Abstract
Probiotics play a vital role in clinical applications for the treatment of diarrhea, obesity and urinary tract infections. Phytate, an anti-nutrient, chelates essential minerals that are vital for human health. In the past few decades, research reports emphasize extensively on phytate degradation in animals. There is a growing need for finding alternate strategies of phytate utilization in human, as they are unable to produce phytase. At this juncture, probiotics can be utilized for phytase production to combat mineral deficiency in humans. The main focus of this review is on improving phosphate bioavailability by employing two approaches: supplementation of (1) fermented food products that contain probiotics and (2) recombinant phytase producing bacteria. In addition, several factors influencing phytase activity such as bacterial viability, optimal pH, substrate concentration and specificity were also discussed.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0647-3) contains supplementary material, which is available to authorized users.
Keywords: Probiotic, Anti-nutrient, Phytate, Phytase, Fermented foods
Introduction
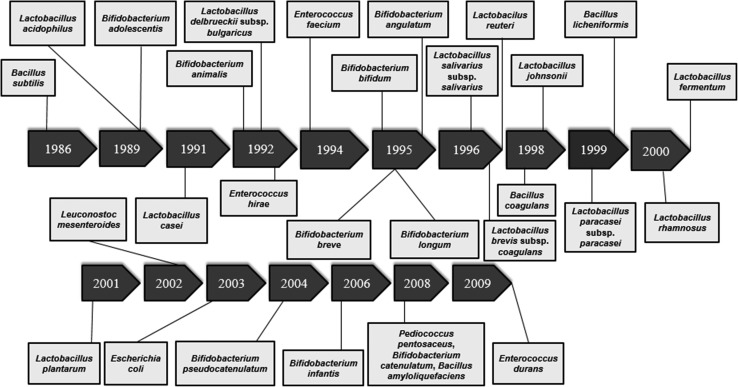
The joint initiative of Food and Agricultural Organization (FAO) as well as the World Health Organization (WHO), defined probiotics as mono or mixed cultures of “live microorganisms which when administered in adequate amounts beneficially affect the host” [1]. The history of probiotics dates back to 1907 when Russian scientist Ellie Metchnikoff postulated the idea of using lactic acid bacteria for modulating intestinal flora. Since then intensive research was undertaken in the field of probiotics for improving human health and the timeline of these applications are presented in Fig. 1.
Fig. 1.
Timeline of probiotics
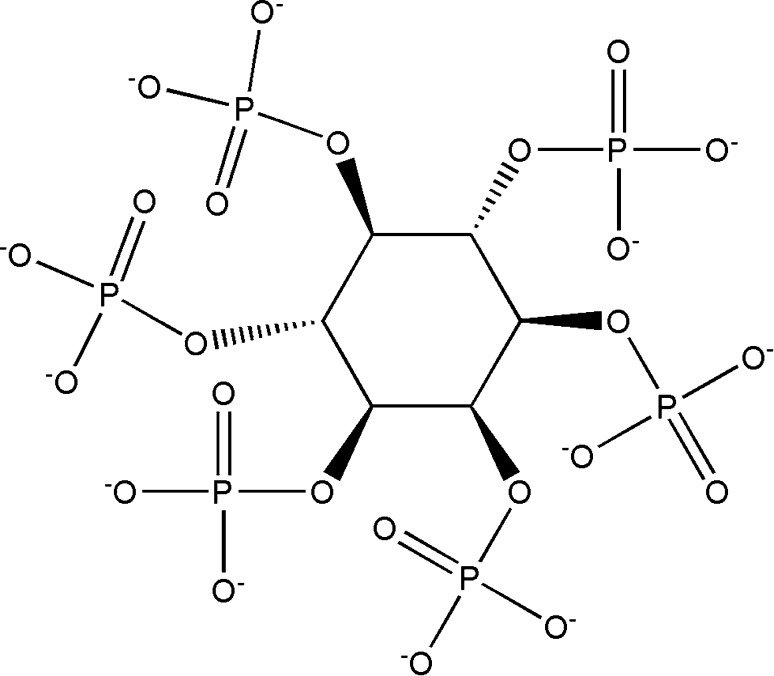
Phytate also known as myo-inositol hexaphosphate is the principal storage form of phosphorus in plant-based foods.
The chemical structure of phytate consists of six phosphate groups (five of them present in equatorial position with the last one placed in axial position) attached to its inositol ring. The overall negative charge contributed by phosphates (Fig. 2) help in chelating divalent and trivalent metal ions. Therefore, phytate acts as an anti-nutrient and limit mineral bioavailability in human. Despite causing a major nutritional deficiency, phytate can be regarded for its beneficial effects especially in treating colon cancer, AIDS, Alzheimer’s disease, Arthritis and Parkinson’s diseases [2].
Fig. 2.
Chemical structure of phytate (retrieved from PubChem)
Phytases of plants and microorganisms degrade phytate into inositol and free orthophosphates. These enzymes differ in their structure. Plant-based phytases predominantly exist in alpha/beta form whereas, bacterial phytases belong to the beta class of proteins [3].
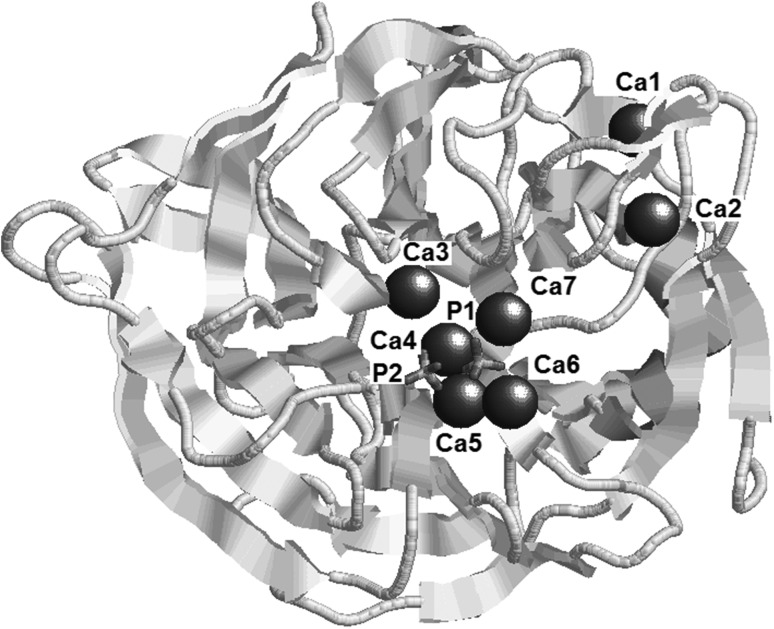
The crystal structure of bacterial phytase consists of antiparallel β-sheets (Fig. 3). The active site of enzyme consists of “catalytic” (phosphate 1) and “affinity” (phosphate 2) binding sites. The two phosphates of the substrate (phytate) bind to these sites causing structural conformations via both ionic and hydrogen bonding. Then the catalytic site is activated by three Ca2+ ions, which are vital for substrate binding. Besides, three more Ca2+ bind to the affinity site that improves the substrate specificity and overall stability of the enzyme. Binding of phosphate ions also activates the presence of a seventh Ca2+ ion in the structure. Catalysis is initiated by an attack of hydroxide ion of water molecule on the first phosphate group, triggering its cleavage. Thereafter, every alternate phosphate is cleaved leading to the final product. Phytases cleave equatorial phosphate groups readily as compared to axial ones [4]. Probiotic bacteria can be efficiently utilized for phytate utilization as compared to their plant-based counterparts owing to the former’s increased substrate specificity and catalytic efficiency [5].
Fig. 3.
Structural configuration of phytase (PDB id: 1H6L)
Humans have a monogastric digestive system which lacks sufficient phytase producing bacteria in their intestine for phytate utilization. This leads to a serious nutritional deficiency in the host. The undigested phytate from food passes into the soil and lead to environmental phosphate pollution [2].
Hence, this review is focused on probiotic bacteria which are capable of degrading phytate in humans.
Probiotic Selection Criteria
Probiotics have been used for centuries due to their broad spectrum of biological activities in human. For bacteria to be used as probiotics, they must be non-pathogenic, non-invasive, non-carcinogenic, adhere to intestinal epithelium, resistant to gastric acidity, stable in food matrix during intestinal digestion, aid in immunomodulation, and colonize for a stable time period. Moreover, they must have verified strain properties, be effective against the specified target and exhibit bile salt hydrolase activity [6].
These probiotics can be administered through enteral route or by enema. Enteral route is widely preferred for consumption of fermented food products. Whereas, an enema is preferred for fecal microbiota transplantation (FMT) [7]. The review is focused only on fermented food products for administering probiotics via an enteral route as it is cost-effective and convenient when compared to FMT [8, 9]. The choice of fermented foods that contain viable bacteria, which are capable of degrading phytate are listed in Table 1.
Table 1.
Fermented food products as the source of probiotics
| Microorganisms | Fermented foods | References |
|---|---|---|
| Bacillus subtilis (natto) N-77, B. subtilis CF92, Bacillus coagulans IDCC 1201 | Commercial natto (traditional Japanese food), fermented soybean, pharmaceuticals | [10–12] |
| Lactobacillus strains acidophilus 16A, brevis 14G, fermentum 6E, plantarum DC400 | Italian sourdough | [13] |
| L. acidophilus BS, L. casei 1 K, L. casei DSM20011, L. fermentum DSM 20052, L. plantarum 110 | Commercial fermented milk, cheese, fermented beets, fermented plant food | [14] |
| L. acidophilus 1C5, 4C1 and 1C3 and 5C2, L. caseiImmunitass 4D2, 4D1 and Lactobacillus rhamnosus 4C3, 2C2, T, 3C15 and 3C23, L. caseiShirota 6D1, 6D2, Bifidobacterium longum T, Lactobacillus delbreuckii 5D1, Bifidobacterium bifidum MF, L. plantarum 6C1, 1D1, 3C12, 1D2, 6C3, 1C1, 6C4, 6C5 | Dairy and pharmaceutical products | [15] |
| L. acidophilus, L. fermentum and L. plantarum | Fermented sorghum–Irish potato gruel | [16] |
| L. casei, L. fermentum, L. plantarum and Pediococcus pentosaceus | (Eragostis tef) Atmit | [17] |
| L. casei NRRL B-1445, L. delbrueckii NRRL B-445, L. fermentum NRRL B-4524, L. fermentum NRRL B-4524, Leuconostoc mesenteriodes NRRL B-512F, P. pentosaceus NRRL B-14009 and L. plantarum NRRL B-4496 | Natural vegetable fermentation | [18] |
| L. casei MF50, 54, L. fermentum MF25, P. pentosaceus MF32, 33 and 35 and L. plantarum MF79 | Ethiopian injera (African soft pancake) | [19] |
| Enterococcus sp. hirae, faecium and durans | Indian fermented soybean foods | [20] |
| E. faecium RJ16 | Goat cheese | [21] |
| E. faecium A86, L. plantarum H5, L. plantarum L3 | Pizza dough, sourdough, sausages | [22] |
| L. brevis, L. plantarum | Southern Italian sourdough | [23] |
| L. brevis, P. pentosaceus, E. durans and L. plantarum | Fermented Himalayan vegetables | [24] |
| L. brevis | Hatay boiled cheese | [25] |
| Lactobacillus reuteri and L. plantarum | Iranian Sangak bread | [26] |
| Lactobacillus pentosus, L. fermentum, L. brevis and L. plantarum Lb 29, L10 | Carper berry | [27] |
| L. plantarum strains 17bp30, 17bp31, 17bp48, Mb25, Mb26, 17bp29, Mb46, Mb50, Mb61, Mb67 and Lactobacillus paracasei strains C3-70, C3-89 | Spanish farmhouse cheese | [28] |
| L. brevis G11, G25, L. fermentum N33, N25 and L. plantarum A6 | Fermented corn | [29] |
| L. plantarum, L. mesenteriodes | Moroccan sourdough bread | [30] |
| L. plantarum | Greek dry fermented sausages | [31] |
| L. acidophilus, L. plantarum and L. mesenteroides | Iranian Sangak bread | [32] |
| L. plantarum | Fermented food (Shalgam) | [33] |
| L. plantarum, L. mesenteroides | Italian Cornetto di Matera sourdough | [34] |
| P. pentosaceus KTU05-8 and KTU05-9 | Wholemeal wheat bread | [35] |
| L. acidophilus EF7, L. plantarum 299v, L. rhamnosus GG B103, L. reuteri M 14-C, CF2 7-F, DSM 20016, SD 2112, MM7 and MM2-3 | Sweet potato | [36] |
| L. plantarum DPC2739, L. plantarum W723 | Dairy products, Sorghum-Ogi | [37, 38] |
Probiotic bacteria in fermented food products have an impact on human health. Probiotics isolated from a wide variety of functional foods ranging from sourdough to fermented bread, dairy products are capable of effective phytate degradation thereby aiding in improved mineral uptake in humans.
In general, probiotics competitively bind to the host intestinal epithelium and stimulate an immune response by activating specific signaling cascades and cell-based reactions. Each bacterial genera has a unique action mechanism for the corresponding biological functions.
Probiotics belonging to Bacilli group are gram-positive, facultative aerobes which produce non-pathogenic spores. The spores play an important role in increased shelf-life of the probiotic product as they are thermostable, recalcitrant to a wide range of pH fluctuations and viable under extreme intestinal conditions. The presence of an outer thick peptidoglycan layer protects bacterial spores from extreme heat, organic acids, lysosomal degradation and helps them in several beneficial activities on human health including treatment of rheumatoid arthritis and blood clotting.
Escherichia coli are gram-negative probiotic microbes which predominately participate in anti-inflammatory activity through modulation of the host immune system. They are also helpful in treatment of colitis and constipation.
Microbes belonging to genera Lactobacilli, Enterococci, Bifidobacteria, Pediococcus and Leuconostoc represents a group of non-pathogenic, facultative anaerobes which are commonly present in the human gut. In addition to their gastric viability, they efficiently colonize and have prolonged epithelial adherence. They also produce bacteriocins which competitively inhibit other pathogenic bacteria and maintain a healthy balance of beneficial microbes. These bacteria find applications ranging from preparation of fermented food products to clinical therapeutics such as inflammatory bowel disease (IBD), diarrhea, and cholesterol regulation.
Functional Foods for Phytase Production
Fermented foods like cheese, sausages and caper berry contain viable lactic acid producing bacteria that colonizes in the human intestine. In addition, it enables the sustenance of enzyme source and stability by continuous multiplication. Probiotic bacteria from fermented foods recorded high phytate degradation (Table S-1, see supplementary material). Fermented soybean containing Bacillus subtilis produced phytase activities ranging up to 1,354,906.6 U/mL.
Lower phytase, phosphatase activities were recorded for Lactobacilli sp. grown in sweet potato base medium (SPM) with sodium phytate, p-nitrophenyl phosphate as substrate. Lactobacillus casei 1 K produced highest phosphatase activity of 162,119.2 U/mL with 0.48% phytate hydrolysis.
The level of phytase activity in vivo depends on factors like; the concentration of phytate, bacterial viability, optimum pH, the accessibility of phytate, presence of inorganic phosphate and other organic acids. Human intestine normally maintains a constant temperature of 37 °C with pH ranging from 5 to 7. Probiotic bacteria isolated from humans showed efficient phytate degradation (Table S-2, see supplementary material).
Bifidobacterium sp. BIF longum 12R, catenulatum 31S and breve 211 were isolated from human infants and adults after consumption of whole wheat bread. They produced high specific phytase activities of 6.92, 6.59 and 6.57 U/mg at pH 7.2. L. reuteri CECT 9025 produced the highest phosphatase activity in modified MRS medium containing sodium phytate.
In addition to above-mentioned parameters, molecular mass of phytase, substrate specificity and the presence of minerals also influence phytate degradation in the human intestine.
Microbial phytases may be monomeric or dimeric depending on the conformational state of the enzyme. The characteristics of phytases and phosphatases from probiotic bacteria are tabulated (Table 2), where most of them contain monomeric chains. The exception being L. brevis, which has dimeric chains each with different molecular masses 73 and 34 kDa, respectively.
Table 2.
Characteristics of probiotic phytases and phosphatases
| Enzyme | Microorganisms | Substrate specificity | Molecular mass (kDa) | References |
|---|---|---|---|---|
| Phytase | B. subtilis (natto) N-77 | Sodium phytate | 38 | [10] |
| B. subtilis CF92 | Sodium phytate (high), α-naphthyl phosphate and Adenosine triphophosphate (ATP) (low) | 46 | [11] | |
| B. coagulans IDCC 1201 | Sodium phytate | – | [12] | |
| L. brevis | – | 73, 34 | [25] | |
| L. plantarum | – | 46 | [33] | |
| Phosphatase | L. plantarum NRRL B-4496 | Acetyl phosphate, sodium phytate, p-nitrophenyl phosphate and α-d-glucose-1 phosphate | – | [18] |
| – | 52 | [29] | ||
| L. plantarum DPC2739 | – | 27 | [37] |
Phosphorylated compounds are generated during digestion of food, mineral metabolism and their presence in the intestinal cavity may interfere with phytate degradation. Most of the bacterial phytases are highly specific towards sodium phytate.
Functional foods contain a good amount of trace elements and minerals important for human nutrition. These metal ions in different concentrations might affect probiotic phytate degradation. The effect of different minerals on phytase activity are tabulated (Table 3).
Table 3.
Effect of minerals on phytate degradation
| Microorganisms | Activators | Inhibitors | References |
|---|---|---|---|
| B. subtilis (natto) N-77 | Ca2+ | Zn2+, Cd2+, Ba2+, Cu2+, Fe3+ and Al3+ | [10] |
| B. subtilis CF92 | – | Mn2+, Zn2+, Fe2+, Cu2+, Mg2+ and Co2+ | [11] |
| B. coagulans IDCC 1201 | Co2+ | – | [12] |
| L. plantarum | – | Ca2+, Hg2+, Mg2+, Mn2+, Zn2+, Ni2+, Cu2+, Co2+ and Fe2+ | [33] |
| L. plantarum DPC2739 | Fluoride, hexametaphosphate at 0.5 mM and orthophosphate, tripolyphosphate and pyrophosphate at 5 mM (phosphatase) | [37] |
Ca2+ plays an important role in stimulating, stabilizing bacterial phytases at optimum concentrations. However, excess Ca2+ can lead to competitive inhibition of enzymatic activity by binding to the enzymatic active site. The presence of inorganic phosphate also inhibits phytate degradation.
Role of Recombinant Phytases and Their Expression
Oral consumption of fermented food products and FMT are effective strategies for probiotic delivery. However, these strategies are associated with certain disadvantages including lack of site specific action and short-term efficacy. This may be overcome by recombinant phytases (Table 4).
Table 4.
Recombinant phytases
| Source of phytase gene | Vector plasmid | Host microorganism | References |
|---|---|---|---|
| B. subtilis NCDC-070, NCIM-2712 | Ins T/A | E. coli JM-109 | [39] |
| B. longum subsp. infantis and B. pseudocatenulatum | pNGPHYpseudso, pNGPHYlongum | L. casei | [40] |
| B. licheniformis PB-13 | pET32a(+) | E. coli BL21 | [41] |
| E. coli | pET22b | Pichia pastoris | [42] |
| B. amyloliquefaciens DSM 1061 | B. amyloliquefaciens DSM 1061 | [43] |
Probiotics belonging to Bacilli genera are efficiently utilized for expression of cloned phytases. Phytase from B. licheniformis, when expressed in E. coli efficiently degraded phytate producing a phytase activity of 0.96 U/mL with a recombinant protein having a molecular mass of 66 kDa.
Lactic acid bacteria (LAB) can be effectively used for recombinant phytase expression [44]. They are safe, cost-effective and also yield enzyme with high purity and stability. On the contrary, high species diversity, obscurity in the route of administration, and stringent monitoring due to lack of clinical trial data pose critical challenges in employing these recombinant probiotic phytases [45].
Well-known probiotics belonging to genus Bacilli and Lactobacilli are capable of adherence and colonization in the human intestine. Most often they are inferred by their high cell counts. They produce bacteriocins, which competitively inhibit pathogens thereby aiding overall improvement of gut microbiome. Moreover, consumption of fermented foods containing viable bacteria provides a scope for phytate degradation.
Conclusion and Future Scope
Phytases from probiotics provide a solution for phosphate utilization in humans. This can be achieved by consuming fermented food products, an ideal vehicle for delivering probiotics. It was noted that L. brevis and B. subtilis in fermented food products are capable of producing higher amount of phytase. Most of the probiotic phytases are very specific towards phytate and are stimulated by Ca2+ ions for effective activity. Alternatively, recombinant phytases can also be used for intestinal phytate utilization. Despite the wide usage of probiotics, it is yet to be approved by US Food and Drug Administration (FDA). Therefore, more research needs to be done on improvising probiotic strains for phytase production as well as optimizing probiotic dosage for phytate utilization.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Dr. V.Ramachandra Murty (Professor, Department of Biotechnology, Manipal Institute of Technology) for his timely inputs and help.
Compliance with Ethical Standards
Conflict of interest
None.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-017-0647-3) contains supplementary material, which is available to authorized users.
Contributor Information
P Priyodip, Email: priyodip.paul@learner.manipal.edu, Email: priyodip6@gmail.com.
P Y Prakash, Email: prakash.py@manipal.edu, Email: prakashpy123@yahoo.co.in.
S Balaji, Email: s.balaji@manipal.edu, Email: biobalagi@gmail.com.
References
- 1.FAO and WHO (2002) Joint working group report, “Guidelines for the evaluation probiotics in food”
- 2.Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B. 2008;9:165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei XG, Porres JM, Mullaney EJ, Brinch-Pedersen H. Phytase: source, structure and application. In: Polaina J, MacCabe AP, editors. Industrial enzymes-structure, function and applications. Netherlands: Springer; 2007. pp. 505–529. [Google Scholar]
- 4.Shin S, Ha NC, Oh BC, Oh TK, Oh BH. Enzyme mechanism and catalytic property of beta propeller phytase. Structure. 2001;9:851–858. doi: 10.1016/S0969-2126(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 5.Konietzny U, Greiner R. Bacterial phytase: potential application, in vivo function and regulation of its synthesis. Braz J Microbiol. 2004;35:11–18. doi: 10.1590/S1517-83822004000100002. [DOI] [Google Scholar]
- 6.Shewale RN, Sawale PD, Khedkar CD, Singh A. Selection criteria for probiotics: a review. Int J Probiotics Prebiotics. 2014;9:17–22. [Google Scholar]
- 7.Hudson LE, Anderson SE, Corbett AH, Lamb TJ. Gleaning insights from fecal microbiota transplantation and probiotic studies for the rational design of combination microbial therapies. Clin Microbiol Rev. 2017;30:191–231. doi: 10.1128/CMR.00049-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WJ, Lattimer LDN, Stephen S, Borum ML, Doman DB. Fecal Microbiota Transplantation: a review of emerging indications beyond relapsing Clostridium difficile toxin colitis. Gastroenterol Hepatol. 2015;11:24–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92:112–127. doi: 10.1016/j.jhin.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu M. Purification and characterization of phytase from Bacillus suhtilis (natto) N–77. Biosci Biotechnol Biochem. 1992;56:1266–1269. doi: 10.1271/bbb.56.1266. [DOI] [Google Scholar]
- 11.Hong SW, Chu IH, Chung KS. Purification and biochemical characterization of thermostable phytase from newly isolated Bacillus subtilis CF92. J Korean Soc Appl Biol Chem. 2011;54:89–94. doi: 10.3839/jksabc.2011.012. [DOI] [Google Scholar]
- 12.Lee SH, Kwon HS, Koo KT, Kang BH, Kim TY. Characterization of phytase from Bacillus coagulans IDCC 1201. Korean J Microbiol Biotechnol. 2006;34:28–34. [Google Scholar]
- 13.De Angelis M. Phytase activity in sourdough lactic acid bacteria: purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int J Food Microbiol. 2003;87:259–270. doi: 10.1016/S0168-1605(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 14.Haros M, Bielecka M, Honke J, Sanz Y. Phytate-degrading activity in lactic acid bacteria. Pol J Food Nutr Sci. 2008;58:33–40. [Google Scholar]
- 15.Khodaii Z, Mehrabani Natanzi M, Naseri MH, Goudarzvand M, Dodson H, Snelling A. Phytase activity of lactic acid bacteria isolated from dairy and pharmaceutical probiotic products. Int J Enterpathog. 2013;1:12–16. [Google Scholar]
- 16.Adegbehingbe KT. Effect of starter cultures on the anti-nutrient contents, minerals and viscosity of Ogwo, a fermented sorghum–Irish potato gruel. Int Food Res J. 2015;22:1247–1252. [Google Scholar]
- 17.Urga K, Keshava N, Narasimha HV. Effects of natural and mixed culture of lactobacilli fermentation on in vitro iron and zinc bioavailability in tef (Eragrostis tef) atmit. Bull Chem Soc Ethiop. 1997;11:101–109. [Google Scholar]
- 18.Zamudio M, Gonzalez A, Medina J. Lactobacillus plantarum phytase activity is due to non-specific acid phosphatase. Lett Appl Microbiol. 2001;32:181–184. doi: 10.1046/j.1472-765x.2001.00890.x. [DOI] [PubMed] [Google Scholar]
- 19.Fischer MM, Egli IM, Aeberli I, Hurrell RF, Meile L. Phytic acid degrading lactic acid bacteria in tef-injera fermentation. Int J Food Microbiol. 2014;190:54–60. doi: 10.1016/j.ijfoodmicro.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Chettri R, Tamang JP. Functional properties of Tungrymbai and Bekang, naturally fermented soybean foods of North East India. Int J Fermented Foods. 2014;3:87–103. doi: 10.5958/2321-712X.2014.01311.8. [DOI] [PubMed] [Google Scholar]
- 21.Abriouel H, Lucas R, Ben Omar N, Valdivia E, Maqueda M, Martínez-Cañamero M, Gálvez A. Enterocin AS-48RJ: a variant of enterocin AS-48 chromosomally encoded by Enterococcus faecium RJ16 isolated from food. Syst Appl Microbiol. 2005;28:383–397. doi: 10.1016/j.syapm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Anastasio M, Pepe O, Cirillo T, Palomba S, Blaiotta G, Villani F. Selection and use of phytate-degrading LAB to improve cereal-based products by mineral solubilization during dough fermentation. J Food Sci. 2010;75:M28–M35. doi: 10.1111/j.1750-3841.2009.01402.x. [DOI] [PubMed] [Google Scholar]
- 23.Reale A, Mannina L, Tremonte P, Sobolev AP, Succi M, Sorrentino E, Coppola R. Phytate degradation by lactic acid bacteria and yeasts during the wholemeal dough fermentation: a 31 P NMR study. J Agric Food Chem. 2004;52:6300–6305. doi: 10.1021/jf049551p. [DOI] [PubMed] [Google Scholar]
- 24.Tamang JP, Tamang B, Schillinger U, Guigas C, Holzapfel WH. Functional properties of lactic acid bacteria isolated from ethnic fermented vegetables of the Himalayas. Int J Food Microbiol. 2009;135:28–33. doi: 10.1016/j.ijfoodmicro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Sumengen M, Dincer S, Kaya A. Phytase production from Lactobacillus brevis. Turk J Biol. 2012;36:533–541. [Google Scholar]
- 26.Didar Z, Khodaparast MHH. Effect of different lactic acid bacteria on phytic acid content and quality of whole wheat toast bread. JFBT. 2011;1:1–10. [Google Scholar]
- 27.Pulido RP, Omar NB, Abriouel H, López RL, Cañamero MM, Guyot J, Gálvez A. Characterization of lactobacilli isolated from caper berry fermentations. J Appl Microbiol. 2007;102:583–590. doi: 10.1111/j.1365-2672.2006.03067.x. [DOI] [PubMed] [Google Scholar]
- 28.Lavilla-Lerma L, Pérez-Pulido R, Martínez-Bueno M, Maqueda M, Valdivia E. Characterization of functional, safety, and gut survival related characteristics of Lactobacillus strains isolated from farmhouse goat’s milk cheeses. Int J Food Microbiol. 2013;163:136–145. doi: 10.1016/j.ijfoodmicro.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Roger T, Léopold TN, Funtong MCM. Nutritional properties and antinutritional factors of corn paste (Kutukutu) fermented by different strains of lactic acid bacteria. Int J Food Sci. 2015 doi: 10.1155/2015/502910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaoui A, Faid M, Belhcen R. Effect of natural starters used for sourdough bread in Morocco on phytate biodegradation. East Mediterr Health J. 2003;9:141–147. [PubMed] [Google Scholar]
- 31.Papamanoli E, Tzanetakis N, Litopoulou-Tzanetaki E, Kotzekidou P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003;65:859–867. doi: 10.1016/S0309-1740(02)00292-9. [DOI] [PubMed] [Google Scholar]
- 32.Najafi MA, Rezaei K, Safari M, Razavi SH. Use of sourdough to reduce phytic acid and improve zinc bioavailability of a traditional flat bread (Sangak) from Iran. Food Sci Biotechnol. 2012;21:51–57. doi: 10.1007/s10068-012-0007-3. [DOI] [Google Scholar]
- 33.Sumengen M, Dincer S, Kaya A. Production and characterization of phytase from Lactobacillus plantarum. Food Biotechnol. 2013;27:105–118. doi: 10.1080/08905436.2013.781507. [DOI] [Google Scholar]
- 34.Zotta T, Ricciardi A, Parente E. Enzymatic activities of lactic acid bacteria isolated from Cornetto di Matera sourdoughs. Int J Food Microbiol. 2007;115:165–172. doi: 10.1016/j.ijfoodmicro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Cizeikiene D, Juodeikiene G, Bartkiene E, Damasius J, Paskevicius A. Phytase activity of lactic acid bacteria and their impact on the solubility of minerals from wholemeal wheat bread. Int J Food Sci Nutr. 2015;66:736–742. doi: 10.3109/09637486.2015.1088939. [DOI] [PubMed] [Google Scholar]
- 36.Hayek SA, Shahbazi A, Worku M, Ibrahim SA. Enzymatic activity of Lactobacillus grown in a sweet potato base medium. Br Microbiol Res J. 2014;4:509–522. doi: 10.9734/BMRJ/2014/5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magboul AA, McSweeney PL. Purification and characterization of an acid phosphatase from Lactobacillus plantarum DPC2739. Food Chem. 1999;65:15–22. doi: 10.1016/S0308-8146(98)00255-6. [DOI] [Google Scholar]
- 38.Onipede GO, Banwo K, Ogunreni OR, Sanni A. Influence of starter culture lactic acid bacteria on the phytic acid content of Sorghum-Ogi (an indigenous cereal gruel) Ann Food Sci Technol. 2014;15:121–134. [Google Scholar]
- 39.Bawane R, Tantwai K, Rajput LPS, Kadam-Bedekar M, Kumar S, Gontia I, Tiwari S. Molecular analysis of phytase gene cloned from Bacillus subtilis. Adv Stud Biol. 2011;3:103–110. [Google Scholar]
- 40.García-Mantrana I, Monedero V, Haros M. Reduction of phytate in soy drink by fermentation with Lactobacillus casei expressing phytases from Bifidobacteria. Plants Food Hum Nutr. 2015;70:269–274. doi: 10.1007/s11130-015-0489-2. [DOI] [PubMed] [Google Scholar]
- 41.Kumar V, Sangwan P, Verma AK, Agrawal S. Molecular and biochemical characteristics of recombinant β-Propeller phytase from Bacillus licheniformis strain PB-13 with potential application in Aquafeed. Appl Biochem Biotechnol. 2014;173:646–659. doi: 10.1007/s12010-014-0871-9. [DOI] [PubMed] [Google Scholar]
- 42.Wua T, Chen C, Cheng Y, Ko T, Lin C, Lai H, Huang T, Liu J, Guo R. Improving specific activity and thermostability of Escherichia coli phytase by structure-based rational design. J Biotechnol. 2014;175:1–6. doi: 10.1016/j.jbiotec.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Shao R, Wang Z, Yan X. Improving the neutral phytase activity from Bacillus amyloliquefaciens DSM 1061 by site-directed mutagenesis. Appl Biochem Biotechnol. 2015;175:3184–3194. doi: 10.1007/s12010-015-1495-4. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Fruitos E. Lactic acid bacteria: a promising alternative for recombinant protein production. Microb Cell Fact. 2012;11:157. doi: 10.1186/1475-2859-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyszyńska A, Kobierecka P, Bardowski J, Jagusztyn-Krynicka EK. Lactic acid bacteria—20 years exploring their potential as live vectors for mucosal vaccination. Appl Microbiol Biotechnol. 2015;99:2967–2977. doi: 10.1007/s00253-015-6498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.