Abstract
Mangrove is a complex ecosystem that contains diverse microbial communities, including rare actinobacteria with great potential to produce bioactive compounds. To date, bioactive compounds extracted from mangrove rare actinobacteria have demonstrated diverse biological activities. The discovery of three novel rare actinobacteria by polyphasic approach, namely Microbacterium mangrovi MUSC 115T, Sinomonas humi MUSC 117T and Monashia flava MUSC 78T from mangrove soils at Tanjung Lumpur, Peninsular Malaysia have led to the screening on antibacterial, anticancer and neuroprotective activities. A total of ten different panels of bacteria such as Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300, ATCC 70069, Pseudomonas aeruginosa NRBC 112582 and others were selected for antibacterial screening. Three different neuroprotective models (hypoxia, oxidative stress, dementia) were done using SHSY5Y neuronal cells while two human cancer cells lines, namely human colon cancer cell lines (HT-29) and human cervical carcinoma cell lines (Ca Ski) were utilized for anticancer activity. The result revealed that all extracts exhibited bacteriostatic effects on the bacteria tested. On the other hand, the neuroprotective studies demonstrated M. mangrovi MUSC 115T extract exhibited significant neuroprotective properties in oxidative stress and dementia model while the extract of strain M. flava MUSC 78T was able to protect the SHSY5Y neuronal cells in hypoxia model. Furthermore, the extracts of M. mangrovi MUSC 115T and M. flava MUSC 78T exhibited anticancer effect against Ca Ski cell line. The chemical analysis of the extracts through GC–MS revealed that the majority of the compounds present in all extracts are heterocyclic organic compound that could explain for the observed bioactivities. Therefore, the results obtained in this study suggested that rare actinobacteria discovered from mangrove environment could be potential sources of antibacterial, anticancer and neuroprotective agents.
Keywords: Microbacterium mangrovi MUSC 115T, Sinomonas humi MUSC 117T, Monashia flava MUSC 78T, Antibacterial, Anticancer, Neuroprotective
Introduction
Actinobacteria is common soil inhabitant and have a high proportion of total microbial biomass in soil [1]. They are considered as the most economically significant as well as biotechnologically valuable microbe, producing bioactive compounds including antibiotics, antimicrobial, anticancer, antitumor, enzyme, enzyme inhibitors and immunosuppressive agents [2]. Actinobacteria such as Streptomyces are excellent producer of bioactive compounds especially secondary metabolites [3–8]. Over 10, 000 bioactive compounds were derived from actinobacteria species, 7600 (76%) compounds were derived from Streptomyces while 2400 (24%) compounds isolated from rare actinobacteria [9]. Unfortunately, repeated isolation of known compounds and a reduced hit-rate of novel compounds have limited the development of new and effective drugs to treat ever increasing human diseases. [10–12]. At the same time, the arising multi drug resistance (MDR) pathogen and other deadly diseases caused the dramatic increase in demand to look for new compounds [13] from other sources such as rare actinobacteria.
Previously, the numbers of rare actinobacteria being discovered were low, as compared to Streptomyces. This is due to the facts that they are difficult to isolate, cultivate and maintain under conventional conditions [14]. However, the number of novel rare actinobacteria is increasing, from only 11 genera in 1970 to 220 genera by 2010 [15]. At the time of writing (March 2016), there are approximately 340 genera of rare actinobacteria (www.bacterio.net) discovered from various environment thus demonstrating that rare actinobacteria are widely distributed in the biosphere. According to Goodfellow [16], there are a low number of rare actinobacteria isolated from marine environments such as the mangrove. Thus, mangrove environment has gained attention from the researcher due to its location where it situated at the inter-phase between the terrestrial and marine environment, and have a special condition such as high moisture, high salinity and hypoxia tolerant [17]. This condition breeds many novel microorganism including rare actinobacteria that contained special and unique metabolic pathways to adapt with those conditions and lead to the production of valuable metabolites [17].
The rare actinobacteria strains discovered in previous work were Microbacterium mangrovi MUSC 115T, Sinomonas humi MUSC 117T and Monashia flava MUSC 78T. These strains were isolated from mangrove soils located at Tanjung Lumpur, Peninsular Malaysia. The taxonomic status of these strains was described in previous publications using polyphasic approach [18–20]. Currently, the study of bioactive compounds from mangrove rare actinobacteria become popular as they possess great potential to pharmaceutical industry [3, 10, 14]. An example, Mangamuri et al. [21] reported that the bioactive metabolites from Pseudonocardia endophytica VUK-10 was able to inhibit the growth of Gram-positive and Gram-negative bacteria, yeast, fungi and also exhibited potent cytotoxic activity against human breast adenocarcinoma cell line (MDA-MB-231, MCF-7), human cervical cell line (HeLa), human ovarian cyst adenocarcinoma cell line (OAW-42). Janardhan et al. [22] showed the extracts of strain Nocardiopsis alba isolated from mangrove soil of Nellore regions, Andhra Pradesh, India, exhibited potent total antioxidant property. Novel anticancer and anti-infection compounds are being isolated from mangrove rare actinobacteria, as represented by the discovery of the salinosporomide A, an anti-cancer compound produced by Salinispora tropica [23].
In light of this, the present study was initiated to investigate the extracts of rare actinobacteria collected from Tanjung Lumpur, Peninsular Malaysia for its biological activity such as antibacterial, anticancer or neuroprotective activity.
Materials and Methods
Preparation of Microbacterium mangrovi MUSC 115T, Sinomonas humi MUSC 117T and Monashia flava MUSC 78T Extracts
All novel strains were grown on ISP2 medium for 5 days prior to fermentation process. The fermentation medium used was FM3 [10, 24] with slight modification and autoclaved at 121 °C for 15 min. The strains were cultured at 200 rpm, for 7–10 days at 28 °C. The resulting fermentation media were separated from the mycelium by centrifugation at 4500 rpm at 4 °C for 30 min. The supernatant was collected and subjected to freeze dry process. Upon freeze-drying, the sample was extracted with methanol for 72 h (ratio 3:1; methanol:sample) and the residue was re-extracted under the same condition twice at 24 h interval with ratio of 2:1 and 1:1, respectively. All the methanol-containing extract was filtered and evaporated using a rotary vacuum evaporator and the extract were kept in −20 °C until further analysis [25].
Bacterial Strains
Ten different pathogens were used for the antibacterial screening; namely Acinetobacter calcoaceticus NBRC 13006, Salmonella typhi ATCC 19430, Escherichia coli ATCC 25922, Vibrio parahaemolyticus VP103 (Jeffrey Cheah School of Medicine and Health Science laboratory), Pseudomonas aeruginosa NRBC 11258, Methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300, ATCC 70069, ATCC 33591, ATCC BAA-44, Bacillus subtilis ATCC 31098. The test organisms were maintained on Mueller–Hinton agar (MHA).
Minimal Inhibitory Concentration (MIC) Determination
Minimal inhibitory concentration (MIC) is the lowest concentration of an antimicrobial that able to inhibit the growth of particular bacterium after overnight incubation. The work was performed by the broth microdilution method in 96 well plate as described by Wiegand et al. [26], with slight modification. Chloramphenicol (0.1 mg/mL) was used as positive control and untreated bacterial culture was used as negative control. One hundred microliters aliquot of the bacteria was added into the wells with an approximate inoculum of 1 × 106 CFU/mL, previously prepared as a 0.5 McFarland’s standard. Serial dilutions of the extracts were done to achieve the final concentration of 5, 2.5, 1.25, 0.625 and 0.313 mg/mL. Aliquot (100 μL) of the extract with different concentration was added into each of the wells and incubated at 37 °C for 24 h. The MIC was determined by assessment of turbidity by optical density readings at 600 nm.
Minimal Bactericidal Concentration (MBC) Determination
Minimal bactericidal concentration (MBC) is the lowest concentration of an antimicrobial that prevent the growth of particular microorganism. The MBC was determined by sub-culturing 100 µL from well that exhibited no growth onto MHA and incubated at 37 °C for 24 h.
Cell lines Maintenance and Growth Condition
The human cancer cell lines (HT-29 and Ca Ski) and the neuronal cell lines (SH-SY5Y) involved in this study was maintained in Roswell Park Memorial Institute (RPMI) and Dulbecco’s Modified Eangle Medium (DMEM), respectively, supplemented with 10% fetal bovine serum and 1× antibiotic–antimycotic at 37 °C humidified incubator containing 5% CO2 [6].
Neuroprotective Assay
Cell viability of neuronal cells were determined by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, SHSY-5Y cells were seeded into a microtitre plate at a density of 3 × 104 cells/well and allowed to adhere overnight. 20 µL of each extracts were added into the cells with the final concentration ranging from 6.25 to 200 µg/mL. Catechin (100 µM) (oxidative stress and hypoxia model) or gallic acid (1 µg/mL) (dementia model) were used as a positive control in the experiments of the study. The pre-treated cells were incubated for 2 h followed by either 250 µM hydrogen peroxide (H2O2), 400 µM streptozotocin (STZ) or 5 mM cobalt (II) chloride (CoCl2) treatment for 24 h.
Anticancer Activity of the Extract on Human Cancerous Cells
The effect of extracts on cell viability of human cancer cells lines was determined by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded into a microtitre plate at a density of 5 × 103 cells/well and allowed to adhere overnight. 20 µL of each extracts were added into the wells with the final concentration ranging from 6.25 to 200 µg/mL and incubated at 72 h. Curcumin (3 µg/mL) was included as positive control.
MTT Assays
The MTT assay for neuroprotective and anticancer activities were performed by adding 20 µL of MTT (5 mg/mL) into each well and the plates were incubated at 37 °C containing 5% CO2 for 4 h [25]. After the incubation period, the medium was then aspirated carefully and 100 μL of DMSO was added. The absorbance of the product was determined spectrophotometrically at 570 nm, with 650 nm as reference using a microplate reader. The percentage of cell viability was calculated as follows:
Statistical Analysis
All values expressed as mean ± standard deviations (SD) by Microsoft Excel. Data were analyzed for statistical significance using one-way ANOVA, followed by Dunnett’s test as a post hoc test with GraphPad Prism 6.0 software for Windows (Inc., San Diego, USA).
Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
Gas chromatography–mass spectrometry (GC–MS) analysis was performed in accordance with our previous developed method with slight modification [27, 28]. The instrument used was Agilent Technologies 6980N (GC) equipped with 5979 Mass Selective Detector (MS), HP-5MS (5% phenyl methyl siloxane) capillary column of dimensions 30.0 m × 250 µm and helium as carrier gas at 1 mL/min. The column temperature was programmed initially at 40 °C for 10 min, followed by an increase of 3 °C/min to 250 °C and was kept isothermally for 5 min. The MS was operating at 70 eV. The constituents were identified by comparison of their mass spectral data with those from NIST 05 Spectral Library.
Results and Discussions
Antibacterial Assays
Results of antibacterial activity are represented in Table 1. Showed that all extracts demonstrated good inhibitory activity against different bacteria tested, with MICs values of 2.5 mg/mL, except for P. aeruginosa NRBC 112582, V. parahaemolyticus VP103 and E. coli ATCC 25922, where the inhibitory concentration was 1.25 mg/mL. These finding indicates that the extracts inhibited the growth of the bacteria tested at low concentrations.
Table 1.
Minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and MBC/MIC ratios of the extracts of strains Microbacterium mangrovi MUSC 115T , Sinomonas humi MUSC 117T and Monashia flava MUSC 78T
| Bacterium tested | MUSC 115T | MUSC 117T | MUSC 78T | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| *Staphylococcus aureus ATCC 43300 | 2.5 | >5 | – | 2.5 | >5 | – | 2.5 | >5 | – |
| *Staphylococcus aureus ATCC 70069 | 2.5 | >5 | – | 2.5 | >5 | – | 2.5 | >5 | – |
| *Staphylococcus aureus ATCC 33591 | 2.5 | >5 | – | 2.5 | >5 | – | 2.5 | >5 | – |
| *Staphylococcus aureus ATCC BAA-44 | 2.5 | >5 | – | 2.5 | >5 | – | 2.5 | >5 | – |
| Acinetobacter calcoaceticus | |||||||||
| NBRC 13006 | 2.5 | >5 | – | 2.5 | >5 | – | 2.5 | >5 | – |
| Bacillus subtilis | |||||||||
| ATCC 31098 | 2.5 | >5 | – | 2.5 | >5 | – | 2.5 | >5 | – |
| Pseudomonas aeruginosa | |||||||||
| NRBC 112582 | 1.25 | 5 | 4 | 1.25 | >5 | – | 1.25 | >5 | – |
| Salmonella typhi | |||||||||
| ATCC 19430 | 2.5 | 5 | 2 | 2.5 | >5 | – | 2.5 | >5 | – |
| Vibrio parahaemolyticus VP103 | 1.25 | >5 | – | 1.25 | >5 | – | 1.25 | >5 | – |
| Escherichia coli | |||||||||
| ATCC 25922 | 2.5 | 5 | 2 | 1.25 | 5 | 2 | 1.25 | >5 | – |
* Methicillin-resistant Staphylococcus aureus (MRSA)
* (−): not calculated for MBC/MIC as the MBC value was >5 mg/mL
The MBC result in Table 1 presented M. mangrovi MUSC 115T and S. humi MUSC 117T extracts were completely inhibiting the growth of P. aeruginosa NRBC 112582, S. typhi ATCC 19430 and E. coli ATCC 25922 at a concentration of 5 mg/mL. However, a higher concentration might be needed for complete inhibition of the growth of the other bacteria tested.
In addition, some of the extracts displayed bactericidal effects on few numbers of bacteria. According to Ocampo et al. [29], bacteriostatic can be defined as the agent inhibit the growth of bacteria without killing effects, while bactericidal means agents that kill bacteria. An extract is considered as bactericidal when the ratio of MBC/MIC is ≤4 and bacteriostatic when this ratio is >4 [30]. This effect was observed with the M. mangrovi MUSC 115T extract against P. aeruginosa NRBC 112582, S. typhi ATCC 19430, and E. coli ATCC 25922 with the ratios of MBC/MIC equal to 4, 2, and 2 respectively. The S. humi MUSC 117T extract was also bactericidal against E. coli ATCC 25922 with the ratio of MBC/MIC equal to 2. Overall, the extracts of each strains possessed activity to inhibit the growth of bacteria tested.
Neuroprotective Assays
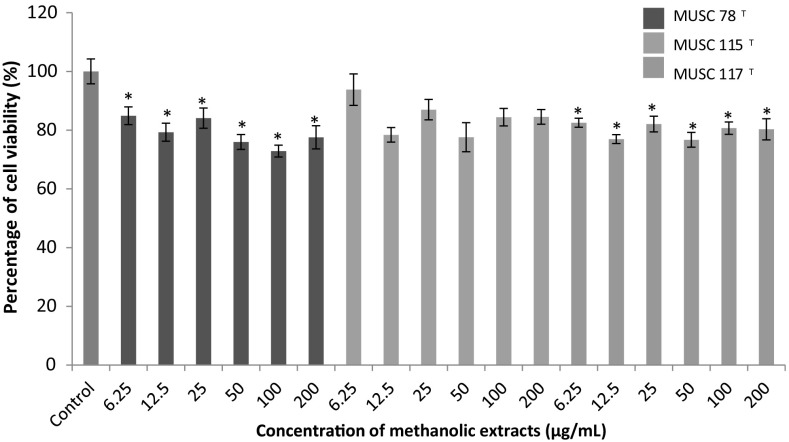
In this study, the neuroprotective assays were performed by using three different experimental models focusing on hypoxia, oxidative stress and dementia. Results of each experimental model were show in Figs. 1, 2 and 3, respectively.
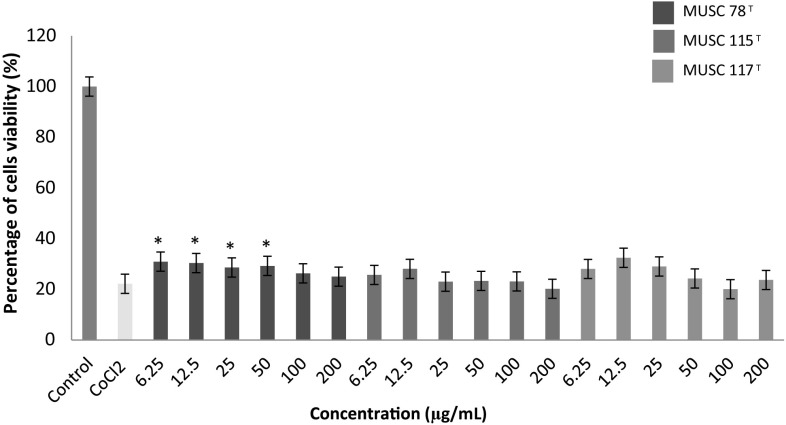
Fig. 1.
The neuroprotective activity of methanolic extracts on the cell viability of SH-SY5Y cells treated with CoCl2. Cells viability was measured using MTT assay. *p < 0.05 indicates statistically significant differences compared to CoCl2 induced cells
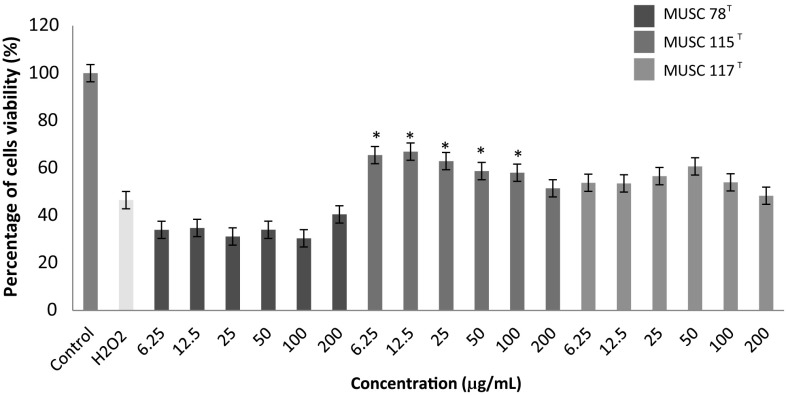
Fig. 2.
The neuroprotective activity of methanolic extracts on the cell viability of SH-SY5Y cells treated with H2O2. Cells viability was measured using MTT assay. *p < 0.05 indicates statistically significant differences compared to H2O2 induced cells
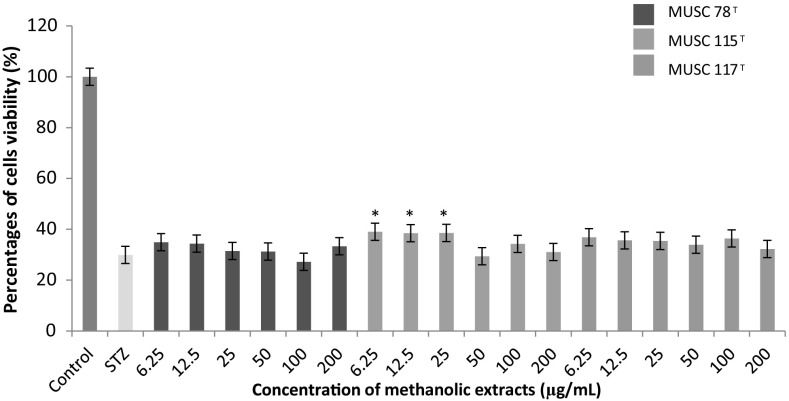
Fig. 3.
The neuroprotective activity of methanolic extracts on the cell viability of SH-SY5Y cells treated with STZ. Cells viability was measured using MTT assay. *p < 0.05 indicates statistically significant differences compared to STZ induced cells
Neuroprotective Property of Extracts on Hypoxia Induced Cytotoxicity
Hypoxia can be defined as the reduction or lack of oxygen in organs, tissues or cells. A common experimental model of hypoxia was created using a transition metal, cobalt (II) chloride (CoCl2) [31, 32]. CoCl2 is a chemical agent that reportedly induces a biochemical and molecular response similar to that observed under low-oxygen conditions in mammalian systems [33]. Beside it is widely used to establish the model of hypoxia in both in vitro and in vivo study. Theoretically, the Co2+ will replace the Fe2+ in heme on the cell surface, thus weaken the oxygen signaling and transport, leading to the generation of reactive oxygen species (ROS) and cell death [34]. In fact, a study done by Lee et al. [35] and Vengellur and LaPres [36] have shown that both hypoxia and cobalt affecting a similar group of genes on a global gene expression level. This implies that the robustness and suitability use of this model of experiment for experimental purposes.
Figure 1 showed that the neuronal cells subjected to CoCl2 exposure showed a significant reduction in viability of cells up to 77.8%. Based on the analysis, the extract of M. flava MUSC 78T was able to protect the neuronal cells from the CoCl2 insult at lower concentration; 6.25–50 µg/mL. The neuroprotective activity reduced when the concentration of the extracts reached at 50 µg/mL. The statistical analysis of S. humi MUSC 117T and M. mangrovi MUSC 115T extracts showed the percentages of cell viability for each concentration tested were not significant when compared to CoCl2 induced cells and concluded that these two extracts were not able to protect neuronal cells from the hypoxia induced neuronal damage.
Neuroprotective Property of Extracts on Oxidative Stress Induced Cytotoxicity
One of the most common methods applied for studying the in vitro neuroprotective activity of antioxidants is H2O2 induced cytotoxicity [37, 38], hence this method was employed to study the extracts of each strain. The insults of H2O2 have been linked to the formation of oxidative stress which is known to cause neurodegenerative diseases such as Alzheimer’s [39] and Parkinson diseases [40]. H2O2 has a short half-life, and its dissociation into hydroxyl and superoxide ions may affect the membrane integrity and leading to cellular damage [39, 41]. In fact, H2O2 has been observed to exert toxic effect on different cell types while neuron was found to be most susceptible to H2O2-induced toxicity [42].
Based on Fig. 2, it was observed that only M. mangrovi MUSC 115T extract was able to protect the neuronal cells against H2O2 challenge at low concentration, 6.25 µg/mL. Furthermore, there is a significant decreased in cell viability in M. mangrovi MUSC 115T extract treated cells from 50 µg/mL to 200 µg/mL as compared to H2O2 control. This indicates the effect of the treatment reached its maximum efficacy at around 12.5 µg/mL. Further increase of treatment will eventually found to be toxic towards the neuronal cells. On the other hand, the S. humi MUSC 117T and M. flava MUSC 78T extracts were found to exhibit no protective activity on SH-SY5Y neuronal cells when challenged by H2O2.
Neuroprotective Property of Extracts on Dementia Induced Cytotoxicity
Dementia is known as a multisystem-related neurodegenerative disorder. A set of symptoms are associated to this disease which include impairment in short- and long-term memory, impairment in thinking, judgment, other disturbance of higher cortical function, or personality change [43]. In order to understand the pathological aspect of dementia in human, researcher have made use of STZ as an inducer in rats to create the experimental model of dementia [44]. It was also commonly utilized in preparing the in vitro dementia model of experiment particularly on SH-SY5Y neuronal cells [45]. The induction of STZ was found to generate excessive free radicals which leading to formation of oxidative stress [46], inflammation [47], abnormal protein [48] and leads to mitochondrial dysfunction and apoptosis in cell [49].
Figure 3 demonstrated the result of neuroprotective activity of extracts on dementia model of experiment. From the data, the percentage of cell viability of SH-SY5Y neuronal cells treated with STZ only was found to be significantly (p < 0.05) reduced up to about 70.0%. The pre-treatment of M flava MUSC 78T and S. humi MUSC 117T extracts on the STZ treated cells showed that both of these extracts were unable to protect SH-SY5Y cells from the STZ induced neuronal damage. However, only M. mangrovi MUSC 115T extracts treatment was found to demonstrating neuroprotective activity at different concentration ranging from 6.25 to 25 µg/mL.
Anticancer Activity of the Extracts on Human Cancerous Cells
Rare actinobacteria, represent a promising reservoir of different kinds of therapeutics drugs. In this study, the anticancer effect of the extracts were tested on two different types of human cancer cell lines; human colon cancer cell lines (HT-29) and human cervical carcinoma cell lines (Ca Ski). The effects of the extract on the tested cancerous cells are shown Figs. 4 and 5.
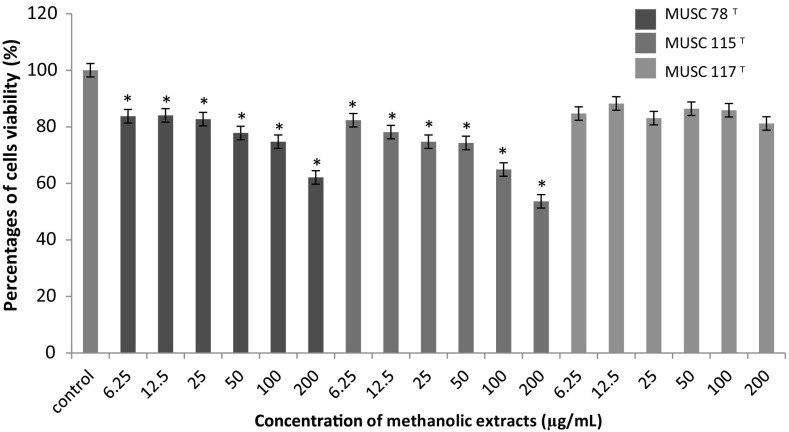
Fig. 4.
The anticancer activity of methanolic extracts on the cell viability of Ca Ski cells. Cells viability was measured using MTT assay. *p < 0.05 indicates statistically significant differences compared to untreated cells
Fig. 5.
The anticancer activity of methanolic extracts on the cell viability of HT-29 cells. Cells viability was measured using MTT assay. *p < 0.05 indicates statistically significant differences compared to untreated cells
All the extracts displayed varying levels of anticancer against the Ca Ski cells (Fig. 4). Interestingly, a dose-dependent response was observed for the M. flava MUSC 78T and M. mangrovi MUSC 115T extracts treatment as there was a significant reduction of the viability of cells when compared to the untreated cells. The Ca Ski cells was found to be the most vulnerable to the treatment of M. mangrovi MUSC 115T extracts with the strongest growth inhibition at high concentration tested (200 µg/mL), seconded by the treatment activity displayed by M. flava MUSC 78T. In the case of S. humi MUSC 117T extract, there was no significant reduction of the viability of Ca Ski cells as compared to control. Overall, M. flava MUSC 78T and M. mangrovi MUSC 115T extracts are effective in inhibiting the growth of Ca Ski cells.
Similarly, the anticancer effect of M. flava MUSC 78T, M. mangrovi MUSC 115T and S. humi MUSC 117T extracts on HT-29 cells were examined as well. Results in Fig. 5 displayed that there is a mild growth inhibition activity of HT-29 cells as the viability of HT-29 cells was significantly reduced especially at the highest concentration of treatment at 200 µg/mL.
Taken altogether, the extracts of M. mangrovi MUSC 115T, S. humi MUSC 117T and M. flava MUSC 78T were shown to be effective in causing cytotoxic effect on this two different cancer cell lines namely human colon cancer cell lines (HT-29) and human cervical carcinoma cell lines (Ca Ski). The results of the studies also demonstrated that two cancer cell lines showed different reaction towards the concentration of extracts tested. M. mangrovi MUSC 115T and M. flava MUSC 78T extracts exhibiting a cytotoxic activity on Ca Ski cells except for S. humi MUSC 117T, meanwhile all the extracts exhibiting a low anticancer activity against HT-29 cells. In general, varying strength at the effect possessed by extracts are most likely affected by the differences in the chemical composition that present in.
Chemical Profiling Analysis
Following the assessment of bioactivities possessed by M. mangrovi MUSC 115T, S. humi MUSC 117T and M. flava MUSC 78T extracts, GC–MS analysis were performed in order to analyze the chemical constituents that present in the extracts. GC–MS is an effective combination of technologies which meant for the analysis of chemical compounds. Basically, the compounds will be separated by GC while MS generates the characteristic mass profile for each of the compounds detected [50, 51]. As shown in Table 2, a total of six chemical compounds were identified in M.mangrovi MUSC 115T extract, ten compounds were detected in S. humi MUSC 117T extract while M. flava MUSC 78T extract analysis yielded a total of twenty compounds. Through GC–MS analysis, the obtained results indicating the majority of the compounds that present in extracts are consisted of organic heterocyclic compounds. These heterocyclic compounds include phenolics, pyrazines, and pyrrolopyrazine.
Table 2.
Compounds identified from Microbacterium mangrovi MUSC 115T, Sinomonas humi MUSC 117T and Monashia flava MUSC 78T by using GS-MS
| Extract | No | Retention time (min) | Compound | Formula | Molecular weight (MW) | Quality (%) |
|---|---|---|---|---|---|---|
| MUSC 115T | 1 | 9.684 | Methyllaurate | C8H10 | 106 | 80 |
| 2 | 44.422 | 2,4-di-tert-butyl phenol | C14H22O | 206 | 97 | |
| 3 | 51.592 | (3R,8aS)-3-methyl-1,2,3,4,6,7,8,8a-octahydropyrrolo[1,2-a]pyrazine-1,4-dione | C8H12N2O2 | 168 | 90 | |
| 4 | 53.188 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 154 | 96 | |
| 5 | 59.008 | 1,4-diaza-2,5-dioxo-3-isobutyl bicyclo[4.3.0]nonane | C11H18N2O2 | 210 | 83 | |
| 6 | 70.761 | 3-benzyl-1,4-diaza-2,5-dioxobicyclo[4.3.0]nonane | C14H16N2O2 | 244 | 76 | |
| MUSC 117T | 7 | 9.936 | Butanoic acid, 3-methyl- | C5H10O2 | 102 | 72 |
| 8 | 10.880 | Butanoic acid, 2-methyl- | C5H10O2 | 102 | 53 | |
| 9 | 44.428 | 2,4-di-tert-butyl phenol | C14H22O | 206 | 95 | |
| 10 | 51.563 | (3R,8aS)-3-Methyl-1,2,3,4,6,7,8,8a-octahydropyrrolo[1,2-a]pyrazine-1,4-dione | C8H12N2O2 | 168 | 90 | |
| 11 | 53.137 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 154 | 96 | |
| 12 | 54.865 | Methyl n-pentadecanoate | C16H32O2 | 256 | 93 | |
| 13 | 59.025 | 1,4-diaza-2,5-dioxo-3-isobutyl bicyclo[4.3.0]nonane | C11H18N2O2 | 210 | 64 | |
| 14 | 59.174 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolo[1,2-a:1′,2′-d]pyrazine | C14H22N2O2 | 250 | 53 | |
| 15 | 61.462 | Methyl 14-methylhexadecanoate | C18H36O2 | 284 | 93 | |
| 16 | 70.749 | pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- | C14H16N2O2 | 244 | 92 | |
| MUSC 78T | 17 | 7.538 | 2-Methylpyrazine | C5H6N2 | 94 | 80 |
| 18 | 9.181 | Pyrrole, 2-methyl- | C5H7N | 81 | 80 | |
| 19 | 13.438 | Pyrazine, 2,5-dimethyl- | C6H8N2 | 108 | 80 | |
| 20 | 17.094 | 2,3,4-Trithiapentane | C2H6S3 | 126 | 72 | |
| 21 | 19.383 | Pyrazine, 2-ethyl-6-methyl- | C7H10N2 | 122 | 60 | |
| 22 | 19.480 | Pyrazine, 2-ethyl-5-methyl- | C7H10N2 | 122 | 95 | |
| 23 | 19.555 | Pyrazine, trimethyl- | C7H10N2 | 122 | 87 | |
| 24 | 24.184 | Pyrazine, 3-ethyl-2,5-dimethyl- | C8H12N2 | 136 | 90 | |
| 25 | 25.900 | 4H-Pyran-4-one, 3-hydroxy-2-methyl- | C6H6O3 | 126 | 70 | |
| 26 | 34.935 | 1H-Indole | C8H7N | 117 | 95 | |
| 27 | 44.439 | 2,4-di-tert-butyl phenol | C14H22O | 206 | 96 | |
| 28 | 45.567 | 1H-Pyrrole, 2-phenyl- | C10H9N | 143 | 87 | |
| 29 | 49.475 | 1-Naphthalenamine, N-ethyl- | C12H13N | 171 | 90 | |
| 30 | 50.213 | 3,4-Dimethyl-2-phenyl-1H-pyrrole | C12H13N | 171 | 72 | |
| 31 | 51.649 | (3R,8aS)-3-Methyl-1,2,3,4,6,7,8,8a-octahydropyrrolo[1,2-a]pyrazine-1,4-dione | C8H12N2O2 | 168 | 90 | |
| 32 | 53.349 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 154 | 97 | |
| 33 | 54.596 | Methyl 13-methyltetradecanoate | C16H32O2 | 256 | 98 | |
| 34 | 57.995 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 93 | |
| 35 | 59.122 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C11H18N2O2 | 210 | 95 | |
| 36 | 70.743 | 3-benzyl-1,4-diaza-2,5-dioxobicyclo[4.3.0]nonane | C14H16N2O2 | 244 | 92 |
Phenolic are a class of organic compound consisting of a hydroxyl group bonded to an aromatic hydrocarbon group. Among other heterocyclic organic compound, phenolic compounds have attracted the attention of researchers as they are well known for their antioxidant and free radical-scavenging abilities. These potent bioactivities are associated with potential beneficial effects on human health [52]. In fact, phenolic compounds have been reported to possess potent antioxidative, anticancer or anticarcinogenic/antimutagenic, antiatherosclerotic, antibacterial, antiviral, and anti-inflammatory activities [53–56]. Through GC–MS analysis, the phenolic compound known as, 2,4-di-tert-butyl phenol (2,4 DTBP) (2, 9, 27) was detected in all of the extracts tested in current study. Literature has shown that 2,4 DTBP can be produced by microorganisms such as fungus [57] and bacteria [58]. For example, this compound has been detected in Pseudomonas monteilii PsF84 and was found to be effective against Fusarium oxysporum [59]. Besides, the existene of this compound in Lactococcus sp was associated to its antifungal and antioxidant properties as well as its cytotoxic activity [58]. It was also reported that, the antibacterial activity of Monochaetia kansensis could be due to the presence of 2,4 DTBP as well [60].
Another group of compound that was found in this study was pyrazines. Pyrazines are known to exist in form of complex structure with the present of nitrogen atoms in their aromatic ring. These compounds are greatly known for their strong odor properties and have been detected in several bacteria. The value of this group of compound reside with theirs bioactivities, as pyrazines are commonly known to exhibit antimicrobial, anticancer, antioxidant as well as neuroprotection properties [6, 61–64]. In current study, the pyrazines compounds; 19, 21, 22, 23 and 24 were found in M. flava MUSC 78T extract. Previous study has shown that compound 19 and 21 were detected in myxobacteria Stigmatella WXNXJ-B which known to exhibit a significant high level of antitumor activities [65]. Meanwhile, compound 22, 23 and 24 were detected previously in Streptomyces antioxidans and were found to have a strong antioxidant activities [64].
The complex structure form with incorporation of one or more pyrrole compound into a pyrazine is normally known as pyrrolopyrazine. The GC–MS characterization analysis have also demonstrated the existence of pyrrolopyrazine compounds in all of the extracts. For example, compounds such as pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (4, 11, 32) and (3R,8aS)-3-methyl-1,2,3,4,6,7,8,8a-octahydropyrrolo[1,2-a]pyrazine-1,4-dione (3, 10, 31) were found as the constituents of the mixture. Literature has shown that these compounds were detected in different Streptomyces species [6, 64, 66] which include Nocardia sp. [67], and Bacillus sp. [68] and was associated to antioxidant activity. Another pyrrolopyrazine compound identified in MUSC 115T and MUSC 78T strains was 3-benzyl-1,4-diaza-2,5-dioxobicyclo[4.3.0]nonane (BDDB) (6, 36). Gohar et al. [69] have reported the present of BDDB in Burkholderia cepacia may responsible for the antibacterial activity against Aeromonas hydrophila, Edwardsiella tarda and Vibrio ordalli. Besides, the detection of BDDB in Streptomyces cacaoi GY525 [70] was believed to contribute for the mortality of second-stage juvenile and hatch inhibition of Meloidogyne incognita. Therefore, the detection of pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- (16) in S. humi MUSC 117T and pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- (35) in M. flava MUSC 78T might contribute for the observed bioactivities. In fact, these compounds were seen to occur in quite a number of different Streptomcyes species which have demonstrated to exhibit a wide range of bioactivities [6, 64, 66, 71, 72]. For example, the present of these compounds in microorganism has been associated to the strong antibacterial activity against E. coli, P. aeruginosa and E. faecalis [73]. Besides, Hong et al. (2008) [74] have also showed 16 was able to inhibit expression of serine/threonine kinase Akt which may be useful for inhibition of cell proliferation and activation of apoptosis activity in cancer cells. Perhaps, the detection of 1,4-diaza-2,5-dioxo-3-isobutyl bicyclo[4.3.0]nonane (6, 17) in M. mangrovi MUSC 115T and S. humi MUSC 117T may explain for the anticancer activity exhibited by these microorganisms. As the present of those compounds in Streptomyces strains of previous studies were suggested to be responsible for the observed cytotoxic effect on human cancer cell line [5, 75]. Overall, majority of the pyrrolopyrazine compounds detected are known to exhibit antioxidant activity. Since antioxidants were suggested to play important role in cellular mechanisms [76], the detection of pyrrolopyrazine compounds in these strains of bacteria could be contributing to the observed cytotoxic effects on cancer cells and neuroprotective effect on SH-SY5Y cells against the insults of H2O2.
Taken altogether, the existence of heterocyclic compounds such as phenolics, pyrazines and pyrrolopyrazines as part of the constituents of M. mangrovi MUSC 115T, S. humi MUSC 117T and M. flava MUSC 78T extracts may account for the observed antibacterial, anticancer activities as well as the neuroprotective properties. Based on current findings, rare actinobacteria may serve as important sources for the potential new drugs development.
Conclusions
The results have demonstrated M. mangrovi MUSC 115T, S. humi MUSC 117T and M. flava MUSC 78T possessed antibacterial, anticancer and neuroprotective activities. The chemical analysis study afforded a further in depth understanding on the mixture of chemical constituents that present in these strains of bacteria. Based on the literature evidences, the occurrence of these chemical compounds might accounted for the observed bioactivities. In short, the current study has showed these novel rare actinobacteria were able to produce a wide range of bioactive compounds which could serve as potential sources for future drug development. Further in depth studies focusing on isolation and characterization of bioactive principle(s) through bioassay-guided isolation is currently undertaking. As we deeply believe the procedure will eventually enabling us to identify the bioactive principle(s) that present in these mixtures and the findings might potentially generate useful knowledge for the future development of new drug(s).
Acknowledgements
Funding was provided by MOSTI eScience Funds (Grant Nos. 02-02-10-SF0215, 06-02-10-SF0300) and University of Malaya for High Impact Research Grant (Grant Nos. H-50001-A000027, A000001-50001).
Contributor Information
Bey-Hing Goh, Email: goh.bey.hing@monash.edu.
Learn-Han Lee, Email: lee.learn.han@monash.edu.
References
- 1.Qin S, Li J, Chen HH, Zhao GZ, Zhu WY, Jiang CL, Xu LH, Li WJ. Isolation, diversity, and antimicrobial activity of rare actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl Environ Microbiol. 2009;75:6176–6186. doi: 10.1128/AEM.01034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manivasagan P, Venkatesan J, Sivakumar K, Kim SK. Marine actinobacterial metabolites: current status and future perspectives. Microbiol Res. 2013;168:311–332. doi: 10.1016/j.micres.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Subramani R, Aalbersberg W. Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol Res. 2012;167:571–580. doi: 10.1016/j.micres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Lee LH, Zainal N, Azman AS, Eng SK, Ab Mutalib NS, Yin WF, Chan KG. Streptomyces pluripotens sp. nov., a bacteriocin-producing streptomycete that inhibits methicillin-resistant Staphylococcus aureus. Int J Syst Evol Microbiol. 2014;64:3297–3306. doi: 10.1099/ijs.0.065045-0. [DOI] [PubMed] [Google Scholar]
- 5.Ser HL, Ab Mutalib NS, Yin WF, Chan KG, Goh BH, Lee LH. Evaluation of antioxidative and cytotoxic activities of Streptomyces pluripotens MUSC 137 isolated from mangrove soil in Malaysia. Front Microbiol. 2015;6:1398. doi: 10.3389/fmicb.2015.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan LTH, Ser HL, Yin WF, Chan KG, Lee LH, Goh BH. Investigation of antioxidative and anticancer potentials of Streptomyces sp. MUM256 isolated from Malaysia mangrove soil. Front Microbiol. 2015;6:1316. doi: 10.3389/fmicb.2015.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ser HL, Law JWF, Chaiyakunapruk N, Jacob SA, Palanisamy UD, Chan KG, Goh BH, Lee LH. Fermentation conditions that affect clavulanic acid production in Streptomyces clavuligerus: a systematic review. Front Microbiol. 2016;7:522. doi: 10.3389/fmicb.2016.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan LTH, Chan KG, Lee LH, Goh BH. Streptomyces bacteria as potential probiotics in aquaculture. Front Microbiol. 2016;7:79. doi: 10.3389/fmicb.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 10.Hong K, Gao AH, Xie QY, Gao H, Zhuang L, Lin HP, Yu HP, Li J, Yao XS, Goodfellow M, Ruan JS. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar Drugs. 2009;7:24–44. doi: 10.3390/md7010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee LH, Zainal N, Azman AS, Eng SK, Goh BH, Yin WF, Ab. Mutalib NS, Chan KG (2014a) Diversity and antimicrobial activities of actinobacteria isolated from tropical mangrove sediments in Malaysia. Sci World J 2014: Article ID: 698178 [DOI] [PMC free article] [PubMed]
- 12.Hamedi J, Imanparast S, Mohammadipanah F. Molecular, chemical and biological screening of soil actinomycete isolates in seeking bioactive peptide metabolites. Iran J Microbiol. 2015;7:23–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Neha S, Sandeep S. Microbial secondary metabolites as potential anti-infective agents. Int J Pharm Sci Rev Res. 2014;2:767–773. [Google Scholar]
- 14.Azman AS, Othman I, Velu SS, Chan KG, Lee LH. Mangrove rare actinobacteria: taxonomy, natural compound, and discovery of bioactivity. Front Microbiol. 2015;6:856. doi: 10.3389/fmicb.2015.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiwari K, Gupta RK. Rare actinomycetes: a potential storehouse for novel antibiotics. Crit Rev Biotechnol. 2012;32:108–132. doi: 10.3109/07388551.2011.562482. [DOI] [PubMed] [Google Scholar]
- 16.Goodfellow M. Selective isolation of Actinobacteria. In: Baltz RH, Davies J, Demain AL, editors. Manual of industrial microbiology and biotechnology. Washington: ASM; 2010. pp. 13–27. [Google Scholar]
- 17.Xu DB, Ye WW, Han Y, Deng ZX, Hong K. Natural products from mangrove actinomycetes. Mar Drugs. 2014;12:2590–2613. doi: 10.3390/md12052590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee LH, Azman AS, Zainal N, Eng SK, Ab Mutalib NS, Yin WF, Chan KG. Microbacterium mangrovi sp. nov., an amylolytic actinobacterium isolated from mangrove forest soil. Int J Syst Evol Microbiol. 2014;64:3513–3519. doi: 10.1099/ijs.0.062414-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee LH, Azman AS, Zainal N, Yin WF, Mutalib NS, Chan KG. Sinomonas humi sp. nov., an amylolytic actinobacterium isolated from mangrove forest soil. Int J Syst Evol Microbiol. 2015;65:996–1002. doi: 10.1099/ijs.0.000053. [DOI] [PubMed] [Google Scholar]
- 20.Azman AS, Zainal N, Ab Mutalib NS, Yin WF, Chan KG, Lee LH. Monashia flava gen. nov., sp. nov., a novel actinobacterium of the family Intrasporangiaceae. Int J Syst Evol Microbiol. 2015 doi: 10.1099/ijsem.0.000753. [DOI] [PubMed] [Google Scholar]
- 21.Mangamuri UK, Vijayalakshmi M, Poda S, Manavathi B, Bhujangarao C, Venkateswarlu Y. Bioactive metabolites produced by Pseudonocardia endophytica VUK-10 from mangrove sediments: isolation, chemical structure determination and bioactivity. J Microbiol Biotechnol. 2015;25:629–636. doi: 10.4014/jmb.1407.07041. [DOI] [PubMed] [Google Scholar]
- 22.Janardhan A, Kumar AP, Viswanath B, Saigopal DVR, Narasimha G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol Res Int. 2014;55:1897–1901. doi: 10.1155/2014/217030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams PG, Buchanan GO, Feling RH, Kauffman CA, Jensen PR, Fenical W. New cytotoxic salinosporamides from the marine Actinomycete Salinispora tropica. J Org Chem. 2005;70:6196–6203. doi: 10.1021/jo050511+. [DOI] [PubMed] [Google Scholar]
- 24.Lee LH, Cheah YK, Sidik SM, Ab Mutalib NS, Tang YL, Lin HP, Hong K. Molecular characterization of Antarctic actinobacteria and screening for antibacterial metabolite production. World J Microbiol Biotechnol. 2012;28:2125–2137. doi: 10.1007/s11274-012-1018-1. [DOI] [PubMed] [Google Scholar]
- 25.Ser HL, Palanisamy UD, Yin WF, Chan KG, Goh BH, Lee LH. Streptomyces malaysiense sp. nov.: a novel Malaysian mangrove soil actinobacterium with antioxidative activity and cytotoxic potential against human cancer cell lines. Sci Rep. 2016;6:24247. doi: 10.1038/srep24247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 27.Supriady H, Kamarudin MNA, Chan CK, Goh BH, Kadir HA. SMEAF attenuates the production of pro-inflammatory mediators through the inactivation of Akt-dependent NF-κB, p38 and ERK1/2 pathways in LPS-stimulated BV-2 microglial cells. J Funct Foods. 2015;17:434–448. doi: 10.1016/j.jff.2015.05.042. [DOI] [Google Scholar]
- 28.Ser HL, Zainal N, Palanisamy UD, Goh BH, Chan KG, Lee LH. Streptomyces gilvigriseus sp. nov., a novel actinobaterium isolated from mangrove forest soil. Antonie Van Leeuwenhoek. 2015;107:1369–1378. doi: 10.1007/s10482-015-0431-5. [DOI] [PubMed] [Google Scholar]
- 29.Ocampo PS, Lazar V, Papp B, Arnoldini M, Wiesch PA, Busa-Fekete R, Fekete G, Pal C, Ackermann M, Bonhoeffer S. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob Agents Chemother. 2014;58:4573–4582. doi: 10.1128/AAC.02463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 31.Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp. 2011;54:2899. doi: 10.3791/2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y-B, Wang X, Meister EA, Gong K-R, Yan S-C, Lu G-W, Ji X-M, Shao G. The effects of CoCl(2) on HIF-1α protein under experimental conditions of autoprogressive hypoxia using mouse models. Int J Mol Sci. 2014;15:10999–11012. doi: 10.3390/ijms150610999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasselli F, Basini G, Bussolati S, Bianco F. Cobalt chloride, a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod Fertil Dev. 2005;17:715–720. doi: 10.1071/RD05059. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Gao D. Overexpression of cytoglobin gene inhibits hypoxic injury to SH-SY5Y neuroblastoma cells. Neural Regen Res. 2013;8:2198–2203. doi: 10.3969/j.issn.1673-5374.2013.23.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SG, Lee H, Rho HM. Transcriptional repression of the human p53 gene by cobalt chloride mimicking hypoxia. FEBS Lett. 2001;507:259–263. doi: 10.1016/S0014-5793(01)02989-1. [DOI] [PubMed] [Google Scholar]
- 36.Vengellur A, LaPres JJ. The role of hypoxia inducible factor 1alpha in cobalt chloride induced cell death in mouse embryonic fibroblasts. Toxicol Sci. 2004;82:638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- 37.Chow JM, Shen SC, Huan SK, Lin HY, Chen YC. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem Pharmacol. 2005;69:1839–1851. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Shivapriya S, Ilango K, Agrawal A, Dubey GP. Effect of Hippophae rhamnoides on cognitive enhancement via neurochemical modulation in scopolamine induced Sprague Dawley rats. Int J Pharm Sci Res. 2014;5:4153–4158. [Google Scholar]
- 39.Triana-Vidal LE, Carvajal-Varona SM. Protective effect of galantamine against oxidative damage using human lymphocytes: a novel in vitro model. Arch Med Res. 2013;44:85–92. doi: 10.1016/j.arcmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Facecchia K, Fochesato LA, Ray SD, Stohs SJ, Pandey S. Oxidative toxicity in neurodegenerative diseases: role of mitochondrial dysfunction and therapeutic strategies. J Toxicol. 2011;2011:683728. doi: 10.1155/2011/683728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konyalioglu S, Armagan G, Yalcin A, Atalayin C, Dagci T. Effects of resveratrol on hydrogen peroxide-induced oxidative stress in embryonic neural stem cells. Neural Regen Res. 2013;8:485–495. doi: 10.3969/j.issn.1673-5374.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuljis RO. Integrative understanding of emergent brain properties, quantum brain hypotheses, and connectome alterations in dementia are key challenges to conquer Alzheimer’s disease. Front Neurol. 2010;1:15. doi: 10.3389/fneur.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salkovic-Petrisic M. Amyloid cascade hypothesis: is it true for sporadic Alzheimer’s disease. Period Biol. 2008;110:17–25. [Google Scholar]
- 45.Wang P, Jiang S, Cui Y, Yue Z, Su C, Sun J, Sheng S, Tian J. The n-terminal 5-MER peptide analogue P165 of amyloid precursor protein exerts protective effects on SH-SY5Y cells and rat hippocampus neuronal synapses. Neuroscience. 2011;173:169–178. doi: 10.1016/j.neuroscience.2010.10.069. [DOI] [PubMed] [Google Scholar]
- 46.Saxena G, Singh SP, Agrawal R, Nath C. Effect of donepezil and tacrine on oxidative stress in intracerebral streptozotocin-induced model of dementia in mice. Eur J Pharmacol. 2008;581:283–289. doi: 10.1016/j.ejphar.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Stuchbury G, Munch G. Alzheimer’s associated inflammation, potential drug targets and future therapies. J Neural Transm. 2005;112:429–453. doi: 10.1007/s00702-004-0188-x. [DOI] [PubMed] [Google Scholar]
- 48.Mandelkow E, von Bergen M, Biernat J, Mandelkow EM. Structural principles of tau and the paired helical filaments of Alzheimer’s disease. Brain Pathol. 2007;17:83–90. doi: 10.1111/j.1750-3639.2007.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samy DM, Ismail CA, Nassra RA, Zeitoun TM, Nomair AM. Downstream modulation of extrinsic apoptotic pathway in streptozotocin-induced Alzheimer’s dementia in rats: erythropoietin versus curcumin. Eur J Pharmacol. 2016;770:52–60. doi: 10.1016/j.ejphar.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 50.Karanja E, Boga H, Muigai A, Wamunyokoli F, Kinyua J, Nonoh J (2010) Growth characteristics and production of secondary metabolites from selected novel Streptomyces species isolated from selected Kenyan national parks. Sci Conf Proc, pp 51–80. http://journals.jkuat.ac.ke/index.php/jscp/article/view/672
- 51.Jog R, Pandya M, Nareshkumar G, Rajkumar S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology. 2014;160:778–788. doi: 10.1099/mic.0.074146-0. [DOI] [PubMed] [Google Scholar]
- 52.Pereira D, Valentão P, Pereira J, Andrade P. Phenolics: from chemistry to biology. Molecules. 2009;14:2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- 53.Han XZ, Shen T, Lou HX. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 54.Baidez AG, Gomez P, Del Rio JA, Ortuno A. Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb: role of phenolic compounds in plant defense mechanism. J Agric Food Chem. 2007;55:3373–3377. doi: 10.1021/jf063166d. [DOI] [PubMed] [Google Scholar]
- 55.Veeriah S, Kautenburger T, Habermann N, Sauer J, Dietrich H, Will F, Pool-Zobel BL. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol Carcinogen. 2006;45:164–174. doi: 10.1002/mc.20158. [DOI] [PubMed] [Google Scholar]
- 56.Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer. 2000;36:1235–1247. doi: 10.1016/S0959-8049(00)00103-9. [DOI] [PubMed] [Google Scholar]
- 57.Lenartowicz P, Kafarski P, Lipok J. The overporoduction of 2,4-DTBP accompanying to the lack of available form of phosphorus during the biodegradative utilization of aminophosphonates by Aspergillus terreus. Biodegradation. 2015;26:65–76. doi: 10.1007/s10532-014-9716-z. [DOI] [PubMed] [Google Scholar]
- 58.Varsha KK, Devendra L, Shilpa G, Priya S, Pandey A, Nampoothiri KM. 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int J Food Microbiol. 2015;11:44–50. doi: 10.1016/j.ijfoodmicro.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 59.Dharni S, Sanchita Maurya A, Samad A, Srivastava SK, Sharma A, Patra DD. Purification, characterization, and in vitro activity of 2,4-Di-tert-butylphenol from Pseudomonas monteilii PsF84: conformational and molecular docking studies. J Agric Food Chem. 2014;62:6138–6146. doi: 10.1021/jf5001138. [DOI] [PubMed] [Google Scholar]
- 60.Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Glob J Pharmacol. 2012;6:65–71. [Google Scholar]
- 61.Premkumar T, Govindarajan S. Antimicrobial study of pyrazine, pyrazole and imidazole carboxylic acids and their hydrazinium salts. World J Microbiol Biotech. 2005;21:479–480. doi: 10.1007/s11274-004-2041-7. [DOI] [Google Scholar]
- 62.Jia J, Zhang X, Hu YS, Wu Y, Wang QZ, Li NN, Wu CQ, Yu HX, Guo QC. Protective effect of tetraethyl pyrazine against focal cerebral ischemia/reperfusion injury in rats: therapeutic time window and its mechanism. Thromb Res. 2009;123:727–730. doi: 10.1016/j.thromres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Baldwin MV, Arikkatt SD, Sindhu TJ, Chanran M, Bhat AR, Krishnakumar K. A review of biological potential of pyrazine and related heterocyclic compounds. World J Pharm Pharmaceut Sci. 2013;3:1124–1132. [Google Scholar]
- 64.Ser HL, Tan LTH, Palanisamy UD, Abd Malek SN, Yin WF, Chan KG, Goh BH, Lee LH. Streptomyces antioxidans sp. nov., a novel mangrove soil actinobacterium with antioxidative and neuroprotective potentials. Front Microbiol. 2016;7:899. doi: 10.3389/fmicb.2016.00899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang DH, Tao WY. Antitumor activity in vitro and volatile components of metabolites from myxobacteria Stigmatella WXNXJ-B. Afr J Microbiol Res. 2009;3:755–760. [Google Scholar]
- 66.Ser HL, Ab Mutalib NS, Yin WF, Chan KG, Goh BH, Lee LH. Evaluation of antioxidative and cytotoxic activities of Streptomyces pluripotens MUSC 137 isolated from mangrove soil in Malaysia. Front Microbiol. 2015;6:1398. doi: 10.3389/fmicb.2015.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma P, Kalita MC, Thakur D. Broad spectrum antimicrobial activity of forest-derived soil Actinomycete, Nocardia sp. PB-52. Front Microbiol. 2016;7:347. doi: 10.3389/fmicb.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gopi M, Dhayanithi NB, Devi KN, Kumar TTA. Marine natural product, Pyrrolo [1,2-a] pyrazine–1,4-dione, hexahydro-(C7H10N2O2) of antioxidant properties from Bacillus species at Lakshadweep archipelago. J Coast Life Med. 2014;2:632–637. [Google Scholar]
- 69.Gohar YM, El-Naggar MMA, Soliman MK, Barakat KM. Characterization of marine Burkholderia cepacia antibacterial agents. J Nat Prod. 2010;3:86–94. [Google Scholar]
- 70.Yoon GY, Lee YS, Lee SY, Park RD, Hyun HN, Nam Y, Kim KY. Effects on Meloidogyne incognita of chitinase, glucanase and a secondary metabolite from Streptomyces cacaoi GY525. Nematology. 2012;14:175–184. doi: 10.1163/138855411X584124. [DOI] [Google Scholar]
- 71.Manimaran M, Gopal JV, Kannabiran K. Antibacterial activity of Streptomyces sp. VITMK1 isolated from mangrove soil of Pichavaram, Tamil Nadu, India. Proc Indian Acad Sci Sect B. 2015 [Google Scholar]
- 72.Narasaiah BC, Leelavathi V, Sudhakar G, Mariyadasu P, Swapna G, Manne AK. Isolation and structural confirmation of bioactive compounds produced by the strain Streptomyces albus CN-4. IOSR J Pharm Biol Sci. 2014;9:49–54. [Google Scholar]
- 73.Melo IS, Santos SN, Rosa LH, Parma MM, Silva LJ, Queiroz SCN, Pellizari VH. Isolation and biological activities of an endophytic Mortierella alpine strain from the Antarctic moss Schistidium antarctici. Extremophiles. 2013;18:15. doi: 10.1007/s00792-013-0588-7. [DOI] [PubMed] [Google Scholar]
- 74.Hong S, Moon BH, Yong Y, Shin SY, Lee YH, Lim Y. Inhibitory effect against Akt of cyclic dipeptides isolated from Bacillus sp. J Microbiol Biotechnol. 2008;18:682–685. [PubMed] [Google Scholar]
- 75.Narendhran S, Rajiv P, Vanathi P, Sivaraj R. Spectroscopic analysis of bioactive compounds from Streptomyces cavouresis KU-V39: evaluation of antioxidant and cytotoxicity activity. Int J Pharm Pharmaceut Sci. 2014;6:322. [Google Scholar]
- 76.Chen HM, Wu YC, Chia YC, Chang FR, Hsu HK, Hsieh YC, Chen CC, Yuan SS. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–171. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]