta (16–30 Hz), and gamma (31–100 Hz) frequency bands. t test, delta t (11) = 2.23, P = 0.047; theta t (11) = 2.815, P = 0.016, alpha t (11) = 2.09, P = 0.059; beta t(11) = 0.61, P = 0.55, gamma t (11) = 0.42, P = 0.45, n = 7 control and 6 mutant mice. Color scale, 70-115 dB. Color scale, 124–130 dB. Data are expressed as mean ± s.e.m.
Spatial memory formation is altered in CCK-specific Erbb4 mutants
We next performed a battery of behavioral tests to evaluate locomotor activity, anxiety responses and cognitive function in CCK-Cre;Erbb4F/F mutants. We found no differences in locomotion or anxiety responses between both genotypes (Supplementary Fig. 10a–d). We also analyzed LFP in relation to several variables of motor behavior in control and CCK-Cre;Erbb4F/F mutant mice, and found no significant differences between both genotypes. These results suggested that the theta power deficits observed in the mutants are independent of the locomotor activity of the mice (Supplementary Fig. 10e–j).
We next used the prepulse inhibition of the startle response test to analyze the ability of CCK-Cre;Erbb4F/F mutant mice to filter out unnecessary information. While the amplitude of the startle response was similar between both genotypes, CCK-Cre;Erbb4F/F mutants failed to inhibit their startle response when the pulse was preceded by a weaker stimulus (Supplementary Fig. 11a). These results suggested that mutant mice have deficits in sensorimotor gating or attention.
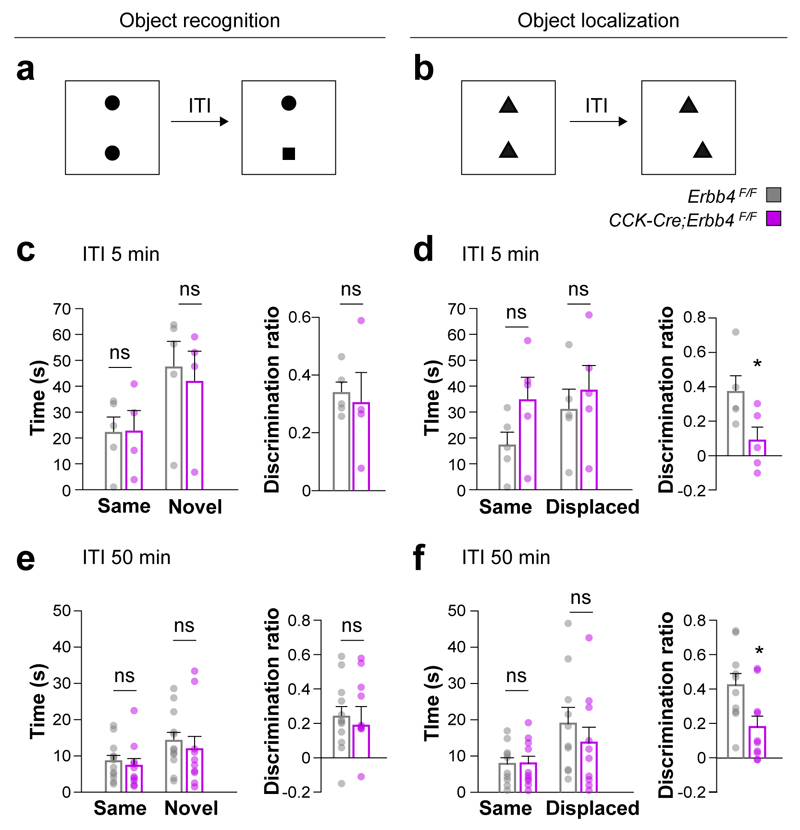
To begin examining cognitive capabilities in CCK-Cre;Erbb4F/F mutants, we used the one-trial novel object recognition and object location tasks. These tests explore hippocampal function and are based on the natural preference of mice towards novelty (Fig. 5a,b). We found that total exploration times during the test phase and discrimination indices for a novel object were similar for both genotypes in both tests (Fig. 5c–f). In object location tasks, however, conditional mutants were less capable to discriminate a displaced object compared to control mice, independently of the retention interval (Fig. 5d,f). These experiments suggest that the impaired wiring and function of CCK+/VGlut3+ interneurons do not interfere with recognition memory in general, but disrupt the ability of conditional mutants to recognize novel spatial configurations, a task that is critically dependent on hippocampal function30, 31.
Figure 5. Deficits in recognition of spatial novelty in Erbb4 conditional mutant mice.
(a, b) Schemes of novel object (a) and object-place (b) recognition tasks. (c–f) Time spent with the two objects during the test phase and discrimination index for novel object recognition (c, e) and object-place (d, f) recognition tasks. t test, t(8) = 0.341, P = 0.743, t test, t(8) = 2.321, P = 0.049, t(20) = 0.439, P = 0.665, t test, t(18) = 2.603, P = 0.018, n = 5 control and 5 mutant mice with an inter-trial interval of 5 min. ITI, inter-trial interval; n = 12 control and 10 mutant mice with an inter-trial interval of 50 min. Data are expressed as mean ± s.e.m.
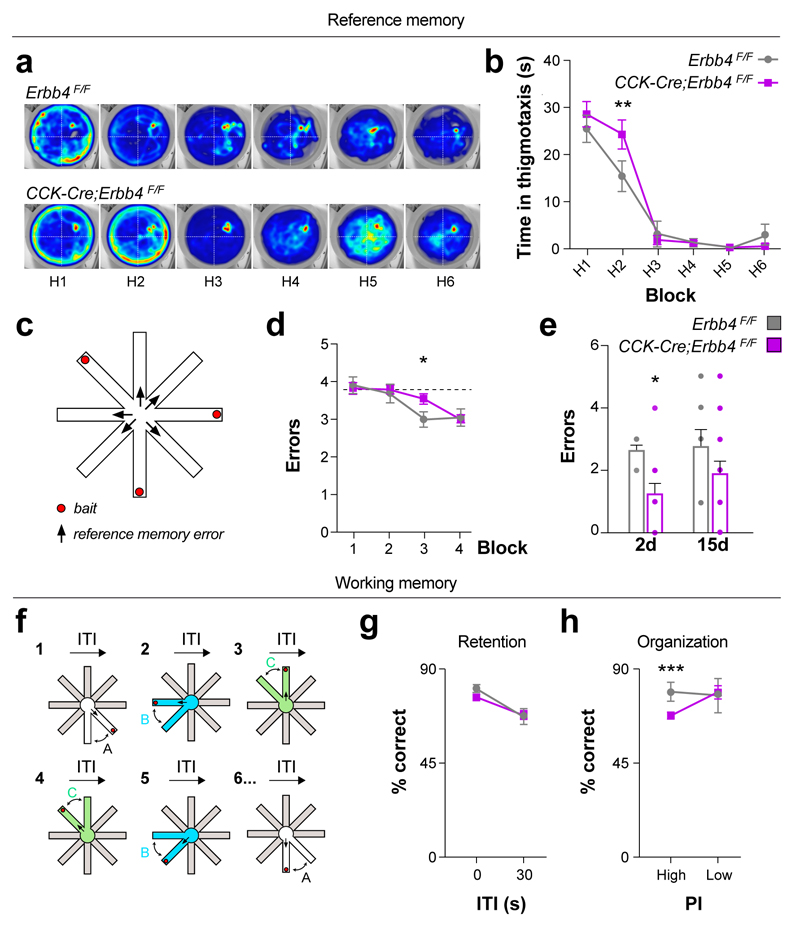
To investigate to what extent spatial information encoding is affected in CCK-Cre;Erbb4F/F mutant mice, we assessed spatial reference memory using the Morris water maze (MWM). The acquisition phase and the probe test analysis failed to reveal prominent differences between both genotypes (Supplementary Fig. 12a–f). However, closer examination revealed that whereas control mice switched from thigmotaxis to full exploration of the maze on the second day, CCK-Cre;Erbb4F/F mutant mice shifted to full exploratory behavior one day later (Fig. 6a,b). This observation suggested that conditional mutants might have a delay in spatial learning.
Figure 6. Delayed acquisition of spatial reference memory and deficits in hippocampal spatial organization in Erbb4 conditional mutant mice.
(a–b) Mice were trained in the Morris Water Maze (MWM) for 6 days (H1-H6) to find a submerged platform located in the center of the NE quadrant. (a) Heatmaps representing the average distribution for a group of tracks from control (upper panel) and Cre;ErbB4F/F mutant groups (lower panel), n = 8 control and 17 mutant mice. Color is mapped to the range of location frequencies in each heatmap separately (blue minimum and red maximum per pixel frequency). (b) Time spent navigating the outer diameter of the tank during the MWM. Two-way repeated measurements ANOVA with Fisher’s LSD correction, t(138) = 2.963, p = 0.0036, n = 8 control and 17 mutant mice (c) Schematic of experiment configuration for the reference memory test in an automated 8-arm radial maze. (d) Learning curve for reference memory test in the automated 8-arm radial maze. Each block corresponds to the average of 2-day training. Two-way ANOVA, time effect F (3, 69) = 9.873, P < 0.001, n = 8 control and 17 mutant mice. Controls reach performance under chance level during the third block and Cre;ErbB4F/F mutant mice were trained two additional days to reach performance under chance level. Two-way ANOVA with Fisher’s LSD correction, t(92)= 2.224, P = 0.0286, n = 8 control and 17 mutant mice (e) Long-term memory retrieval test two and fifteen day after the reference memory training. Genotype effect, Two-way ANOVA F(1, 23) =5.711, P = 0.0254. CCK-Cre;ErbB4F/F mutant mice performed significantly less reference memory errors 2 days after training but not 15 days after training. Two-way ANOVA with Fisher’s LSD correction, t(46) = 2.218, P = 0.0315, n = 8 control and 17 mutant mice. (f) Schematic of experiment configuration for the working memory test in an automated 8-arm radial maze. In a different radial maze setup and after two days of habituation, mice were trained to alternate between three different pairs of arms presented in pseudorandom order. (g) Percent of correct alternation performed by control and Cre;ErbB4F/F mutant mice in sessions with high or low memory retention demand. Both groups scored higher at 0 seconds-ITI than at 30 seconds ITI effect. Two-way ANOVA with Fisher’s LSD correction, F(1, 23) = 19.941, P = 0.0002, genotype effect F(1, 23) = 0.511, P = 0.4819, interaction effect F(1, 23) = 0.993, P = 0.3295, n = 8 control and 17 mutant mice. (h) Percent of correct alternation in trials with high or low hippocampal organization/proactive interference (PI) demand. PI x Genotype Interaction effect Two-way ANOVA with Fisher’s LSD, F=(1, 23) = 5.389, P = 0.0295. CCK-Cre;ErbB4F/F mutant mice performed significantly less correct choices only under high proactive interference demand, Two-way ANOVA, F(1,23)= 14.475, P < 0.001, correction, n = 8 control and 17 mutant mice. Data are expressed as mean ± s.e.m.
To further explore spatial memory, we used the 8-arm radial maze. We first carried out experiments in a conventional version of the radial maze (3/8 baited arms, all arms remaining opened), assessing simultaneously reference and working memory (Supplementary Fig. 12g–i). In this task, reference memory errors (i.e., entries into an unbaited arm) and total errors (reference errors + entries into already visited arms) were higher and showed significance at day 3 in CCK-Cre;Erbb4F/F mutants compared to controls (Supplementary Fig. 12g,h). In contrast, working memory errors (re-entries into an arm) were not significantly different between controls and their mutant littermates (Supplementary Fig. 12i), which indicated that working memory might not be particularly affected in these mutants. Consistently, we did not observe prominent differences between controls and mutants in other working memory tests (Supplementary Fig. 11b,c).
We used two different automated 8-arm radial maze setups to further characterize memory impairments in CCK-Cre;Erbb4F/F mutants (Fig. 6c,f). We analyzed reference memory again by using a radial-maze task (3/8 baited arms) in which re-entries were prevented and found that, similar to our observations in the MWM, CCK-Cre;Erbb4F/F mutants showed a transitory delay of spatial learning (Fig. 6c,d). In contrast, long-term retention of spatial memory was not altered; mutant mice even made fewer errors than controls in the retention test (Fig. 6c,e). Then, we evaluated short-term working memory with an experimental design that allowed us to manipulate the task demand on retention (“which arm was visited last”) and organization (update of successive spatial memories, avoiding interference from irrelevant memories) by using different inter-trial interval conditions (see Methods and Ref. 32). We observed that CCK-Cre;Erbb4F/F mutants have a selective impairment in the organization but not in retention aspects of spatial short-term memory (Fig. 6f–h). These findings indicate that abnormal wiring and function of CCK+/VGlut3+ interneurons do not affect short-term/working memory in general, but selectively disrupt an organizational component, necessary for the fast update of spatial memories. Collectively, our experiments demonstrated that CCK-Cre;Erbb4F/F mice display deficits in hippocampal-dependent learning tasks that require encoding of spatial information and update of novel spatial information during short-term memory, but can consolidate and/or retrieve spatial information normally.
Defective place cells fields in CCK-specific Erbb4 mutants
The previous results prompted us to investigate the characteristics of place cells in CCK-Cre;Erbb4F/F conditional mutants. We implanted tetrodes in the hippocampus and recorded the activity of single units during a pellet-chasing exploration task in the open field. We isolated single units from these recordings and selected those that met specific qualitative and quantitative criteria for place cells (see methods and Supplementary Figs. 13 and 14). We observed that the majority of pyramidal cells displayed spatial selectivity in control mice, but not in CCK-Cre;Erbb4F/F conditional mutants (Fig. 7a,b). Detailed analyses revealed that single units in the mutants have lower spatial coherence and spatial information per spike than in controls (Fig. 7c,d). In contrast, we found that the firing field area and firing rates of pyramidal neurons are increased in CCK-Cre;Erbb4F/F mutants compared to controls (Fig. 7e,f). Similar to our findings during spontaneous exploration (Fig. 4), we found a significant decrease in theta power between both groups, with no changes in theta/delta ratio or theta mean frequency (data not shown). To assess whether the distortion of theta activity could predict place cells properties, we correlated the theta power obtained during the pellet-chasing task with the mean spatial coherence. We found a significant correlation between both parameters (Fig. 7g).
Figure 7. Impaired spatial representation by place cells in the hippocampus of Erbb4 conditional mutant mice.
(a, b) Firing-rate maps showing the discharge of representative control (a) and mutant (b) place cells. Firing fields are organized following centrality and dispersion values for the distribution for each group. Values at the bottom of each firing map indicate the maximal firing rate (red). (c) Spatial coherence values for control and mutant mice. Right panels in c show the distribution of spatial coherences for place cells in control and mutant mice. Mann-Whitney, U=6540, P < 0.001, Mann-Whitney, n = 147 and 131 neurons from 7 control and 6 mutant mice, respectively. (d–f) Mean values for spatial information (Mann-Whitney, U=6551, *** P < 0.001), firing fields (t test, t(276) = -2.75, P = 0.006) and firing rates (Mann-Whitney, U=8309.5, P = 0.048), Mann-Whitney or t test, n = 147 and 131 neurons from 7 control and 6 mutant mice. (g) Correlation between theta power and the spatial coherence of place cells, Pearson, r = 0.707, ** P = 0.006,. Data are expressed as mean ± s.e.m.
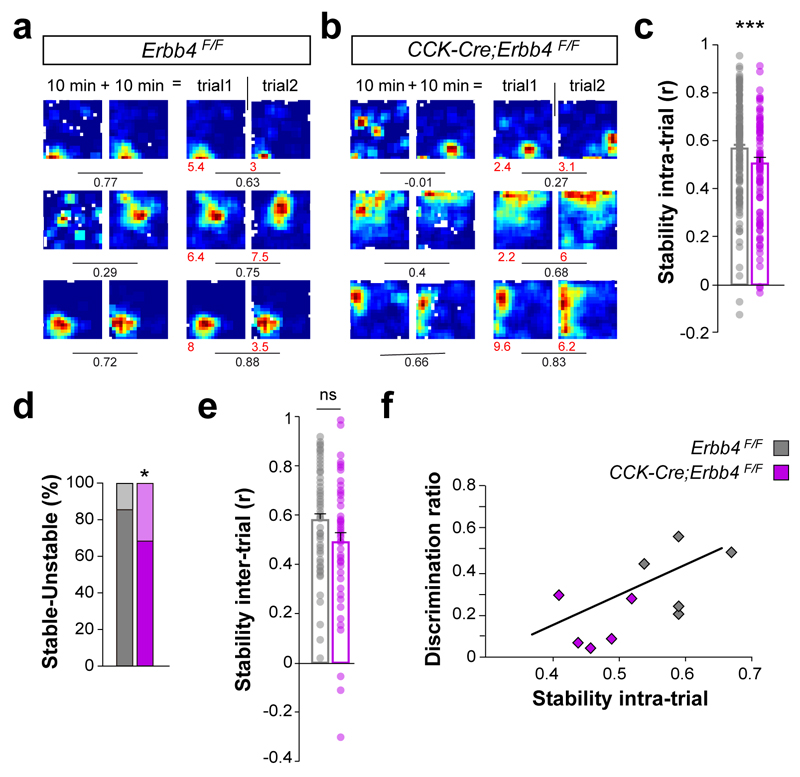
To investigate the stability of place cells in CCK-Cre;Erbb4F/F mutant mice, baseline recordings were divided in two epochs, and pairwise correlations between the firing rate maps were performed for each cell within the same recording session (intra-trial). Place cell stability was significantly lower in conditional mutants compared to controls (Fig. 8a–c). Consistently, we also found a higher percentage of unstable place cells in CCK-Cre;Erbb4F/F mutants than in controls (Fig. 8d). In contrast, stability across different recording sessions (inter-trial) was not significantly different between both genotypes (Fig. 8e). We also noticed that intra-trial stability strongly correlates with performance in the object-place recognition, which suggests that the behavioral abnormalities might be linked to place cell stability (Fig. 8f). Altogether, our results indicated that place cells exhibit lower spatial modulation in CCK-Cre;Erbb4F/F mutants than in controls, which reveals a prominent role for CCK+/VGlut3+ interneurons in circuits processing novel spatial configurations.
Figure 8. Abnormal stability of place cells in Erbb4 conditional mutant mice.
(a, b) Representative firing-rate maps for control (a) and mutant (b) place cells. For each genotype, the first two columns illustrate firing-rate maps for the same 20 min trial, with the first column displaying the first 10 min of the trial and the second column the second 10 min of the trial. Columns three and four show firing rates for two different 20 min trials (trial 1 and trial 2) separated by 5 minutes. Values show maximum firing rate (red) and spatial coherence (black). Values under lines represent the spatial correlation between firing maps above. (c) Intra-trial stability, Mann-Whitney test, U=4948, P < 0.001, n = 147 and 99 cells from 7 controls and 6 mutants, respectively. (d) Percentage of stable and remapping units, χ2 (1) = 6.31, P = 0.012. (e) Inter-trial stability, Mann-Whitney test, U= 1075, P = 0.054, n = 51 and 53 cells from 4 controls and 5 mutants, respectively. (f) Performance in the spatial object recognition was correlated with the intra-trial stability of place cells, Pearson, r = 0.640, * P = 0.046, n = 106 and 118 cells from 5 controls and 5 mutants, respectively. Data are expressed as mean ± s.e.m.
Discussion
Understanding the role that specific classes of GABAergic interneurons play on cortical operations is essential to shed light on the organization of cortical microcircuits. The function of interneurons is largely determined by their spiking properties, synaptic features and specific connectivity, but little is known about the molecular mechanisms that regulate these processes for different classes of interneuron. Here, we identified ErbB4 as an essential regulator of the wiring of CCK+/VGlut3+ interneurons, a population of basket cells whose contribution to cortical function has remained elusive. Genetic manipulation of the normal connectivity of CCK+/VGlut3+ interneurons reveals a critical role for these cells in the regulation of theta oscillatory activity and in the coding of spatial information in the hippocampus. Our findings thus demonstrate that normal integration of CCK+/VGlut3+ interneurons in cortical circuits is required for accurate spatial representations of the environment.
ErbB4 is required for the wiring of CCK+/VGlut3+ basket cells
The fine-tuning of cortical networks is orchestrated by a collection of interneuron classes with distinctive neurochemical and electrophysiological properties and, most notably, axonal targets. Since the location of synaptic contacts largely determines the influence of interneurons on the postsynaptic neuron, it has been suggested that the elaborate organization of inhibitory inputs greatly increases the overall computational power of individual neurons3. Basket cells comprise a major group of interneurons whose synapses surround the cell body and proximal dendrites of their postsynaptic targets, and therefore constitute particularly powerful regulators of the firing of principal cells33. The two main classes of basket cells, PV+ fast spiking and CCK+ regular spiking basket cells, are distinctly wired in cortical circuits34. Previous studies have shown that PV+ cells require ErbB4 to develop and maintain a normal number of excitatory inputs14, 16–18. Here, we found that ErbB4 signaling is also required for the development of a normal complement of excitatory synapses in CCK+ basket cells, which suggests that at least some of the mechanisms controlling the wiring of basket cells are shared. In contrast, while PV+ basket cells do not seem to require ErbB4 to make synapses onto pyramidal cells16, 18, we have found that loss of ErbB4 in CCK+ basket cells prominently decreases the number of synapses that these cells make and impair their ability to inhibit pyramidal cells. This finding is consistent with electron microscopy studies that revealed that ErbB4 is present in about a quarter of the inhibitory synapses contacting the soma of pyramidal cells14.
Abnormal wiring of CCK+/VGlut3 basket cells disrupts theta oscillations
Different classes of cortical interneurons seem to contribute to information processing by timing the activity of specific neural networks and gating information flow in relation to specific behavioral states2, 3. The introduction of new genetic means that provide unprecedented targeting specificity, together with the tools to manipulate interneuron activity in vivo, is greatly accelerating progress in the field9, 10, 35–37. Genetic manipulation of CCK+ interneurons, however, has proven difficult because CCK is also abundantly expressed in pyramidal cells23, 38. Due to the selective expression of ErbB4 in GABAergic cells14, 19, CCK-Cre;Erbb4F/F conditional mutants represent a unique resource to examine the contribution of CCK+/VGlut3+ basket cells to cortical operations. While the developmental nature of the genetic manipulation reported here might lead to deficits that are not directly comparable to the acute probing of CCK+/VGlut3+ basket cells, our study offers important clues to the function of these interneurons.
Previous studies have shown that CCK+ basket cells in the hippocampus fire on the ascending theta phase, when pyramidal cell assemblies emerge21, 39. Conversely, these interneurons are weakly coupled to gamma oscillations in anesthetized rats39. In CCK-Cre;Erbb4F/F conditional mutants, abnormal wiring of CCK+/VGlut3+ basket cells leads to decreased power of low frequency oscillations in freely moving animals during exploration, with no significant changes in gamma frequency. These deficits are likely due to defects in cortical CCK+/VGlut3+ basket cells, because none of the main subcortical generators of theta activity – medial septum, pedunculopontine tegmentum and raphe nuclei28, 29 – are targeted in CCK-Cre mice. In contrast, CA1-projecting neurons in the entorhinal cortex are strongly innervated by CCK+/VGlut3+ basket cells40, and so it is possible that defects in entorhinal CCK+/VGlut3+ basket cells may also contribute to the phenotype described here.
CCK+/VGlut3 basket cells and spatial coding
The majority of hippocampal pyramidal cells discharge at very low frequency during spatial navigation, but when the animal enters into the place field of a specific unit, this neuron increase its firing more than a hundred times41. It has been hypothesized that CCK+ basket cells are well suited to enhance this contrast in firing rate during theta oscillations through a form of short-term plasticity called depolarization-induced suppression of inhibition42. This involves the selective suppression of inhibition specifically in the terminals of CCK+ interneurons innervating the active place cell, while CCK+ interneurons continue to release GABA only to the majority of the silent pyramidal cells. This process is mediated by the spatially restricted action of endocannabinoids released by the active place cell, which act on CCK+ interneurons through presynaptic CB1 receptors22, 25. Our analysis suggests that defective wiring of CCK+/VGlut3+ basket cells leads to abnormal firing fields for place cells in CCK-Cre;Erbb4F/F mutants. It is tempting to speculate that these defects are caused by the failure of CCK+/VGlut3+ basket cells to suppress the activity of neighboring pyramidal cells outside the field of the place cell. In addition, the reduced inhibitory control over PV+ interneurons observed in CCK-Cre;Erbb4F/F mutants may also contribute to the decreased spatial coherence and stability of place cells, because modulation of PV+ activity is linked to the precision of hippocampal spatial representations by place cells and indeed perisomatic inhibition is very effective in regulating spike timing37, 43. Alternatively, a potential alteration of synaptic weights across different subpopulations of cells might alter the necessary temporal dynamics for a stable spatial representation and other memory processes, including spatial remapping. We cannot rule out that deficits in CCK+/ErbB4+ interneurons in the entorhinal cortex and other parahippocampal regions contribute to the stability of the place cells44. At any rate, our results reinforce the notion that CCK+ interneurons modulate spatial coding by pyramidal place cells.
Our conclusions are reinforced by the observation that abnormal wiring of CCK+/VGlut3+ basket cells leads to important hippocampal-dependent behavioral alterations. CCK-Cre;Erbb4F/F conditional mutants exhibit severe deficits in the object location test, which requires fast encoding, and in the radial maze working memory task with maximized organization demand, which requires fast encoding plus updating of spatial memories32, 45. At least some of these defects are due to defective hippocampal function, because bilateral lesions in the entorhinal cortex do not affect object location tasks46, and the function of place cells has been linked to novelty associated with an object location47, 48. We cannot exclude, however, that abnormal function of CCK+/VGlut3+ basket cells in other cortical areas contributes to some extent to the deficient coding of spatial information in the hippocampus and therefore to the behavioral abnormalities observed in CCK-Cre;Erbb4F/F conditional mutants. Considering the links that exist between ErbB4 signaling and psychiatric disorders49, it will be interesting to explore how alterations in CCK interneurons might contribute to the pathophysiology of neurodevelopmental disorders.
Online Methods
Mice
Mice were maintained in a C57B/6 background. CCK-Cre;Erbb4F/F mice were generated by breeding CCK-Cre mice23 with mice carrying loxP-flanked (F) Erbb4 alleles24. VIP-Cre23, RCE (Jackson labs 032037)50 and Ai9/tdTomato line (Jackson labs 007905)51 were used for expression analysis. In some experiments control mice included mice carrying wild type and CCK-Cre alleles. All animal procedures were approved by the ethical committee (UMH-CSIC, Spain and Home Office, UK) and met the guidelines of the local and European regulations and the standards for Use of Laboratory Animals.
Immunohistochemistry and in situ hybridization
For immunohistochemistry, adult (P30 and P60) mice were transcardially perfused and post-fixed for 2h. Immunohistochemistry was performed in 40 μm thick sections as described previously14. Primary antibodies used: mouse anti-β-galactosidase (1:500, Promega #Z3781), anti-ErbB4 (1:300; Thermo Scientific #MA5-12888, specificity tested in the HER4heart; Erbb4-/-), anti-GAD65 (1:500; Millipore #MAB351R), anti-NeuN (1:500; Millipore #MAB377), anti-parvalbumin (1:500; Sigma #P-3088) anti-PSD95 (1:500; NeuroMab #70-028); rabbit anti-CCK8 (1:300; Immunostar, #20078), anti-ErbB4 0618 (1:300, a gift form C. Lai), anti-parvalbumin (1:500; Swant, #PV-25), anti-VGlut3 (1:500, Synaptic systems #135203), anti-VIP (1:1000; Immunostar #20077); guinea pig anti-VGlut1 (1:2000; Chemicon #AB5905) anti-VGlut3 (1:500; Frontier Institute, #NM153725); goat anti-CB1 (1:500; Frontier Institute #Af450-1) and anti-ChAT (1:100; Chemicon #AB144p); chicken anti-GFP (1:1000; Aves Lab #1020). For the reconstruction of neurobiotin-filled axons and staining of VGlut3 boutons, we used a modified protocol described before52. Briefly, 300 μm sections used for patch-clamp electrophysiology, were incubated overnight with 555-Streptavidin (1:100; Molecular Probes), the primary and the secondary antibodies were incubated for 48h and overnight, respectively, at 4 °C. For standard in situ hybridization, brains were post-fixed overnight in 4% PFA and 20 µm sections were hybridized with digoxigenin-conjugated probes as described previously53. cDNA probes were cloned form adult (P30) cortex cDNA into the pCR™II-Topo® vector (Sigma) using the primers described in the Allen Mouse Brain Atlas.
Imaging and quantification
Images were acquired as 8-bit in an inverted Leica TCS-SP8 Confocal at 1024x1024 pixel resolution. For cell co-localization analyses, images were acquired with a 40X 1.4 NA objective and 0.75 digital zoom at 400 Hz speed. For synaptic bouton/cluster analysis, images were acquired with a 100X 1.44 NA objective and 2.2 digital zoom at 200Hz speed. Analysis of synaptic bouton/cluster densities was performed blind to genotype using Fiji (ImageJ) software as described previously16. For double positive bouton analysis, the binaries for both channels were overlaid and those particles ≥ 0.2 μm2 in the co-localization mask were filtered as double positive CB1/VGlut3 boutons. For cell density analysis, CCK and VGlut3 cells were quantified on 40 μm brain sections using Neurolucida software. CCK is also expressed in pyramidal cells, which preclude the quantification of CCK+ interneurons located in the pyramidal cell layer using this technique. To overcome this problem, and given that pyramidal cells seemed to express lower levels of CCK mRNA than interneurons, we carried out experiments with a short exposure to the colorimetric reaction. By doing that, we were able to clearly identify putative CCK+ interneurons in the stratum pyramidale, stratum radiatum and stratum oriens. To confirm that we were detecting most of the interneurons, we also carry out experiments with a longer exposure. At least in the stratum radiatum and oriens, the density of CCK+ cell was the same with short or long exposures to the reaction. For the assessment of axonal synaptic density, we first identified the Neurobiotin-positive/VGlut3 positive neurons and followed up their axons. Axons were distinguished by their small diameter, tortuosity and presence of bouton-like varicosities. We confirmed that bouton-like varicosities contained VGlut3 staining. Then, we restricted our quantification to Neurobiotin-filled varicosities, since VGlut3 staining in the varicosities decreased as the axon penetrated deeper into the tissue. Confocal images were taken using a 100X objective 1.4 NA and Neurolucida software was used for the analysis. Density of varicosities was normalized to 100 μm segment of axon length in those CCK-cells in which more than 100 μm axon could be followed in the slice.
Western blot
Mouse hippocampus were prepared from P30 control and CCK-Cre;Erbb4F/F homogenized in lysis buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5 mM prevanadate with Protease Inhibitor Cocktail (Complete, Mini, Roche). Samples were denatured and run on 10% SDS–PAGE gels. Gels were electrophoretically transferred onto PVDF membranes (Whatman GmbH). Membranes were blocked with 5% BSA (Sigma) in TBS (20 mM Tris-HCl, pH 7.5, 150 mM NaCl) for 1 h and probed with primary antibodies: anti-β III-tubulin (1:2000; Sigma #T8660), anti-GAD65 (1:1000; Millipore #MAB351R) and anti-GAD67 (1:1000; Chemicon #MAB5406), in 1% BSA in TBS + 0.1% Tween20. Subsequently, they were treated with horseradish-peroxidase-conjugated secondary antibodies and ECL western blotting detection reagents (Immobilon, Millipore) Signals were acquired as 16 bit images with a luminescent image analyzer (LAS-1000PLUS; Fujifilm) and quantified with Quantity One 1D Analysis Software (Bio-Rad Laboratories).
Stereotaxic injections
Adeno-associated viruses containing pAAV-DIO:EF1α:hChR2 (H134R)-EYFP:WPRE:hGHpA (a gift from K. Deisseroth, Stanford University) and produced commercially by UNC Vector, serotype AAV2/1 at 4x1012 IFU/ml) were delivered bilaterally to the hippocampus of adult (P60-70) CCK-Cre or CCK-Cre;Erbb4F/F mice anesthetized with isofluorane by stereotaxic injection using pulled glass pipettes. In brief, glass pipettes were back-filled, then placed in a Nanoliter2010 injector and front-filled with virus. Animals were anesthetized in an induction chamber with 5% isofluorane for 3 min, head-fixed in a stereotaxic frame and then maintained at 1.5-2.5% isofluorane. The stereotaxic coordinates to target bilaterally both dorsal and ventral CA1 subfields were: A/P, - 2.3; M/L, ±1.5; D/V, -1.2 and A/P, -3.2; M/L, ±3.8; D/V, 2.5 (from Bregma). Volume (110 nl at each stereotaxic coordinate) and speed (75 nl/min) of the injections was controlled by a WPI Micro4 pump. The pipette was retracted from the brain after a 5 min waiting period to allow diffusion. After suturing and disinfecting with betadine, mice were injected 100µl of buprenorphine 0.03mg/ml) and allowed to recover for 2-4 weeks post-injection before in vitro-electrophysiology experiments.
In vitro patch clamp recordings
Postnatal day (P) 60-70 mice were deeply anesthetized with sodium pentobarbital and transcardially perfused with ice-cold N-Methyl-D-Glucamine (NMDG)-based cutting solution containing (in mM): 93 NMDG, 2.5 KCl, 1.2, NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 Sodium Ascorbate, 2 Thiourea, 3 Sodium Pyruvate, 10 MgSO4, 0.5 CaCl2 adjusted to pH of 7.3 with HCl. After brain dissection, 300 m transverse hippocampal slices were cut using a vibratome (Leica) in the same ice-cold NMDG-based solution. After cutting, slices were allowed to recover for 15 minutes at 33 °C. Slices were then transferred to room temperature holding artificial cerebrospinal fluid (hACSF), where they were incubated for an additional 45 minutes and throughout the day, before recordings. hACSF contained (in mM): 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 Glucose, 5 Sodium Ascorbate, 2 Thiourea, 3 Sodium Pyruvate, 2 MgSO4, 2 CaCl2.
Patch clamp recordings
Slices were transferred to a chamber continuously superfused with recording (r) ACSF heated to 34 °C. rACSF contained (in mM): 127 NaCl, 2.5 KCl, 0.6 NaH2PO4, 26 NaHCO3, 13 Glucose, 1.3 MgSO4, 2 CaCl2. Pyramidal neurons (stratum pyramidale) or putative CCK interneurons (stratum radiatum) from hippocampal CA1 areas were viewed with infrared-differential interference optics (Hamamatsu camera controller) through a 40x water-immersion objective (Olympus). Patch microelectrodes (4–8 MΩ) were pulled from borosilicate glass (1.5 mm outer diameter x 0.86 mm inner diameter; Harvard Apparatus) using a vertical P10 puller (Narishige).
Voltage clamp recordings
To measure sIPSCs, sEPSCs, and evoked IPSCs we used a cesium-based intracellular solution containing (in mM): 135 Cs-methanesulfonate, 8 KCl, 10 HEPES, 4 Mg-ATP, 0.4 Na-GTP, 5 QX-314, 0.1 spermine, 0.5 EGTA. Cells were kept under current-clamp or voltage-clamp configuration with an Axoclamp 200A amplifier operating in fast mode. Data were filtered on-line at 2 kHz, and acquired at a 20 kHz sampling rate using pClamp 6.0.2 software (Molecular Devices). Spontaneous events were analyzed using Mini-Analysis (Synaptosoft) and current clamp recordings were analyzed with Clampfit 10.2.
In vitro optogenetics
All optogenetic experiments were conducted under 40 μM D-APV and 40 μM CNQX (Tocris). Photostimulation consisted of full-field flashes (1 ms, 0.2 Hz) delivered by TTL square-pulse activation of a Cool LED light source (473 nm) through a 40x water-immersion objective (Olympus), with the field of view centered in the middle of stratum radiatum directly beneath or above the recorded cell, respectively, for pyramidal or CCK interneuron recordings. Photostimulation intensity at the slice was systematically varied between 0.05 mW/mm2 and 21.2 mW/mm2, with 10-20 trials recorded per stimulation intensity. Recordings were targeted to EYFP-rich areas of CA1, indicating recombination of the conditional EYFP-ChR2 vector. Optogenetically-evoked spikes were recorded in fluorescently labeled CCK interneurons in stratum radiatum under current-clamp (in mM, 130 Gluconate, 5 KCl, 10 HEPES, 2 MgCl2, 10 Sodium Phosphocreatine, 2 Na2-ATP and 0.4 Na-GTP) in order to allow neurobiotin (1mg/ml) labeling and post-recording confirmation of CCK immunoreactivity. Current-voltage curves for ChR2 photocurrents were obtained by recording from ChR2-expressing CA1 pyramidal neurons under voltage-clamp, in the presence of 40 μM D-APV and CNQX, as well as 20 μM GABAzine (Tocris). Optogenetically-evoked IPSCs were recorded from pyramidal neurons in voltage-clamp at a holding potential (+10 mV) that resulted in exclusively negative direct photocurrents, with 10 μM AM-251 being used to prevent depolarization-induced suppression of inhibition54. Evoked IPSCs were detected within a 50 ms time-window after stimulus onset and the peak amplitude of the earliest IPSC was measured. Evoked IPSCs were readily distinguishable from spontaneous events due to their predictable and reliable trial-to-trial latency after stimulus onset. For minimal stimulation experiments, photostimulation intensity was reduced until no IPSCs could be evoked. At this point, stimulation intensity was increased until a combination of failures and consistent amplitude IPSCs could be observed. Under our experimental conditions this transition was sharp, with most cells showing a change from 0 to 60-70% successful trials within a 0.03 mW/mm2 increment in irradiance.
Behavioral analysis
Behavioral studies were performed with male adult (P60) mutant mice and control littermates. Mice were housed in standard cages in a 12 h dark/light cycle. Water and rodent chow were available ad lib during all the battery tests with the exception of the rewarded alternation test. All tests were performed during the light phase of the light/dark cycle by trained observer blind to genotype. The open field, object recognition, Y-maze, elevated plus maze and dark/light box were videotaped using a computer-assisted data acquisition system (Smart, PANLAB, Spain). With the exception of the sequential object recognition task48, all behavioral tests were separated at least by 24 h. The order of tests were as follows, open field, object recognition, spontaneous alternation in Y-maze, elevated plus maze, rewarded alternation in T-maze, dark-light box, PPI. Two additional batches of non-genotyped mice were tested exclusively in spatial learning and memory related tasks, using a classical automated radial maze (Ethovision) and a water maze using a computer-assisted tracking system (Imetronic or Viewpoint), and genotyped following the last behavioral task.
Open field and habituation
The open field consisted of a white acrylic glass arena of 48 x 48 x 30 cm under uniform lightning conditions 25 LUX. Mice were allowed to explore the arena for 10 min. The arena was delimited into two regions using the SMART software: zone 1, the central square area of 25 x 25 cm equidistant from the walls and zone 2 which are the remaining borders. The time spent, velocity as well as transitions between the two zones was monitored as explained above. Habituation to the open field was performed four days after first exposure to the arena in the same conditions as described before.
Novel object recognition task
Object recognition task was performed in the same environmental conditions as in the open field. During the training session, mice were allowed to individually explore two objects for 10 min and time spent exploring each object was recorded. Then, mice were returned back to the home cage and the open field and object were cleaned to avoid olfactory cues. After a determined inter-trial interval (50 min), the mouse was placed back into the same open field in which one of the familiar objects was replaced by a novel object. The mice did not show any object preference before trials. Mice were excluded from the analysis only if the total time of exploration for each mouse was lower than 5 seconds in the test session. Object exploration was defined as the mice being within 2 cm of an object, directing its nose at the object, and being involved in active exploration such as sniffing. A discrimination index (Id) was calculated to measure recognition memory:
Spatial object recognition task
Object-place recognition task was performed in the same environmental conditions as the open field. During the training session, mice were allowed to explore for 10 min two objects placed in parallel in the center of the arena at 10 cm of the open field walls. Between the training and test session the mice were placed back into their home cage and the arena as well as objects were cleaned to avoid olfactory cues. After a 50 min delay, one object randomly selected was displaced 10 cm away from its previous position in the arena in a random direction but keeping the same distance to the closest wall and during the test phase, mice were allowed to explore for additional 10 min. Mice were excluded from the analysis only if the total time of exploration for each mouse was lower that 5 seconds in the test session. A discrimination index (Id) was calculated to measure recognition memory:
Sequential object and object-place recognition task
To exclude a possible phenotype in novel/ displaced object recognition due to the session block when the tasks were performed, a combination of object and object-place recognition tasks was performed sequentially on the same day similarly to the paradigm described before48. Mice were allowed to explore twice for 10 min two objects in the arena. Then, a spatial object recognition task was performed displacing an object 10 cm away from its previous position in the arena and exploration was recorded for 10 min. Finally, one of the familiar objects were substituted by a novel object and exploration was allowed and recorded for another 10 min. Along all the trials, inter-trial interval of 5 min was applied during which the animals where placed back into their home cage and the objects as well as the arena were cleaned. The objects were presented and displaced in a counterbalanced order to avoid spontaneous object preference.
Morris water maze (MWM)
Testing was performed in a circular tub of 150 cm diameter filled with opaque water (21ºC, rendered opaque by a non-toxic white cosmetic adjuvant) as previously described55. Mice were trained to swim to a submerged platform (14 cm diameter, 1 cm below the water surface) using visual cues placed distally (at least 1 m from the maze walls) on black curtains surrounding the maze. Data were collected with a videocamera fixed to the room’s ceiling and connected to a computerized tracking system (Viewpoint) located in an adjacent room. To assess performance, the escape latency, distance to the target, time spent on thigmotaxis, and swim speed were analyzed as outcome measures for each session with Ethovision XT software (Noldus).
In the acquisition training, mice were trained for 2 daily trials with a cutoff of 60 s. Once on the platform, they were allowed to remain 10 s on it. When mice did not find the platform, the experimenter led them to it. The platform was placed in the middle of the quadrant NE and mice were released randomly from different starting points (NW, SW or SE) facing the wall.
In the probe testing, the probe trials were designed to examine the extent of spatial discrimination learning at the end of the last day of acquisition training. To achieve this, the platform was removed from the pool and the mice were allowed to navigate for 60 s. The percentage of distance that the mice spent exploring the target quadrant (where the platform was located during the hidden platform training) was measured over the 60 sec trial.
In the allocentric navigation test, we assayed after the probe test, whether mice were using the visual cues to locate the platform. To test this, all distal cues and platform were rotated in the same direction one quadrant and mice were allowed to find the platform during 60 sec.
Classical 8-arm radial maze
A fully open 8-arm radial maze was used to assess for both working and reference memory using a single task. The classical 8-arm radial maze was made of a transparent Plexiglas maze with 22 cm long and 7 cm wide radial arms with 20 cm high walls. The maze was located in a sound proof room with white walls and a constant illumination of 60 LUX. Distal visual cues of different colors, shapes and volumes where placed on the walls at 1 m distance from the center maze. Mice were weighted daily and food was progressively restricted (small chow pieces of 1.5-2.5 g/mouse), to maintain 85-90% of the free-feeding body weight throughout the experiment. Habituation to the maze was performed during two days prior to the test. For assessment of spatial reference and working memory, the bait (dry milk pellets) was placed in three arms (1, 2 and 4) and the mice were allowed to freely explore until they collected all the three baits. Mice performed two trials a day and the maze was cleaned between each trial. Every day, the maze was rotated randomly 45, 90 or 180º to dissociate potential intra-maze cues from the distal cues and surrounding environment. In addition, at the end of each arm, a false bottom was filled with the bait to avoid that mice perform spatial entries guided by the bait’s odor. Following Olton’s definition, entries into an arm that has been visited previously constituted working memory errors, whereas entries into an arm that is never baited constitute a reference memory error.
Working memory test, memory retention and interference assessment in an automatized radial maze
We used 3 fully automatized 8-arm radial maze made of grey Plexiglas (Imetronic, Pessac, France), as described before32, 45. Mice were food deprived as previously described for the classical 8-arm radial maze, and habituated during two days prior to the working memory training, as described before32. Working memory test started the following day. We used a working memory task designed to assess for mnemonic retention and organization/update of spatial information as previously described32, 45. Mice are assigned six arms grouped into three pairs (A, B, C) and have to concomitantly store different pieces of spatial information related to three different events32: the last visited arm in each. Each training session consisted of a pseudorandom presentation of the arm-pairs (20 trials for each session). The location of the food varied according to an alternation rule in each arm i.e., every time the arm-pair A is opened, the bait is loaded into the last non-visited arm. Mice remember which arm of a given pair is visited in the last trial until the next presentation of the same pair takes place. This task requires that mice retain the memory for the last visited arm in each pair of arms independently and update each of this stored information to alternate its choices between the two arms of each pair across repetitions. To assess for memory retention and organization, 3 sessions were performed with different inter-trial intervals (ITI) between the presentations of the pairs of arms (0 sec and 30 sec ITI). The task therefore implies to retain in memory of the last arm visited in any given trial n within one of the 3 pairs (the ‘sampling trial”) until the next trial within that same pair, n+1 (the “testing” trial). Performance under low or high retention demand was assessed by using a short (i.e. 0 sec) or long (i.e. 30sec) ITI, respectively. This task also implies organization of spatial information to overcome interference between repetitions in order not to confuse the n trial with the previous one within the same pair n-1 (the “proactively interfering” trial). Thus, the organizational difficulty of the trials depends on the proactive interference, determined by the proximity between the sampling trial n and the proactively interfering trial n-1. Within each the 0 sec ITI-testing condition, trials with low vs. high proactive interference (PI) level were distinguished. High interference occurs when a presentation of a pair of arms has been performed with one or less presentations of other pairs in between (0-1 intervening trials). This results in a more frequent presentation of the pair and requires the last spatial location visited by the animal to be updated in shorter time (high proactive interference). Low interference occurs when the presentation of the pair of arms is intervened by 2-4 trials in other pairs, therefore requiring an update more spaced in time.
Reference memory test in an automatized radial maze
This task is designed to specifically assess reference memory, the ability to acquire a cognitive map i.e. spatial information stable in time about the general layout of a particular environment. The reference memory task took place in a new room with an automatized 8-arm radial maze setup not explored previously (technical description described above). Animals were deprived as previously described and habituated to the new radial maze setup to collect all baits from the 8-arms. The task to assess reference memory consisted of baiting constantly 3 out of 8 arms separated by 90 and 135º (1, 3 and 6). Mice performed 2 trials/day. For each trial they were allowed to explore each arm for a single time only until the 3 baits were collected. Closing the arm automatically after the visit prevented re-entries. Learning criterion was achieved when the average of number of errors for each group was under the chance level (# error probability = 3.79 errors when for 8 arms 3 are correct and 5 counted as error). For long-term memory retrieval, mice were left in the housing room for 2 or 15 days with ad libitum rodent chow. They were food restricted 12-16 h before the beginning of the memory retrieval test. Reference memory retrieval test consisted of a single trial.
Spontaneous alternation task in Y-maze
Testing was carried out in a transparent Y-maze (cm each arm). Mice were placed into the center of the maze and allowed to freely explore for 8 min. The sequence of mouse entries was recorded. Entries in each arm were counted only if 100% of mouse body including the tail passed into maze’s arm. The alternation behavior was quantified as described before16.
Rewarded alternation task in T-maze
Spatial working memory was assessed with a rewarded alternation task on a continuous black T-maze in the same environmental conditions as before but with distal spatial cues. Before the pre-training period animals were habituated for 8 min to a black T-maze (dimensions). Light sources in the room were adjusted in order to have the same light intensity in every arm of the maze (25 LUX). Chocolate milk was used as reward, which was fed in the home cages the same day of habituation to avoid hyponeophagia. After habituation, mice were food deprived as previously described for the classical 8-arm radial maze. Then, animals were pre-trained daily to the T-maze until the consumption of the reward reached 80%. For spatial non-matching to place testing each trial consisted of a sample run and a choice run as described before43. Learning alternation task was performed with 20 randomized left-right trials or to a maximum time of 50 min per animal. Mice were tested in a counterbalanced order and interval of 10 sec was set between sample and choice runs. Criterion point for a correct trial was that the whole animal (including tip of the tail) entered the rewarded arm. Maze was cleaned between trials to avoid any potential odor cues. Mice selected for the statistical analysis were those reaching 80% alternation at the end of the training period.
Elevated plus maze
Similar to previous work16, the plus maze consisted of four arms (50 x 10 cm) elevated 50 cm above the floor: two enclosed arms with black acrylic glass walls (30 cm high) and two open (wall-free) arms joined through a central platform (10 x 10 cm). Indirect illumination provided 60 LUX onto the open arms and 15 LUX onto the closed arms. Mice were place in the center of the maze facing a closed arm and their behavior was recorded and tracked for 5 min with a camera above the maze coupled to the Smart tracking system (PANLAB, Spain). This allowed for automatic assessments of the time spent and the number of entries in the different compartments.
Dark/light box
The dark/light box test is aimed to assess for anxiety behavior. This test is based on the innate aversion of mice to avoid strong illuminated areas and on the spontaneous exploratory behavior of rodents in response to a novel environment56. We used two boxes of the same size (25 x 25 cm): one open box with direct illumination of 170 LUX-intensity (light box) and a opaque black acrylic box (dark box). Mice were place in the facing the dark box and their behavior was recorded for 10 min and video tracked in the light box. Time spent in the light box as well as number of transitions between the two chambers was quantified.
Prepulse inhibition (PPI)
Startle responses and inhibition of startle responses after presentation of a non-startling pulse (prepulse) was measured in an animal acoustic startle response system (Harvard Apparatus) as described before57. Mice were habituated to a transparent plastic-restrainer and to the conditioning chamber without background noise twice for 8 min the day prior to the PPI test. Presentation of acoustic pulse and pre-pulse stimuli was controlled by the Startle interface (Harvard Apparatus) which recorded the responses from the accelerometer. Tests session consisted to 5 min acclimatization period to a background noise at 70 dB followed by three 20 ms initial pulse stimuli at 120 dB to determine the initial amplitude of acoustic startle response. Test sessions consisted of four different trial types presented 12 times in a pseudo-random order at a 7-23 s inter-trial variable interval: pulse-only stimulus of 120 dB and three different 20 ms pre-pulse trials of 10, 15 and 20 dB above the background noise (70 dB) followed by a 20 ms startle pulse of 120 dB 100 ms later. The average value for each type of trial across the twelve blocks was used to calculate the percent of PPI after the following formula:
In vivo recordings on freely moving mice
8 control and 10 mutant male mice between 60 to 90 days of age were implanted with micro-drives (Axona ltd) with four independent screws were loaded with tetrodes (12 mm tungsten wire, California Fine Wire Company, Grover Beach, CA, USA). Electrodes were implanted under isofluorane anesthesia and buprenorphine analgesia through a craniotomy above the hippocampus of control and CCK-Cre;Erbb4F/F mutant male mice as described before16. Recordings were performed between the 60 to 120 days of age. Animals with misplaced electrodes or showing lesions were left out of the analysis. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in our previous publications. Data collection and analysis were not performed blind to the conditions of the experiment.
Data acquisition
As in previously studies16, 58, electrophysiological recordings were obtained using 16-channel head stage (gain x1), with an infrared light-emitting diode (LED) to track mouse position (Axona Ltd, UK). Signals were amplified (400 to 1000x), band pass filtered (0.3 Hz to 24 KHz, Dacq system, Axona, UK) and recorded at 48 KHz/24 bit precision. These recordings were initially used to determine electrode location previous to the implementation of the recording protocols. For single unit recordings, signal was band-pass filtered between 380 Hz and 6 KHz. Spikes were recorded whenever the signal was 3-4 times above background noise, typically 20-30 μV and stored in windows of 1 ms duration (200 ms before threshold and 800 ms after threshold detection). Same channels were also recorded in continuous mode at frequency of 4.8 KHz and band-pass filtered between 0-3-2.4 KHz.
Local field potential recordings
During recording sessions, animals were examined for their basal activity while they were in their home cages. Tetrodes were lowered until typical hippocampal activity was observed. Ripple power, unit activity and theta power were used as electrophysiological landmarks to determine electrode location. Recordings were performed at the pyramidal cell layer of CA1 of hippocampus. Anteroposterior, and mediolateral coordinates were similar in all animals. Dorsoventral location was verified in CA1 pyramidal cell layer by the presence of flat ripples. Then mice were placed in a black square area (50 cm x 50 cm) made of Plexiglas and recorded while freely exploring for 20 min. For experimental homogeneity data included for band power analysis and theta-gamma relationship was obtained during the first exploratory session in the open field. To correlate spatial coherence with theta power, we used LFP recordings obtained during the pellet-chasing experiments.
Place cells recordings
Animals were food-deprived up to 85-90% of their original weight before recording sessions. Small pellets of food were thrown in every 20 s to random locations within the open field, keeping the animal in continuous locomotion, thereby allowing a complete sampling of the environment. For sequential object and object-place recognition task in implanted animals, a similar pellet-chasing paradigm was followed and mice underwent the same object recognition test previously described48.
Data Analysis
Local field potential was analyzed using Cronux (codes available at http://chronux.org/) and custom-written MatLab (Mathworks, Natick, MA, USA) codes (available in SI, updates of scripts on reasonable request). Raw recordings were FIR filtered (<1.2 KHz) and down sample to 2.4 KHz. Similarly to previous work16, data obtained in the open field recordings was used to characterize the local field potential. Running speed was computed based on the coordinates of the animal’s position and epochs above 5 cm/s were included for analysis obtaining epochs of similar behaviors in both groups. To visualize the power spectrum in relationship to the speed of movement the spectral power (in decibels 10·log10) and spectrogram was built using the Thomson multi-taper method. Then, a sliding window with 50% overlap, yielding a frequency resolution of 1 Hz was used. For group comparisons, periodograms were plotted and the power (mV2) for different bands was calculated and then filtered by epochs in which the speed was above 5 cm/s as described before16. Complementary analysis was performed to eliminate delta related artifacts but including all behavioral states. We carried out a segmentation of the LFP in windows of 1 s and included for the analysis epochs in which the theta/delta ratio was above 3. We confirmed that theta power was significantly lower in the CCK-Cre;Erbb4F/F.
Unit isolation
Single-unit activity was isolated using cluster cutting techniques developed for data acquired with tetrodes, TINT (Axona, St. Albans, UK). Principal components, peak-trough distance, voltage at time t, peak height, trough, time of peak and time of trough in each electrode were used for unit isolation. Klustakwik was used for initial discrimination of clusters and manual refinement was performed using principal components, spike amplitude and other parameters. To verify cluster quality isolation the probability of cluster overlapping was calculated as described before59, 60 (Supplementary Fig. 13). Spike trains were analyzed by generating interval time histograms and temporal auto-correlogram (Supplementary Fig. 14). Only units with no spikes in the refractory period of the inter spike time histogram (1-2 ms), and with spike amplitudes 3-4 times above background noise, typically 20-30 μV, were included. Putative pyramidal cells and interneurons were differentiated following previous criteria58, 60 (Supplementary Fig. 13).
Firing rate maps
MatLab (Mathworks, Natick, MA, USA) codes (available as SI and updates of scripts on reasonable request) were developed for place cell activity analysis. Firing-rate maps were assembled as described previously58, 61. Pixel maps were converted to a bin matrix with a bin size of 2.5 cm x 2.5 cm (Supplementary Fig. 13). Firing rate in each bin was determined by a smoothing process using overlapping squares of 7.5 cm x 7.5 cm as described before58, 62. Firing fields were plotted as a contour map reflecting the frequency of firing; colors were interpolated from the top firing bins down to the lowest firing area by scaling them in decreasing intervals of the peak firing and giving them a color scale: red highest frequency, dark blue, lowest frequency.
The spatial information content was quantified using the Skaggs information index over the smoothed map62. This index calculates the spatial information per spike:
Where λi is the mean firing rate in bin i, λ is the overall mean firing rate and Pi is the occupancy probability of bin i.
Spatial coherence is a spatial autocorrelation measurement from which a correlation coefficient is calculated between the rate for each bin, and the average rate of the eight surrounding bins. Firing fields were defined as a group of 6 contiguous bins, where the firing frequency was above the mean firing frequency plus the standard error of the firing matrix. The maximal firing frequency of this group of bins had to be above 1 Hz. For those units displaying more than one firing field, firing field size was computed as the sum of the existing firing fields and expressed as the percentage of the arena size occupied by the firing field calculated using the smoothed firing matrix. In-field maximum frequency was computed as the maximum firing field frequency of the smoothed firing map. Mean frequency was computed as the total number of spikes divided by the total recording time to provide the average session firing rate expressed in Hz.
To classify units as having spatially-related activity, a randomized distribution was calculated using the values obtained in the original unsmoothed firing map and respecting the position matrix (n =1000). Although we observed low spatial information per spike in some of the place cells selected, we verified that the spatial coherence obtained from the original map compared with the randomized distribution was the most efficient strategy to determine spatial firing on single cell basis. Thus, for a unit to be regarded as being spatially modulated, cells had to display a spatial coherence above 99% of the randomized distribution58. This same random distribution was used to obtain stability index between the original and a random distribution. To determine the spatial stability of place cells during the intra-trial and across different trials we only compared units recorded during trials in which the at least the 70% of the arena was covered. Thus, for units to be regarded as stable they had to be above the 99% of cross-correlation between the original and the randomized distribution (Supplementary Fig. 14).
Statistics
SPSS software (IBM Corp) was used for statistical analysis and all data are presented as mean ± s.e.m. Biological replicates (n values are different populations derived from different brains from different litters) were analyzed to assess biological variability and reproducibility of data. In general, no statistical methods were used to pre-determine sample sizes but chosen sample sizes were similar to those reported in previous publications16 or to those generally employed in the field. No randomization was used. Differences were considered significant when P < 0.05. To obtain unbiased data, experimental mice from both genotypes were processed together and quantifications were performed blinded to the genotype. Data was analyzed with parametric tests, t test or ANOVA, when data sets met assumptions of normality (Kolmogorov-Smirnov, K-S test) and homoscedasticity (Levene test). Also non-parametric tests for independent groups, Mann-Whitney, and χ2 tests were applied when normality was not met or when comparing probability distributions.
Data availability
Custom codes are available on SI file titled “Supplementary Software”. The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Supplementary Material
Acknowledgments
We are very thankful to Cristina Garcia-Frigola for scientific advice and support, Marian Fernández, Diana Baeza and Victor Rodríguez-Millán for technical assistance, Trinidad Gil and Francisco Navarrete for lab support, and J. Z. Huang for mouse colonies (CCK-Cre and VIP-Cre). We thank Cathy Fernandes for guidance during an early phase of behavioral experiments at King’s College London, Shaam Al Abed for technical advice and stimulating discussions on behavioral experiments performed at the Magendie Institute, and Cindie Leteneur for help with the behavioral experiments. We are grateful to Liset Menéndez de la Prida and Miguel Maravall for critically reading early versions of this manuscript, and members of the Marín and Rico laboratories for stimulating discussions and ideas. Supported by grants from Fundación Alicia Koplowitz and the European Research Council (ERC-2012-StG 310021) to B.R., from the European Research Council (ERC-2011-AdG 293683) to O.M., from the Spanish Government (CONSOLIDER CSD2007-00023) and Lilly Research Awards Program to B.R. and O.M, and from the French Government (ANR-10-EQX-008-1) to A.M. O.M. and B.R. are Wellcome Trust Investigators.
Footnotes
Authors Contribution
I.P. performed cell, synaptic, biochemical and behavior experiments and analyzed data. J.B. performed in vivo electrophysiology recordings and analyzed data. A.M.S. carried out in vitro electrophysiology recordings and analyzed data. A.M. contributed to the behavior analysis. A.F. contribute with resources. I.P. O.M. and B.R. wrote the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Ascoli GA, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science (New York, N.Y.) 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartos M, Elgueta C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol. 2012;590:669–681. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Tricoire L, et al. A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. J Neurosci. 2011;31:10948–10970. doi: 10.1523/JNEUROSCI.0323-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somogyi J, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19:552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- 8.Valero M, et al. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18:1281–1290. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 12.Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyaya B, Baho E, Huang ZJ, Schachner M, Di Cristo G. Neural cell adhesion molecule-mediated Fyn activation promotes GABAergic synapse maturation in postnatal mouse cortex. J Neurosci. 2013;33:5957–5968. doi: 10.1523/JNEUROSCI.1306-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 15.Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Pino I, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–1168. doi: 10.1016/j.neuron.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Ting AK, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JM, et al. Development of GABA circuitry of fast-spiking basket interneurons in the medial prefrontal cortex of erbb4-mutant mice. J Neurosci. 2013;33:19724–19733. doi: 10.1523/JNEUROSCI.1584-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vullhorst D, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron. 2013;78:498–509. doi: 10.1016/j.neuron.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klausberger T, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raastad M, Storm JF, Andersen P. Putative Single Quantum and Single Fibre Excitatory Postsynaptic Currents Show Similar Amplitude Range and Variability in Rat Hippocampal Slices. Eur J Neurosci. 1992;4:113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 28.Gogolak G, Stumpf C, Petsche H, Sterc J. The firing pattern of septal neurons and the form of the hippocampal theta wave. Brain Res. 1968;7:201–207. doi: 10.1016/0006-8993(68)90098-x. [DOI] [PubMed] [Google Scholar]
- 29.Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- 30.Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- 31.Stupien G, Florian C, Roullet P. Involvement of the hippocampal CA3-region in acquisition and in memory consolidation of spatial but not in object information in mice. Neurobiol Learn Mem. 2003;80:32–41. doi: 10.1016/s1074-7427(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 32.Al Abed AS, et al. Estradiol enhances retention but not organization of hippocampus-dependent memory in intact male mice. Psychoneuroendocrinology. 2016;69:77–89. doi: 10.1016/j.psyneuen.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong C, Soltesz I. Basket cell dichotomy in microcircuit function. J Physiol. 2012;590:683–694. doi: 10.1113/jphysiol.2011.223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matyas F, Freund TF, Gulyas AI. Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci. 2004;19:1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Kruglikov I, Huang ZJ, Fishell G, Rudy B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci. 2013;16:1662–1670. doi: 10.1038/nn.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royer S, et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci. 2012;15:769–775. doi: 10.1038/nn.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgunder JM, Young WS., 3rd Cortical neurons expressing the cholecystokinin gene in the rat: distribution in the adult brain, ontogeny, and some of their projections. J Comp Neurol. 1990;300:26–46. doi: 10.1002/cne.903000104. [DOI] [PubMed] [Google Scholar]
- 39.Lasztoczi B, Tukker JJ, Somogyi P, Klausberger T. Terminal field and firing selectivity of cholecystokinin-expressing interneurons in the hippocampal CA3 area. J Neurosci. 2011;31:18073–18093. doi: 10.1523/JNEUROSCI.3573-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga C, Lee SY, Soltesz I. Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci. 2010;13:822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 42.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 43.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 45.Mingaud F, et al. Retinoid hyposignaling contributes to aging-related decline in hippocampal function in short-term/working memory organization and long-term declarative memory encoding in mice. J Neurosci. 2008;28:279–291. doi: 10.1523/JNEUROSCI.4065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hales JB, et al. Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep. 2014;9:893–901. doi: 10.1016/j.celrep.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Delcasso S, Lee I. Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J Neurosci. 2011;31:16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24:773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 51.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fogarty MJ, Hammond LA, Kanjhan R, Bellingham MC, Noakes PG. A method for the three-dimensional reconstruction of Neurobiotin-filled neurons and the location of their synaptic inputs. Front Neural Circuits. 2013;7:153. doi: 10.3389/fncir.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flames N, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau MM, et al. The planar polarity protein Scribble1 is essential for neuronal plasticity and brain function. J Neurosci. 2010;30:9738–9752. doi: 10.1523/JNEUROSCI.6007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 57.Ortega-Alvaro A, et al. Differential Pharmacological Regulation of Sensorimotor Gating Deficit in CB1 Knockout Mice and Associated Neurochemical and Histological Alterations. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brotons-Mas JR, Montejo N, O'Mara SM, Sanchez-Vives MV. Stability of subicular place fields across multiple light and dark transitions. Eur J Neurosci. 2010;32:648–658. doi: 10.1111/j.1460-9568.2010.07308.x. [DOI] [PubMed] [Google Scholar]
- 59.Bellistri E, Aguilar J, Brotons-Mas JR, Foffani G, de la Prida LM. Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J Physiol. 2013;591:2667–2686. doi: 10.1113/jphysiol.2013.251892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox SE, Ranck JB., Jr Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41:399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- 61.Jeffery KJ, Gilbert A, Burton S, Strudwick A. Preserved performance in a hippocampal-dependent spatial task despite complete place cell remapping. Hippocampus. 2003;13:175–189. doi: 10.1002/hipo.10047. [DOI] [PubMed] [Google Scholar]
- 62.Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE. Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus. 1994;4:410–421. doi: 10.1002/hipo.450040404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Custom codes are available on SI file titled “Supplementary Software”. The data that support the findings of this study are available from the corresponding authors upon reasonable request.