Abstract
Objectives
The HEART Pathway combines a decision aid and serial contemporary cardiac troponin I (cTnI) measures to achieve >99% sensitivity for major adverse cardiac events (MACE) at 30 days and early discharge rates >20%. However, the impact of integrating high-sensitivity troponin (hs-cTn) measures into the HEART Pathway has yet to be determined. In this analysis we compare test characteristics of the HEART Pathway using hs-cTnI, hs-cTnT, or cTnI.
Design & Methods
A secondary analysis of participants enrolled in the HEART Pathway RCT was conducted. Each patient was risk stratified by the cTn-HEART Pathway (Siemens TnI-Ultra at 0- and 3-hours) and a hs-cTn-HEART Pathway using hs-cTnI (Abbott) or hs-cTnT (Roche) at 3-hours. The early discharge rate, sensitivity, specificity, and negative predictive value (NPV) for MACE (death, myocardial infarction, or coronary revascularization) at 30 days were calculated.
Results
hs-cTnI measures were available on 133 patients. MACE occurred in 11/133 (8%) of these patients. Test characteristics for the HEART Pathway using serial cTnI vs 3 hour hs-cTnI were the same: sensitivity (100%, 95%CI: 72–100%), specificity (49%, 95%CI: 40–58%), NPV (100%, 95%CI: 94–100%), and early discharge rate (45%, 95%CI: 37–54%). The HEART Pathway using hs-cTnT missed one MACE event (myocardial infarction): sensitivity (91%, 95%CI: 59–100%), specificity (48%, 95%CI: 39–57%), NPV (98%, 95%CI: 91–100%), and early discharge rate (45%, 95%CI: 37–54%).
Conclusions
There was no difference in the test characteristics of the HEART Pathway whether using cTnI or hs-cTnI, with both achieving 100% sensitivity and NPV. Use of hs-cTnT with the HEART Pathway was associated with one missed MACE.
Keywords: troponin, chest pain, acute coronary syndrome, HEART Pathway
1. Introduction
Accelerated diagnostic pathways (ADPs), such as the HEART Pathway, objectively combine variables from the patient’s history, electrocardiogram findings, and cardiac troponin (cTn) measures to risk stratify patients with acute chest pain. These tools are being increasingly used by Emergency Department (ED) providers and have been incorporated into the guidelines for the early risk stratification of patients with acute chest pain [1].
The HEART Pathway is designed to identify patients who can be safely discharged from the ED without stress testing or coronary angiography. To be considered low-risk and eligible for early discharge the HEART Pathway requires a HEART Score of 0–3 and normal serial contemporary cTn at 0 and 3 hours [2–4]. In a recently completed clinical trial, the HEART Pathway significantly increased early discharges and decreased hospital lengths of stay and objective cardiac testing (stress testing and coronary angiography) compared to the usual care group. Reductions in healthcare utilization outcomes were achieved by the HEART Pathway without any low-risk patients experiencing adverse cardiac events at 30 days [4].
While the HEART Pathway has demonstrated excellent sensitivity for adverse cardiac events using contemporary cTn, many health systems in Europe, Canada, and the Asia-Pacific Region are using high sensitivity troponin (hs-cTn) assays, and approval of hs-cTn assays in the United States is expected soon. Recent studies suggest that hs-cTn measures should be used within the context of an ADP [5, 6]. However, the impact of integrating hs-cTn measures into the HEART Pathway has yet to be determined. The objective of this secondary analysis is to determine the test characteristics of the HEART Pathway using hs-cTnI and hs-cTnT assays compared to a contemporary cTnI assays.
2. Materials and Methods
2.1 Study design
A pre-planned secondary analysis of participants enrolled in the HEART Pathway Randomized Controlled Trial was conducted. Participants were enrolled from September 2012, through February 2014, and all gave written informed consent at the time of study entry. The HEART Pathway trial was approved by the sponsoring organization’s Internal Review Board and was registered with clinicaltrials.gov (clinical trial number NCT01665521).
Methods of the HEART Pathway trial have been previously described [4]. Adults presenting to the ED with symptoms suggestive of acute coronary syndrome (ACS) without ST-elevation on ECG were enrolled. Patients were randomized with equal probability to risk stratification using the HEART Pathway or usual care (based on American College of Cardiology/American Heart Association guidelines). In the HEART Pathway arm, ED providers used a clinical decision aid, the HEART (History ECG Age Risk factors Troponin) score, paired with serial cTn measures at 0 and 3 hours to guide disposition decisions.
2.2 Study setting
Participants were enrolled from the ED of (institution name withheld for review), an academic tertiary care center located in the Piedmont Region of North Carolina. The ED is staffed by board certified/eligible emergency physicians 24 hours a day, 7 days a week who directly provide patient care and oversee care delivered by residents, and advanced practice clinicians. During the trial enrollment period ED patient volume consisted of approximately 104,000 encounters per year. The cardiac testing modalities that were routinely available to study participants included exercise stress echocardiogram, dobutamine stress echocardiogram, coronary computed tomography angiography, stress nuclear imaging, stress cardiac magnetic resonance imaging, and invasive coronary angiography.
2.3 Participants
Patients ≥21 years old presenting with symptoms suggestive of ACS were screened for enrollment 6 days per week excluding Saturday (80 hours/week). Patients for whom their provider order an ECG and troponin for the evaluation of ACS were eligible for participation. Patients were excluded for new ST-segment elevation ≥ 1mm, hypotension, life expectancy <1 year, a non-cardiac medical, surgical, or psychiatric illness determined by the provider to require admission, prior enrollment, non-English speaking, and incapacity or unwillingness to consent.
2.4 Data collection
2.4.1 Patient data
Data elements were collected prospectively in accordance with Standardized Reporting Guidelines [7], standards of Good Clinical Practice, and Key Data Elements and Definitions [8]. Electronic medical records (EMR) were used as the primary source for variables reliably contained in the medical record. For data elements not reliably present in the EMR, study coordinators used REDCap data collection templates to prospectively collect and store data from the patients and their care providers.
Following the index visit and at 30 days, a structured record review was completed. At 30 days a telephone interview using a validated scripted follow-up dialogue was conducted to identify and clarify events since discharge.[9] Events occurring at out-of-network health care facilities were confirmed using a structured review of medical records requested from the outside facility. Participants with a record of ongoing visits in the EMR were considered to have complete follow-up information and were classified based on available data in the medical record. Participants without ongoing visits were considered lost to follow-up at the point of last contact. The Social Security Death Master File was used to search for patients unable to be reached by phone or without EMR data. When a discrepancy between a participant’s self-reported event and the medical record was identified, the medical record was considered accurate.
2.4.2 cTn Measurement
All study participants had serum cTn measurements performed using the institutional core-lab contemporary assay: the ADVIA Centaur platform TnI-Ultra™ (Siemens, Munich, Germany). This assay has a 99th percentile of the upper reference limit (URL) and 10% coefficient of variation (CV) at 0.040 µg/L (40 ng/L), which was also the clinical threshold for detection of myocardial injury during the study period. Per study protocol, the contemporary cTnI measures were obtained at 0 and 3 hours after the patient was evaluated by the ED clinical team and these results were used for clinical and research purposes.
At 3 hours an additional blood specimen for research purposes only was collected in a 10ml lithium heparin plasma tube on willing participants (n=259). Following collection, blood was centrifuged at 3000 ×g Relative Centrifugal Force at 4 degrees Celsius for 15 minutes. Aliquots (1 ml) of plasma were transferred into cryovials and stored in a −70 degree Celsius Freezer. Samples were shipped on dry ice to Fred Apple’s laboratory for hs-cTnI and hs-cTnT analysis. Blood samples were tested using the Abbott ARCHITECT stat hs-cTnI (Abbott Laboratories, Abbott Park, IL, USA) which has URL of 34 ng/L for males and 16 ng/L for females, a limit of detection of 1.9 ng/L, and a 10% CV at 6 ng/L. Samples were also tested using the Roche Elecsys 2010 hs-cTnT (Roche Diagnostics, Risch-Rotkreuz, Switzerland), which has a URL of 14 ng/L, limit of detection of 5 ng/L, and 10% CV at 13 ng/L. Previously established gender specific URLs of 20 ng/L for males and 13 ng/L for females were used for this analysis [10]. A sensitivity analysis was conducted using different gender specific URLs (15.5 ng/L for males and 9 ng/L for females) established by Saenger, et al [11]. For this analysis all cTnI values above the URL, or any hs-cTn value above the gender specific URL were considered consistent with myocardial injury and “high risk” when utilized as part of the HEART Pathway. Given that these samples were tested for hs-cTn asynchronously with clinical care, results were not available to the patient’s medical care team.
2.4.3 HEAR Scores
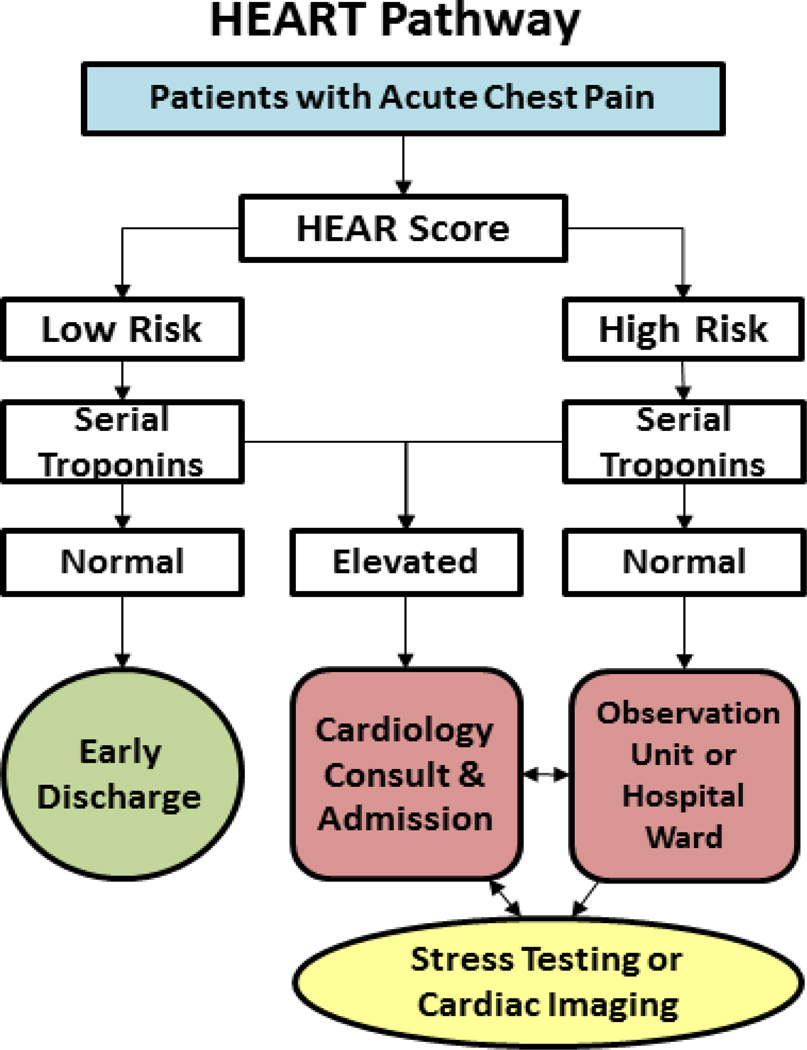
Participants randomized to the HEART Pathway arm were risk stratified by attending ED providers using the History, Electrocardiogram, Age, and Risk factors (HEAR) components of the HEART score [12–15], and serial cTn measures at 0 and 3 hours. To complete a HEAR score, the provider utilized the participant’s ECG and a study worksheet at the patient’s bedside. Patients were risk stratified (as low-risk or at-risk) based on the HEAR score and cTn results, utilizing the three different assays discussed above (see Figure 1). Patients were considered low-risk if HEAR scores were 0–3 and cTn results were below the URL. Patients with a HEAR score ≥4 or a cTn above the URL regardless of HEAR score were considered at-risk. Based on our prior analyses, a HEAR score of 3 or less with two negative cTn measures (a low-risk assessment by the HEART Pathway) is associated with a <1% risk of MACE at 30 days [2–4].
Figure 1.
HEART Pathway algorithm
2.5 Study Measures
MACE was defined as the composite of death, myocardial infarction (MI), or coronary revascularization within 30 days of presentation, based on the standardized reporting guidelines and key data elements and definitions [7, 8]. The Universal Definition of Myocardial Infarction was used to define myocardial infarction; a gradual rise of cTnI above the URL with a CV <10% and gradual fall with at least one of the following: a. ischemic symptoms; b. new pathologic Q waves on the ECG; c. acute ischemic ECG changes (ST segment elevation or depression); d. myocardial imaging demonstrating a new regional wall motion abnormality [16]. Coronary revascularization was defined as coronary artery bypass grafting, stent placement, or other percutaneous coronary intervention. A consensus of two reviewers (CDM, BCH), blinded to HEART Pathway risk assessments, adjudicated elements required to determine the occurrence of MACE. Adjudicators were provided index and discharge records including participant’s cTnI measures, follow-up call information, outside records obtained from follow-up, and study definitions. Disagreements were settled by the two reviewers achieving consensus or the involvement of a third reviewer.
2.6 Data Analysis
The percentage of patients identified as safe for early discharge (low-risk) by the three different cTn assays and the HEART Pathway was calculated. The sensitivity, specificity, and positive and negative predictive values for MACE were also determined. Exact 95% binomial confidence intervals were computed. Patients with incomplete follow up were considered to be free of 30-day MACE events. The performance of the HEART Pathway with each assay was compared using McNemar’s test and net reclassification improvement (NRI). The HEART Pathway RCT was powered to detect a 15% difference in objective cardiac testing between randomization arms with 80% power at the 5% two-sided level of significance and an expected loss to follow up rate of 10%. Statistical analysis was performed using SAS 9.4 (Cary, North Carolina).
3. Results
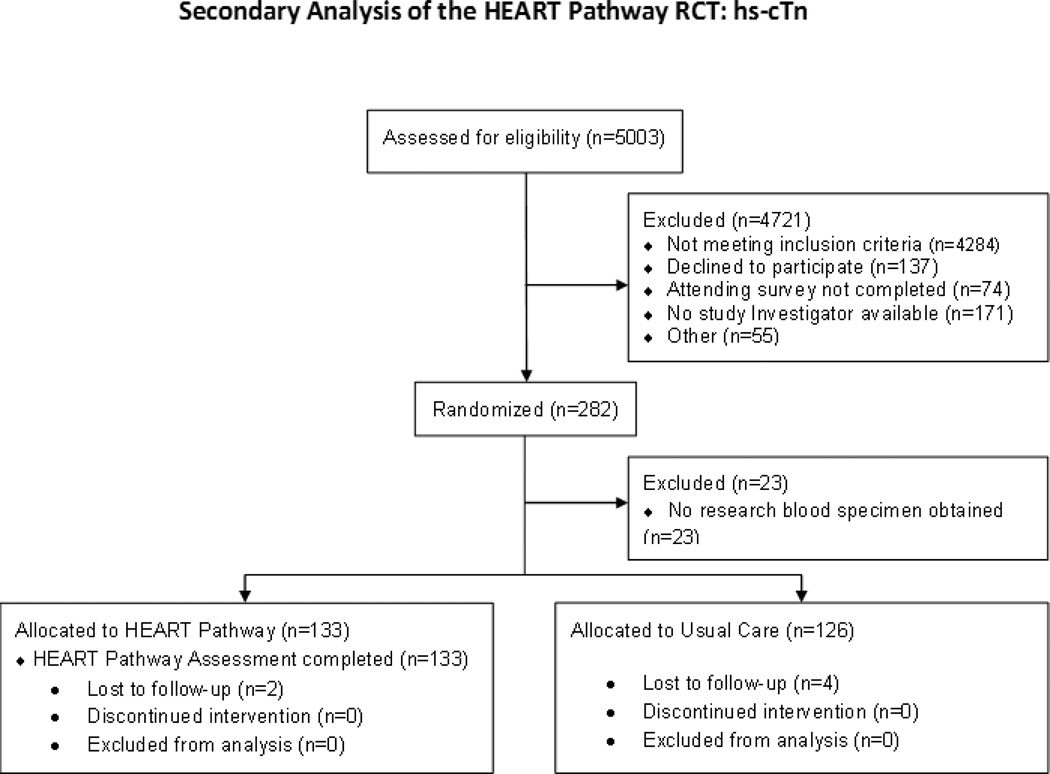
From September 2012 to February 2014, 282 patients with symptoms suggestive of ACS were enrolled in the HEART Pathway RCT, of which 259 had blood specimens available for hs-cTn analysis. In the HEART Pathway, arm blood samples were available on 133/141 for hs-cTn measurement (Figure 2). Follow-up for 30 day events was complete for 97.7% (253/259) of participants and 98.4% (131/133) of patients randomized to the HEART Pathway arm. MACE occurred in 20/259 (7.7%) patients in the main cohort: there were no deaths, 14 patients had MI, and 6 patients had coronary revascularization without MI. In the HEART Pathway arm, MACE occurred in 11/133 (8.2%): with no deaths, 7 MIs, and 4 revascularizations without MI. Patient characteristics are summarized in Table 1. When used without a HEAR Score the cTnI, hs-cTnI, and hs-cTnT assays had low sensitivity (60%, 65%, and 60% respectively) for MACE (with most missed events classified as coronary revascularization without MI). Performance of the cTnI, hs-cTnI, and hs-cTnT assays in the entire cohort (n=259) is presented in Table 2. The sensitivity analysis comparing different hs-cTnT gender specific URLs is presented in Appendix 1.
Figure 2.
Enrollment flow diagram
Table 1.
Characteristics of the Cohort and HEART Pathway Arm.
| Patient Characteristics | Main Cohort N=259 |
HEART Arm N=133 |
|---|---|---|
| Age—mean ±SD* | 53.6 + 12.0 | 53.6 + 12.0 |
| Gender | ||
| Female | 145 (56.0) | 77 (57.9) |
| Race‡‡ | ||
| Caucasian | 173 (66.8) | 87 (65.4) |
| African American | 81 (31.3) | 43 (32.3) |
| Asian | 1 (0.4) | 1 (0.8) |
| Native American | 2 (0.8) | 1 (0.8) |
| Other | 2 (0.8) | 1 (0.8) |
| Ethnicity | ||
| Hispanic | 5 (1.9) | 1 (0.8) |
| Not Hispanic | 254 (98.1) | 132 (99.2) |
| Risk Factors | ||
| Current smoking | 66 (25.5) | 37 (27.8) |
| Recent Cocaine (last 90 days) | 6 (2.3) | 3 (2.3) |
| Hypertension | 145 (56.0) | 74 (55.6) |
| Hyperlipidemia | 115 (44.4) | 60 (45.1) |
| Diabetes | 54 (20.9) | 31 (23.3) |
| Family history of coronary disease | 98/257 (38.1) | 44/132 (33.3) |
| BMI >30 (kg/m2) | 137 (52.9) | 67 (50.4) |
| TIMI risk score >1 | 116 (44.8) | 59 (44.4) |
| Prior Coronary Disease | 46 (17.8) | 24 (18.1) |
| Prior MI | 41 (15.8) | 20 (15.0) |
| Prior PCI | 32 (12.4) | 14 (10.5) |
| Prior CABG | 10 (3.9) | 7 (5.3) |
| Prior Cerebral Vascular Disease | 11 (4.3) | 3 (2.3) |
| Prior Peripheral Vascular Disease | 8 (3.1) | 4 (3.0) |
| Insurance status | ||
| Insured | 194/257 (75.5) | 100/133 (75.2) |
| Private | 126 (65.0) | 64 (64.0) |
| Medicare | 40 (20.6) | 21 (21.0) |
| Medicaid | 28 (14.4) | 13 (13.0) |
| Uninsured | 63/257 (24.5) | 33/133 (24.8) |
Table 2.
Test characteristics of the cTnI, hs-cTnI and hs-cTnT for MACE in the entire cohort.
| cTn Assay | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|
| cTnI at 0 hrs | 50.0% (27.2–72.8%) |
96.2% (93.0–98.3%) |
52.6% (28.9–75.6%) |
95.8% (92.5–98.0%) |
| cTnI at 3 hrs* | 60.0% (32.2–83.7%) |
99.1% (96.7–99.9%) |
81.8% (48.2–97.7%) |
97.2% (94.1–99.0%) |
| hs-cTnI at 3 hrs | 65.0% (40.8–84.6%) |
95.4% (91.9–97.7%) |
54.2% (32.8–74.5%) |
97.0% (94.0–98.8%) |
| hs-cTnT at 3 hrs | 60.0% (36.1–80.9%) |
93.3% (89.4–96.1) |
42.9% (24.5–62.8%) |
96.5% (93.3–98.5%) |
Excludes 2/259 patients without second cTn measure, and 29/259 with second clinical cTn measure at ≥6 hour
The HEART Pathway had the same test characteristics for MACE using serial cTnI measures or a 3-hour hs-cTnI measure. Both identified 60/133 patients (45.1%, 95% CI 36.5–54.0%) as low-risk. Of these low-risk patients, none had MACE events at 30 days, yielding a sensitivity and NPV of 100% (95% CI 71.5–100% and 95% CI 94.0–100% respectively). Using hs-cTnT, the HEART Pathway identified the same proportion of patients as low-risk (60/133, 45.1%, 95% CI 36.5–54.0%). Of these patients, one patient had an adjudicated index NSTEMI, yielding a sensitivity of 90.9% (95% CI 58.7–99.8%) and NPV of 98.3% (95% CI 91.1–100%). The performance characteristics of the HEART Pathway using the different assays are summarized in Table 3. The use of cTnI or hs-cTnI for the HEART Pathway compared to hs-cTnT, was able to correctly reclassify 1 patient without MACE as low-risk and 1 patient with MACE as high-risk, producing a NRI of 0.099 (95% CI −0.072, 0.270). The test characteristics of the HEART Pathway using the 3 different assays were similar (p=1.0).
Table 3.
Performance characteristics of the HEART Pathway using cTnI, hs-cTnI, and hs-cTnT,
| Risk Stratification Strategy | % Low-Risk (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|---|
| HEART Pathway cTnI | 45.1% (36.5–54.0%) |
100% (71.5–100%) |
49.2% (40.0–58.4%) |
15.1% (7.8–25.4%) |
100% (94.0–100%) |
| HEART Pathway hs-cTnI | 45.1% (36.5–54.0%) |
100% (71.5–100%) |
49.2% (40.0–58.4%) |
15.1% (7.8–25.4%) |
100% (94.0–100%) |
| HEART Pathway hs-cTnT | 45.1% (36.5–54.0%) |
90.9% (58.7–99.8%) |
48.4% (39.2–57.6%) |
13.7% (6.8–23.8%) |
98.3% (91.1–100%) |
4. Discussion
Results of this secondary analysis demonstrate that the HEART Pathway has high sensitivity and NPV for 30-day MACE whether a contemporary or high sensitivity cTn assay using gender specific cut points is utilized. Use of serial cTnI measures at 0 and 3 hours or a single 3-hour hs-cTnI with the HEART Pathway was associated with 100% sensitivity and NPV for 30-day MACE. Use of the HEART Pathway with the hs-cTnT assay resulted in misclassifying one patient with MACE as low-risk. The test characteristics for the HEART Pathway were similar regardless of the assay used. However, it is important to note that this study was not powered to detect small differences, so further evaluation is needed to definitively conclude that the performance of the HEART Pathway which each of these assays is the same.
Numerous studies have demonstrated that hs-cTn assays are more sensitive for the early detection and early rule out of MI than contemporary cTn assays [17–19]. For example, Neumann et al., have confirmed that hs-cTnI measured at baseline and 1 hour after presentation have a very high negative (99.0%) and positive predictive value (87.1%) for AMI; enabling rapid, safe treatment [20]. However, the improved sensitivity of these newer assays comes at the cost of specificity [21]. hs-cTn assays are less specific than contemporary assays for MI, as more patients with non-ACS conditions have hs-cTn elevations that are missed with the contemporary assay [22]. While patients with hs-cTn elevations have increased downstream mortality, regardless of cause [23, 24], the optimal testing and management strategies for non-ACS patients with hs-cTn elevations are unclear. Therefore, some have been concerned about the impact of integrating hs-cTn assays into an ADP, such as the HEART Pathway, which has previously demonstrated a sensitivity >99% for 30-day MACE using serial contemporary cTn measures. They have hypothesized that hs-cTn use could decrease specificity and lead to more patients with diagnostic and therapeutic dilemmas [25, 26].
Our results suggest that the test characteristics of the HEART Pathway were similar regardless of the assay utilized. In fact, use of the cTnI and hs-cTnI assays with the HEART Pathway resulted in identical test characteristics; 100% sensitivity and 49% specificity for 30-day MACE. Based on prior studies of the HEART Pathway, high sensitivity for MACE was expected [2–4]. However, it is particularly interesting that the specificity and proportion of patients identified as low-risk by the HEART Pathway were not decreased by hs-cTnI assay use. All of the patients in our cohort with elevated hs-cTn measurements were identified as high-risk based on their HEAR Score or contemporary cTnI results. These findings are consistent with the results of Aldous et, al. which demonstrated no significant difference in the sensitivity or specificity for MACE of an ADP when using a new generation point of care cTnI versus a hs-cTnI assay [27]. The finding from our study are significant given the current lack of availability of hs-cTn assays in the US and the concern that lack of these assays significantly hampers our ability to perform chest pain risk stratification. Furthermore, this study suggests the HEART Pathway may be utilized without modification in places where hs-cTnI assays are currently available and in the US (when available).
Use of a hs-cTnT assay with the HEART Pathway resulted in a lower sensitivity and specificity for MACE than use of the cTnI and hs-cTnI assays. However, these differences were small, because they resulted from the change in risk classification of just 2 patients. The HEART Pathway using hs-cTnT missed one patient with an adjudicated diagnosis of a Type II non-ST-segment elevation MI. This patient, who is described in Table 4, had a HEAR score of 2 and an undetectable (<3 ng/L) hs-cTnT measure at 3 hours, but had an elevated cTnI and hs-cTnI at the same blood draw. Recent studies have demonstrated the possibility that autoantibodies interfere with the measurement of cTnT by blocking the immunoreactivity of anti-cTnT antibodies to the I-T-C complex released from the myocardium after injury; thus resulting in falsely low concentrations in blood [28]. This one case of missed MACE resulted in a sensitivity of 91% for MACE at 30 days. In addition, one patient with a HEAR Score <3 had an elevated hs-cTnT result despite normal cTnI and hs-cTnI results. This resulted in a slightly lower specificity for MACE (48.4%) for the HEART Pathway using hs-cTnT. The reclassification of one patient without MACE as low-risk and 1 patient with MACE as high-risk resulted in a net reclassification index near zero with a 95% confidence including zero (indicating that no significant reclassification occurred).
Table 4.
Characteristics of the patient with missed MACE using the HEART Pathway with hs-cTnT.
| Age | Sex | Race | CAD history |
HEAR Score |
1st cTnI ng/L (URL) |
2nd cTnI ng/L (URL) |
hs-cTnI ng/L (URL) |
hs-cTnT ng/L (URL) |
Event Type |
Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| 36 | Male | Caucasian | None | 2 | 10 (40) |
71 (40) |
35.1 (34) |
<3 (20) |
Type II NSTEMI |
MI thought to be secondary to a tachydysrhythmia. No coronary angiography was performed. |
CAD= coronary artery disease, MACE= major adverse cardiac events, NSTEMI=Non ST-segment elevation Myocardial Infarction
Use of hs-cTn assays alone, without the HEART Pathway, yielded poor sensitivity for 30-day MACE underscoring the importance of using these biomarkers within the context of an ADP. This is consistent with several prior studies suggesting that these biomarkers should be combined with an ADP to achieve sufficient sensitivity for 30-day adverse events. For example, in the APACE cohort, a study of 909 patients with serial hs-cTnI measures at 0 and 2 hours resulted in a sensitivity of 82.7% sensitivity for 30-day ACS events [5]. However, when hs-cTnI results used as part of an ADP (in combination with a clinical decision aid and ECG data), the sensitivity for adverse events increased to >99%. Another recent study, by Body et al., reported that hs-cTnT measurements along with a non-ischemic ECG (but without additional clinical data) resulted in an adverse event (death, MI, and all coronary revascularization events) rate of 3.6%, well above the missed event rate that most providers find acceptable [6, 29, 30].
Most of the adverse events missed by the hs-cTn assays in this analysis were revascularization events without MI. While coronary revascularization improves mortality and re-infarction rates among patients with an acute MI, studies are less clear regarding the role of revascularization in patients without acute MI [31]. Among patients with stable coronary artery disease, the COURAGE trial and recent systematic reviews have failed to show a decreased risk of MI or death among patients receiving revascularization compared to medical management [32–35]. Many have interpreted these results as evidence that ED risk stratification tool, such as ADPs and biomarkers, do not need to detect patients with revascularization events. However, it is important to consider that the COURAGE trial’s control group is medical management not early discharge from the ED. Patients who are discharged from the ED based on a low-risk ADP assessment are unlikely to be started on maximal medical management. Therefore, it should not be assumed that an ADP identifying these patients as low-risk will result in equivalent outcomes.
In order to enhance the value of care for patients with acute chest pain it is important that we have tools capable of safely identifying low-risk patients for early discharge from the ED, focusing cardiac testing and hospitalizations on higher-risk patients. The ability of the HEART Pathway to identify patients for early discharge is likely to reduce costs and radiation exposure (from stress testing and angiography), and decrease false positive and non-diagnostic testing. As the US healthcare system switches to value based payment models, tools which can avoid potentially unnecessary testing and hospitalizations will be increasingly implemented. The results of this analysis when viewed in the context of our prior studies suggests that whether high-sensitivity assays or contemporary assays are used, the HEART Pathway can enhance the value of chest pain care. However, the HEART Pathway’s ability to identify 44–45% of patients as low-risk leaves substantial room for improvement. New decision aids or modifications of the HEART Pathway should pursue an increase in the proportion of patients identified as low-risk while maintaining a sensitivity >99% for MACE.
A relatively small sample size and enrollment from a single center may limit the generalizability of this analysis. A low occurrence of MACE rate in our cohort results in wide confidence intervals around the point estimate of sensitivity and limits comparisons between the different assays used for the HEART Pathway. Furthermore, the large number of patients screened and excluded from enrollment in the HEART Pathway RCT may have produced a selection bias. In the HEART Pathway RCT, baseline blood samples were not collected for hs-cTn analysis. This prevents us from evaluating the HEART Pathway using a serial (0- and 3-hour) hs-cTn strategy versus serial cTnI measures. In addition, the 3-hour blood sample collected for hs-cTn analysis was not collected in 23 patients enrolled, which may have introduced a selection bias. Another potential limitation of this analysis is incomplete follow-up on 4 patients, which may have caused underestimation of MACE. However, none of these patients were found in the Social Security Death Master File. Furthermore, the likelihood of MACE occurring shortly after discharge among these patients seems low given that all of the known MACE events occurred during the index visit.
5. Conclusions
In conclusion, there were no differences in the test characteristics of the HEART Pathway whether using cTnI or hs-cTnI, with both achieving 100% sensitivity and NPV for 30-day MACE and a specificity of 49%. While confirmation in a larger cohort is needed, our data suggests that the HEART Pathway may be utilized without modification in places where hs-cTnI assays are already available and when they become available in the U.S. In addition, the performance of the HEART Pathway when using hs-cTnT was very similar to the performance of the HEART Pathway using hs-cTnI or cTnI. When used alone (without the HEART Pathway), hs-cTn measures at 3 hours were insufficiently sensitive for MACE, mostly missing patients with revascularization events. This study provides additional evidence that hs-cTn assays should be used in the context of an ADP.
Highlights.
The HEART Pathway using cTnI or hs-cTnI achieved 100% sensitivity and NPV for MACE.
HEART Pathway test characteristics using hs-cTnT were similar to cTnI and hs-cTnI.
Use of the HEART Pathway with hs-cTnT did result in one missed MACE.
Without the HEART Pathway, hs-cTn measures were insufficiently sensitive.
Acknowledgments
Funding
This study was funded by the AHA Clinical Research Program (12CRP12000001 and 13CRP17090055) and in part by the Minneapolis Medical Research Foundation. Supplies provided by Abbott Laboratories and Roche Diagnostics. Dr. Mahler receives research funding from the AAMC/Donaghue Foundation, Duke Endowment, Abbott Point of Care, and NHLBI (1 R01 HL118263-01, L30 HL120008). Use of Research Electronic Data Capture was supported by the Wake Forest Translational Science Institute via a grant from National Center for Catalysis Research (M01 RR007122).
Abbreviations
- cTn
cardiac troponin
- MACE
major adverse cardiac events
- hs-cTn
high sensitivity cardiac troponin
- RCT
randomized controlled trial
- NPV
negative predictive
- CI
confidence interval
- ADP
accelerated diagnostic pathway
- ED
emergency department
- ACS
acute coronary syndrome
- EMR
electronic medical record
- MI
myocardial infarction
- URL
upper reference limit
- NRI
net reclassification improvement
Appendix 1
Comparison of the test characteristics of the hs-cTnT assay using different gender-specific URLs suggested as by Apple et al. (Male: 20 ng/L, Female: 13 ng/L) and Saenger et al. (Male: 15.5 ng/L, Female: 9 ng/L)
Test characteristics of the hs-cTnT at 3 hours for MACE in the entire cohort using gender specific URLs from Apple et al vs Saenger et al.*
| URL | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|
| Apple et al. | 60.0% (36.1–80.9%) |
93.3% (89.4–96.1%) |
42.9% (24.5–62.8%) |
96.5% (93.3–98.5%) |
| Saenger et al. | 70.0% (45.7–88.1%) |
87.9% (83.0–91.7%) |
32.6% (19.1–48.5%) |
97.2% (94.1–99.0%) |
Table excludes 2/259 patients without second cTn measure, and 29/259 with second clinical cTn measure at ≥6 hour
Performance characteristics of the HEART Pathway using hs-cTnT gender specific URLs from Apple et al vs Saenger et al.
| URL | % Low-Risk (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
|---|---|---|---|---|---|
| Apple et al. | 45.1% (36.5–54.0%) |
90.9% (58.7–99.8%) |
48.4% (39.2–57.6%) |
13.7% (6.8–23.8%) |
98.3% (91.1–100%) |
| Saenger et al. | 45.1% (36.5–54.0%) |
90.9% (58.7–99.8%) |
48.4% (39.2–57.6%) |
13.7% (6.8–23.8%) |
98.3% (91.1–100%) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Connor RE, Al Ali AS, Brady WJ, Ghaemmaghami CA, Menon V, Welsford M, Shuster M. Part 9: Acute Coronary Syndromes: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132(18 Suppl 2):S483–S500. doi: 10.1161/CIR.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 2.Mahler SA, Hiestand BC, Goff DC, Jr, Hoekstra JW, Miller CD. Can the HEART Score Safely Reduce Stress Testing and Cardiac Imaging in Patients at Low Risk for Major Adverse Cardiac Events? Crit Pathw Cardiol. 2011;10(3):128–133. doi: 10.1097/HPC.0b013e3182315a85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahler SA, Miller CD, Hollander JE, Nagurney JT, Birkhahn R, Singer AJ, Shapiro NI, Glynn T, Nowak R, Safdar B, Peberdy M, Counselman FL, Chandra A, Kosowsky J, Neuenschwander J, Schrock JW, Plantholt S, Diercks DB, Peacock WF. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168(2):795–802. doi: 10.1016/j.ijcard.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahler SA, Riley RF, Hiestand BC, Russell GB, Hoekstra JW, Lefebvre CW, Nicks BA, Cline DM, Askew KL, Elliott SB, Herrington DM, Burke GL, Miller CD. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circulation. Cardiovascular quality and outcomes. 2015;8(2):195–203. doi: 10.1161/CIRCOUTCOMES.114.001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen L, Mueller C, Parsonage WA, Wildi K, Greenslade JH, Twerenbold R, Aldous S, Meller B, Tate JR, Reichlin T, Hammett CJ, Zellweger C, Ungerer JP, Rubini Gimenez M, Troughton R, Murray K, Brown AF, Mueller M, George P, Mosimann T, Flaws DF, Reiter M, Lamanna A, Haaf P, Pemberton CJ, Richards AM, Chu K, Reid CM, Peacock WF, Jaffe AS, Florkowski C, Deely JM, Than M. Validation of high-sensitivity troponin I in a 2-hour diagnostic strategy to assess 30-day outcomes in emergency department patients with possible acute coronary syndrome. J Am Coll Cardiol. 2013;62(14):1242–1249. doi: 10.1016/j.jacc.2013.02.078. [DOI] [PubMed] [Google Scholar]
- 6.Body R, Mueller C, Giannitsis E, Christ M, Ordonez-Llanos J, de Filippi CR, Nowak R, Panteghini M, Jernberg T, Plebani M, Verschuren F, French JK, Christenson R, Weiser S, Bendig G, Dilba P, Lindahl B T.-A. Investigators. The Use of Very Low Concentrations of High-sensitivity Troponin T to Rule Out Acute Myocardial Infarction Using a Single Blood Test. Acad Emerg Med. 2016;23(9):1004–1013. doi: 10.1111/acem.13012. [DOI] [PubMed] [Google Scholar]
- 7.Hollander JE, Blomkalns AL, Brogan GX, Diercks DB, Field JM, Garvey JL, Gibler WB, Henry TD, Hoekstra JW, Holroyd BR, Hong Y, Kirk JD, O'Neil BJ, Jackson RE, Aufderheide T, Christenson J, Collins S, Fesmire FM, Green GB, Lindsell CJ, Peacock WF, Pollack CV, Zalenski R. Standardized reporting guidelines for studies evaluating risk stratification of emergency department patients with potential acute coronary syndromes. Ann Emerg Med. 2004;44(6):589–598. doi: 10.1016/S0196064404012806. [DOI] [PubMed] [Google Scholar]
- 8.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJJ, Weintraub WS, Mitchell KR, Morrisson SL, Anderson HV, Cannom DS, Chitwood WR, Jr, Cigarroa JE, Collins-Nakai RL, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, Mckay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes: A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, American College of Emergency Physicians, American Heart Association, Cardiac Society of Australia & New Zealand, National Heart Foundation of Australia, Society for Cardiac Angiography and Interventions, and the Taiwan Society of Cardiology. J Am Coll Cardiol. 2001;38(7):2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 9.Kline JA, Mitchell AM, Runyon MS, Jones AE, Webb WB. Electronic medical record review as a surrogate to telephone follow-up to establish outcome for diagnostic research studies in the emergency department. Acad Emerg Med. 2005;12(11):1127–1133. doi: 10.1197/j.aem.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58(11):1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 11.Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, Giannitsis E, Gustafson S, Handy B, Katus H, Melanson SE, Panteghini M, Venge P, Zorn M, Jarolim P, Bruton D, Jarausch J, Jaffe AS. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clinica chimica acta; international journal of clinical chemistry. 2011;412(9–10):748–754. doi: 10.1016/j.cca.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16(6):191–196. doi: 10.1007/BF03086144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Backus BE, Six AJ, Kelder JC, Mast TP, van den Akker F, Mast EG, Monnink SH, van Tooren RM, Doevendans PA. Chest pain in the emergency room: a multicenter validation of the HEART Score. Crit Pathw Cardiol. 2010;9(3):164–169. doi: 10.1097/HPC.0b013e3181ec36d8. [DOI] [PubMed] [Google Scholar]
- 14.Backus BE, Six AJ, Kelder JC, Bosschaert MA, Mast EG, Mosterd A, Veldkamp RF, Wardeh AJ, Tio R, Braam R, Monnink SH, van Tooren R, Mast TP, van den Akker F, Cramer MJ, Poldervaart JM, Hoes AW, Doevendans PA. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153–2158. doi: 10.1016/j.ijcard.2013.01.255. [DOI] [PubMed] [Google Scholar]
- 15.Six AJ, Cullen L, Backus BE, Greenslade J, Parsonage W, Aldous S, Doevendans PA, Than M. The HEART score for the assessment of patients with chest pain in the emergency department: a multinational validation study. Crit Pathw Cardiol. 2013;12(3):121–126. doi: 10.1097/HPC.0b013e31828b327e. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 17.Hollander JE, Than M, Mueller C. State-of-the-Art Evaluation of Emergency Department Patients Presenting With Potential Acute Coronary Syndromes. Circulation. 2016;134(7):547–564. doi: 10.1161/CIRCULATIONAHA.116.021886. [DOI] [PubMed] [Google Scholar]
- 18.de Lemos JA. Increasingly sensitive assays for cardiac troponins: a review. JAMA. 2013;309(21):2262–2269. doi: 10.1001/jama.2013.5809. [DOI] [PubMed] [Google Scholar]
- 19.Reichlin T, Cullen L, Parsonage WA, Greenslade J, Twerenbold R, Moehring B, Wildi K, Mueller S, Zellweger C, Mosimann T, Rubini Gimenez M, Rentsch K, Osswald S, Muller C. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med. 2015;128(4):369–379. e4. doi: 10.1016/j.amjmed.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Neumann JT, Sorensen NA, Schwemer T, Ojeda F, Bourry R, Sciacca V, Schaefer S, Waldeyer C, Sinning C, Renne T, Than M, Parsonage W, Wildi K, Makarova N, Schnabel RB, Landmesser U, Mueller C, Cullen L, Greenslade J, Zeller T, Blankenberg S, Karakas M, Westermann D. Diagnosis of Myocardial Infarction Using a High-Sensitivity Troponin I 1-Hour Algorithm. JAMA cardiology. 2016;1(4):397–404. doi: 10.1001/jamacardio.2016.0695. [DOI] [PubMed] [Google Scholar]
- 21.Apple FS, Jaffe AS, Collinson P, Mockel M, Ordonez-Llanos J, Lindahl B, Hollander J, Plebani M, Than M, Chan MH B.-M. International Federation of Clinical Chemistry Task Force on Clinical Applications of Cardiac. IFCC educational materials on selected analytical and clinical applications of high sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4–5):201–203. doi: 10.1016/j.clinbiochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 23.Sarkisian L, Saaby L, Poulsen TS, Gerke O, Jangaard N, Hosbond S, Diederichsen AC, Thygesen K, Mickley H. Clinical Characteristics and Outcomes of Patients with Myocardial Infarction, Myocardial Injury, and Nonelevated Troponins. Am J Med. 2016;129(4):446 e5–446 e21. doi: 10.1016/j.amjmed.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Cediel G, Gonzalez-Del-Hoyo M, Carrasquer A, Sanchez R, Boque C, Bardaji A. Outcomes with type 2 myocardial infarction compared with non-ischaemic myocardial injury. Heart. 2016 doi: 10.1136/heartjnl-2016-310243. [DOI] [PubMed] [Google Scholar]
- 25.Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124(21):2350–2354. doi: 10.1161/CIRCULATIONAHA.111.023697. [DOI] [PubMed] [Google Scholar]
- 26.Marini MG, Cardillo MT, Caroli A, Sonnino C, Biasucci LM. Increasing specificity of high-sensitivity troponin: new approaches and perspectives in the diagnosis of acute coronary syndromes. Journal of cardiology. 2013;62(4):205–209. doi: 10.1016/j.jjcc.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Aldous S, Mark Richards A, George PM, Cullen L, Parsonage WA, Flaws D, Florkowski CM, Troughton RW, O'Sullivan JW, Reid CM, Bannister L, Than M. Comparison of new point-of-care troponin assay with high sensitivity troponin in diagnosing myocardial infarction. Int J Cardiol. 2014;177(1):182–186. doi: 10.1016/j.ijcard.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Vylegzhanina AV, Kogan AE, Katrukha IA, Antipova OV, Kara AN, Bereznikova AV, Koshkina EV, Katrukha AG. Anti-Cardiac Troponin Autoantibodies Are Specific to the Conformational Epitopes Formed by Cardiac Troponin I and Troponin T in the Ternary Troponin Complex. Clin Chem. 2016 doi: 10.1373/clinchem.2016.261602. [DOI] [PubMed] [Google Scholar]
- 29.Than M, Herbert M, Flaws D, Cullen L, Hess E, Hollander JE, Diercks D, Ardagh MW, Kline JA, Munro Z, Jaffe A. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department?: a clinical survey. Int J Cardiol. 2013;166(3):752–754. doi: 10.1016/j.ijcard.2012.09.171. [DOI] [PubMed] [Google Scholar]
- 30.Stopyra JP, Mahler SA. Ready for a Risk Stratification Robot? Acad Emerg Med. 2016 doi: 10.1111/acem.13038. [DOI] [PubMed] [Google Scholar]
- 31.Simoons ML, Windecker S. Controversies in cardiovascular medicine: Chronic stable coronary artery disease: drugs vs. revascularization. Eur Heart J. 2010;31(5):530–541. doi: 10.1093/eurheartj/ehp605. [DOI] [PubMed] [Google Scholar]
- 32.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS C.T.R. Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 33.Katritsis DG, Ioannidis JP. PCI for stable coronary disease. N Engl J Med. 2007;357(4):414–415. doi: 10.1056/NEJMc071317. author reply 417–8. [DOI] [PubMed] [Google Scholar]
- 34.Katritsis DG, Ioannidis JP. Percutaneous coronary intervention versus conservative therapy in nonacute coronary artery disease: a meta-analysis. Circulation. 2005;111(22):2906–2912. doi: 10.1161/CIRCULATIONAHA.104.521864. [DOI] [PubMed] [Google Scholar]
- 35.Bucher HC, Hengstler P, Schindler C, Guyatt GH. Percutaneous transluminal coronary angioplasty versus medical treatment for non-acute coronary heart disease: meta-analysis of randomised controlled trials. BMJ. 2000;321(7253):73–77. doi: 10.1136/bmj.321.7253.73. [DOI] [PMC free article] [PubMed] [Google Scholar]