Abstract
Nitrilases are commercial biocatalysts used for the synthesis of plastics, paints, fibers in the chemical industries, pharmaceutical drugs and herbicides for agricultural uses. Nitrilase hydrolyses the nitriles and dinitriles to their corresponding carboxylic acids and ammonia. They have a broad range of substrate specificities as well as enantio-, regio- and chemo-selective properties which make them useful for biotransformation of nitriles to important compounds because of which they are considered as ‘Green Catalysts’. Nitriles are widespread in nature and synthesized as a consequence of anthropogenic and biological activities. These are also present in certain plant species and are known to cause environmental pollution. Biotransformation using native organisms as catalysts tends to be insufficient since the enzyme of interest has very low amount in the total cellular protein, rate of reaction is slow along with the instability of enzymes. Therefore, to overcome these limitations, bioengineering offers an alternative approach to alter the properties of enzymes to enhance the applicability and stability. The present review highlights the aspects of producing the recombinant microorganisms and overexpressing the enzyme of interest for the enhanced stability at high temperatures, immobilization techniques, extremes of pH, organic solvents and hydrolysing dintriles to chiral compounds which may enhance the possibilities for creating specific enzymes for biotransformation.
Keywords: Biotransformation, Bioremediation, Green catalyst, Nitrilases, Thermostable
Introduction
Nitriles are the cyanide containing compounds wide spread in the nature and produced by various plants in form cyanogenic glycosides, cyanolipids, ricine, phenylacetonitrile, b-cyanoalanine etc. as well as by certain animals and insects as metabolic products [1]. Anthropogenic activities have also a major contribution in the production of large amounts of nitrile compounds [2]. Nitriles are toxic in nature and are known to cause environmental pollution. The main supply of cyanide in soil and water is by the release of effluents with a range of inorganic cyanide and nitriles. The widespread use of herbicides containing the Nitrile group such as 2,6-dichloro-benzonitrile and bromoxynil (3,5-dibromo-4 hydroxybenzonitrile) has also resulted in soil pollution. In addition, automobile exhaust also contributed to nitrile pollution. Nitrite toxicity is mostly related to abdominal pain, convulsions, labored breathing, sore throat, unconsciousness and kidney damage. There are various reports on use of enzymes and technologies based on quorum sensing, gene editing, computational modelling, thermostable enzymes, technological improvements which are paving new ideas for relevant enzymes and can be crucial for utilizing nitriliases through bioengineering [3–10]. Moreover the use of nitrous and monooxygenases for bioremediation is also well described along with similar related studies in towards describing interactions through nitrogen assimilation technologies [11–15].
Nitrile compounds can be degraded in nature through microbial activities or in the presence of strong acids or base. Chemical degradation of nitriles produces the corresponding carboxylic acid and ammonia along with a large amount of intermediates. Biological degradation involves nitrile hydrolysing enzymes that can metabolize different nitrile compounds into their corresponding acids. Nitrile hydrolysing enzymes, include, nitrilases (EC 3.5.5.1), nitrile hydratase (EC 4.2.1.84), and amidase (EC 3.5.1.4). Theses enzymes are widely exploited for the synthesis of industrially important amides and organic acids. Nitrilase enzyme catalyses biotransformation of nitriles to corresponding acid and ammonia in a single step pathway, whereas, nitrile hydratase initially converts nitrile into corresponding amide which further converted to carboxylic acid and ammonia in the presence of enzyme amides (Fig. 1).
Fig. 1.
Nitrile hydrolysing enzyme catalysed reactions
Historical Background and Producers of Nitrilases
Nitrilase enzymes belong to nitrilase superfamily having cysteine, glutamine and lysine at their catalytic sites with an additional sulfhydryl group which is necessary for biological activity and hence, nitrilases are also known as thiol enzymes [16]. Over the past few years, various nitrile degrading enzymes were reported in a large number of bacteria (Rhodococcous, Arthrobacter, Pseudomonas, Bacillus, Nocardia, Klebsiella etc.), yeasts (Candida, Pichia, Saccharomyces, Hanseniaspora, Debaryomyces, Geotrichum, Williopsis, Torulopsis, Exophiala, Kluyveromyces, Aureobasidium, Cryptococcus,and Rhodotorula,), fungi (Aspergillus, Fusarium, Penicillum, Gibberella etc.) and plants (Brassicaceae, Gramineae, Cruciferae, and Musaceae). Nitrilases in plants are involved in NAD+ synthesis, polyamine biosynthesis and pyrimidine catabolism [17–19]. Plant nitrilase is also involved in conversion of Indole 3-Aceto Nitrile to Indole 3-Aetic Acid (IAA, Auxin), β-cyano-l-alanine to aspartic aid. Microbial nitrilases are involved in nitrogen utilization, nitrile detoxification and modulation of hormone signalling pathway and in gene regulation. These are present everywhere in the environment; soil environment, thermal spring and plant growth promoting rhizobacteria (PGPR). These enzymes have biotechnological importance because of their high potential to degrade several nitrile compounds to commercially valuable products under different in vivo and in vitro conditions. A number of bacteria with certain physiological conditions have been explored as potential producers of nitrilase and listed in Table 1. It has been observed that most of the nitrilases are inducible in nature and expressed in a wide range of temperature and pH.
Table 1.
Microorganisms producing nitrilases
| Microorganisms | Nature of expression | Substrate specificity (Nitriles) | Optimum temperature (°C)/pH | Reference |
|---|---|---|---|---|
| Fusarium solani IMI196840 | Inducible (2-cyanopyridine) | Aromatic | 40–45 °C/8 | [23] |
| Nocardia globerula NHB-2 | Inducible (propionitrile) | Aromatic | 35 °C/7.5 | [24] |
| Rhodococcus rhodochrous BX2 | Inducible (isovaleronitrile) | Aromatic | 45 °C/7.6 | [25] |
| Rhodococcus rhodochorus PA-34 | Inducible (propionitrile) | Aromatic | 35 °C/7.5 | [26] |
| Bacillus pallidus DAC 521 | Inducible (benzonitrile) | Aromatic | 65 °C/7.6 | [27] |
| Aspergillus niger K10 | Inducible (2-cyanopyridine) | Aromatic | 45 °C/8.0 | [28] |
| Pyrococcus abyssi | Inducible (isopropyl-β-d-thiogalactopyranoside) | Aliphatic | 60–80 °C/6.0–8.0 | [20] |
| Streptomyces sp. MTCC 7546 | Inducible (benzonitrile) | Mono and di aliphatic nitrile | 50 °C/7.0–7.4 | [29] |
| Fusarium solani O1 | Inducible (2-cyanopyridine) | Aromatic | 40–45 °C/8.0 | [30] |
| Pseudomonas fluorescens Pf-5. | Inducible | Aliphatic | 45 °C/7.0 | [31] |
| Geobacillus pallidus RAPc8 | Inducible (isopropyl-β-d-thiogalactoside) | Aromatic | 37 °C/8.0 | [21] |
| Rhodobacter sphaeroides LHS-305 | Inducible (acetonitrile) | Aromatic | 30 °C/9.0 | [32] |
| Rhodobacter sphaeroides LHS-305 | Inducible (acetonitrile) | Aromatic | 40 °C/7.0 | [33] |
| Burkholderia cenocepacia J2315 | Inducible | Aromatic | 30 °C/8.0 | [34] |
| Pyrococcus sp. M2D13 | Inducible | Aromatic | 85 °C/7.5 | [35] |
| Stenotrophomonas maltophilia AC21 | Inducible (acetonitrile) | Acetonitrile | 45 °C/8.0 | [36] |
Nitrilase are being exploited for their applicability in different industrial sectors, and it is constantly increasing in order to improve stability of enzymes to carry out catalysis at elevated temperatures, which generally provides rapid reaction rates, organic solvents stability, regeneration of enzymes and storage. The biotransformation using native organisms as catalysts have tended to be insufficient since, the enzyme of interest is very low in the amount of the total cellular proteins and reaction rate is slow and therefore, to overcome these limitations, alteration in the properties of enzymes through bioengineering is the best way which resulted in producing the recombinant microorganisms or over expressing the enzyme of interest in a recombinant organism for their improved stability. Few studies in this direction were attempted where a recombinant Pyrococcus abyssi nitrilase was over expressed in E. coli [20]. The nitrilase from Geobacillus pallidus RAPc8 was cloned and recombinant enzyme produced both acid and amide products from the nitrile substrates [21]. There is a recent report on role of nitrilase in IAA pathway towards plant beneficial activities [22].
Types of Nitrilases
Nitrilase superfamily consists of CN-hydrolases which include enzymes that catalyse non-peptide carbon–nitrogen bonds. According to sequence uniqueness at their active sites, the members of nitrilase superfamily are separated into 13 branches. The 1st branch includes nitrilase, along with cyanide hydratase and cyanide dihydratase and the remaining 12 branches showed amidase activity where aliphatic amidase comes under 2nd branch, amino-terminal amidase belongs to 3rd branch, biotinidase, b-ureidopropionase and carbamylase falls under 4th, 5th and 6th branch respectively. Branch 7th and 8th consisted of prokaryotic and eukaryotic NAD+ synthetase, which is associated with the amidase domain to utilize the ammonia liberated from glutamine as a source of nitrogen for NAD+ synthesis, whereas branch 9th includes apolipoprotein N-acyltransferase and catalyze reverse amidase reaction. The branch 10th, 11th, 12th and 13th consisted of Nit and NitFhit, NB11, NB12 and non-fused outliners respectively [37]. Among all the branches, nitrilase is the most characterized member of the nitrilase superfamily with several instances recognized across kingdoms.
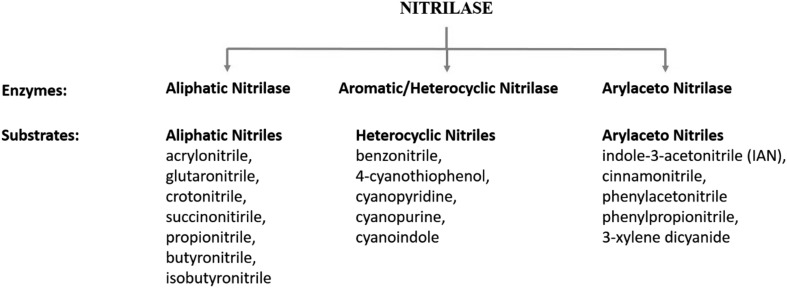
As per their substrate specificity, nitrilases are further categorized into aliphatic nitrilases, aromatic nitrilases and arylacetonitrilases (Fig. 2) [16]. Some novel nitrilases showed broad substrate specificity hydrolyze more than one nitriles [35]. Extremely substrate specific nitrilase was also detected from Streptomyces sp. MTCC 7546 [38].
Fig. 2.
Classification of nitrilases according to their substrate specificity
Bioengineering of Nitrilase Systems
Several studies showed operational instability in nitrilase catalysis and works are being carried out for developing novel nitrilase systems that could withstand extreme conditions of temperature, solvents and high substrate concentration etc. There are a number of ways to obtain stable enzymes with enhanced properties like isolation from extremophilic microorganisms, stabilization of unstable enzymes by modification through immobilization and chemical modification and genetic manipulation and cloning in suitable hosts. Immobilization of nitrilase producing sources and purified enzymes makes biotransformation more cost-effective and repeated reaction along with recovery of catalyst [39–41].
Genetic and enzyme engineering are widely applied for developing novel enzymes with improved stability to elevated temperatures, pH, oxidizing substances and tolerant to organic solvents. Cloning and expression of genomic information into a suitable and rapidly growing host, and site directed mutagenesis have potentials for constructing the particular enzyme with enhanced properties essential for biotransformation. Bromoxynil specific enzyme nitrilase gene (bxn) was cloned and expressed in E. coli as a host organism [42]. Later, a nitrilase encoding gene from A. thaliana was identified, followed by sequencing and expression [22]. It has been reported that specific activity was raised to 35% along with reduction in Km for thiopene-2-acetonitrile by gene manipulation in nitrilase expressed by A. faecalis JM3 [43].
A nitrilase from A. facilis 72 W involved in regio-selective biotransformation showed a 15-fold increased activity over native strain when cloned and expressed under controlled conditions [44] and further specific activity was enhanced to 125 fold during glycolic acid synthesis after optimization of fermentation process [45].
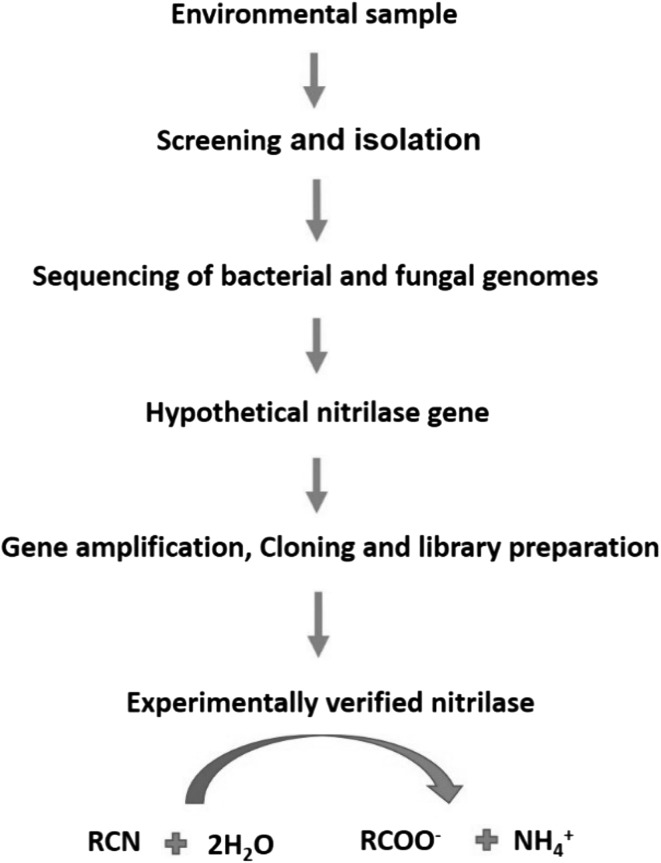
The cloning and expression of a thermoactive nitrilase from P. abyssi in E. coli resulted in creation of a nitrilase with a half-life (t1/2) of 6 h at 90 °C. In case of a recombinant fungal nitrilase from A. Niger, the specific activity was increased two fold. [46]. Further, certain fungal nitrilases from Gibberella, Neurospora, and Aspergillus were expressed in E. coli [47]. Recently, one study was carried out where enantio-selectivity and activity of nitrilase from Burkholderia cenocepacia BCJ2315 enhanced by the use of site-directed mutagenesis [34]. In another recent study, the recombinant nitrilase of Fusarium proliferatum AUF-2 catalysed aliphatic, heterocyclic and aromatic nitriles respectively [48]. Site directed mutagenesis has also attracted the nitrilase modification at the genetic level. Point mutation, single amino acid exchange can alter the properties of the enzyme like specific activity, specificity, selectivity etc. Induced mutation of amino acid tyrosine at 142th position in the nitrilase gene of R. rhodochrous ATCC 33278 demonstrated broad substrate specificity and hydrolyses aromatic nitriles as well as aliphatic nitriles [49]. Figure 3 reveals a brief of the cloning and expression of nitrilase enzyme.
Fig. 3.
Schematic representation of cloning and expression of isolated nitrilase gene
Genetically Engineered Nitrile Producing Microorganisms
Nitrilase producing microorganisms have been proved as an excellent tool to remediate nitrile pollutant from various ecological niches [50]. The Nitrile bioremediation technique is based on three major processes: natural attenuation, bioaugmentation and biostimulation [51]. Natural attenuation is the simplest process of bioremediation in which different samples of soil, water and sediments are monitored only for fluctuations in pollutant concentrations to authenticate the natural pollutant biotransformation is active or not. Biostimulation is a process to stimulate the bioremediation rates by providing some physical or chemical stimulus. Bioaugmentation is the process in which population of contaminant degrading organisms is increased to accelerate the bioremediation rates [52].
During the last fifty years there is considerable increase in toxic pollutants and xenobiotic compounds in environments due to agricultural and industrial activities. There is a number of nitrile compounds as explained in previous sections which are hazardous to the flora and fauna of our ecosystem. Native microfloara is not sufficient to degrade all these toxic nitrile compounds. Various type of nitrilase producing microorganism requires a number of metabolic pathways for complete biodegradation of nitrile pollutants. Further, it is not possible to inhabit such a diverse range of microorganisms to an ecological niche to carry out metabolic reaction that functions to degrade the nitriles compounds.
However, different microbial consortia found at different polluted sites may act as a good source of genetic diversity for several nitriles degrading metabolic pathways. Likewise with the aid of genetically modified microorganisms, it is possible to incorporate various nitriles degrading metabolic pathways into a single microbial strain. Genetically engineered microorganisms (GEMs) are produced through a multidisciplinary approach which includes the involvement of several disciplines such as biochemistry, Bioinformatics, genomics, molecular biology, Organic chemistry, microbiology, and proteomics. The use of native microorganisms is preferred to produce GEMs instead of foreign microbial strains as these indigenous microbes are more prone to interact with the population and can withstand to the complex stressful environmental conditions [53]. There are some reports available where genetically modified microorganisms have been proven as a more efficient bioremediation agent than wild microbial strain applied to eliminate the recalcitrant compounds [54].
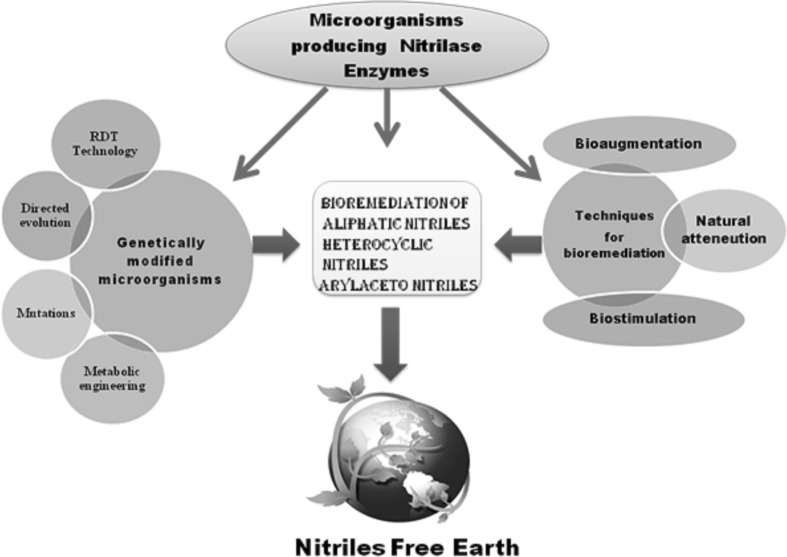
There are a number of methods to obtain genetically engineered nitrilase producing microorganisms such as directed evolution, recombinant DNA Technology, mutations and metabolic engineering, etc. The engineered nitrilase producing organisms has better operational stability like temperature stability, pH stability, wide range of substrate to act upon, higher nitrilase production which are best suitable for the in situ bioremediation of nitrile compounds.
The directed evolution approach has been applied to Alcaligenes faecalis nitrilase (NITAf) to obtain engineered nitrilase with increased activity at its optimal pH as well as improved fitness at low pH values [55]. Another report site directed mutagenesis has been used to engineer Nitrilase PpL19 from Pseudomonas psychrotolerans by Sun et al., 2015 [56]. The nitrilase gene from Acidovorax facilis ZJB09122 was also expressed in Escherichia coli (DE-3) to improve nitrilase activity and production by Zhang et al., 2014 [57]. There are various strategies to improve nitrilase production and their activity together with operational stability. A compilation of different molecular techniques and bioremedial processes has been depicted in Fig. 4.
Fig. 4.
An overview of using genetically modified microorganisms in bioremediation of nitrile compounds
Commercial Applications of Nitrilases
Biotransformation and biocatalysis have gained increasing interest in recent years due to their environmental friendly catalysts i.e. green catalysts, high activities and tremendous selectivity and specificity. Nautiluses are being used for the synthesis of chiral substances besides production of different acids. Chemical manufacturers are increasingly using these enzymes for the synthesis of fine chemicals which are commercially important and pharmaceutically active. The traditional chemical method to produce important carboxylic acids from nostrils has a number of challenges such as the requirement of harsh environment, generation of undesired by-products and low selectivity, as the nitrile bond is very stable. In contrast, microbial degradation of nitriles for the production of compounds of commercial interests has been considered as a proficient way of eliminating extremely toxic nitriles. Nitrilases can be used in synthesis of some key carboxylic acids like p-amino benzoic acid, acrylic acid, nicotinic acid, indole-3-acetic acid, mandelic acid, glycolic acid and 4-hydroxyphenyl acetic acid etc. without altering the functional groups for various uses [38, 41, 58–60]. Nitrilases are also employed for industrial production of acrylamide, nicotinamide and certain other amino acids [61]. In recent years, the nitrile hydrolysing enzymes are being investigated for its use in textile processing unit for improving the quality of fibers minimizing the practice of harsh chemicals for processing purposes [62]. These systems have also been employed for degradation of cyanide group containing herbicides that enter the food chain of the ecosystem. Table 2 provides the commercial production of certain fine chemicals synthesized from nitrilase systems.
Table 2.
Major nitrilase catalysed products
| Producers | Location | Trade products |
|---|---|---|
| Lonza | China | Nicotinic acid |
| Degussa, BASF | Germany | Acrylic acid/esters |
| Nepra, Reilly industries | USA | Niacin/Niacor |
| Mistubishi Rayon | Japan | R-(-mandelic acid) |
Conclusions
Several microorganisms are involved in metabolism of different nitriles, though the degree of hydrolysis varied. Further, these biocatalysts are recognised for synthesis of several pharmaceuticals and other fine chemicals. In view of the great potential of nitrilase systems in generation of chiral compounds and continuing demand of green catalytic practice, attempts have been made to investigate its possible application at industrial scale. The applications of recombinant technology, protein engineering and enzyme technology along with structure and function prediction of nitrilases have led to enhanced stability at operational conditions, high specific activity and selectivity to some extent. Though recent developments in molecular biology broadened the scope of nitrilase catalysis, studies are further needed to utilize these versatile enzymes to their full potential.
Most of the nitrile substrates are poorly soluble in aqueous environments and the use of organic solvents as co-solvents raise the solubility of substrates, enhancing the enzyme substrate reaction. Nevertheless, most of the organic solvents were noted to denature the enzyme protein minimizing the activity of nitrilase. Therefore, nitrilases catalysis in organic solvents needs further investigations. High throughput selection is generally used for the isolation of better biocatalysts with improved property. The rational protein design and three dimensional structure predictions of nitrilases could be a further work for designing a biocatalyst that can hydrolyze a wide range of substrates with tremendous operational stability. Exploring better biocatalysts with improved catalysis is of immense importance for sustainability of industrial process. Hence, the significance of screening and isolation of microorganisms for desired enzymes will always be there.
Acknowledgements
Tesnim Arfi duly acknowledges Senior Research Fellowship of Moulana Azad National Fellowship, U. G. C, Government of India (F1-17.1/2012-13/MANF-2012-13-MUS-JHA-9035), New Delhi, India for pursuing Ph.D.
References
- 1.Sewell BT, Berman MN, Meyers PR, Jandhyala D, Benedik MJ. The cyanide degrading nitrilase from Pseudomonas stutzeri AK61 is a two-fold symmetric, 14-subunit spiral. Structure. 2003;11(11):1413–1422. doi: 10.1016/j.str.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Li Y, Cheng X, Feng L, Xi C, Zhang Y. Immobilization of Rhodococcus rhodochrous BX2 (an acetonitrile-degrading bacterium) with biofilm-forming bacteria for wastewater treatment. Bioresour Technol. 2013;131:390–396. doi: 10.1016/j.biortech.2012.12.140. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Marin-Navarro J, Shukla P. Thermostable microbial xylanases for pulp and paper industries: trends, applications and further perspectives. World J Microbiol Biotechnol. 2016;32(2):1–10. doi: 10.1007/s11274-015-2005-0. [DOI] [PubMed] [Google Scholar]
- 4.Singh PK, Joseph J, Goyal S, Grover A, Shukla P. Functional analysis of the binding model of microbial inulinases using docking and molecular dynamics simulation. J Mol Model. 2016;22(4):1–7. doi: 10.1016/j.jmgm.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Baweja M, Nain L, Kawarabayasi Y, Shukla P. Current technological improvements in enzymes towards their biotechnological applications. Front Microbiol. 2016;7:965. doi: 10.3389/fmicb.2016.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta SK, Shukla P. Advanced technologies for improved expression of recombinant proteins in bacteria: perspectives and applications. Crit Rev Biotechnol. 2016;36(6):1089–1098. doi: 10.3109/07388551.2015.1084264. [DOI] [PubMed] [Google Scholar]
- 7.Baweja M, Singh PK, Shukla P (2015) Enzyme technology, functional proteomics and systems biology towards unraveling molecular basis for functionality and interactions in biotechnological processes. In: Shukla P (ed) Frontier discoveries and innovations in interdisciplinary microbiology. Springer, Berlin, pp 207–212. doi: 10.1007/978-81-322-2610-9
- 8.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31(2):224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2014;37(2):121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 10.Kalia VC, Purohit HJ. Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol. 2008;35(5):403–419. doi: 10.1007/s10295-007-0300-y. [DOI] [PubMed] [Google Scholar]
- 11.Nigam VK, Shukla P. Enzyme based biosensors for detection of environmental pollutants—a review. J Microbiol Biotechnol. 2015;25(11):1773. doi: 10.4014/jmb.1504.04010. [DOI] [PubMed] [Google Scholar]
- 12.Shukla P, Nigam V, Gupta R, Singh A, Kuhad RC (2013) Sustainable enzyme technology for environment: biosensors for monitoring of pollutants and toxic compounds. In: Kuhad RC, Singh A (eds) Biotechnology for environmental management and resource recovery. Springer India, New Delhi, pp 69–76. doi: 10.1007/978-81-322-0876-1
- 13.Singh PK, Imam J, Shukla P (2014) In-silico approach in bioremediation, microbial biodegradation and bioremediation. In: Das S (ed) Microbial biodegradation and bioremediation, 1st edn. Elsevier. doi: 10.1016/B978-0-12-800021-2.00027-3
- 14.Dubey KK, Kumar P, Singh PK, Shukla P (2014) Exploring prospects of mono-oxygenases based bio-catalyst in xenobiotics and their computational modeling. In: Das S (ed) Microbial biodegradation and bioremediation, 1st edn. Elsevier. doi: 10.1016/B978-0-12-800021-2.00027-3
- 15.Kumar V, Baweja M, Singh PK, Shukla P. Recent developments in systems biology and metabolic engineering of plant-microbe interactions. Front Plant Sci. 2016;7:1421. doi: 10.3389/fpls.2016.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podar M, Eads JR, Richardson TH. Evolution of a microbial nitrilase gene family: a comparative and environmental genomics study. BMC Evol Biol. 2005;5:42–46. doi: 10.1186/1471-2148-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janowitz T, Kneifel H, Piotrowski M. Identification and characterization of plant agmatine iminohydrolase, the last missing link in polyamine biosynthesis of plants. FEBS Lett. 2003;544:258–261. doi: 10.1016/S0014-5793(03)00515-5. [DOI] [PubMed] [Google Scholar]
- 18.Baumann S, Sander A, Gurnon JR, Yanai-Balser GM, VanEtten JL, Piotrowski M. Chlorella viruses contains genes encoding a complete polyamine biosynthetic pathway. Virology. 2007;360:209–217. doi: 10.1016/j.virol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piotrowski M, Janowitz T, Kneifel H. Plant C-N hydrolases and the identification of a plant N-carbamylputrescine amidohydrolase involved in polyamine biosynthesis. J Biol Chem. 2003;278:1708–1712. doi: 10.1074/jbc.M205699200. [DOI] [PubMed] [Google Scholar]
- 20.Mueller P, Egorova K, Vorgias CE, Boutou E, Trauthwein H, Verseck S, Antranikian G. Cloning, overexpression, and characterization of a thermoactive nitrilase from the hyperthermophilic archaeon Pyrococcus abyssi. Prot Exp Pur. 2006;47:672–681. doi: 10.1016/j.pep.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Williamson DS, Dent KC, Brandon WW, Varsani A, Frederick J, Thuku RN, Cameron RA, Heerden JHV, Cowan DA, Sewell T. Structural and biochemical characterization of a nitrilase from the thermophilic bacterium, Geobacillus pallidus RAPc8. Appl Microbiol Biotechnol. 2010;88:143–153. doi: 10.1007/s00253-010-2734-9. [DOI] [PubMed] [Google Scholar]
- 22.Shao J, Li S, Zhang N, Cui X, Zhou X, Zhang G, Shen Q, Zhang R. Analysis and cloning of the synthetic pathway of the phytohormone indole-3-acetic acid in the plant-beneficial Bacillus amyloliquefaciens SQR9. Microb Cell Fact. 2015;14(1):130. doi: 10.1186/s12934-015-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vejvoda V, Kubac D, Davidova A, Kaplan O, Sulc M, Sveda O, Chaloupkova R, Martinkova L. Purification and characterization of nitrilase from Fusarium solani IMI196840. Pro Biochem. 2010;45(7):1115–1120. doi: 10.1016/j.procbio.2010.03.033. [DOI] [Google Scholar]
- 24.Sharma NN, Sharma M, Kumar H, Bhalla TC. Nocardia globerula NHB-2: bench scale production of nicotinic acid. Pro Biochem. 2006;41(9):2078–2081. doi: 10.1016/j.procbio.2006.04.007. [DOI] [Google Scholar]
- 25.Fang S, An X, Liu H, Cheng Y, Hou N, Feng L, Huang X, Li C. Enzymatic degradation of aliphatic nitriles by Rhodococcus rhodochrous BX2, a versatile nitrile-degrading bacterium. Biores Technol. 2015;185:24–28. doi: 10.1016/j.biortech.2015.02.078. [DOI] [PubMed] [Google Scholar]
- 26.Bhalla TC, Miura A, Wakamoto A, Ohba Y, Furuhasi K. Asymmteric hydrolysis of α-aminonitriles to optically active amino acids by a nitrilase of Rhodococcus rhodochrous PA-34. Appl Microbiol Biotechnol. 1992;37:184–190. doi: 10.1007/BF00178168. [DOI] [Google Scholar]
- 27.Alamatawah QA, Cramp R, Cowan DA. Characterization of an inducible nitrilase from a thermophilic Bacillus. Extremophiles. 1999;3:283–291. doi: 10.1007/s007920050129. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan O, Nikolau K, Pisvejcova CA, Martinkova L. Hydrolysis of nitriles and amides by filamentous fungi. Enz Microb Technol. 2006;38:260–264. doi: 10.1016/j.enzmictec.2005.07.022. [DOI] [Google Scholar]
- 29.Khandelwal AK, Nigam VK, Chaudhury B, Mohan MK, Ghosh P. Optimization of nitrilase production from a new thermophilic isolate. J Chem Technol Biotechnol. 2007;82:646–651. doi: 10.1002/jctb.1721. [DOI] [Google Scholar]
- 30.Vejvoda V, Kaplan O, Bezouska K, Pompach P, Sulc M, Cantarella M, Benada O, Uhnakova B, Rinagelova A, Wahl SL, Fischer L, Kren V, Martinkova L. Purification and characterization of a nitrilase from Fusarium solani O1. J Mol Catal B Enzym. 2008;50:99–106. doi: 10.1016/j.molcatb.2007.09.006. [DOI] [Google Scholar]
- 31.Kim J, Tiwari MK, Moon H, Jeya M, Ramu T, Oh D, Kim I, Lee J. Identification and characterization of a novel nitrilase from Pseudomonas fluorescens Pf-5. Appl Microbiol Biotechnol. 2009;83:273–283. doi: 10.1007/s00253-009-1862-6. [DOI] [PubMed] [Google Scholar]
- 32.Yang C, Wang X, Wei D. A new nitrilase-producing strain named Rhodobacter sphaeroides LHS-305: biocatalytic characterization and substrate specificity. Appl Biochem Biotechnol. 2011;165:1556–1567. doi: 10.1007/s12010-011-9375-z. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Li Guinan, Li Mingyang, Wei Dongzhi, Wang X. A novel nitrilase from Rhodobacter sphaeroides LHS-305: cloning, heterologous expression and biochemical characterization. World J Microbiol Biotechnol. 2014;30:245–252. doi: 10.1007/s11274-013-1445-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Gao W, Sun H, Chen L, Zhang L, Wang X, Wei D. Protein engineering of a nitrilase from Burkholderia cenocepacia J2315 for efficient and enantioselective production of (r)-o-chloromandelic acid. Appl Environ Microbiol. 2015;81(24):8469–8477. doi: 10.1128/AEM.02688-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennett GV, Blamey JM. A new thermophilic nitrilase from an antarctic hyperthermophillic microorganism. Front Bioeng Biotechnol. 2016;4:1–9. doi: 10.3389/fbioe.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badoei-Dalfard A, Karami Z, Ramezani-pour N. Nitrilase induction and characterization from a newly isolated Stenotrophomonas maltophilia AC21 and its application for bench scale production of nicotinic acid from 3-cyanopyridine. J Mol Catal B Enzym. 2016 [Google Scholar]
- 37.Vergne-Vaxelaire C, Bordier F, Fossey A, Besnard-Gonnet M, Debard A, Mariage A, Salanoubat M. Nitrilase activity screening on structurally diverse substrates: providing biocatalytic tools for organic synthesis. Adv Synth Catal. 2013;355(9):1763–1779. doi: 10.1002/adsc.201201098. [DOI] [Google Scholar]
- 38.Brenner C. Catalysis in the nitrilase superfamily. Curr Opinion Struc Biol. 2002;12:775–782. doi: 10.1016/S0959-440X(02)00387-1. [DOI] [PubMed] [Google Scholar]
- 39.Winkler M, Kaplan O, Vejvoda V, Klempier N, Martinkova L. Biocatalytic application of nitrilases from Fusarium solani O1 and Aspergillus niger K10. J Mol Catal B Enzym. 2009;59:243–247. doi: 10.1016/j.molcatb.2008.06.012. [DOI] [Google Scholar]
- 40.Agarwal A, Nigam VK. Nitrilase mediated conversion of indole-3-acetonitrile to indole-3-acetic acid. Biocatal Agric Biotechnol. 2014;3(4):351–357. [Google Scholar]
- 41.Raj J, Singh N, Prasad S, Seth A, Bhalla TC. Bioconversion of benzonitrile to benzoic acid using free and entrapped cells of Nocardia globerula NHB-2. Acta Microbiol Immunol Hung. 2007;54:79–88. doi: 10.1556/AMicr.54.2007.1.8. [DOI] [PubMed] [Google Scholar]
- 42.Kabaivanova L, Dimitrov P, Boyadzhieva I, Engibarov S, Dobreva E, Emanuilova E. Nitrile degradation by free and immobilized cells of the thermophile Bacillus sp. UG-5B, isolated from polluted industrial water. World J Microbiol Biotechnol. 2008;24:2383–2388. doi: 10.1007/s11274-008-9757-8. [DOI] [Google Scholar]
- 43.Nigam VK, Agarwal A, Sharma M, Ghosh P, Choudhury B. Bioconversion of 3-cyanopyridine to nicotinic acid by a thermostable nitrilase. Res J Biotechnol. 2009;41:33–36. [Google Scholar]
- 44.Chauhan S, Wu S, Blumerman S, Fallon RD, Gavagan JE, DiCosimo R, Payne MS. Purification, cloning, sequencing and over-expression in Escherichia coli of a regioselective aliphatic nitrilase from Acidovorax facilis 72W. Appl Microbiol Biotechnol. 2003;61:118–122. doi: 10.1007/s00253-002-1192-4. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Fogiel AJ, Petrillo KL, Jackson RE, Parker KN, DiCosimo R, Ben-Bassat A, O’Keefe DP, Payne MS. Protein engineering of nitrilase for chemoenzymatic production of glycolic acid. Biotechnol Bioeng. 2008;99:717–720. doi: 10.1002/bit.21643. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan O, Bezouska K, Plihal O, Ettrich R, Kulik N, Vanek O, Kavan D, Benada O, Malandra A, Sveda O, Vesela A, Inagelova A, Slamova K, Cantarella M, Felsberg J, Duskova J, Dohnalek J, Kotik M, Kren V, Martinkova L. Heterologous expression, purification and characterization of nitrilase from Aspergillus niger K10. BMC Biotechnol. 2011;11:1–15. doi: 10.1186/1472-6750-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Kaplan O, Bezouska K, Malandra A, Vesela A, Petrickova A, Felsberg J, Rinagelova A, Kren V, Martinkova L. Genome mining for the discovery of new nitrilases in filamentous fungi. Biotechnol Lett. 2011;33:309–312. doi: 10.1007/s10529-010-0421-7. [DOI] [PubMed] [Google Scholar]
- 48.Yusuf F, Rather IA, Jamwal U, Gandhi SG, Chaubey A. Cloning and functional characterization of nitrilase from Fusarium proliferatum AUF-2 for detoxification of nitriles. Funct Integr Genom. 2015;15(4):413–424. doi: 10.1007/s10142-014-0430-z. [DOI] [PubMed] [Google Scholar]
- 49.Yeom SJ, Kim HJ, Lee JK, Kim DE, Oh DH. An amino acid at position 142 in nitrilase from Rhodococcus rhodochrous ATCC 33278 determines the substrate specificity for aliphatic and aromatic nitriles. Biochem J. 2008;415:401–407. doi: 10.1042/BJ20080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demnerova K, Mackova M, Spevakova V, Beranova K, Kochankova L, Lovecka P, Ryslava E, Macek T. Two approaches to biological decontamination of groundwater and soil polluted by aromatics characterization of microbial populations. Int Microbiol. 2005;8:205–211. [PubMed] [Google Scholar]
- 51.Olaniran AO, Pillay D, Pillay B. Biostimulation and bioaugmentation enhances aerobic biodegradation of dichloroethenes. Chemosphere. 2006;63:600–608. doi: 10.1016/j.chemosphere.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 52.Fantroussi El, Agathos S. Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol. 2005;8:268–275. doi: 10.1016/j.mib.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Singh JS, Abhilash PC, Singh HB, Singh RP, Singh DP. Genetically engineered bacteria: an emerging tool for environmental remediation and future perspectives. Gene. 2011;480(1–2):1–9. doi: 10.1016/j.gene.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 54.de Lorenzo V. Recombinant bacteria for environmental release: what went wrong and what we have learnt from it. Clin Microbiol Infect. 2009;15:63–65. doi: 10.1111/j.1469-0691.2008.02683.x. [DOI] [PubMed] [Google Scholar]
- 55.Schreiner U, Hecher B, Obrowsky S, Waich K, Klempier N, Steinkellner G, Gruber K, Rozzell JD, Glieder A, Winkler M. Directed evolution of Alcaligenes faecalis nitrilase. Enzyme Microb Technol. 2010;47(4):140–146. doi: 10.1016/j.enzmictec.2010.05.012. [DOI] [Google Scholar]
- 56.Sun H, Wang H, Gao W, Chen L, Kai W, We D. Directed evolution of nitrilase PpL19 from Pseudomonas psychrotolerans L19 and identification of enantiocomplementary mutants toward mandelonitrile. Biochem Biophys Res Commun. 2015;468(4):820–825. doi: 10.1016/j.bbrc.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 57.Zhang XH, Liu ZQ, Xue YP, Zheng YG. Activity improvement of a regioselective nitrilase from Acidovorax facilisand its application in the production of 1-(cyanocyclohexyl) acetic acid. Process Biochem. 2014;49(12):2141–2148. doi: 10.1016/j.procbio.2014.08.018. [DOI] [Google Scholar]
- 58.Janowitz T, Trompetter I, Piotrowski M. Evolution of nitrilases in glucosinolate-containing plants. Phytochemistry. 2009;70:1680–1686. doi: 10.1016/j.phytochem.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 59.Arfi T, Agarwal A, Nigam VK. Bioproduction of nicotinic acid. Int J Pharm Technol. 2013;5(2):2622–2631. [Google Scholar]
- 60.Piotrowski M. Primary or secondary? Versatile nitrilases in plant metabolism. Phytochemistry. 2008;69:2655–2667. doi: 10.1016/j.phytochem.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 61.Gong JS, Lu ZM, Li H, Shi JS, Zhou ZM, Xu ZH. Nitrilases in nitrile biocatalysis: recent progress and forthcoming research. Microb Cell Fact. 2012;11:1–18. doi: 10.1186/1475-2859-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S, Gao H, Chen J, Wu J. Surface modification of polyacrylonitrile fibre by nitrile hydratase from Corynebacterium nitrilophilus. Appl Biochem Biotechnol. 2014;174:2058–2066. doi: 10.1007/s12010-014-1186-6. [DOI] [PubMed] [Google Scholar]