Introduction
Adversity experienced during sensitive periods of development may “get under the skin” and become embedded in children’s physiology and deleteriously affect health outcomes1,2. Poverty experienced in early childhood has been linked to cardiovascular disease, hypertension, impaired immune functioning, as well as mental health disorders such as anxiety, depression, and schizophrenia3. One way adversity may impact biological mechanisms affecting health outcomes is by recalibrating the stress response system4. While various biological systems are implicated in the stress response system, the autonomic nervous system (ANS) in particular has received attention because it indexes the calibration of the stress response system to early environmental influences and may represent an etiological pathway to poor cardiac and mental health outcomes5,6.
Research has largely focused on the detrimental health effects of environmental influences, such as poverty, on health and ANS functioning (e.g.,7,8). However, scarce research has focused on protective factors that could mitigate the effects of poverty. Maternal responsivity is one such protective factor, which has been linked with the development of physiological homeostasis in children9,10,11. Research that identifies modifiable protective factors could help shape treatment strategies for young children and families living in conditions of poverty.
Biological processes, such as cardiac function assessed via ANS activation, have the potential to yield new information about the way stress associated with poverty becomes engrained in one’s physiology. The parasympathetic and sympathetic branches of the ANS flexibly coordinate (via co-active, co-inhibited, reciprocal, or uncoupled patterns of activation12) to influence stress responding, cardiac functioning, and dually innervate overall heart rate13. Given that both branches of the ANS influence heart rate, it is useful to examine more specific indices of parasympathetic and sympathetic activation as well. First, there is vagal tone, a marker of parasympathetic influence on the heart that is non-invasively measured via respiratory sinus arrhythmia (RSA). The parasympathetic branch is responsible for maintaining homeostasis, and serves to calm an individual and decrease heart rate after the experience of an acute stressor. Higher resting levels of RSA are thought to be adaptive and have been positively associated with social interaction, attention, emotional functioning, and behavioral control14,15,16,17. The second index of specific cardiac function, pre-ejection period (PEP), assesses the influence of the sympathetic nervous system, also known as the fight-or-flight system. The sympathetic branch of the ANS is charged with mounting a response to stress or challenge in the environment, and leads to increases in (and less variability in) heart rate. Activation of the sympathetic branch increases readiness to respond to threat3. PEP is a time-based measure of contractility of the heart (milliseconds), with shorter PEP intervals indicating greater sympathetic activation18,19. Thus, studying the patterns of activation of both the parasympathetic and sympathetic branches of the ANS across early life can yield much more precise information regarding the physiological mechanisms by which children’s early experiences become biologically embedded. Despite this, most studies of infant and child ANS functioning have focused solely on the parasympathetic branch, given theories surrounding its involvement in early life attention, affect regulation, coping, and communication (e.g.,20,21).
An extensive literature documents an association between early adversity and subsequent physiological reactivity (see for review22,23–26) which suggests that physiological functioning can serve as an indicator of prior exposure to adversity and canalization of stress physiology through experience27. Such experiential canalization has implications for subsequent physiological responsivity or adaptability to context28. While the majority of this research has focused on reactivity as an indicator of early life adversity, it also could be that resting set points are affected by early adversity. Research illuminates the far-reaching implications of early childhood poverty on subsequent child development, regardless of whether there is later upward mobility, and suggests that childhood poverty is linked with increased sympathetic activity indicating increased stress on heart function29. However, one longitudinal examination conducted with the present study sample examined the effects of prenatal socioeconomic status on postnatal ANS reactivity and found that socioeconomic adversity marginally (p = .05) predicted PEP reactivity from 6 months to 5 years of age30, such that socioeconomic adversity predicted blunted PEP reactivity across time. These results indicate that even assessed prenatally, socioeconomic adversity can affect subsequent ANS trajectories of development. While that examination focused specifically on ANS reactivity, it is likely that socioeconomic adversity also affected children’s resting ANS functioning, which could help contextualize the effects on ANS reactivity (e.g., resting ANS levels could reduce the bandwidth available for reactivity, under the law of initial values31).
There is reason to focus specifically on the influence of adversities such as low SES on ANS development during the first five years of life. These early years have been identified as a period during which stress physiology circuits can be permanently programmed depending on environmental influences5,32. Research demonstrates that during the last trimester of pregnancy and first year of an infant’s life, the ANS rapidly changes33,34, indicating a time of increased plasticity along the trajectory of development. Given this increased plasticity, both positive and negative environmental influences exerted during this early time frame may become biologically embedded into the physiology of a child and alter trajectory of subsequent ANS development4,28. Low SES represents a cue of unpredictability in the environment which can shift psychobiological set points to become more sensitized to environment4. Such biological embedding can serve to recalibrate physiological set resting or basal activity within an individual as the result of conditional adaptation to environment, or the evolved ability of an organism to modify its developmental trajectory to match the social and physical environment. This recalibration is a part of allostasis, or the process of achieving stability through physiological or behavioral change35, which can have subsequent implications for how an organism interfaces with the environment in the future, mediating the individual’s adjustment to both positive and negative events4,28.
Positive environmental influences experienced during early childhood also may also alter ANS functioning and subsequent developmental trajectories during this time of plasticity. Both longitudinal and cross-sectional research show that maternal caregiving plays a pivotal role in programming early stress response systems, with more emotionally supportive parenting linked to healthier stress systems36. Some of these studies have focused on Hypothalamic-Pituitary-Adrenal (HPA) functioning, a hormonal index of stress functioning (e.g.,21,36,37), whereas others have investigated the influence of positive factors on ANS functioning.
Many studies have focused specifically on parasympathetic activation in association with maternal behavior, due to its link to emotion and coping (e.g., polyvagal theory,20,38). Two studies that indexed resting parasympathetic activation found that higher maternal responsivity was a significant predictor of higher resting parasympathetic activation (a more adaptive profile,39) and parental conflict was associated with reduced parasympathetic activation (a less adaptive profile,40). Longitudinal studies of vagal tone have also revealed important associations across childhood. Calkins and Keane (2004) documented extensive stability in children’s resting and reactive RSA from 2 to 4.5 years of age, and a second study using data from the present sample showed moderate stability in RSA from six to 60 months in Mexican American children41. A third large-scale longitudinal investigation examined vagal tone and maternal parenting practices when children were 2–4 years old42. This study revealed stability in parasympathetic regulation and parenting, but also showed that less well-regulated child physiology and parenting practices interacted to predict maternal use of restrictive parenting practices across childhood, highlighting the importance of considering proximal effects of parenting in conjunction with physiological development.
Of the fewer studies that have focused on the link between maternal responsivity and profiles of ANS functioning (i.e., sympathetic and parasympathetic function), most have examined ANS reactivity rather than resting ANS. For example, one cross-sectional study found that insensitive caregiving was associated with increased sympathetic activation and less parasympathetic reactivity to the still-face paradigm in 6 month olds6. Another study examined the role of social support on the co-regulation between parasympathetic vagal reactivity and sympathetic reactivity to stressors in the lab in 4–5 year olds living in poverty43. This study found that parasympathetic reactivity predicted attenuated sympathetic reactivity, but only in socially supportive contexts, thus highlighting the importance of social support for children living in poverty. Research is still needed to understand the effects of poverty and maternal responsivity on the development of resting parasympathetic and sympathetic function, especially in early childhood.
Much of the existing knowledge has been derived from predominantly European American samples, and no known research on the relationship between resting ANS functioning, poverty, and maternal sensitivity has been conducted among Mexican-origin children, or any other Latino ethnic group. Mexican American children may represent a particularly vulnerable population, as Latino youth in the U.S. experience poverty at disproportionate rates compared to children from other ethnic groups44,45. While the effects of stress appear to be additive in European American populations, such that more stressors predict greater influence over physiological functioning, research with other ethnic minority populations suggests interactive effects exist such that the cumulative stressors of living in low resource environments can cause an exponential effect on physiological functioning46. For example, prior research has shown that for African American youth residing in impoverished environments, lower or blunted physiological profiles may be the most advantageous so the individual is only responsive to the most extreme or intense stressors in the environment.46 Thus, we may expect to see the effects of low SES and poor maternal responsivity to be compounded (interactive) for Mexican American youth. The addition of poor maternal responsivity with low SES may compound the stress load on physiological mechanisms causing maladaptive resting ANS states (parasympathetic withdrawal, sympathetic activation, and higher heart rate). On the other hand, recent theoretical models emphasize the importance of positive familial interactions to protect children from the effects of stressful environmental influences47,48, and thus may predict adaptive ANS states (less parasympathetic withdrawal, less sympathetic activation, and lower heart rate) even in the face of low SES. While these interactive effects have been demonstrated in other ethnic minority samples (e.g., African American), the interactive effects of maternal sensitivity and SES have yet to be examined among Mexican origin youth. This research is of upmost importance to public health, as Mexican American children comprise the greatest number of Latino youth in the United States, and therefore, represent an important subpopulation of Latinos for whom poverty and other associated stressors are particularly salient.
The current study examined both the additive and interactive influences of socioeconomic status (SES) and maternal responsivity (measured at 1 year of age as a proxy for early childhood exposure) on ANS functioning at ages 1, 3.5, and 5 years among an impoverished Mexican American sample of children of primarily immigrant parents. We focused on resting ANS functioning, rather than reactivity to challenge tasks. We sought to provide clarity about whether and how SES and maternal responsivity relate to children’s resting ANS functioning, which could have direct effects on responsivity to environmental input and subsequent responsivity based on the law of initial values31. It was hypothesized that low SES would predict a trajectory of higher resting HR, increasing sympathetic activation, and increasing parasympathetic withdrawal across time, with the most divergent effects on these indices by age 5, which is a profile consistent with increased stress on the heart at rest. In contrast, it was expected that maternal responsivity would predict an adaptive trajectory of ANS functioning including lower HR, less sympathetic activation, and more parasympathetic activation, again with the most exacerbated effects by age 5. Finally, it was predicted that there would be an interaction between SES and maternal responsivity, such that the impact of SES on ANS functioning would vary with different levels of maternal responsivity. We expected that low maternal responsivity would be related to a stronger relationship between SES and resting ANS functioning (e.g., high SES relates to better ANS functioning even in the face of low maternal responsivity), while high maternal responsivity will be related to a weakened relationship between SES and resting ANS functioning (e.g., high maternal responsivity will weaken the deleterious impact of low SES on ANS functioning).
Methods
Participants were drawn from an ongoing birth cohort study aimed at investigating the association of environmental exposures with children’s health and development, called Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS). Participants are primarily Mexican-origin families living in an agricultural community in Monterey County, California. Detailed methods of the CHAMACOS cohort have been described previously (e.g., [omitted for blind review]). Briefly, 1800 pregnant women were screened for eligibility between October 1999 and 2000 at six prenatal clinics serving primarily farmworker families. Pregnant women were eligible if they were 1) 18 years of age or older, less than 20 weeks gestation, 2) Spanish- or English-speaking, 3) eligible for California’s low-income health insurance program (Medi-Cal), 4) receiving prenatal care, and 5) planning to deliver at the county hospital. Of the 1130 eligible women, 601 women initially enrolled, 527 were followed through delivery and 418 of their infants were seen at 1 year of age (336 of whom completed the ANS protocol and had scorable data). Of these infants, 330 (136 had ANS data at 3.5 years) were followed up at 3.5 years of age and 319 (297 had ANS data at 5 years) were seen again at 5 years. This examination utilized data from participants who completed at least one wave of the ANS protocol. Although ANS data were also collected at 6 months of age, this wave of ANS data was not included in our analyses as there were issues of temporal order (our predictor variables were assessed at 1 year of age and after). Our final sample included 336 Mexican American children and their mothers. Analyses indicated no differences between children who were included versus excluded on pertinent study variables.
During the 1-year visit, a 7-min ANS protocol was administered in the office or in a mobile van/recreational vehicle. At the 3.5 and 5-year visit, children completed a 15-minute ANS protocol in the office. Each visit included a home visit, where trained observers completed the Home Observation for Measurement of the Environment (HOME; see measures below). The HOME was also highly correlated across time (r = .34, p < 0.001 comparing 1 and 5-year visits) indicating that using the 1 year wave data was a good proxy for level of maternal responsivity across early childhood.
Measures
Home Observation for Measurement of the Environment (HOME)
The HOME was created as an objective measure to assess the quality and quantity of stimulation in the home environment within a natural context that is widely used in epidemiological research 49. The Infant-Toddler version of the HOME consists of 45-item scale with response choices that are binary (Yes/No). Higher total HOME scores indicate a more enriched home environment. There is a vast literature linking HOME scores to children’s cognitive development, attachment status, medical conditions, psychopathology (of both children and their parents), and parental substance abuse see50 for review. The Emotional and Verbal Responsivity of Primary Caregiver subscale was used in analyses to measure maternal responsivity. This subscale is comprised of 11 items that are rated by observation of the dyad across the span of an hour in the home. Example items include: “Mother caresses or kisses child at least once during the visit” and “Mother spontaneously vocalizes to child at least twice during the visit.” This measure in particular has been linked to infant attachment status, with mothers rated high on this scale having more securely attached infants51.
Socioeconomic Status (SES)
SES was assessed using a factor that was comprised of three variables assessed at age one. The first variable included in the SES factor was family income-poverty ratio, which was calculated by taking the family’s reported monthly household income, divided by number of people supported by that income, and then dividing by the federal poverty threshold ascertained from U.S. Bureau of the Census, Current Population Survey at the time of collection (the year of age 1 data collection in our sample occurred in 2000). The second variable included in the SES factor was household crowding, which was calculated by using the number of people living in the home divided by the number of rooms in the home. Finally, the third variable included in the SES factor was a variable categorizing whether the family received food stamps/Supplemental Nutrition Assistance Program benefits (y/n). A number of other socioeconomic variables were considered for the factor (including father’s highest education, mother’s highest education, and language spoken at home) but a principal components analysis with varimax rotation indicated that only these three variables (poverty, crowding, food stamps) held together. The factor accounted for 41.57% of variance. Of note, the income-to-poverty ratio was highly correlated across the 1, 3.5, and 5-year visits (r = .35, p < 0.001; comparing the ratio at 1 year to 5 years of age). Thus the 1 year visit was used as a proxy for overall SES during early childhood.
Resting ANS
Children participated in a standardized protocol where continuous measures of impedance cardiography, electrocardiography (ECG), and respirations were measured. Band electrodes were used to collect the ANS data at 1 year of age (for details see [omitted for blind review]) and spot electrodes at 3.5 and 5 years52. The four impedance spot electrodes were placed on the neck (x2) and trunk (x2) and three electrodes were placed in a lead II configuration to collect ECG measures53. After the bands/spots electrodes were in place, the experimenter verified the quality of the ECG and impedance waveforms for the first 5 minutes of the protocol during which time no data were analyzed. Data were acquired at 1 year of age using the Minnesota Impedance Cardiograph HIC-2000 and at 3.5 and 5 years of age using the Biopac MP150, as the HIC-2000 was no longer available. Continuous measures of heart rate (HR), ECG, Zo (basal impedance), and dZ /dt (first derivative of the impedance signal) were collected during the protocol. A 4-mA AC current at 100 Hz was passed through the two outer, current electrode bands/spots and Zo and dZ/dt signals were acquired from the two inner voltage-recording bands/spots. Data were filtered, extracted, and then scored using the ANS suite software at 1 year52,54 and MindWare Technologies LTD (Gahanna, Ohio) at 3.5 and 5 years of age. The sampling rate was 1000 Hz and the data were scored in 60-second intervals.
Data cleaning procedures involved examining for outliers (> 3 SD) minute-by-minute and in summary scores (there were no outliers), and a child’s data were deleted if more than 25% of the task minutes were unscorable and not included in present analyses (n = 15 in the current sample). The first two minutes of the ANS protocol at each time point included a resting measure. During the 1-year visit, children listened to a lullaby, and during the 3.5 and 5-year visits, children listened to a non-stressful story read aloud. The two minutes of the rest measure were averaged at each time point and used in longitudinal analyses for the current study.
Respiratory sinus arrhythmia (RSA), a measure of the parasympathetic nervous system, is the periodic oscillation in sinus rhythm occurring at the frequency of respiration and manifested as an increase in heart rate (HR) with inspiration and a decrease in HR during expiration. Respiratory sinus arrhythmia (RSA) was derived in accord with recommendations of the Society for Psychophysiological Research (SPR) committee on heart rate variability55 (SPR Committee Report) by the MindWare HRV analytical software package. The EKG signal was digitized (1000 Hz) and an interbeat interval (IBI) series was derived by a peak- identification algorithm that identifies the peak of the R wave as the fiducial point. Artifacts were flagged by statistical algorithms, including that of Berntson et al.56, and were then checked visually and edited as necessary according to the guidelines of the SPR Committee Report. The IBI series was then converted to a time series by resampling at fixed time intervals with interpolation57. The time series was linearly detrended to minimize nonstationaries in the data58. The residual series was then tapered with a Hamming window and submitted to the Fast Fourier Transform (FFT) module of LabVIEW (National Instruments, Austin, TX) to derive the spectral distribution. RSA was quantified as the natural log of the integral power within the respiratory frequency bandwidth (0.24 to 1.04 Hz at 1 year of age and 0.15 to 0.80 Hz at 3.5 and 5 years of age)59–61.
PEP, derived from impedance cardiography, is the period between the electrical invasion of the ventricular myocardium (Q wave of the ECG) and the opening of the aortic valve (B point). PEP was quantified as the time interval in milliseconds from the onset of the ECG Q wave to the B point of the dZ/dt wave62. The B point was automatically derived by the MindWare analysis software by means of a validated algorithm that used the time interval of the Q point to the maximum point of the dZ/dt wave to estimate the location of the B point63. For each subject, ECG and impedance data were ensemble averaged for each minute to produce estimates of the PEP.
The interbeat interval (IBI) series was derived by a peak-identification algorithm that identifies the peak of the R wave as the fiducial point. Artifacts were flagged by statistical algorithms, including that of Berntson et al.56, and were then visually inspected and edited as necessary according to the guidelines of the SPR Committee Report and previously described methods56,63. Trained research assistants visually inspected each minute of ECG data to identify the R peaks marked by the MindWare software algorithm as an outlier. If the research assistant determined that a R peak was coded incorrectly or not coded, the research assistant added or deleted a R peak mark.
Analyses
Main analyses were conducted using Hierarchical Linear Modeling (HLM v6.05)64 to account for the inherent nesting of the waves of ANS data collected within the individual (level-1) and across individuals (level-2). Hierarchical Linear Modeling (HLM) allows for the simultaneous modeling of ANS levels (intercept) and the changes in ANS functioning across time (slope). Each ANS indicator was modeled separately (i.e., HR, RSA, and PEP), and each model indexed the intercept and slope across three time points (age in months during assessment at wave 1, 3.5, and 5 years) as the outcome of interest (YANS). At level-1, ANS intercept was centered at the participant’s age in months during the third wave of data (approximately 5 years of age) because, theoretically, we hypothesized that the effects of low SES and maternal responsivity at 1 year would potentiate divergences between individuals on emerging ANS trajectories, resulting in the most striking individual differences at age 5. Thus, the best fitting model also included a variable estimating the slope across time as a function of age in months (β1Slope) for each ANS index (HR, RSA, and PEP). Intercept was a fixed variable, whereas slope was specified to allow for random effects.
Once a level-1 or within-individual equation is established, level-1 predictors can become outcomes-of-interest at level-2. Cross-level interactions capture how individual difference factors impact level-1 associations, specifically ANS trajectories at rest and intercept centered at age 5. Analyses focused on SES and maternal responsivity at age 1 as individual difference predictors capable of impacting ANS. Below is an example of the HLM model.
Level-1 Model
Level-2 Model
Results
The majority of the participants’ mothers were born in Mexico (87.5%). Twenty-five percent of the sample reported receiving food stamps when their children were 1 year of age, and mean housing density was 1.64, SD = 0.74. Mean income to poverty ratio was 0.91 (SD = 0.49). To contextualize these results, the poverty line is at 1, and anything below 1 would be living below the poverty level. Thus, the average family in this sample was living at 9% below the poverty level at 1 year of age. As mentioned, maternal responsivity was highly correlated across time (r = .34, p < 0.001) indicating that using the 1 year wave data likely provided a good proxy for level of exposure across early childhood.
Preliminary analyses investigated the effects of child’s sex and mother’s country of birth as covariates on the level 1 ANS models. As shown in Table 1, these covariates did not significantly affect the parameters of the base model, and thus were not included in main analyses. Although mother’s country of birth trended towards being related to PEP functioning at age 5, inclusion of this covariate in PEP analyses did not alter the pattern of results or model fit and was thus excluded for the sake of parsimony.
Table 1.
HLM analyses of potential covariates on the ANS base models (N=336)
| Variables | B0 Intercept | B1 Slope |
|---|---|---|
| HR | ||
| Child’s Sex | −2.23 | .01 |
| Mother’s Birth Country | 2.17 | .05 |
| RSA | ||
| Child’s Sex | −.10 | −.01 |
| Mother’s Birth Country | .07 | .01 |
| PEP | ||
| Child’s Sex | −.41 | −.03 |
| Mother’s Birth Country | −2.95+ | −.04 |
Note: + < .10
The level 1 model demonstrated moderate between-person variability for HR, RSA, and PEP (30%, 32%, and 43%, respectively, and also serves as estimates of interclass correlation) and substantial variability within person (70%, 68%, 57%, respectively). The base model for heart rate demonstrated that resting heart rate tended to decrease over time (β = −0.83, p < 0.001), whereas RSA increased (β = 0.06, p < 0.001) and PEP lengthened with age (β = 0.25, p < 0.001), which are the expected trends with normal development41. Table 2 lists the beta weights for each predictor of ANS functioning.
Table 2.
Resting ANS intercept at age 5 and ANS trajectories from 1 to 5 years of age (N=336)
| Variables | B0-Intercept | B1 - Slope |
|---|---|---|
| HR | ||
| SES | .77 | .06** |
| Responsivity | −2.53** | −.04 |
| SES × Responsivity | −1.36+ | – |
| RSA | ||
| SES | −.06 | −.01 |
| Responsivity | .11 | .01* |
| SES × Responsivity | .23** | – |
| PEP | ||
| SES | −1.09* | −.04* |
| Responsivity | .49 | .06** |
| SES × Responsivity | .63 | – |
Note: – fixed, + < .10,
p < .05,
p < .01
The interaction effect in prediction of slope was fixed in each model (–) if they were poor predictors of the model and affected model fit.
Main Effects of SES
Partial support was found for hypotheses predicting lower SES associated with less adaptive ANS functioning across trajectories and intercept age 5. Low SES was associated with a trajectory of high and more slowly decreasing resting heart rate (β = 0.06, p < 0.01; Figure 1), and a higher and flatter increase in resting PEP across early childhood (β = −.04, p < 0.05), indicating that children with lower SES demonstrated less change in sympathetic activation at rest over the first few years of life. Low SES was also associated with shorter PEP (β = −1.09, p < 0.05) at age 5. However, SES was not significantly associated with heart rate at age 5, and SES did not significantly impact RSA trajectory from 1 to 5 years of age or intercept at age 5.
Figure 1.
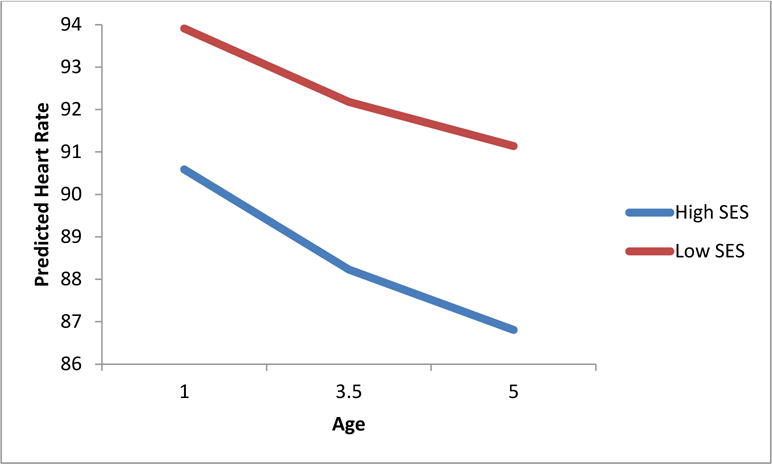
SES predicting children’s resting heart rate trajectory from 1 to 5 years of age (β = 0.06, p < 0.01)
Main (Additive) Effects of Maternal Responsivity
Partial support was found for hypotheses predicting higher maternal responsivity would be associated with more adaptive ANS functioning across trajectories and intercept at age 5. High maternal responsivity significantly predicted more steeply increasing trajectories of resting RSA levels, such that the higher the level of maternal responsivity, the steeper the RSA increase up to age 5 (β = 0.01, p < 0.05). Further, higher maternal responsivity predicted a steeper increase in resting PEP trajectory (β = 0.06, p < 0.01), indicating that children with more responsive mothers demonstrated less sympathetic activation at rest across the first years of life (Figure 2). Maternal responsivity did not relate to heart rate trajectory, but was associated with a lower resting heart rate at age 5 (β = −2.53, p < 0.01). No association was found between maternal responsivity and PEP or RSA intercept at age 5.
Figure 2.
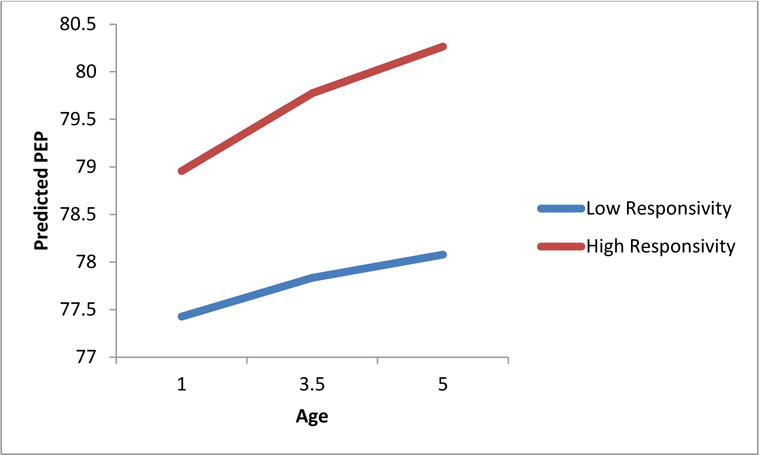
Maternal responsivity predicting children’s resting PEP trajectory from 1 to 5 years of age (β = 0.06, p < 0.01).
Interactive Effects
It was predicted that maternal responsivity and SES would interact to predict the relationship between SES and ANS functioning. Results suggest only partial support for this hypothesis. A significant interaction between SES and maternal responsivity (β = 0.23, p < 0.01) on RSA intercept at age 5 was found (Figure 3), but there was no interaction predicting the ANS trajectory. Children with low SES measured at age 1 had higher resting levels of RSA at age 5 if also coupled with high maternal responsivity. The interaction between SES and maternal responsivity was not significant for PEP or heart rate trajectories or intercepts at age 5.
Figure 3.
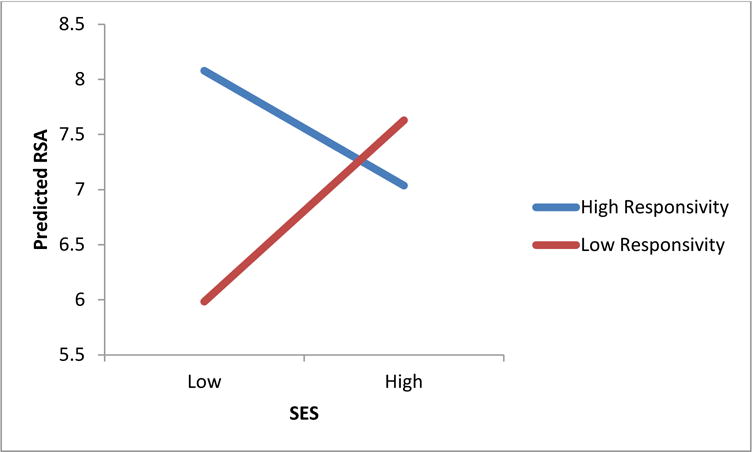
Interaction between maternal responsivity and family SES predicting children’s resting RSA at 5 years of age (β = 0.23, p < 0.01)
Discussion
The current study examined whether low SES in early childhood “gets under the skin” of children to influence their trajectories of resting heart rate, RSA, and PEP (from 1 to 5 years of age) and levels of ANS activation at age 5 (intercept). The protective effects of maternal responsivity were also examined to assess whether high maternal responsivity influences resting heart function, and whether there was an interaction between SES and maternal responsivity. Hypotheses were partially supported. SES related to heart rate and PEP resting trajectories (and PEP at age 5) in the predicted directions, and maternal responsivity related to RSA and PEP resting trajectories, but the interaction between these two environmental variables predicted only parasympathetic activation (resting RSA) at age 5. Higher maternal responsivity appeared to offset the negative effects of low SES on RSA, and this pattern of early experiences was particularly important for children’s resting RSA levels at age 5.
The CHAMACOS study provided an optimal test of our hypotheses because our sample is characterized by high poverty, yet had sufficient variability within this high poverty sample to assess the effects of SES on ANS functioning. Moreover, scarce research has examined the psychophysiological effects of poverty on Mexican American youth, and in particular, how low SES “gets under the skin” for this vastly understudied population. Given the large numbers of Mexican-origin youth who are living in poverty, and the growing number of Mexican American youth living in the US, this population merits more attention65.
The parasympathetic nervous system undergoes momentous shifts during the first year of life (see for review,34). Given the links between vagal tone and emotional and psychological well-being, it is physiologically meaningful that environmental inputs with high emotional value would shape a child’s developing resting vagal tone during this developmental window of physiological change, and that the parasympathetic resting set point would have relationships to children’s heart and psychological health as they develop. Such adaptive calibration to environmental contexts, whereby environmental inputs biologically embed themselves (for the better in this case) into the child’s basal physiology, have implications for future interactions with the social surround and environment4. Indeed, our results fit nicely with prior research showing the important role mothers play during the first year of life in supporting the development of their children’s physiological homeostasis, which is associated with healthy socio-emotional and cognitive development10,11,66. Such a high quality social environment is likely to foster healthy sensitivity to environmental cues, as well as appropriate physiological response and recovery from daily stressors that are encountered4.
While there are well-known and influential theories about vagal tone development in children (e.g.,67), less is generally known about sympathetic development during these young ages and in particular in response to adversities and protective factors. Interestingly, our results showed that SES had a significant effect on both trajectory of change and age 5 resting sympathetic activation, such that low SES at 1 year of age predicted a flatter increase in resting PEP intervals (i.e., PEP remained shorter across time) from 1 to 5 years of age, and also shorter resting PEP at age 5 (i.e., increased sympathetic activation). These results are in line with other research that has found increased sympathetic activity associated with childhood poverty29. Given that PEP indexes the “fight or flight” branch of the ANS, it may be more sensitive to the effects of adversity than vagal tone. Given the higher levels of activation across time associated with low SES, it would not be surprising if these children have blunted reactivity to stress given the law of initial values31. Fortunately, maternal responsivity had an opposing effect on resting PEP trajectory, predicting steeper gains in resting PEP length (i.e., reduced sympathetic activation). The interaction between maternal responsivity and SES was nonsignificant for PEP. More research is needed to assess the effects of prolonged activation of the sympathetic branch as it could be prone to burn-out over time, similar to its’ hormonal counterpart, cortisol, which is also released in times of fight-or-flight (e.g.,68).
While low SES was not directly related to RSA resting trajectory from one to five years of life, the link between SES and resting RSA levels in our sample at age 5 was differentiated by level of maternal responsivity. In other words, higher maternal responsivity not only predicted how the child’s parasympathetic activation developed from 1 to 5 years of age, but also served to offset against the long-term deleterious effects of low SES, at least by 5 years of age. Thus, maternal responsivity appears to have consequences for children’s parasympathetic development, in line with other studies relating parasympathetic activity with maternal responsivity39,40. This finding supports the notion that the ANS is especially plastic during early childhood, and environmental inputs during this time can continue to influence ANS functioning throughout early development5,32.
Finally, it is important to note that the most well known indicator of ANS functioning, heart rate, is innervated by both the parasympathetic and sympathetic branches and thus, it may be more meaningful to contextualize our heart rate results in light of what we have discussed above. Maternal responsivity and SES had distinct relationships with which branch of the ANS that they appear to influence. In particular, results of this study demonstrate an RSA-specific set of effects for maternal responsivity, and PEP-specific effects for SES (setting aside heart rate). These effects are related to the chronicity of SES and the potentially more situational experience of maternal responsivity, or related to the proximal nature of maternal responsivity and the more distal effects of SES context elements of the environment69. If the parasympathetic branch is shaped more by social engagement and can be protected to an extent by positive influences, and sympathetic branch is more responsive to stressful circumstances, then heart rate may be best viewed as an indicator of the overall tax an individual’s body experiences as a result of ANS responses in the parasympathetic and sympathetic branches. Regardless, these results give insight into the differential effects of certain kinds of experiences even if the interaction was fairly specific to RSA levels at age 5.
The following limitations should be highlighted. First, this sample was living in moderate to extreme poverty in an agricultural area, and thus these results do not generalize to all Mexican Americans or other racial/ethnic groups. While the sample was optimal for testing the effects of maternal responsivity in low SES environments, future research should attempt to replicate these results in Mexican Americans with more financial resources as well as with other ethnic groups. Second, there are other standardized measures of maternal responsivity that could be employed in future research. However, a large body of epidemiological research has been conducted using the HOME thus allowing for comparison across studies. Third, it should be noted that we changed systems of ANS measurement and data collection after year 1 of the study, which may have impacted our results. However, prior research from this dataset has shown within-person stability of resting ANS measures across these ages despite the changes in equipment (omitted for blind review). Finally, the current study did not model chronicity of poverty or maternal responsivity across the time points. Thus, our model does not disentangle the first year of life from the subsequent years as our measures of SES and maternal responsivity were highly correlated across time and the factor structure limited abilities to decipher individual changes across time due to standardization within each time point. However, our study does provide support for the notion that early poverty and parental responsiveness relate to ANS trajectories of development across the window of early development.
Despite these limitations, this study had a number of strengths. The results of the current study highlight the importance of mother-child interactions, in particular maternal responsivity, in offsetting the deleterious effects of low SES on cardiovascular health and autonomic function from 1 to 5 years of age. These findings provide support for preventative programming aimed at targeting maternal-child interaction among families living in high poverty environments. In particular, programs targeting Mexican American youth should include a strong focus on family interactions, especially maternal responsivity, in order to support resiliency and mitigate the deleterious impact of low SES. As Mexican American children constitute the largest group of youth living in poverty in the U.S.44, these results have important implications for preventative programming with a potentially wide reach. Further studies are needed to replicate these findings across other ethnic groups at high risk for poverty to better understand if maternal responsivity is an appropriate target for prevention programming more generally. Given that poverty is widespread in the U.S. and is not easily modifiable, prevention efforts to improve the maternal-infant relation could provide a useful pivot point.
Beyond individual-level interventions, policy implications could include allowing for or facilitating more parental involvement with their children early in life, including extending Family Medical Leave Act laws. Parents might further find hope knowing that they have control over something that could buffer the effects of poverty so that it does not “get under the skin” and cause detrimental effects to their children’s health. Poverty as a social determinant of health could also be intervened upon, which could reduce health disparities among Mexican-American families who experience poverty at a disproportional rate compared to other groups45. For example, the Earned Income Tax Credit creates dramatically different income levels for working families, and changes in this policy over time have been shown to have impacts on child health and development70. Future research could assess for change in ANS resting states as a function of such individual and policy-level interventions among high poverty populations.
Highlights.
Experiences during early childhood shape autonomic nervous system (ANS)
development
Maternal responsivity may moderate the effects of social economic status (SES)
Resting ANS was collected at 1, 3.5, and 5 years of age and modeled across time
Low SES predicted maladaptive resting ANS trajectories
Maternal responsivity predicted adaptive ANS trajectories
Acknowledgments
The authors would like to thank the parents and children who participated in the CHAMACOS study from the prenatal period to seven years of age. We also wish to thank the CHAMACOS and UCSF staff who worked on the study. Finally, we would like to acknowledge Dr. Elizabeth Shirtcliff and Dr. Jenn-Yun Tien for their consultation on the statistical analyses used in this article.
Funding Source: This publication was made possible by the National Institute of Child Health and Development (NICHD) ARRA 445211-33252-01 grant HD058091, National Institute for Environmental Health Sciences (NIEHS) grant ES009605, and U.S. Environmental Protection Agency (US EPA) grants R826709011 and RD83171001. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 2.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 3.Alkon A, Wolff B, Boyce WT. Poverty, Stress, and Autonomic Reactivity. In: Rosalind King VM, editor. The Oxford Handbook of Poverty and Child Development. New York, New York: Oxford University Press Inc.; 2012. [Google Scholar]
- 4.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and biobehavioral reviews. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Bergh BR. Developmental programming of early brain and behaviour development and mental health: a conceptual framework. Dev Med Child Neurol. 2011;53(Suppl 4):19–23. doi: 10.1111/j.1469-8749.2011.04057.x. [DOI] [PubMed] [Google Scholar]
- 6.Enlow BM, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, Wright RJ. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Human Development. 2014;90(7):377–385. doi: 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters SF, Boyce WT, Eskenazi B, Alkon A. The impact of maternal depression and overcrowded housing on associations between autonomic nervous system reactivity and externalizing behavior problems in vulnerable Latino children. Psychophysiology. 2016;53(1):97–104. doi: 10.1111/psyp.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkon A, Wolff B, Boyce WT. Poverty, Stress, and Autonomic Reactivity. In: Rosalind King VM, editor. The Oxford Handbook of Poverty and Child Development. New York, New York: Oxford University Press Inc.; 2012. [Google Scholar]
- 9.Shonkoff JP, Garner AS. The Committee on Psychosocial Aspects of Child and Family Health, The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 10.Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Dev. 1993;64(5):1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 11.Spangler G, Schieche M, Ilg U, Maier U, Ackermann C. Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Dev Psychobiol. 1994;27(7):425–437. doi: 10.1002/dev.420270702. [DOI] [PubMed] [Google Scholar]
- 12.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev. 1991;98(4):459–487. doi: 10.1037/0033-295x.98.4.459. [DOI] [PubMed] [Google Scholar]
- 13.Mezzacappa E, Tremblay RE, Kindlon D, et al. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. J Child Psychol Psychiatry. 1997;38(4):457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 14.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: a longitudinal study. Child Dev. 1995;66(5):1360–1384. [PubMed] [Google Scholar]
- 16.Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: stability, continuity, and implications for childhood adjustment. Dev Psychobiol. 2004;45(3):101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- 17.Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2007;74(2):286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. J Dev Behav Pediatr. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood A. Use of impedance cardiography in cardiovascular reactivity research. In: Blascovich J, Katkin ES, editors. Cardiovascular reactivity to psychological stress and disease. Washington, DC: American Psychological Association; 1995. pp. 157–199. [Google Scholar]
- 20.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, Cox MJ. Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Dev. 2009;80(1):209–223. doi: 10.1111/j.1467-8624.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 22.Obradovic J. How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Dev Psychopathol. 2012;24(2):371–387. doi: 10.1017/S0954579412000053. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin KA, Alves S, Sheridan MA. Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Dev Psychobiol. 2013 doi: 10.1002/dev.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair C, Berry D, Mills-Koonce R, Granger D, Investigators FLP Cumulative effects of early poverty on cortisol in young children: moderation by autonomic nervous system activity. Psychoneuroendocrinology. 2013;38(11):2666–2675. doi: 10.1016/j.psyneuen.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Propper CB, Holochwost SJ. The influence of proximal risk on the early development of the autonomic nervous system. Developmental Review. 2013;33(3):151–167. [Google Scholar]
- 26.Sturge-Apple ML, Suor JH, Davies PT, Cicchetti D, Skibo MA, Rogosch FA. Vagal Tone and Children’s Delay of Gratification Differential Sensitivity in Resource-Poor and Resource-Rich Environments. Psychological Science. 2016;27(6):885–893. doi: 10.1177/0956797616640269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair C, Raver CC. Child development in the context of adversity: experiential canalization of brain and behavior. Am Psychol. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 29.Evans GW, Kim P. Childhood Poverty, Chronic Stress, Self-Regulation, and Coping. Child Development Perspectives. 2013;7:43–48. [Google Scholar]
- 30.Alkon A, Boyce WT, Tran L, Harley KG, Neuhaus J, Eskenazi B. Prenatal adversities and Latino children’s autonomic nervous system reactivity trajectories from 6 months to 5 years of age. PLoS One. 2014;9(1):e86283. doi: 10.1371/journal.pone.0086283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- 32.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 33.Porges SW, Furman SA. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant Child Dev. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 36.Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant KA, McMahon C, Reilly N, Austin MP. Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant responses to the still-face procedure. Infant Behav Dev. 2010;33(4):453–462. doi: 10.1016/j.infbeh.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Hastings PD, Nuselovici JN, Utendale WT, Coutya J, McShane KE, Sullivan C. Applying the polyvagal theory to children’s emotion regulation: Social context, socialization, and adjustment. Biol Psychol. 2008;79(3):299–306. doi: 10.1016/j.biopsycho.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan LA, Evans L, Monk C. Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: can prenatal programming be modified? Early Hum Dev. 2008;84(4):249–256. doi: 10.1016/j.earlhumdev.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore GA. Parent conflict predicts infants’ vagal regulation in social interaction. Dev Psychopathol. 2010;22(1):23–33. doi: 10.1017/S095457940999023X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alkon A, Boyce WT, Davis NV, Eskenazi B. Developmental changes in autonomic nervous system resting and reactivity measures in Latino children from 6 to 60 months of age. J Dev Behav Pediatr. 2011;32(9):668–677. doi: 10.1097/DBP.0b013e3182331fa6. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy AE, Rubin KH, Hastings PD, Maisel B. Longitudinal relations between child vagal tone and parenting behavior: 2 to 4 years. Dev Psychobiol. 2004;45(1):10–21. doi: 10.1002/dev.20013. [DOI] [PubMed] [Google Scholar]
- 43.Wolff BC, Wadsworth ME, Wilhelm FH, Mauss IB. Children’s vagal regulatory capacity predicts attenuated sympathetic stress reactivity in a socially supportive context: evidence for a protective effect of the vagal system. Dev Psychopathol. 2012;24(2):677–689. doi: 10.1017/S0954579412000247. [DOI] [PubMed] [Google Scholar]
- 44.Fund CsD. The State of Hispanic Children in America. Washington, D.C.: Children’s Defense Fund; 2014. [Google Scholar]
- 45.Brown A, Patten E. Statistical Portrait of Hispanics in the United States, 2012. Washington, D.C.: Pew Hispanic Center; 2014. [Google Scholar]
- 46.Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev Psychopathol. 2011;23(4):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev Psychopathol. 2011;23(1):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- 48.Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 49.Elardo R, Bradley R, Caldwell BM. Relation of Infants Home Environments to Mental Test Performance from 6 to 36 Months - Longitudinal Analysis. Child Development. 1975;46(1):71–76. [Google Scholar]
- 50.Totsika V, Sylva K. The Home Observation for Measurement of the Environment Revisited. Child and Adolescent Mental Health. 2004;9:25–35. doi: 10.1046/j.1475-357X.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 51.Network NECCR. Child-care and family predictors of preschool attachment and stability from infancy. Developmental Psychology. 2001;37:847–862. [PubMed] [Google Scholar]
- 52.Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT. Developmental and contextual influences on autonomic reactivity in young children. Dev Psychobiol. 2003;42(1):64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- 53.Allen MT, Matthews KA. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology. 1997;34(3):329–339. doi: 10.1111/j.1469-8986.1997.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 54.Cacioppo J, Uchino B, Berntson G. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiol. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- 55.Berntson GG, Bigger JT, Jr, Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 56.Berntson G, Quigley K, Jang J, Boysen S. A conceptual approach to artifact identification: Application to heart period data. Psychophysiology. 1990;27:568–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 57.Berntson G, Cacioppo J, Quigley K. The metrics of cardiac chronotropism: Biometric perspectives. Psychophysiology. 1995;32:162–171. doi: 10.1111/j.1469-8986.1995.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 58.Litvack D, Oberlander T, Carney L, Saul J. Time and frequency domain methods for heart rate variability analysis: a methodological comparison. Psychophysiology. 1995;32:492–504. doi: 10.1111/j.1469-8986.1995.tb02101.x. [DOI] [PubMed] [Google Scholar]
- 59.Bar-Haim Y, Marshall P, Fox N. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Developmental Psychobiology. 2000;37:44–56. doi: 10.1002/1098-2302(200007)37:1<44::aid-dev6>3.0.co;2-7. 2000. [DOI] [PubMed] [Google Scholar]
- 60.Bazhenova OV, Plonskaia O, Porges SW. Vagal reactivity and affective adjustment in infants during interaction challenges. Child Dev. 2001;72(5):1314–1326. doi: 10.1111/1467-8624.00350. [DOI] [PubMed] [Google Scholar]
- 61.Weiner OM, McGrath JJ. Test-Retest Reliability of Pediatric Heart Rate Variability: A Meta-Analysis. Journal of Psychophysiology. 2016:1–23. doi: 10.1027/0269-8803/a000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 63.Lozano D, Norman G, Knox D, et al. Where to B in dZ/dt. Psychophysiology. 2007;44:113–119. doi: 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 64.Raudenbush SW. HLM 6: Hierarchical Linear and Nonlinear Modeling. Scientific Software International, Inc.; 2004. [Google Scholar]
- 65.Lopez G. Hispanics of Mexican origin in the United States, 2013. Washington, DC: Pew Hispanic Center; 2015. [Google Scholar]
- 66.Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17302–17307. doi: 10.1073/pnas.1121259109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Porges SW. The Polyvagal Theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 68.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80(3):265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard University Press; 1979. [Google Scholar]
- 70.Evans WN, Garthwaite CL. Giving Mom a Break: The Impact of Higher EITC Payments on Maternal Health. American Economic Journal: Economic Policy, American Economic Association. 2014;6(2):258–290. [Google Scholar]


