Abstract
Introduction
Patients with hypertension often require a combination of three antihypertensive agents to achieve blood pressure control, but very few single-pill triple combinations are available. The aim of this study was to determine whether a single-pill triple combination of perindopril, indapamide, and amlodipine was as effective as a dual-pill combination of perindopril/indapamide plus separate amlodipine at reducing blood pressure in patients with uncontrolled, essential hypertension.
Methods
This international, multicenter, open-label, randomized controlled trial was conducted in men or women aged ≥18 years old with confirmed essential hypertension (SBP ≥140 and <160 mmHg and DBP ≥90 and <100 mmHg), uncontrolled on maximal dose antihypertensive monotherapy or with a single dose of dual therapy. Patients were randomly assigned to: single-pill triple combination of perindopril 5 mg/indapamide 1.25 mg/amlodipine 5 mg (Per/Ind/Aml) or dual-pill combination perindopril 5 mg/indapamide 1.25 mg + amlodipine 5 mg (Per/Ind + Aml) once daily for 12 weeks. The primary endpoint was change in office supine SBP and DBP from baseline to week 12. The proportion of responders defined as those with normalized BP (SBP <140 mmHg and DBP <90 mmHg), and/or decrease of SBP ≥20 mmHg, and/or decrease of DBP ≥10 mmHg at week 12 (W12) compared with baseline was also assessed. Secondary efficacy endpoints included change in office supine SBP and DBP, response, and BP control at weeks 4 and 8. The tolerability of the treatments was also assessed.
Results
A total of 148 patients were randomized: 75 to Per/Ind/Aml and 73 to Per/Ind + Aml. Mean supine SBP and DBP were 149.1 ± 4.7 and 94.1 ± 3.1 mmHg, respectively, with no relevant between-group difference. At week 12, both triple-therapy regimens were associated with clinically significant reductions in SBP compared with baseline (−21.5 ± 11.7 and −20.0 ± 12.9 mmHg, respectively). Reductions in office supine DBP were also clinically significant (−15.3 ± 7.8 and −14.8 ± 9.0 mmHg, respectively). The proportion of treatment responders was high in both groups: 89.2 and 87.1%, respectively. The reduction in office supine SBP/DBP was already evident at week 4 and maintained for the duration of the study in both groups. The majority of patients were treatment responders at week 4 (89.2 and 82.9%, respectively) and had achieved BP control (87.8 vs. 78.6%, respectively), which was maintained until week 12 in both treatment groups. Both treatments were well tolerated with no between-group differences.
Conclusions
In adult patients with uncontrolled essential hypertension on treatment, single-pill triple-combination therapy with Per/Ind/Aml is as effective as the same dose dual-pill combination of Per/Ind + Aml. Both treatments were associated with clinically significant BP reductions compared with baseline and were well tolerated.
Clinical trials number: http://www.controlled-trials.com ISRCTN: 16442558.
Funding: Les Laboratoires Servier.
Electronic supplementary material
The online version of this article (doi:10.1007/s40119-017-0085-7) contains supplementary material, which is available to authorized users.
Keywords: Amlodipine, Antihypertensive, Indapamide, Perindopril, Single-pill combination, Uncontrolled essential hypertension
Introduction
Hypertension is a major modifiable risk factor for cardiovascular disease and stroke and there is global agreement among current hypertension management guidelines that the majority of patients with uncomplicated hypertension should be treated to a blood pressure (BP) goal of systolic blood pressure (SBP) <140 mmHg and diastolic blood pressure (DBP) <90 mmHg [1–6].
Monotherapy can effectively reduce BP in only a limited number of patients and the majority will therefore require treatment with two or more agents to reach target levels [7]. In a meta-analysis of SBP reductions in 42 trials (N = 10,698), the combination of any two medications from different BP-lowering drug classes was approximately five times more effective than doubling the dose of a single drug [8].
The use of triple-combination therapy, targeting three different mechanisms of arterial hypertension pathogenesis, is becoming increasingly used to provide optimal BP control [8–13] with fewer dose-related adverse effects. In addition, each agent in the combination can lessen adverse effects of other components [14]. This may counteract the tendency of some physicians to prescribe lower than guideline-recommended doses of anti-hypertensive drugs because of concerns over tolerability, and the inevitable suboptimal dosing and inadequate dose titration required to achieve target BP goals [15, 16].
When triple-combination therapy is required, the European Society of Cardiology/European Society of Hypertension guidelines [1] and other guidelines [3, 17–20] recommend the use of an angiotensin-converting enzyme (ACE) system inhibitor or angiotensin II receptor blocker (ARB) with a calcium channel blocker (CCB) and a diuretic as rational and effective. One such combination is perindopril, indapamide, and amlodipine. In addition to additive effects on BP control, each component of this combination has demonstrated protection of target organs at risk from hypertension including the heart, renal system, brain, and vasculature, in a wide range of patients with hypertension. The three components are also complementary in terms of tolerability: perindopril reduces the peripheral edema that is a dose-limiting side effect of CCBs [14]; amlodipine reduces ACE inhibitor-related cough by decreasing prostaglandin synthesis that is induced by bradykinin [21]; and indapamide is a metabolically neutral diuretic [22]. Each individual component is associated with a large evidence base of use in arterial hypertension, and single-pill combinations have also been extensively evaluated: perindopril/indapamide has shown efficacy in patients with recurrent stroke [23], diabetes mellitus [24], and in octogenarians with hypertension [25]; perindopril/amlodipine combination has been studied in detail in patients with hypertension at high cardiovascular risk [26].
Given the extensive experience with these agents as mono and dual therapy it was hypothesized that combining the three agents into a single-pill would maintain the established efficacy and safety of these agents while reducing dosing complexity. Knowing that some physicians may be reluctant to prescribe fixed-dose triple-therapy combinations due to concerns over tolerability, the aim of the present study was to evaluate the clinical efficacy and safety of a triple combination of perindopril 5 mg/indapamide 1.25 mg/amlodipine 5 mg in a single-pill versus dual-pill combination perindopril 5 mg/indapamide 1.25 mg plus amlodipine 5 mg over 12 weeks, in treated patients with uncontrolled hypertension.
Methods
Study Design
The study was an international, multicenter, open-label, randomized controlled trial conducted at 13 centers in Russia and four centers in Serbia. The study protocol was approved by independent Ethics Committees, in accordance with the local regulations in each of the countries and complied with the Declaration of Helsinki, current Good Clinical Practice guidelines (including source documents archiving), and local laws and regulations (ISRCTN registration number: 16442558). All participants provided informed consent before enrolment. Patients were randomized 1:1 to treatment by a computer-generated allocation schedule using a permuted block method (with a block size of four) by the clinical biostatistics department of the study sponsor. Randomization was stratified by study center.
Participants
Eligible patients were men or women of any ethnic origin, aged ≥18 years old, treated with antihypertensive monotherapy at maximal dose or with a single dose of dual therapy other than study treatment (perindopril or indapamide or amlodipine), and with confirmed essential, uncontrolled, hypertension on treatment (SBP ≥140 and <160 mmHg and DBP ≥90 and <100 mmHg at two separate visits: selection and inclusion). During the run-in period (1 week) patients continued their current antihypertensive therapy. Uncontrolled hypertension was confirmed at the inclusion visit. Patients were excluded if they had any contraindications to perindopril, indapamide or amlodipine, or any of the following conditions: complicated hypertension (known stage III or IV hypertensive retinopathy); macroalbuminuria; diabetes; moderate or severe renal failure (creatinine clearance 30–59 and <30 ml/min, respectively); known complicated liver disease; recent disease (previous 6 months) such as cerebrovascular disease (ischemic stroke, cerebral hemorrhage, transient ischemic attack); recent ventricular rhythm disorders (except isolated extrasystoles); and known or suspected symptomatic orthostatic hypotension (defined as a reduction in SBP ≥20 mmHg and/or in DBP ≥10 mmHg from supine to standing-up, maintained for the first 3 min of standing-up).
Intervention
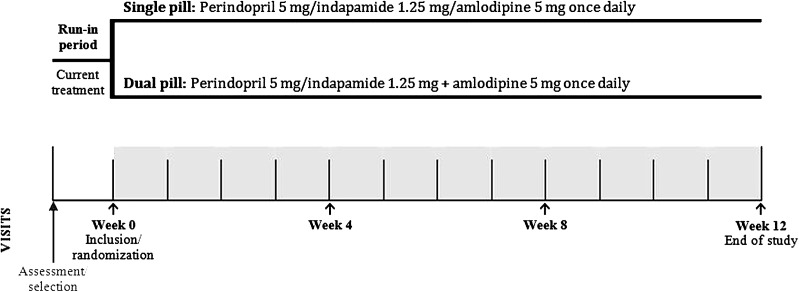
At the inclusion visit, patients were randomly assigned to one of the following treatment groups: a single-pill triple combination of perindopril 5 mg/indapamide 1.25 mg/amlodipine 5 mg (1 tablet/day orally in the morning before breakfast), or a dual-pill combination of perindopril 5 mg/indapamide 1.25 mg + amlodipine 5 mg (1 tablet each/day orally in the morning before breakfast) (Fig. 1). Treatment allocation was balanced, non-centralized, non-adaptive and stratified by center. It was performed according to the allocation list sent to each site (blocks of size 4). The treatment numbers were assigned in order, sequentially, according to the list. The treatment period was 12 weeks.
Fig. 1.
Study design
Study Assessments
All office BP measurements were witnessed and performed at each visit (weeks 0, 4, 8, 12), on the same arm, using a validated automatic OMRON blood pressure measurement device (Model 705CP-II, Omron Healthcare Inc., Vernon Hills, IL, USA) according to ESH guidelines [1]: BP was recorded three times at 1–2-min intervals in the supine position after 10 min of rest. The mean of the last two consecutive measurements was used as the BP level at the study visit. Office SBP and DBP values were measured at baseline prior to the first drug intake and at all post-baseline visits at trough (i.e., 24 ± 3 h after the last intake). The response criteria were the proportion of patients with normalized BP (SBP <140 mmHg and DBP <90 mmHg), and/or decrease of SBP ≥20 mmHg, and/or decrease of DBP ≥10 mmHg at week 12 (W12) compared with baseline. In order to identify orthostatic hypotension, two additional measurements of SBP and DBP in a standing position were performed 1 and 3 min after rising from a supine position.
Safety assessments were performed at inclusion and W12 and included complete laboratory examinations by local laboratories: blood biochemistry; hematology and urine check for proteinuria. Dyslipidemia was qualified by the investigator based on the patient’s medical history or according to treatment or laboratory values and coded using the MedDRA dictionary (version 17.0). Total cholesterol was evaluated during the study (at baseline and at study end) and any significant abnormal value was reported as an adverse event. At W4 only creatinine, creatinine clearance, uric acid and potassium were assessed. Orthostatic hypotension and heart rate were evaluated at every visit.
Statistical Methods
Descriptive statistics were provided for study outcome and safety analyses. The full analysis set (FAS) was used for evaluation of the primary endpoint. Based on the intention-to-treat principle, the FAS corresponds to randomized patients having received at least one dose of study treatment and having a value at baseline and at least one post-baseline value for office supine SBP and DBP over the W0–W12 period. The per protocol set (PPS) corresponds to patients from the FAS without relevant protocol violation(s) that could have affected the evaluation of supine SBP and DBP criteria.
For all primary and secondary endpoints, the treatment effect was estimated, as well as its accuracy: estimate of the between-group difference, standard error of the estimate, and two-sided 95% confidence interval (CI) of the estimate.
The primary efficacy outcome was change from baseline to last post-baseline value in office supine SBP and DBP over the W0–W12 period. The response to antihypertensive treatment expressed as the rate of responders at last post-baseline visit over W0–W12 period was also assessed.
A number of secondary efficacy endpoints were also evaluated including: change from baseline to post-baseline value in office supine SBP and DBP over the W0–W4 and W0–W8 periods; response to antihypertensive treatment expressed as the rate of responders at each post-baseline visit over the W0–W4 and W0–W8 periods; and BP control expressed as the proportion of patients with BP control at post-baseline visit over W0–W12 period and at each post-baseline visit W4 and W8.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
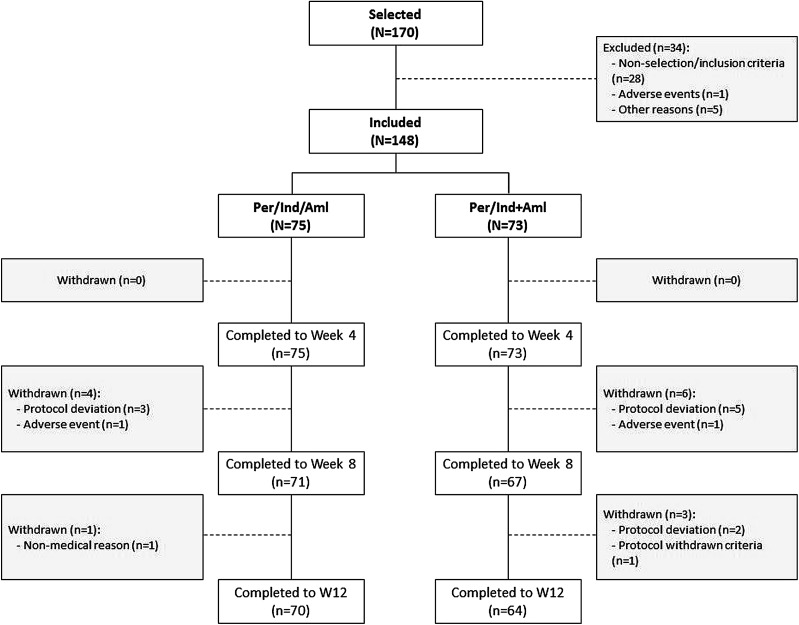
A total of 148 patients were randomized: 75 to the perindopril 5 mg/indapamide 1.25 mg/amlodipine 5 mg group (Per/Ind/Aml) and 73 to the perindopril 5 mg/indapamide 1.25 mg + amlodipine 5 mg group (Per/Ind + Aml). The number of patients completing the study was 134: 70 (Per/Ind/Aml) and 64 (Per/Ind + Aml). Fourteen patients were withdrawn from the study (five in the Per/Ind/Aml group and nine in Per/Ind + Aml). The main reason for withdrawal was protocol deviation for 10 patients (three in Per/Ind/Aml, seven in Per/Ind + Aml). The number of patients withdrawn due to adverse events was low (1 patient in each treatment group). Protocol deviations were mainly related to timing of blood pressure measures with no relevant between-group differences. The trial profile is illustrated in Fig. 2.
Fig. 2.
Trial profile illustrating selected/included patients
Baseline Characteristics
Baseline characteristics are summarized in Table 1 and no major differences were observed between the two groups. Mean age was 56 years and a large proportion was at least 60 years old (44%). Just over half of the patients were women (53%). All patients presented with essential hypertension with an average duration of 99 months (range 2–471 months). A family history of hypertension was reported in 70% of patients. Other relevant medical history included a higher frequency of dyslipidemia in the Per/Ind/Aml group than in the Per/Ind + Aml group: 53.3 versus 43.8%. Frequent cardiovascular risk factors included smoking (18%) and alcohol consumption (24%).
Table 1.
Baseline demographic characteristics in the randomized set
| Baseline characteristic | Per/Ind/Aml (n = 75) | Per/Ind + Aml (n = 73) | Global (n = 148) |
|---|---|---|---|
| Age (years) | 57.2 ± 10.6 | 55.6 ± 10.9 | 56.4 ± 10.8 |
| Over 60 years (%) | 46.7 | 41.1 | 43.9 |
| Women (%) | 53.3 | 52.1 | 52.7 |
| BMI (kg/m2) | 27.8 ± 3.3 | 27.9 ± 2.9 | 27.8 ± 3.1 |
| Duration of hypertension (months) | 103.5 ± 89.7 | 94.1 ± 78.7 | 98.9 ± 84.3 |
| Family history of hypertension (%) | 68.0 | 72.6 | 70.3 |
| Dyslipidemia (%) | 53.3 | 43.8 | 48.7 |
| Smoker (%) | 18.7 | 17.8 | 18.2 |
| Alcohol consumption (%) | 26.7 | 20.6 | 23.7 |
| Previous treatment for hypertension (%) | |||
| Monotherapy (%) | 31.1 | 24.3 | 27.8 |
| Bi-therapy (%) | 68.9 | 75.7 | 72.2 |
| Agents involved (%) | |||
| ACEI or ARB | 93.3 | 98.6 | 95.9 |
| Diuretic | 61.3 | 68.5 | 64.9 |
| CCB | 14.7 | 9.6 | 12.2 |
| Concomitant treatment | |||
| Lipid modifying agents | 32.0 | 26.0 | 29.1 |
| Antithrombotic agents | 22.7 | 21.9 | 22.3 |
At inclusion, all patients were receiving at least one antihypertensive treatment (27.8% monotherapy and 72.2% dual therapy). The most common were agents acting on the renin–angiotensin system (96%) and diuretics (65%). Other concomitant treatments were mainly lipid modifying agents (29%) and antithrombotic agents (22%).
No relevant between-group differences were observed in vital signs. Mean supine SBP and DBP at inclusion were 149 and 94 mmHg, respectively. Mean heart rate was 77 bpm.
Adherence
The mean treatment duration was 82.2 ± 13.8 days for the period W0–W12 in the FAS. Mean treatment adherence was very good at 97.6 ± 6.4%, and all patients but one had an overall adherence between 80 and 120%. No relevant between-group differences were observed in adherence.
Primary Endpoints
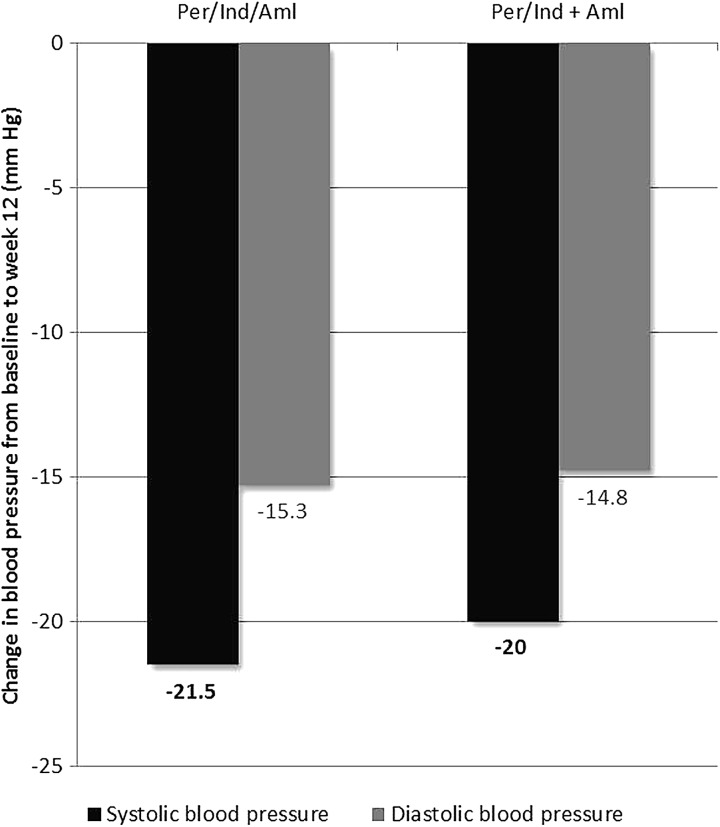
A decrease in office supine SBP was observed in both treatment groups for last post-baseline value compared with baseline, even though patients were already receiving antihypertensive therapy before entry into the study (monotherapy at maximal dose or a single dose of dual therapy other than study treatment). The changes were similar in both treatment groups: −21.5 ± 11.7 mmHg in the Per/Ind/Aml group versus −20.0 ± 12.9 mmHg in the Per/Ind + Aml group; mean (SE) between-group difference in office supine SBP −1.4 (1.8) mmHg, 95% CI [−5.1; 2.2] (Table 2) (Fig. 3).
Table 2.
Change in office supine blood pressure from baseline to week 12
| Per/Ind/Aml (n = 74) | Per/Ind + Aml (n = 70) | |
|---|---|---|
| SBP (mm Hg) | ||
| Baseline (mean ± SD) | 149.1 ± 4.9 (95% CI [147.9; 150.1]) | 149.0 ± 4.7 (95% CI [147.9; 150.1]) |
| Week 12 | 127.6 ± 11.1 (95% CI [125.0; 130.1]) | 129.0 ± 11.7 (95% CI [126.2; 131.8]) |
| Week 12−baseline | −21.5 ± 11.7 (95% CI [−24.3; −18.8]) | −20.0 ± 12.9 (95% CI [−23.1; −17.0]) |
| Estimatea ± SD | −1.4 ± 1.8 (95% CI [−5.1; 2.2]) | |
| DBP (mm Hg) | ||
| Baseline (mean ± SD) | 94.1 ± 3.0 (95% CI [93.4; 94.8]) | 94.1 ± 3.1 (95% CI [93.4; 94.9]) |
| Week 12 | 78.8 ± 8.0 (95% CI [76.9; 80.6]) | 79.3 ± 8.4 (95% CI [77.3; 81.3]) |
| Week 12−baseline | −15.3 ± 7.8 (95% CI [−17.1; −13.5]) | −14.8 ± 9.0 (95% CI [−17.0; −12.7]) |
| Estimatea ± SD | −0.5 ± 1.3 (95% CI [−3.1; 2.0]) | |
Comparison between groups during the study period (week 12−baseline) for the full analysis set (n = 144)
Aml amlodipine, CI confidence interval, Per perindopril, Ind indapamide, SD standard deviation
aEstimate (±standard deviation) of the difference in adjusted mean changes from baseline to last post-baseline value until week 12 [Per/Ind/Aml]−[Per/Ind + Aml] using a general linear model with treatment, baseline, and center as covariates
Fig. 3.
Change in office supine blood pressure from baseline to week 12 with triple-drug antihypertensive therapy containing perindopril, indapamide, and amlodipine in patients with uncontrolled hypertension (SBP ≥140 and <160 mmHg and DBP ≥90 and <100 mmHg at two separate visits: selection and inclusion). Comparison between single-pill (Per/Ind/Aml, n = 74) and dual-pill combinations (Per/Ind + Aml, n = 70). Aml amlodipine, Per perindopril, Ind indapamide
Results for office supine DBP followed the same trend with a decrease over time and similar changes in both treatment groups: −15.3 ± 7.8 mmHg in the Per/Ind/Aml group versus −14.8 ± 9.0 mmHg in the Per/Ind + Aml group; mean (SE) between-group difference −0.5 (1.3) mmHg, 95% CI [−3.1; 2.1] (Table 2) (Fig. 3). For both office supine SBP and DBP results for the per protocol set (PPS) were similar to the FAS.
A response to antihypertensive treatment at the last post-baseline value was observed for the majority of patients in both treatment groups, and the frequency of responders was similar: 89.2% in the Per/Ind/Aml group versus 87.1% in the Per/Ind + Aml group; mean (SE) between-group difference 2.1% (5.4), 95% CI [−8.5; 12.6] in the FAS. A similar trend was observed in the PPS.
Similar changes in SBP and DBP were also observed in the two treatment groups for the monotherapy and dual therapy subgroups (Supplementary Tables S1 and S2).
Secondary Endpoints
For the secondary endpoints at intermediate visits, results for the PPS were similar to the FAS and only the latter are presented. Most of the changes in SBP and DBP were observed by the first visit at week 4 and maintained over the course of the study, and were similar in both groups (Supplementary Figures S1 A and S1B). At W4, SBP decreased by −19.8 mmHg in the Per/Ind/Aml group versus −19.0 mmHg in the Per/Ind + Aml group; mean (SE) between-group difference −0.7 (1.6) mmHg, 95% CI [−3.9; 2.5]. For DBP, the corresponding values were −14.6 versus −14.7 mmHg, respectively; mean (SE) between-group difference 0.04 (1.32) mmHg, 95% CI [−2.6; 2.7]. These decreases in BP were maintained throughout the study. Accordingly, most patients were responders from the W4 visit (89.2 versus 82.9%, respectively; mean (SE) between-group difference 6.3% (5.8), 95% CI [−5.0; 17.7]. At W8, the percentage of responders was 91.9% versus 92.9%, respectively; mean (SE) between-group difference −1.0% (4.4), 95% CI [−9.6; 7.7].
Control of supine BP (defined as SBP <140 mmHg and DBP <90 mmHg) was reached in most patients in both treatment groups for the post-baseline value at W4, W8, and W12. In the majority of patients, control of supine BP was attained at W4 and maintained until W12 in both treatment groups. At W4, 87.8% of patients in the Per/Ind/Aml group versus 78.6% in the Per/Ind + Aml group achieved control of supine BP; mean (SE) between-group difference 9.3% (6.20), 95% CI [−2.89; 21.43]. W8 values were 85.1 versus 88.6%, respectively; mean (SE) between-group difference −3.4% (5.62), 95% CI [−14.45; 7.57]. W12 values were 81.1 versus 80.0%, respectively; mean (SE) between-group difference 1.1% (6.60), 95% CI [−11.86; 14.02].
Safety
Safety was evaluated in all patients who took at least one dose of the study drug (safety set). Emergent adverse events (EAEs) were reported in 18.7% patients in the Per/Ind/Aml group and 21.9% patients in the Per/Ind + Aml group. The most frequent EAEs in the Per/Ind/Aml group were hypokalemia (2.7%) and nasopharyngitis (2.7%), and in the Per/Ind + Aml group the most frequent EAEs were hyperuricemia, and an increase in potassium, and blood urea (2.7% each); none were serious. There were no reports of peripheral edema or cough.
EAEs leading to treatment withdrawal were reported in one patient in each treatment group: hypertension in the Per/Ind/Aml group and rash (considered treatment-related) in the Per/Ind + Aml group. Neither event was serious and both patients recovered.
In this study, patients were carefully followed for any signs of emergent hypotension and orthostatic hypotension. Only one patient reported an emergent hypotension in the Per/Ind + Aml group. This EAE was not serious and was not considered treatment-related. None of the patients were affected by a symptomatic orthostatic hypotension.
Treatment-related EAEs were reported in only a few patients: (4.0%) in the Per/Ind/Aml group and (6.8%) in the Per/Ind + Aml group. Those that were reported were in accordance with those listed in the summary of product characteristics for perindopril, indapamide, and amlodipine.
Blood biochemistry results showed that the rate of patients affected by potentially clinically significant abnormal (PCSA) values was low in both treatments groups (<5%) and mostly detected for creatinine clearance (low value, whatever the formula used) in (4.2%) patients in the Per/Ind/Aml group and (4.4%) patients in the Per/Ind + Aml group. However, all these patients already had a baseline creatinine clearance <90 ml/min or ml/min/1.73 m2 and were aged ≥60 years except for one patient aged less than 60 years in the Per/Ind + Aml group.
Discussion
As expected, this study confirms the similar and clinically significant efficacy in reducing office supine BP of both regimens with reductions of 21.5 and 20.0 mmHg (SBP) and 15.3 and 14.8 (DBP) for the Per/Ind/Aml and Per/Ind + Aml groups, respectively. These reductions were achieved in a population with treated hypertension remaining uncontrolled on maximal dose monotherapy or single-dose dual therapy (mean baseline 149.1/94.1 mmHg), and both treatment groups used low doses of the active components (perindopril 5 mg, indapamide 1.25 mg, amlodipine 5 mg). The majority of patients were responders: 89.2% (Per/Ind/Aml) and 87.1% (Per/Ind + Aml). The results indicate that therapy with therapeutic doses of three component antihypertensive therapies: ACE inhibitor (perindopril), CCB (amlodipine) and diuretic (indapamide) allows BP targets to be achieved in eight out of ten patients previously uncontrolled on mono or dual therapy.
Analysis of the secondary endpoints revealed that achievement of BP control was rapid. The full effect on supine SBP and DBP was attained by W4 and then maintained until the end of the study. The percentage of responders was already very high at W4, with a rate of 89% in the Per/Ind/Aml group compared with 83% in the Per/Ind + Aml group, and more importantly the rate of hypertension control was 88 versus 79%, respectively. Rapid attainment of target BP levels is an important goal of therapy and can lead to improved cardiovascular outcomes. Results from several major cardiovascular clinical trials suggest that the risk of vascular events is significantly influenced by the time it takes to achieve BP control [26–29].
Both triple-therapy regimens showed good tolerability and safety in the current study. The adverse events reported were in accordance with the known safety profile of the component drugs (perindopril, indapamide and amlodipine). Importantly there was no increased risk of hypotension with the use of triple therapy and no report of any peripheral edema or cough.
The study confirms previous observations from the PIANIST study [22] conducted in a high-risk population, in whom target BP levels were also achieved in more than 80% patients who had previously taken two antihypertensive drug combinations, albeit at higher doses of the three components than in the present study. The results suggest that the triple-therapy Per/Ind/Aml combination is suitable for a wide spectrum of patients with hypertension at both low and high cardiovascular risk.
Neither the present study nor the PIANIST trial was designed to evaluate long-term outcomes. However, a post hoc analysis of data from ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation) hints at possible advantages of a triple combination. In the blood pressure arm of ADVANCE, 11, 140 patients with type 2 diabetes were randomly assigned to a fixed combination of perindopril/indapamide (4/1.25 mg) or placebo. Of these, 3427 patients were also receiving a CCB at baseline [30]. Active treatment reduced the relative risk of death by 28% (95% CI [10%; 43%]) among patients with CCB at baseline compared with 5% (−12 to 20%) among those without CCB (P homogeneity = 0.02). The relative risk reduction for major cardiovascular events was 12% (−8 to 28%) versus 6% (−10 to 19%) for those with and without CCB at baseline although the difference was not statistically significant (P homogeneity = 0.38). The benefit on total and cardiovascular death was seen independent of BP reduction because the group on CCBs had a 4.7 mm Hg SBP reduction, whereas the non-CCB group had a 6.2 mm Hg reduction. The treatment benefits were observed despite a higher risk profile among the patients receiving CCB, including a longer duration of type 2 diabetes, more elevated SBP and DBP, higher rates of antihypertensive drug treatment, and higher rates of previous cardiovascular disease. There was no detectable increase in adverse effects in those receiving a CCB.
The greater BP reductions achieved with antihypertensive drug combinations means that lower drug doses are required to reach BP goals with subsequent minimized adverse effects. Furthermore, one component of an antihypertensive combination therapy can reduce the occurrence of adverse events caused by a second component with a different mechanism of action. For example, thiazide diuretics lower BP by reducing volume, which may activate the renin–angiotensin–aldosterone system (RAAS) as a compensatory mechanism and in turn raise BP; a RAAS blocker counteracts this effect and provides additive BP control. RAAS blockers may also reduce the risk of CCB-induced edema by beneficial effects on post-capillary dilation and hydrostatic pressure [31].
There are currently two other approved single-pill triple-therapy combinations available in Europe: olmesartan/amlodipine/hydrochlorothiazide [32] and valsartan/amlodipine/hydrochlorothiazide [33]. However, these agents have no long-term outcome data and neither contains a diuretic that has proven efficacy in a clinical outcome trial, such as indapamide [23, 25, 30] which is recommended by cardiology experts because of its strong morbidity and mortality data [34–38]. As the first single-pill triple-therapy combination incorporating an ACE inhibitor, Per/Ind/Aml fulfills a therapeutic need for many patients currently benefiting from an ACE-inhibitor based therapy who require an additional therapy with single pill. Clinically, ACE inhibitors and ARBs may appear similar. Both are used to treat cardiovascular risk factors and both reduce BP, stroke and symptoms of heart failure. However, the existence of clinical differences, in particular with regards to cardiovascular risk reduction, have been demonstrated by a number of authors. Recently, several meta-analyses have compared the effects of ACE inhibitors and ARBs demonstrating that ACE inhibitors prevent coronary events with reductions in cardiovascular morbidity and mortality endpoints while ARBs are, at best, efficient for the prevention of stroke [39]. One of these, which was fundamentally robust in terms of data quality and numbers analyzed (73,100 patients), showed that ACE inhibitors, but not ARBs, were associated with mortality reduction [40]. This finding was confirmed in a further meta-analysis, performed in 108,212 patients without heart failure, but at high cardiovascular risk [41]. Parallel meta-analyses of ACE inhibitor and ARB trials vs. placebo or other active comparator, and meta-regression analyses that have adjusted for BP within the trials, have demonstrated that ACE inhibitors reduce the risk of myocardial infarction and mortality above and independently of BP lowering, whereas ARBs do not, reinforcing the need for an ACE inhibitor in a triple-therapy combination [42]. The meta-analysis of Ferrari et al. showed that perindopril-based trials accounted for a substantial part of the all-cause and cardiovascular mortality reduction observed with RAAS inhibitors in hypertension [39].
A further benefit of a single-pill triple combination is that it reduces the complexity of the treatment regimen. The use of single-pill combinations has been shown to markedly improve patient adherence [43–45]. Improved adherence is associated with greater BP control [46, 47] and reduced cardiovascular events [48]. People with hypertension often suffer other comorbid conditions that may necessitate taking multiple medications. Healthcare providers and patients may therefore be less willing to accept additional pills to treat one condition or to increase the dose of a single agent because of concerns about increased side-effects. Single-pill triple-combination therapy can help overcome this clinical inertia by reducing dosing complexity at the same time as improving efficacy and tolerability [49–51].
Limitations
Our study is subject to potential limitations. While randomized, the study was not conducted double blind according to the purpose of the study and its exploratory nature which compared fixed versus free combination regimens on a limited number of patients, consequently, no sample size calculation was performed and no formal power calculation were made. The type I error for statistical analyses was set at α = 5% using a two-tailed test and no interpretation of statistical tests was provided. Due to the design of such randomized clinical trials, the enhanced compliance/adherence to treatment that would be expected with fixed combination cannot be determined; only a clinical study conducted in daily medical practice could address this issue. However, control of hypertension was demonstrated from the week 4 visit in 88% of patients on the single-pill triple-drug combination compared with 79% on dual therapy + amlodipine. Finally, only the lowest dose of this fixed combination was evaluated (Per/Ind/Aml 5/1.25/5 mg). Two higher, single-pill, triple-therapy dosages (10/2.5/5 and 10/2.5/10 mg) are also available on the market.
Conclusions
In patients with uncontrolled essential hypertension, single-pill triple therapy with perindopril 5 mg/indapamide 1.25 mg/amlodipine 5 mg was as effective as the same dose of a dual-pill combination of perindopril/indapamide plus separate amlodipine with earlier achievement of BP control.
Both dosing regimens produced clinically significant reductions in SBP and DBP at 1 month which were maintained until the end of the study. The majority of patients in both treatment groups responded early to the antihypertensive therapy and achieved BP control. Both treatment regimens were well tolerated.
Until now, the only choice for physicians wanting to initiate single-pill triple combinations was an ARB-based therapy [1, 2, 6]. Patients already treated with an ACE inhibitor-based dual therapy who required three drugs in a single-pill were obliged to switch to other therapeutic classes. Perindopril/indapamide/amlodipine is currently the only triple-drug therapy incorporating an ACE inhibitor in a single pill, providing an evidence-based drug combination supported by international guidelines and recommendations for the treatment of hypertension.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study and article processing charges were supported by Les Laboratoires Servier. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Editorial assistance was provided by Jenny Grice and funded by Les Laboratoires Servier.
Sergey V. Nedogoda and Vesna J. Stojanov were supported for writing the study by Les Laboratoires Servier.
Disclosures
Sergey V. Nedogoda has received consulting fees or honorarium from Novartis, Sanofi-Aventis, Servier, AstraZeneca, Boehringer Ingelheim and Bayer. He has also received payment for lectures from Novartis, MSD, Sanofi-Aventis, Pfizer, Servier, Boehringer Ingelheim, Bayer, AstraZeneca, Abbott, and Takeda.
Vesna J. Stojanov has no conflicts of interest to declare.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/A487F0607C763559.
References
- 1.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. Guidelines for the Management of Arterial Hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 2.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 3.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Clinical Guidelines CG127. 2011. [PubMed]
- 5.Benoit G, Feber J, Harris KD, Poirier L, Padwal RS, For the Canadian Hypertension Education Program The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2015;31:549–568. doi: 10.1016/j.cjca.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Tykarski A, Narkiewicz K, Gaciong Z, Polish Society of Hypertension, et al. 2015 guidelines for the management of hypertension. Recommendations of the Polish Society of Hypertension. Arterial Hypertens. 2015;19:53–83.
- 7.Mancia G, De Backer G, Dominiczak A, The task force for the management of arterial hypertension of the European Society of Hypertension, The task force for the management of arterial hypertension of the European Society of Cardiology, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536.
- 8.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy vs. monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Leung AA, Nerenberg K, Daskalopoulou SS, CHEP Guidelines Task Force et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569–588. doi: 10.1016/j.cjca.2016.02.066. [DOI] [PubMed] [Google Scholar]
- 10.Gradman AH. Rationale for triple-combination therapy for management of high blood pressure. J Clin Hypertens (Greenwich). 2010;12:869–878. doi: 10.1111/j.1751-7176.2010.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elijovich F, Laffer C. A role for single-pill triple therapy in hypertension. Ther Adv Cardiovasc Dis. 2009;3:231–240. doi: 10.1177/1753944709334947. [DOI] [PubMed] [Google Scholar]
- 12.Black HR. Triple fixed-dose combination therapy. Back to the past. Hypertension. 2009;54:19–22. doi: 10.1161/HYPERTENSIONAHA.109.132688. [DOI] [PubMed] [Google Scholar]
- 13.Neutel JM, Smith DH. Hypertension management: rationale for triple therapy based on mechanisms of action. Cardiovasc Ther. 2013;31:251–258. doi: 10.1111/1755-5922.12015. [DOI] [PubMed] [Google Scholar]
- 14.Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med. 2011;124:128–135. doi: 10.1016/j.amjmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Chung N, Baek S, Chen MF, et al. Expert recommendations on the challenges of hypertension in Asia. Int J Clin Pract. 2008;62:1306–1312. doi: 10.1111/j.1742-1241.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 16.Remme WJ, McMurray JJ, Hobbs FD, et al. Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J. 2008;29:1739–1752. doi: 10.1093/eurheartj/ehn196. [DOI] [PubMed] [Google Scholar]
- 17.Liu L. 2010 Chinese guidelines for the management of hypertension. Chin J Cardiol. 2011;39:579–616. [PubMed] [Google Scholar]
- 18.Sociedade Brasileira de Cardiologia (SBC). 7a Diretriz Brasileira de Hipertensão Arterial. Arq Bras Cardiol. 2016;107(3 Suppl.3):1–83. [DOI] [PMC free article] [PubMed]
- 19.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Eur Heart J. 2016 doi: 10.1007/s12529-016-9583-6. [DOI] [PubMed] [Google Scholar]
- 20.Russian Society on Hypertension All-Russian Scientific Society of Cardiologists (VNOK). Diagnosis and treatment of arterial hypertension. Systemnye Hypertensii. 2010;3:5–26. [Google Scholar]
- 21.Fogari R, Zoppi A, Tettamanti F, Malamani GD, Tinelli C, Salvetti A. Effects of nifedipine and indomethacin on cough induced by angiotensin-converting enzyme inhibitors: a double-blind, randomized, cross-over study. J Cardiovasc Pharmacol. 1992;19:670–673. [PubMed] [Google Scholar]
- 22.Tóth K, Investigators PIANIST. Antihypertensive efficacy of triple combination perindopril/indapamide plus amlodipine in high-risk hypertensives: results of the PIANIST study (Perindopril-Indapamide plus AmlodipiNe in high rISk hyperTensive patients) Am J Cardiovasc Drugs. 2014;14:137–145. doi: 10.1007/s40256-014-0067-2. [DOI] [PubMed] [Google Scholar]
- 23.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 24.ADVANCE Collaborative Group Effect of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomized controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 25.Beckett N, Peters R, Fletcher AE, For the HYVET Study Group, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. [DOI] [PubMed]
- 26.Dahlof B, Sever P, Poulter N, For the ASCOT investigators, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–90. [DOI] [PubMed]
- 27.Julius S, Kjeldsen SE, Weber M, VALUE trial group et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 28.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 29.Jamerson K, Bakris GL, Dahlöf B, ACCOMPLISH Investigators et al. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Press. 2007;16:80–86. doi: 10.1080/08037050701395571. [DOI] [PubMed] [Google Scholar]
- 30.Chalmers J, Arima H, Woodward M, et al. Effects of combination of perindopril, indapamide, and calcium channel blockers in patients with type 2 diabetes mellitus: results from the Action In Diabetes And Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) trial. Hypertension. 2014;63:259–264. doi: 10.1161/HYPERTENSIONAHA.113.02252. [DOI] [PubMed] [Google Scholar]
- 31.de la Sierra A. Mitigation of calcium channel blocker-related oedema in hypertension by antagonists of the renin-angiotensin system. J Hum Hypertens. 2009;23:503–511. doi: 10.1038/jhh.2008.157. [DOI] [PubMed] [Google Scholar]
- 32.Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32:1252–1269. doi: 10.1016/j.clinthera.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Calhoun DA, Crikelair NA, Yen J, Glazer RD. Amlodipine/valsartan/hydrochlorothiazide triple combination therapy in moderate/severe hypertension: secondary analyses evaluating efficacy and safety. Adv Ther. 2009;26:1012–1023. doi: 10.1007/s12325-009-0077-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen P, Chaugai S, Zhao F, Wang DW. Cardioprotective effect of thiazide-like diuretics: a meta-analysis. Am J Hypertens. 2015;28:1453–1463. doi: 10.1093/ajh/hpv050. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan N. Treatment of hypertension: drug therapy—diuretics. In: Kaplan NM, editor. Clinical hypertension, 8th ed. Philadelphia, USA: Lippincott Williams and Wilkins; 2002. p. 241–252.
- 36.Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S. Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring. A meta-analysis of randomized trials. J Am Coll Cardiol. 2011;57:590–600. doi: 10.1016/j.jacc.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 37.NICE—Clinical Guidelines Hypertension: the clinical management of primary hypertension in adults—NCGC (National Clinical Guideline Centre) 127. 2011.
- 38.Roush GC, Ernst ME, Kostis JB, Tandon S, Sica DA. Head-to-head comparisons of hydrochlorothiazide with indapamide and chlorthalidone antihypertensive and metabolic effects. Hypertension. 2015;65:1041–1046. doi: 10.1161/HYPERTENSIONAHA.114.05021. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari R, Rosano GM. Not just numbers, but years of science: putting the ACE inhibitor—ARB meta-analyses into context. Int J Cardiol. 2013;166:286–288. doi: 10.1016/j.ijcard.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone-system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savarese G, Costanzo P, Cleland JG, et al. A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013;61:131–142. doi: 10.1016/j.jacc.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Strauss MH, Hallb AS. The divergent cardiovascular effects of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on myocardial infarction and death. Prog Cardiovasc Dis. 2016;58:473–482. doi: 10.1016/j.pcad.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816. [DOI] [PubMed] [Google Scholar]
- 44.Dragomir A, Cote R, Roy L, et al. Impact of adherence to antihypertensive agents on clinical outcomes and hospitalization costs. Med Care. 2010;48:418–425. doi: 10.1097/MLR.0b013e3181d567bd. [DOI] [PubMed] [Google Scholar]
- 45.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719. doi: 10.1016/j.amjmed.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12:239–245. doi: 10.18553/jmcp.2006.12.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yiannakopoulou EC, Papadopulos JS, Cokkinos DV, et al. Adherence to antihypertensive treatment: a critical factor for blood pressure control. Eur J Cardiovasc Prev Rehabil. 2005;12:243–249. doi: 10.1097/00149831-200506000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299. [DOI] [PubMed] [Google Scholar]
- 49.Heisler M, Hogan MM, Hofer TP, Schmittdiel JA, Pladevall M, Kerr EA. When more is not better: treatment intensification among hypertensive patients with poor medication adherence. Circulation. 2008;117:2884–2892. doi: 10.1161/CIRCULATIONAHA.107.724104. [DOI] [PubMed] [Google Scholar]
- 50.Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47:345–351. doi: 10.1161/01.HYP.0000200702.76436.4b. [DOI] [PubMed] [Google Scholar]
- 51.Basile J, Neutel J. Overcoming clinical inertia to achieve blood pressure goals: the role of fixed-dose combination therapy. Ther Adv Cardiovasc Dis. 2010;4:119–127. doi: 10.1177/1753944709356012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.