Abstract
The present study evaluates the probiotic properties of three Lactobacillus plantarum strains MJM60319, MJM60298, and MJM60399 possessing antimicrobial activity against animal enteric pathogens. The three strains did not show bioamine production, mucinolytic and hemolytic activity and were susceptible to common antibiotics. The L. plantarum strains survived well in the simulated orogastrointestinal transit condition and showed adherence to Caco-2 cells in vitro. The L. plantarum strains showed strong antimicrobial activity against enterotoxigenic Escherichia coli, Shiga toxin-producing E. coli, Salmonella enterica subsp. enterica serovar Typhimurium, Choleraesuis and Gallinarum compared to the commercial probiotic strain Lactobacillus rhamnosus GG. The mechanism of antimicrobial activity of the L. plantarum strains appeared to be by the production of lactic acid. Furthermore, the L. plantarum strains tolerated freeze-drying and maintained higher viability in the presence of cryoprotectants than without cryoprotectants. Finally, the three L. plantarum strains tolerated NaCl up to 8% and maintained >60% growth. These characteristics of the three L. plantarum strains indicate that they could be applied as animal probiotic after appropriate in vivo studies.
Keywords: Lactobacillus plantarum, Antimicrobial, Enteric pathogens, NaCl tolerance, Probiotic
Introduction
Gastrointestinal infections caused by Escherichia coli serotypes and Salmonella enterica serovars are among the major problems hampering livestock production. These pathogens are gram-negative, rod-shaped and belong to the family Enterobacteriaceae and commonly occur in the intestine of warm-blooded animals. Gastrointestinal infections by E. coli and Sal. enterica strains cause illness and death in weaned piglets, young calves and in poultry. E. coli pathotypes such as enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), and enterohemorrhagic E. coli (EHEC) cause diarrhea in animals such as pig, sheep, goat, dog and horse [1]. Sal. enterica subsp. enterica cause severe infections in animals with crucial serotypes being Sal. enterica subsp. enterica serovar Typhimurium and Sal. enterica subsp. enterica serovar Choleraesuis [2]. Salmonella Typhimurium infection in pigs results in inflammation in the gut, diarrhoea and may lead to sepsis [3], whereas serovar Choleraesuis causes septicaemia in pigs [2]. Sal. enterica subsp. enterica serovar Gallinarum biotype Gallinarum cause fowl typhoid in broiler chickens and causes severe economic losses in the poultry industry [4]. The symptoms of fowl typhoid in chickens include anorexia, diarrhea, dehydration and decreased egg production [5].
Several of these disease-causing pathogens are potentially transferred from animals to humans through contact, undercooked meat and other animal products causing outbreaks. Cattle, sheep, goats and poultry are the common cause of such outbreaks, which resulted in a total of 55 enteric disease outbreaks in the United States alone between the years 1991–2005 [6]. Livestock animals are more prone to infection by enteric pathogens due to the crowded and unhygienic environment in animal production systems. Management of these diseases were accomplished with the use of human clinical antibiotics as growth-promoters in animal production systems [7]. However, due to the evolution of antibiotic resistant pathogens, several countries have now banned the use of antibiotics as feed-additives and growth promoters [8]. This scenario has led to the search for alternative strategies for disease prevention and control in livestock animals.
In recent years, there has been interest in the use of probiotics as alternative to antibiotics in the prevention and control of diseases in animal farms. Probiotics are live microbes, which when administered in adequate amount confer beneficial effects in the host animal [9]. Several strains of Lactic acid bacteria (LAB) and Bifidobacteria, which are common inhabitants of animal intestine, are recognized as probiotics. In order to be considered a probiotic, a microbe should satisfy several criteria recommended by the FAO/WHO, which are survival in the gastrointestinal passage, colonization of the intestine, and suppression of intestinal pathogens, modulation of the host intestinal microflora beneficially and stimulation of the host immune system [9]. Additionally, the microbe should also satisfy safety criteria to be used in animals [9].
Probiotics have been shown to be effective in the suppression of enteric pathogen infection in several livestock animals. Probiotic Lactobacillus casei was reported to inhibit the adhesion of enterotoxigenic E. coli K99 to the intestinal mucosa in gnotobiotic lambs [10]. Bifidobacterium lactis Bb12 and Lactobacillus rhamnosus LGG in combination was reported to be probiotic and prevented enteric pathogen adhesion to pig intestinal mucosa [11]. Probiotic Lactobacillus plantarum B2984 was shown to enhance antibody-based immune response against Salmonella Typhimurium in pigs [3]. Probiotics have also been shown to reduce mycotoxin levels in chicken feed when used as a feed-additive [12]. Meta-analysis of randomised controlled trials showed that probiotic supplementation increased body weight gain and feed efficiency in pigs [13] and in broiler chickens [14].
In the present study three Lactobacillus plantarum strains such as MJM60319, MJM60298, and MJM60399 were characterized for their putative probiotic activity, particularly for use against infection in livestock caused by gastrointestinal pathogens such as enterotoxigenic and Shiga toxin-producing E. coli, Sal. enterica serovars Typhimurium, Choleraesuis and Gallinarum for the general health of the livestock animals such as swine, cattle and poultry.
Materials and Methods
Isolation of LAB Strains and Safety Assessment
Lactic acid bacteria (LAB) strains were isolated from fermented cow milk called “Thayir”, a yogurt-type dairy product collected from the Indian state of Tamil Nadu. The LAB were isolated using de Man, Rogasa and Sharpe (MRS) medium. LAB were isolated based on morphology and subcultured in MRS medium. The LAB were then screened for safety assessments such as hemolytic activity, bioamine production, mucin degradation and antibiotic susceptibility to European Food Safety Authority (EFSA) recommended antibiotics.
Biogenic Amine Production Test
The biogenic amine production of the isolates was determined using decarboxylase medium (0.5% Tryptone, 0.5% yeast extract, 0.5% beef extract, 0.25% NaCl, 0.05% glucose, 0.1% tween 80, 0.02% MgSO4, 0.005% MnSO4, 0.004% FeSO4, 0.2% Ammonium citrate, 0.001% Thiamine, 0.2% K2HPO4, 0.01% calcium carbonate, 0.005% pyridoxal 5-phosphate, 1% Amino acid, 0.006% bromocresol purple, 2% agar, pH 5.3) as described previously [15]. The amino acids were l-tyrosine, l-histidine, l-ornithine, l-arginine, l-phenylalanine, l-lysine or l-tryptophan. Control medium was without any amino acids.
Mucin Degradation Test
Mucin degradation was studied using 0.3% porcine stomach mucin supplied agarose medium with or without glucose [16]. The basic composition of the medium included (g L−1): tryptone—7.5; casitone—7.5; yeast extract—3.0; meat extract—5.0; NaCl—5.0; K2HPO4·3H2O—3.0; KH2PO4—0.5; MgSO4·7H2O—0.5; cysteine HCl—0.5; resazurin—0.002. d-(+)-glucose—3, purified hog gastric mucin—0.5, and agarose—1.5. The pH of medium was adjusted to 7.2 ± 0.2 with 2 N NaOH. Ten microlitre of 24-h viable bacterial cultures were inoculated onto the surface of the agarose medium in a petri dish. The plates were incubated at 37 °C anaerobically (BBL Gas Pack System) for 72 h and subsequently stained with 0.1% Amido black in 3.5 M acetic acid for 30 min. They were then washed with 1.2 M acetic acid until the mucin lysis zone (discoloured halo) around the colony of positive control cultures (faecal flora) appeared. The mucin degradation activity was defined as the size of the mucin lysis zone. Bacillus sp. from human faeces was used as the positive control.
Hemolytic Activity
Hemolytic activity of LAB strains was assessed with Columbia blood agar base (BD, Difco) containing 5% defibrinated sheep blood. A zone of clearance around the colony in blood agar indicates positive for hemolytic activity otherwise negative.
Susceptibility of LAB to Antibiotics
The susceptibility of LAB to antibiotics was determined by twofold broth microdilution method [17]. The MIC values obtained for the LAB strains were compared with the EFSA cutoff values to determine the antibiotic susceptibility.
Phylogenetic Analysis of Strains MJM60319, MJM60298, and MJM60399
Phylogenetic analysis of strains MJM60319, MJM60298, and MJM60399 was done according to our previous report [18]. Genomic DNA from the three strains was isolated using commercial kit (GeneALL, Seoul, Republic of Korea). The16S rRNA gene sequences of the strains were analyzed according to our previous report [18]. The sequences were compared with 16 rRNA gene sequences in the Eztaxon database using BLAST program and the neighbour joining phylogenetic tree was constructed using MEGA 6 software [19].
GTG5-PCR Fingerprint
The (GTG)5-rep-PCR fingerprinting was performed using the primer (5′-GTGGTGGTGGTGGTG-3′) [20]. A 20 μl total PCR reaction mixture consisted of 1 μl genomic DNA (50 ng), primer 1 μl (100 pmol), and 18 μl sterile deionized water added to maxime i-Taq PCR premix tubes (iNtRON Biotechnology, INC, Gyeonggi-do, Korea). The PCR reaction was performed following the condition: initial denaturation at 95 °C (7 min), denaturation at 90 °C (30 s), annealing at 40 °C (1 min), extension at 65 °C (8 min) and final extension at 65 °C (16 min). The PCR products were visualised using 2% agarose gel electrophoresed using 1× TBE buffer at 130 V for 4 h.
Antimicrobial Activity
The anti-microbial activity of strains MJM60319, MJM60298, and MJM60399 was determined against animal pathogens such as Salmonella Typhimurium KCTC2514 (Sal. enterica subsp. enterica serovar. Typhimurium), Sal. Choleraesuis KCTC2932 (Sal. enterica subsp. enterica serovar. Choleraesuis), Sal. Gallinarum KCTC2931 (Sal. enterica subsp. enterica serovar. Gallinarum biotype Gallinarum), Escherichia coli O138 KCTC2615, E. coli O1 KCTC2441, and E. coli K99 KCTC 2617. The bacterial culture filtrate was concentrated tenfold in a rotary vacuum evaporator and dissolved in sterile distilled water. One hundred microliter of concentrated crude culture filtrate was loaded on to 8 mm paper disc and dried. The disc was placed on the pathogen swabbed LB agar plates and incubated for 18 h and the zone of inhibition was measured. The entire assay was performed in triplicates to check the reproducibility.
Analysis of the Production of d, l-Lactate by Strains MJM60319, MJM60298, and MJM60399
The strains were cultured in MRS liquid medium for 24 h at 37 °C. The culture supernatant was analyzed by d, l-lactate by an enzymatic method using the d, l-Lactate assay kit (Megazyme, Ireland, Cat.No. K-DLATE) following the manufacturer’s protocol.
Physiological Characteristics
Tolerance of Strains MJM60319, MJM60298, and MJM60399 to Oro-Gastrointestinal Transit Condition
Oro-gastro intestinal transit tolerance assay was performed as previously described [21] with minor modification. The LAB strains were initially subjected to oral stress by treating the bacterial cells (1 × 109 mL−1) for 10 min in electrolyte solution (g L−1: NaCl, 6.2; KCl, 2.2; CaCl2, 0.22; NaHCO3, 1.2) [22] containing 150 mg L−1 lysozyme. The cells were removed from oral stress solution by centrifugation at 1800×g for 5 min and subjected to gastric stress by incubating in gastric electrolyte solution containing 0.3% pepsin at pH 3 for 1 h. Then, the cells were removed by centrifugation and incubated for 120 min in the intestinal electrolyte solution (g L−1: NaCl, 5; KCl, 0.6; CaCl2, 0.25) [22] containing 0.1% pancreatin, 0.3% bile oxgall, and pH 7. Strains incubated in PBS without stress were used as control. The cell viability was determined at each step by plating the cells in MRS medium, incubated at 37 °C and counting the number of colony forming units (CFU) after 48 h.
Adhesion of Strains MJM60319, MJM60298, and MJM60399 to Caco-2 Cell Line
Caco-2 cell line was purchased from KCTC, Republic of Korea and routinely cultured in MEM high glucose medium supplemented with 20% (v/v) heat inactivated fetal bovine serum and 1 unit antibiotics, penicillin–streptomycin. For the adhesion assay, well differentiated 21 days cultured Caco-2 cell line was used. Adhesion assay was performed according to the method described previously [23] using the LAB cell concentration of 6.2 ± 0.5 Log CFU mL−1 per well. After adherence assay, the non-adhered LAB was removed by washing thrice with PBS (pH 7.2). The adhered LAB and Caco-2 cells were detached by adding 1 ml of 0.05% (v/v) Triton-X 100 and CFU of adhered bacteria was calculated by serial dilution and spreading the bacterial suspension of appropriate dilution on MRS agar plates. The percentage of adherence was calculated using the formula (% Adhesion = [CFU of adhered bacteria per mL/CFU of initially added bacteria per mL] × 100).
Technological Characteristics
Study of Sodium Chloride Tolerance of Strains MJM60319, MJM60298, and MJM60399
To check the survival of LAB strains in NaCl concentration, 1 mL of 0.5 OD bacterial culture was inoculated into MRS broth containing 1–10% NaCl and incubated for 24 h. The bacterial culture inoculated into MRS broth without NaCl was used as control. After incubation, the bacterial growth was measured at absorbance 600 nm using 96-well plate reader (infinite M200® PRO, Tecan Austria GmbH, Untersbergstr). The percentage growth was calculated using formula (% growth = [AC − AT/AC × 100). Where, AC is the absorbance of control at 600 nm and AT is the absorbance of samples grown in MRS broth containing different NaCl concentrations.
Survival of Strains MJM60319, MJM60298, and MJM60399 to Freeze-Drying
The initial viable bacterial count was adjusted to 10.08 ± 0.32 Log CFU mL−1 with or without 10% cryoprotectant (1:1 ratio of skim milk powder and maltodextrin), frozen at −80 °C and freeze dried under reduced pressure (5 mbar) using a freeze drier (IlShin lab Co., ltd, Republic of Korea) for 18 h. After freeze-drying, the samples were rehydrated using sterile distilled water. The percentage viability was calculated by comparing viable bacteria (CFU/mL) after freeze-drying relative to the initial number of bacteria (CFU/mL) subjected to freeze-drying.
Results
Safety Assessment of the LAB Strains
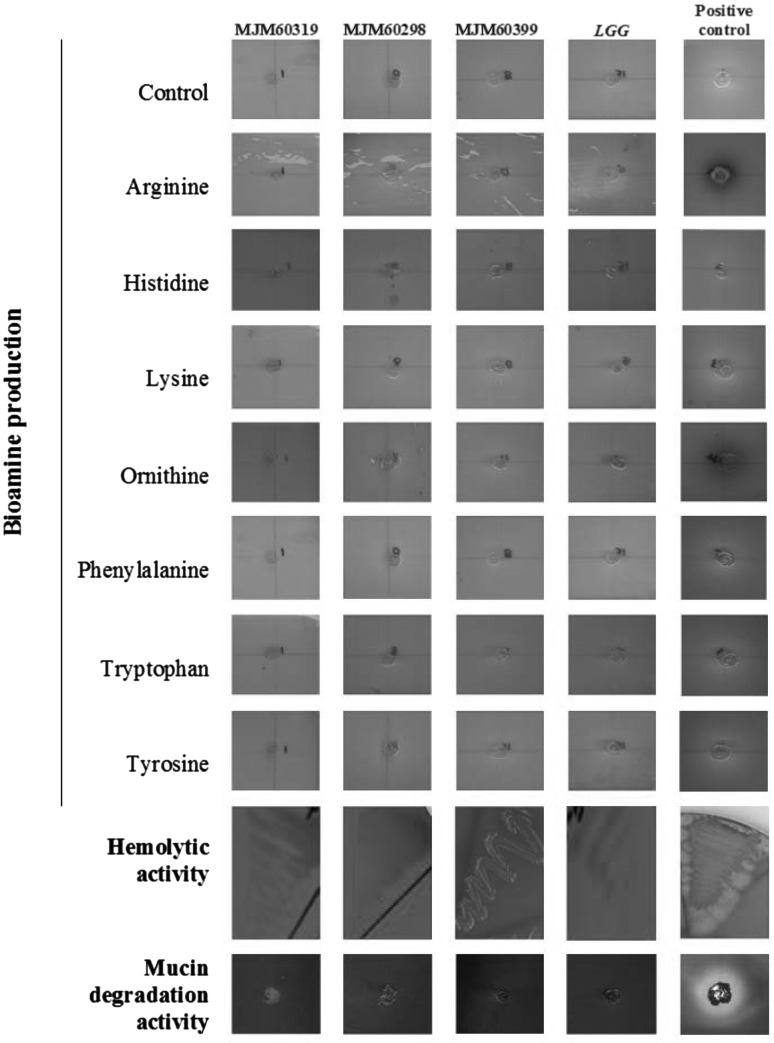
Assessment of bioamine production, hemolytic and mucinolytic activity showed that the L. plantarum strains MJM60319, MJM60298, and MJM60399 do not possess any of these characteristics (Fig. 1). The antibiotic susceptibility data for the three strains are given in Table 1. The three strains were susceptible to all the antibiotics tested in this study (Table 1) and does not pose problem of antibiotic-resistance.
Fig. 1.
Safety assessment of L. plantarum strains. Bioamine production was tested using seven different amino acids with a Lactobacillus brevis strain from our collection as positive control. Hemolytic activity was tested using 5% sheep blood supplied Columbia agar. Mucin degradation activity was tested using 3% mucin (from porcine stomach) supplemented medium. For hemolytic activity and mucin degradation activity a Bacillus isolate from a human faeces was used as positive control. L. rhamnosus GG was used as the probiotic positive control
Table 1.
Antibiotic susceptibility (MIC of antibiotics) of L. plantarum strains MJM60319, MJM60298, and MJM60399
| Antibiotics | MIC (µg ml−1) | EFSA cutoff (µg ml−1) | ||
|---|---|---|---|---|
| MJM60319 | MJM60298 | MJM60399 | ||
| Chloramphenicol | 2 | 2 | 2 | 8 |
| Tetracycline | 16 | 16 | 16 | 32 |
| Erythromycin | 0.5 | 0.5 | 0.5 | 1 |
| Ampicillin | 1 | 1 | 1.5 | 2 |
Identification of the LAB Strains
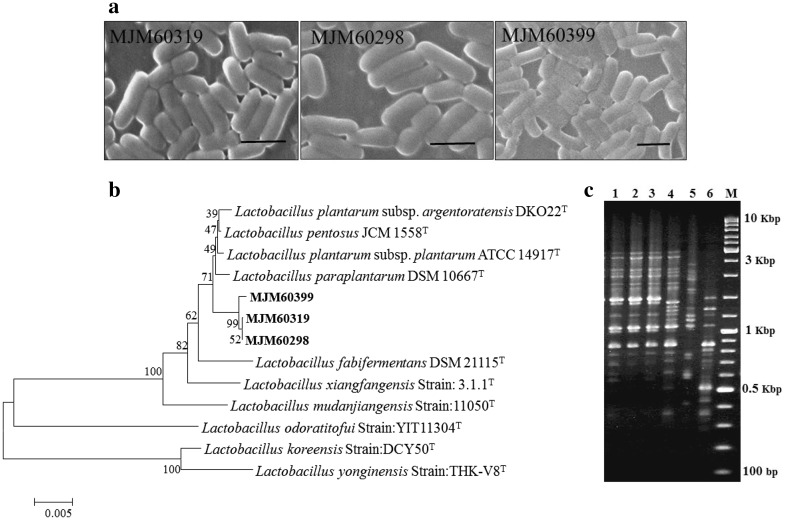
Microscopic examination of the LAB strains showed rod-shaped cells (Fig. 2a). BLAST search of the 16S rRNA gene sequence of MJM60319, MJM60298, and MJM60399 using the EzTaxon database revealed that the strain showed more than 99% similarity to L. plantarum. Phylogenetic analysis of the 16S rRNA gene sequence of the three LAB strains showed that they belong to the L. plantarum group (Fig. 2b). (GTG)5-rep-PCR fingerprinting of the three strains compared with that of the type strains Lactobacillus plantarum ssp. plantarum KACC 11451; 5, Lactobacillus paraplantarum KACC 12373; 6, Lactobacillus pentosus KACC 12428 revealed that strains MJM60319, MJM60298, and MJM60399 belonged to L. plantarum. These strains were deposited in the Korean Agricultural Culture Collection (KACC), Republic of Korea with the strain numbers KACC92111P for MJM60319, KACC92110P for MJM60298 and KACC92112P for MJM60399.
Fig. 2.
Identification of strains MJM60319, MJM60298, and MJM60399. a Morphology of strains MJM60319, MJM60298, and MJM60399 observed by scanning electron microscopy. Scale bar indicates 1 µm, b phylogenetic tree constructed based on 16S rDNA sequence of the strain. The strains MJM60319, MJM60298, and MJM60399 possesses 99% similarity with L. plantarum. c GTG5 PCR fingerprinting analysis of LAB strains. 1, MJM60319; 2, MJM60298; 3, MJM60399; 4, L. plantarum ssp. plantarum KACC 11451; 5, L. paraplantarum KACC 12373; 6, L. pentosus KACC 12428; M, Marker: 100–10,000 bp GeneRulerTM DNA ladder mix (Fermentas)
Antimicrobial Activity of the L. plantarum Strains
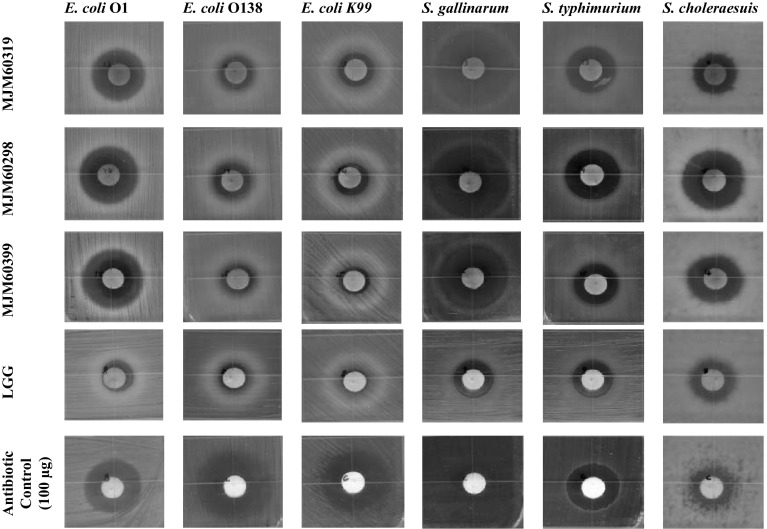
The three L. plantarum strains MJM60319, MJM60298 and MJM60399 showed good but varying level of antimicrobial activity against the various pathogens tested, which is given in Fig. 3. The antimicrobial activity was compared to that of commercial probiotics L. rhamnosus and antibiotic control such as chloramphenicol, streptomycin and tetracycline. The three L. plantarum strains showed a relatively better activity compared to L. rhamnosus GG (Fig. 3). Additionally, the L. plantarum strains showed the strongest activity against Salmonella Gallinarum and good activity against Salmonella Typhimurium, Salmonella Choleraesuis and E. coli O1 (Fig. 3). The three L. plantarum strains showed relatively weak activity against E. coli O138 and E. coli K99 (Fig. 3).
Fig. 3.
Antimicrobial activity of L. plantarum strains MJM60319, MJM60298, and MJM60399 in comparison with probiotic and antibiotic control against enteric animal pathogens. One hundred microliter of crude culture filtrate was used for antimicrobial activity
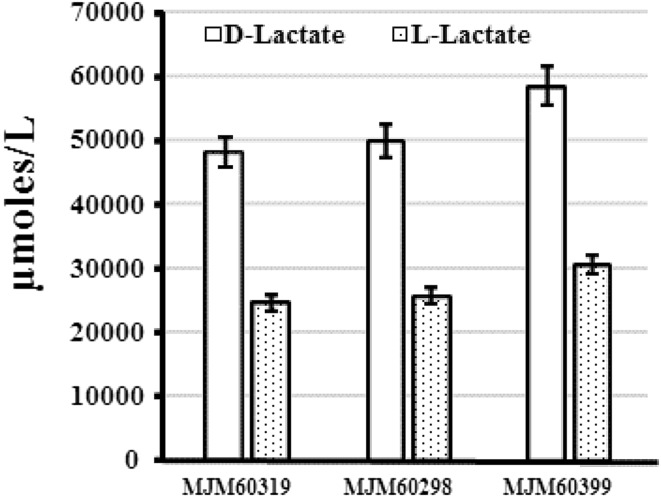
Lactate Production by L. plantarum Strains
Strains MJM60319, MJM60298, and MJM60399 produced both d- and l-lactate (Fig. 4). However, all these strains produced higher amount of d-Lactate than l-lactate (Fig. 4).
Fig. 4.
Production of d- and l-lactate by L. plantarum strains MJM60319, MJM60298, and MJM60399
Physiological Characterization
Orogastrointestinal Transit Tolerance of the L. plantarum Strains
The strains MJM60319, MJM60298, and MJM60399 demonstrated higher tolerance to oral stress for 10 min and subsequent exposure to gastric stress pH 3 for 1 h. We observed loss in viability of three strains after exposure to gastric stress pH 3 for 1 h followed by intestinal stress for 120 min. The viability loss is quite usual as most strains loose viability at pH 2. All the strains maintained >5.5% viability after the OGT assay (Table 2).
Table 2.
Viability of L. plantarum strains MJM60319, MJM60298 and MJM60399 after orogastrointestinal transit assay
| LAB strains | % of viable cells after OGT assay |
|---|---|
| MJM60319 | 5.7 |
| MJM60298 | 6.1 |
| MJM60399 | 6.9 |
Adherence of L. plantarum Strains to Caco-2 Cells
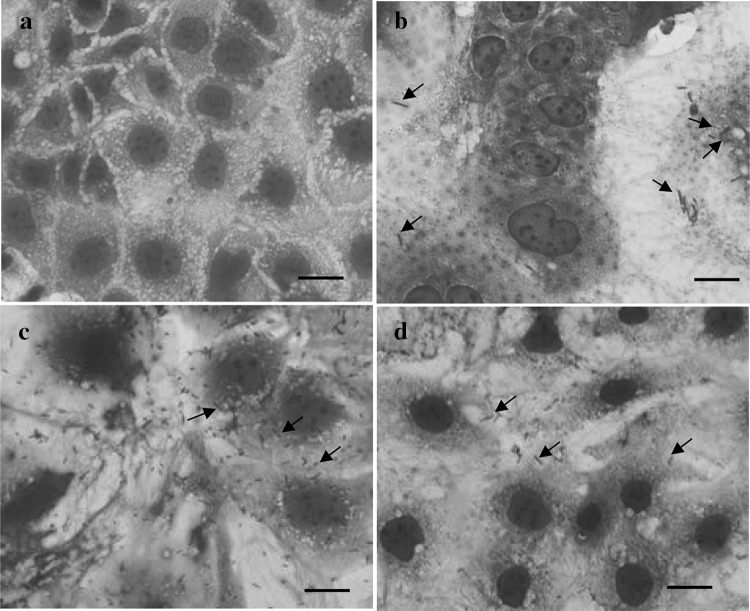
The three L. plantarum strains MJM60319, MJM60298, and MJM60399 showed good adherence to Caco-2 cell monolayer (Fig. 5).
Fig. 5.
Adherence of L. plantarum strains to Caco-2 cell line. a Control cells, b MJM60319, c MJM60298, d MJM60399. Arrows indicate bacterial adherence to Caco-2 cell surface. Scale bar indicates 10 µm
Technological Characterization
Growth of L. plantarum Strains in the Presence of NaCl
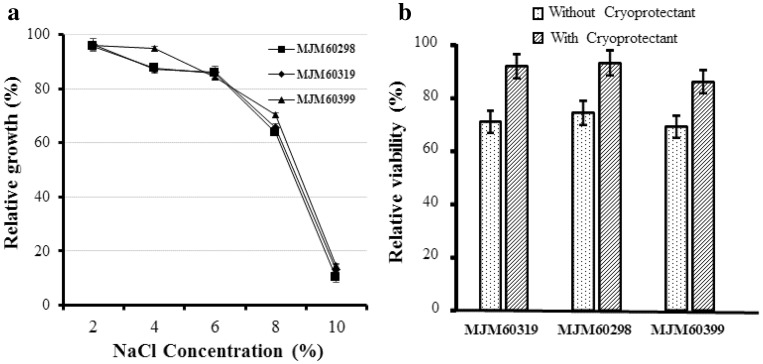
All the strains showed decreasing pattern of growth at the increasing concentration of NaCl (Fig. 6a). The L. plantarum strains MJM60319, MJM60298, and MJM60399 tolerated sodium chloride concentration up to 8% with the viability of 60% eventually the viability was decreased to 10% at the 10% NaCl concentration (Fig. 6a).
Fig. 6.
Growth of L. plantarum strain MJM60319, MJM60298, and MJM60399 in the presence of NaCl. Viability of L. plantarum strains MJM60319, MJM60298, and MJM60399 after freeze-drying with or without cryoprotectant
Tolerance of L. plantarum Strains to Freezing Stress
The relative viability of the strains MJM60319, MJM60298 and MJM60399 in freeze dried samples was higher in the presence of cryoprotectant than without cryoprotectant indicating better protection of the cells from freeze-drying with cryoprotectant than absence of any cryoprotectant (Fig. 6b).
Discussion
In the present study, three L. plantarum strains MJM60319, MJM60298, and MJM60399 were evaluated for their antimicrobial activity against animal enteric pathogens and their probiotic properties were evaluated to utilize the L. plantarum strains as probiotic feed supplement for maintaining general health of the livestock animals. These strains were compared with the well-known commercial probiotic strain L. rhamnosus GG.
New strains intended to be used as probiotic must be sufficiently characterized according to FAO/WHO and EFSA guidelines. Accordingly, the probiotic strains must not possess any transferable antibiotic resistant genes. For evaluating the antibiotic susceptibility, EFSA [24] has recommended MIC cut-off value for common antibiotics and strains be labelled susceptible or resistant to these antibiotics when the strains are used as feed supplements. The strains used in this study has MIC value within the cut-off values recommended by EFSA and are considered safe for use in animal feed supplements.
Additionally, the probiotic strain must not produce toxic substances such as biogenic amines. Biogenic amines such as tyramine, histamine and tryptamine are produced in the rumen by rumen microbes by the decarboxylation of amino acids [25]. Among these biogenic amines, histamine has been linked with the development of laminitis [25]. Additionally, the use of biogenic amine producing strains as probiotics in feed supplements could lead to the production of biogenic amines in feed, which are off-flavours and decrease feed intake and growth of livestock animals [26]. Biogenic amines have also been implicated in post weaning colibacillosis in pigs [27]. Hence, it is important to select non-bioamine producing strains for use as probiotics. In our present study, the three L. plantarum strains did not show biogenic amine production in any of the amino acids tested indicating that these are non-bioamine producing strains and could be used in animal feed.
Mucin degradation and hemolytic activity are other important safety test as several pathogenic microbes were known to have these virulence factors [28, 29]. Probiotic microbes are preferred not to have these activities [18]. The L. plantarum strains used in this study did not possess mucinolytic and hemolytic activities.
Probiotic microbes are ingested live and must reach the colon alive where they exert their function. In order to reach the colon alive and in desirable population the probiotic microbe must survive the harsh conditions in the gastrointestinal tract. To assess the ability of the L. plantarum strains to survive the gastrointestinal tract, we simulated the conditions in vitro following the method of [21], which evaluated the performance of mutant strains of L. plantarum. The L. plantarum strains used in this study tolerated the OGT condition and all the strains showed a viability of greater than 5.5% at the end of the OGT assay. This viability is higher compared to previously reported L. plantarum strains [30] and can be improved through microencapsulation and formulations that stabilize the L. plantarum cells.
Apart from these essential characteristics probiotics must possess specific functional characteristics. This study evaluated the antimicrobial activity of the L. plantarum strains against enterotoxigenic E. coli (ETEC), Shiga toxin-producing E. coli (STEC), Salmonella Typhimurium, Choleraesuis and Gallinarum. The L. plantarum strains exhibited strong activity against all the serovars of Salmonella and an E. coli serotype. These L. plantarum strains are useful as animal probiotics in the suppression of these pathogens. The antimicrobial activity of lactic acid bacteria can be by the production of organic acid (lactic and acetic acid), hydrogen peroxide, ethanol, diacetyl, acetaldehyde, acetoine, reuterin, reutericyclin and bacteriocin [31]. The antimicrobial activity of L. plantarum strains used in this study could be due to the production of organic acids. The strains were shown to produce d- and l-lactate, which inhibit several Gram-negative pathogens such as E. coli and [32, 33]. Salmonella. The L. plantarum strains used in this study also showed good adherence to Caco-2 cell line in vitro, which indicate that these strains can efficiently colonize the intestine and deliver their antimicrobial activity.
Additionally, probiotic strains must tolerate production conditions such as high or low temperature used in spray-drying or freeze-drying to make powdered formulations and tolerance to NaCl for cross-stress tolerance to heat [34]. The probiotic must maintain desirable viability and cell count during storage and hence selection of the strains based on the technological properties is essential. Also, Lactobacillus strains with NaCl tolerance has been shown to effectively suppress pathogenic bacteria in silage fermentation when co-treated with NaCl [35]. Hence, selection of probiotic strains with NaCl tolerance is useful in silage fermentations. Furthermore, the addition of cryoprotectants during freeze drying of lactobacilli has been used to help overcome inactivation during drying and stabilization during storage [34]. The present study demonstrated the viability of the L. plantarum strains during freeze-drying with or without cryoprotectants. The L. plantarum strains maintained higher viability in the presence of cryoprotectants, which is in accordance with a previous study, where freeze-dried Lactobacillus bulgaricus maintained high viability during storage at −20 °C for 10 months with cryoprotectants [36]. The L. plantarum strains also showed good tolerance to NaCl, maintaining more than 60% growth at 8% NaCl, which is higher than previously reported Lactobacillus strains [35]. This property of the L. plantarum strains of this study make them useful in silage fermentation with NaCl as additive to suppress the growth of harmful butyric acid producing bacteria.
Conclusion
The three L. plantarum strains characterized in this study demonstrated potential probiotic properties relevant to the livestock production industry. These strains could be used as probiotics to control infections caused by Salmonella and E. coli strains after appropriate animal study. Additionally, due to their NaCl tolerance these strains could be used with NaCl in silage fermentation to suppress pathogenic microbial growth.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01133402)” Rural Development Administration, Republic of Korea.
Footnotes
Sasikumar Arunachalam Palaniyandi and Karthiyaini Damodharan have contributed equally to this work.
Contributor Information
Joo-Won Suh, Phone: 82-31-330-6190, Email: jwsuh@mju.ac.kr.
Seung Hwan Yang, Phone: 82-61-659-7306, Email: ymichigan@jnu.ac.kr.
References
- 1.Garmendia J, Frankel G, Crepin VF. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect Immun. 2005;73:2573–2585. doi: 10.1128/IAI.73.5.2573-2585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunol Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 3.Naqid IA, Owen JP, Maddison BC, Gardner DS, Foster N, Tchórzewska MA, La Ragione RM, Gough KC. Prebiotic and probiotic agents enhance antibody-based immune responses to Salmonella Typhimurium infection in pigs. Anim Feed Sci Technol. 2015;201:57–65. doi: 10.1016/j.anifeedsci.2014.12.005. [DOI] [Google Scholar]
- 4.Van Immerseel F, Studholme DJ, Eeckhaut V, Heyndrickx M, Dewulf J, Dewaele I, Van Hoorebeke S, Haesebrouck F, Van Meirhaeghe H, Ducatelle R, Paszkiewicz K, Titball RW. Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine. 2013;31:4940–4945. doi: 10.1016/j.vaccine.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Kwon YK, Kim A, Kang MS, Her M, Jung BY, Lee KM, Jeong W, An BK, Kwon JH. Prevalence and characterization of Salmonella Gallinarum in the chicken in Korea during 2000 to 2008. Poult Sci. 2010;89:236–242. doi: 10.3382/ps.2009-00420. [DOI] [PubMed] [Google Scholar]
- 6.Steinmuller N, Demma L, Bender JB, Eidson M, Angulo FJ. Outbreaks of enteric disease associated with animal contact: not just a foodborne problem anymore. Clin Infect Dis. 2006;43:1596–1602. doi: 10.1086/509576. [DOI] [PubMed] [Google Scholar]
- 7.Osei Sekyere J. Antibiotic types and handling practices in disease management among pig farms in Ashanti region, Ghana. J Vet Med. 2014;2014:531952. doi: 10.1155/2014/531952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tellez G, Pixley C, Wolfenden RE, Layton SL, Hargis BM. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res Int. 2012;45:628–633. doi: 10.1016/j.foodres.2011.03.047. [DOI] [Google Scholar]
- 9.FAO/WHO (2001) Expert consultation on health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization [online]. http://www.fao.org/3/a-a0512e.pdf. Accessed 01 June 2016
- 10.Bomba A, Kravjansk I, Kagtel R, Herich R, Juhasova Z, Cizek M, Kapitancik B. Inhibitory effects of Lactobacillus casei upon the adhesion of enterotoxigenic Escherichia coli K99 to the intestinal mucosa in gnotobiotic lambs. Small Rumin Res. 1996;23:199–206. doi: 10.1016/S0921-4488(96)00905-4. [DOI] [Google Scholar]
- 11.Collado MC, Grzeskowiak L, Salminen S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr Microbiol. 2007;55:260–265. doi: 10.1007/s00284-007-0144-8. [DOI] [PubMed] [Google Scholar]
- 12.Slizewska K, Piotrowska M. Reduction of ochratoxin A in chicken feed using probiotic. Ann Agric Environ Med. 2014;21:676–680. doi: 10.5604/12321966.1129913. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann JA, Rossler E, Blajman JE, Romero-Scharpen A, Astesana DM, Olivero CR, Berisvil AP, Signorini ML, Zbrun MV, Frizzo LS, Soto LP. Effects of probiotics in swines growth performance: a meta-analysis of randomised controlled trials. Anim Feed Sci Technol. 2016;219:280–293. doi: 10.1016/j.anifeedsci.2016.06.021. [DOI] [Google Scholar]
- 14.Blajman JE, Frizzo LS, Zbrun MV, Astesana DM, Fusari ML, Soto LP, Rosmini MR, Signorini ML. Probiotics and broiler growth performance: a meta-analysis of randomised controlled trials. Br Poult Sci. 2014;55:483–494. doi: 10.1080/00071668.2014.931930. [DOI] [PubMed] [Google Scholar]
- 15.Bover-Cid S, Holzapfel WH. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int J Food Microbiol. 1999;53:33–41. doi: 10.1016/S0168-1605(99)00152-X. [DOI] [PubMed] [Google Scholar]
- 16.Zhou JS, Gopal PK, Gill HS. Potential probiotic lactic acid bacteria Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) do not degrade gastric mucin in vitro. Int J Food Microbiol. 2001;63:81–90. doi: 10.1016/S0168-1605(00)00398-6. [DOI] [PubMed] [Google Scholar]
- 17.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 18.Damodharan K, Lee YS, Palaniyandi SA, Yang SH, Suh JW. Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front Microbiol. 2015;6:768. doi: 10.3389/fmicb.2015.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vuyst L, Camu N, De Winter T, Vandemeulebroecke K, Van de Perre V, Vancanneyt M, De Vos P, Cleenwerck I. Validation of the (GTG)(5)-rep-PCR fingerprinting technique for rapid classification and identification of acetic acid bacteria, with a focus on isolates from Ghanaian fermented cocoa beans. Int J Food Microbiol. 2008;125:79–90. doi: 10.1016/j.ijfoodmicro.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Bove P, Gallone A, Russo P, Capozzi V, Albenzio M, Spano G, Fiocco D. Probiotic features of Lactobacillus plantarum mutant strains. Appl Microbiol Biotechnol. 2012;96:431–441. doi: 10.1007/s00253-012-4031-2. [DOI] [PubMed] [Google Scholar]
- 22.Marteau P, Minekus M, Havenaar R, Huis in’t Veild JH. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Diary Sci. 1997;80:1031–1037. doi: 10.3168/jds.S0022-0302(97)76027-2. [DOI] [PubMed] [Google Scholar]
- 23.Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W. Aggregation and adhesion properties of 22 Lactobacillus strains. J Dairy Sci. 2013;96:4252–4257. doi: 10.3168/jds.2013-6547. [DOI] [PubMed] [Google Scholar]
- 24.EFSA Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012;10:2740. [Google Scholar]
- 25.Wang DS, Zhang RY, Zhu WY, Mao SY. Effects of subacute ruminal acidosis challenges on fermentation and biogenic amines in the rumen of dairy cows. Livest Sci. 2013;155:262–272. doi: 10.1016/j.livsci.2013.05.026. [DOI] [Google Scholar]
- 26.Canibe N, Virtanen E, Jensen BB. Effect of acid addition to pig liquid feed on its microbial and nutritional characteristics. Livest Sci. 2007;108:202–205. doi: 10.1016/j.livsci.2007.01.094. [DOI] [Google Scholar]
- 27.Kiarie E, Slominski BA, Nyachoti CM. Tissue fatty acid profiles, plasma biochemical characteristics and cecal biogenic amines in piglets fed diets containing flaxseed and carbohydrase enzymes. Livest Sci. 2009;121:1–6. doi: 10.1016/j.livsci.2008.05.009. [DOI] [Google Scholar]
- 28.Roberton AM, Corfield AP. Mucin degradation and its significance in inflammatory conditions of the gastrointestinal tract. In: Tannock GW, editor. Medical importance of the normal microflora. Dordrecht: Springer; 1999. pp. 222–261. [Google Scholar]
- 29.Colina A-R, Aumont F, Deslauriers N, Belhumeur P, Repentigny LD. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect Immun. 1996;64:4514–4519. doi: 10.1128/iai.64.11.4514-4519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pringsulaka O, Rueangyotchanthana K, Suwannasai N, Watanapokasin R, Amnueysit P, Sunthornthummas S, Sukkhum S, Sarawaneeyaruk S, Rangsiruji A. In vitro screening of lactic acid bacteria for multi-strain probiotics. Livestock Sci. 2015;174:66–73. doi: 10.1016/j.livsci.2015.01.016. [DOI] [Google Scholar]
- 31.Šušković J, Kos B, Beganović J, Pavunc AL, Habjanič K, Matošić S. Antimicrobial activity—the most important property of probiotic and starter lactic acid bacteria. Food Technol Biotechnol. 2010;48:296–307. [Google Scholar]
- 32.Raftari M, Jalilian FA, Abdulamir AS, Son R, Sekawi Z, Fatimah AB. Effect of organic acids on Escherichia coli O157:H7 and Staphylococcus aureus contaminated meat. Open Microbiol J. 2009;3:121–127. doi: 10.2174/1874285800903010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hussain G, Rahman A, Hussain T, Uddin S, Ali T. Citric and lactic acid effects on the growth inhibition of E. coli and S. typhymurium on beef during storage. Sarhad J Agric. 2015;31:183–190. doi: 10.17582/journal.sja/2015/31.3.183.190. [DOI] [Google Scholar]
- 34.Ross RP, Desmond C, Fitzgerald GF, Stanton C. Overcoming the technological hurdles in the development of probiotic foods. J Appl Microbiol. 2005;98:1410–1417. doi: 10.1111/j.1365-2672.2005.02654.x. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y, Ohmomo S, Ogawa M, Kumai S. Effect of NaCl-tolerant lactic acid bacteria and NaCl on the fermentation characteristics and aerobic stability of silage. J Appl Microbiol. 1997;83:307–313. doi: 10.1046/j.1365-2672.1997.00229.x. [DOI] [PubMed] [Google Scholar]
- 36.Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol Prog. 2004;20:248–254. doi: 10.1021/bp034165y. [DOI] [PubMed] [Google Scholar]