Abstract
Gene specific DNA based sensors have potential applications for rapid and real time monitoring of hybridization signal with the target nucleic acid of pathogens. Different types of DNA based sensors and their applications have been studied for rapid and accurate detection of pathogens causing human diseases. These sensors are based on surface plasmon resonance, quantum-dots, molecular beacons, piezoelectric and electrochemical etc. Curbing epidemics at an early stage is one of the massive challenges in healthcare systems. Timely detection of the causative organism may provide a solution to restrain mortality caused by the disease. With the advent of interdisciplinary sciences, bioelectronics has emerged as an effective alternative for disease diagnostics. Gene specific DNA sensors present themselves as cost-effective, sensitive and specific platforms for detection of disease causing pathogens. The mini review explores different transducer based sensors and their potential in diagnosis of acute and chronic diseases.
Keywords: DNA sensors, E. coli, M. tuberculosis, N. meningitidis, Pathogens, S. pyogenes, S. typhi
Introduction
Integration of transducers in biomaterials have enabled generation of interfaces capable of eliciting signals and have been aptly addressed as biosensors Fig. 1. Biosensors, also known as bio-electromechanical systems can be classified on the basis of label, type of transducer and general configuration. Biosensors can be made based on their affinity and functionality. Affinity based on analyte recognition, dissociation constant (Kd) forms an evaluative factor in conforming the sensitivity of the sensor platform. The analytes recognition may also result in change in three dimensional structure which is further transduced as a signal.
Fig. 1.
Process flow of biosensing mechanism
Complementarity of the target molecule with the capture molecule forms the basis of shape-specific binding. The most familiar types of interaction are receptor-ligand and antibody-antigen binding. Aptamers, proteins, molecular imprints, lectins and antibodies are the different types of recognition elements which can be used as shape specific binding. Aptamers or aptasensors are artificial oligonucleotides (ribo or deoxy ribonucleotides) that at certain conditions (ionic strength, pH, temperature) tertiary structure having complementarity to oligonucleotides, certain proteins and low-molecular-weight compounds. The review highlights the role of different types of DNA biosensors and their practical applications in detection of microorganisms causing pathogenic (infectious) diseases. Dr. Kalia and group have numerous publications on genomic biomarker based identification of several strains of Streptococcus and other bacterial pathogens [1, 2].
Transducers Based Classification
Surface Plasmon Resonance Sensors
In early 1980, the surface plasmon resonance (SPR) based biosensor was first time reported for detection of gases. Since then, SPR sensors have been used for detecting chemical and biological species [3]. SPR occurs when contact of polarized light occurs with electrically conducting surface of prism resulting in generation of electron charge density waves called ‘plasmons’ which reduces the intensity with a specific angle defined as ‘resonance angle’. The resonance angle is proportional to the mass on the surface of the sensor (Fig. 2).
Fig. 2.
Schematic representation of SPR for detection of target ssDNA
Mathematical modeling for detection of DNA hybridization using graphene as a biological recognition element has been performed and it was experimentally inferred that association of a complementary DNA gave distinctly increased resonance frequency and transmittance. Furthermore, it was established sandwiching of each graphene sublayer augmented 95% sensitivity [4]. An SPR system with single stranded (ssDNA) aptamer bioreceptors against human immunoglobulin E (hIgE) was designed and limits of detection and quantification was standardized to 2 and 6 nM. The dissociation constant of aptamer with hIgE was found to be 30.9 ± 2.9 nM. [5]. The SPR sensor has detected up to 1 pM streptavidin and around 700 pM anti-biotin-biotinylated Ag nanoparticles and <7.5 nM anti-DNP -dinitrophenylated AgNPs with high specifity [6].
Fluorescence Based Sensor
Fluorescence resonance energy transfer (FRET) depends on excited states of two molecules in proximity, where emitted wavelength from donor is absorbed and subsequently excites the acceptor molecule Fig. 3.
Fig. 3.
Schematic representation of FRET based DNA sensor
Fluorescent biosensors may be categorized into two forms: analyte sensitive and conformation sensitive. Analyte sensor generates a fluorescent signal based on the interaction between target molecule and fluorophore. Generally, fluorescent intercalating dyes are used in hybridization based sensing technologies. Alternatively, protein networks linked to fluorescent dyes are used to distinguish between ssDNA and dsDNA. One such ‘reagent less biosensor’ utilized single stranded binding protein tagged with a diethylaminocoumarin, was utilized in detecting Escherichia coli using helicase. Similar mechanism was also employed in Bacillus subtilis to monitor the stoichiometric increase in the activity of helicase [7]. Conformation based sensors depend on the change of receptor configuration and thus on the elicitation of the fluorescence response. One such fluorescent genosensor was developed for the detection of PCR products and single-base mismatches. The DNA hairpins with 5′-thiol and 3′-fluorophore labeled was immobilized on gold electrode to devise a sensor. Quenching of the fluorophore was mediated by its proximity to the gold surface, alteration in conformation on binding to the target DNA resulted in an augmentation of fluorescent signal [8].
Another, FRET sensor designed on graphene oxide utilized the synergistic effect of fluorescent dyes fluoroscein (FAM) and SYBR green I for detection of methicillin resistant Staphylococcus aureus (MRSA) by strand displacement reaction. FAM labeled ssDNA was conjugated with both capture and signal probe. On addition of target ssDNA results enhance in fluorescence signal. The limit of detection was found 1–40 nmol L−1 for confirmation of the presence of S. aureus infection [9]. Silica nano particles based platform was also developed using oligonucleotides to detect the presence of S. aureus in whole blood. The secreted nuclease from the S. aureus was measured via a modified oligonucleotide mediated by ‘nanokeepers’. The sensor was fast, specific and highly sensitive for detection of S. aureus [10].
Quantum-Dots Based Sensor
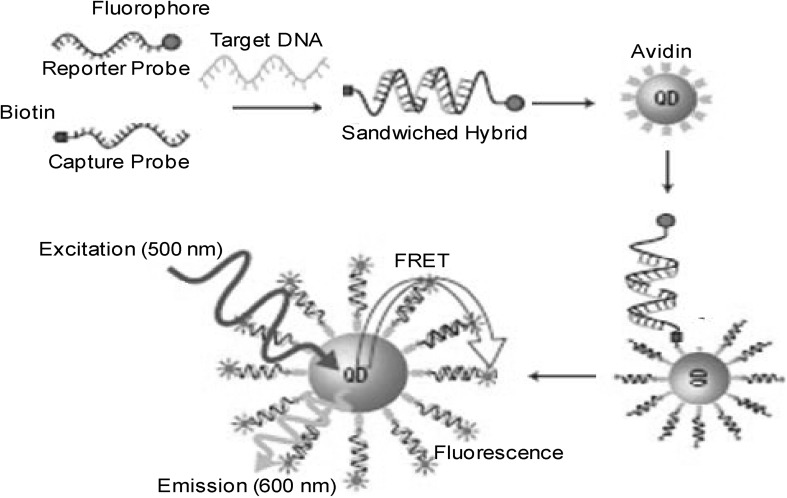
Quantum dots (QDs) are nanoparticles of semiconducting metals (2–10 nm in diameter). Because of its nano-dimensions, QDs display unique optical and electrical properties [11]. QDs emit photon under excitation. Wavelength of photon depends on size of the QDs. Smaller the QDs close to the blue spectrum and bigger closer to red spectrum even beyond visible light. QDs sensors are ultrasensitive nanosensor based on FRET which can detect very low concentrations of DNA without separation of unhybridized DNA (Fig. 4). QDs also concentrate and amplify signals by collecting several targets at nanoscale domain. Unbound DNA do not participate in FRET and produce zero background fluorescence whereas minute quantity (<50 copies) of target DNA produce extremely strong signals. Several types of QDs are available such as CdSe–ZnS, ZnSe, CdS, MnO, ZnO, ZnSe–ZnS, PbS etc. They tend to possess increased photo stability, excellent chemical resistance and can act as excellent donors for FRET based assays. Newer QDs with improved biocompatibility have been thus augmenting the biological applications [12]. A schematic diagram of quantum-dots based sensor is shown in Fig. 4.
Fig. 4.
Schematic diagram of quantum-dots based DNA sensor
A self assembled sensor was developed using QD-maltose binding protein of E. coli. A β-cyclodextrin-QSY9 quencher was found to represses fluorescence emission in the presence of maltose in solution. QD-biomolecule assemblies constructed using these methods enabled synthesis of new hybrid sensing platforms [13]. QD based DNA sensors employ the principle of specificity and complementarity between target-probe oligonucleotides. The ssDNA modified QDs were used as DNA probes. The detection of target relies on the concurrence of two different wavelength fluorescent signals of QDs/DNA target-probe complexes (Fig. 4). The target-probe hybridization causing proximal placement of linked QDs and final detection of target by FRET [14].
The ssDNA probe functionalized cadmium selenide quantum dots (QCdSe) was used to design an impedimetric microfluidic-based nucleic acid sensor for identification of chronic myelogenous leukemia marker. Indium-tin-oxide (ITO) was etched and sealed with polydimethylsiloxane microchannel for fluid control. Langmuir–Blodgett (LB) technique was used to deposit the QCdSe on ITO chips. The probe was fabricated to detect change in concentration via interfacial charge transfer resistance after hybridization. The biosensor exhibited improved linearity in the concentration range of 10−15–10−11 M DNA [15].
Molecular Beacons Sensor
Molecular beacons (MB) are synthetic hairpin oligonucleotides probe with stem-loop structure labeled with fluorophore and quencher on either end of the stem. These probes have the ability to detect specific complementary target nucleotide sequences via hybridization and presents novel non-radioactive mechanism for detection of specific nucleic acid sequences (Fig. 5). MB probes provide high sensitivity and specificity. MB-based assays have enabled easier mechanism of SNP genotyping, reduction in signal-to-noise ratio, and detection of folded RNA and DNA [16]. A biotinylated MB was made and linked to glass through streptavidin or avidin (bridge between MB and glass). The change in fluorescence was monitored due to conformational change in MB after hybridization of target complementary DNA sequence. The fluorophore and quencher are separated and MB gives fluorescence whereas in absence of hybridization no fluorescence is observed due to closeness of fluorophore to quencher.
Fig. 5.
Functioning of MB sensor and subsequent fluorescent signal on excitation
Impedance based DNA biosensor was developed using MB-DNA as probe and thionine as charge neutralizer [17]. Intercalation of thionine into dsDNA was an efficient approach to amplify the signal using impedance technique. Additionally, specificity of sensor was enhanced as compared to traditional unstructured DNA probe.
Beadcons are a new type of MB wherein, the beacons are conjugated on a beaded structure. These discrete beaded structures may be assayed for sensitivity based on flow cytometric methods. Unbound samples of target DNA can be easily distinguished from bound samples due to effective loss in FRET. The method is characterized by shorter response time and ease-of-use, thus proving to be good candidate for a further development of its diagnostic capabilities [18]. Beadcon has also been utilized to detect artificial sequences of Streptococcus pneumoniae and Micrococcus luteus in concentration equivalent to 10−9–10−12 M using a miniaturized fluorescence microscope.
A miRNA based biosensor was developed for monitoring the progression of angiogenesis of endothelial progenitor cells (EPC) treated ischemia. Mir126 NIR (near-infra red) MB consists of synthetic stem loop structure (hairpin) with fluorophore (Cy5) as reporter and quencher (BHQ2) which gives fluorescence upon hybridization [19]. The diagnosis of myelogenous leukemia was also enabled using MB based sensor targeted to identify 278 bp fragment of cancer DNA. The MB can be labeled with tetramethylrhodamine, and 4-(2-methyl-on-amino-azobenzene) benzoate (DABCYL) [20].
Label-free multifunctional MB (LMMB) was also designed by integrating template, polymerization primer, target recognition and G-quadruplex as one entity. Initial hybridization between LMMB and G-hairpin DNA recognition stem triggered conformational change between the G-hairpin and C-hairpin leading to fluorophore SYBR green dye intercalation and subsequent signal amplification. The technique was simple and cost effective. LMMB can perceive single nucleotide mutations and hence can be employed to identify mutant cell lines and opens new prospects for genetic disease therapy [21].
Piezoelectric and Cantilever Based Sensor
Piezoelectric DNA sensor utilizes quartz crystal (silica oxide) that oscillates at a defined frequency at applied oscillating voltage. The method emerged as most simple, cost effective, sensitive and enabled real time label-free detection. The quartz crystal microbalance (QCM) is a sensitive mass-measuring device that allows dynamic monitoring of hybridizations. QCM sensors consist of oscillating crystal with an immobilized DNA probe. DNA hybrid has higher mass and its formation resulted in diminished oscillating frequency (Fig. 6). Cantilever platforms are fabricated in nanoscale dimensions for the label-free detection of biomolecules. Molecular interaction in the cantilever results in quantifiable bending, permitting the measurement of deflection. Microcantilevers composed of miniature silicon and silicon nitride for development of sensors. Piezoelectric-excited, millimeter-sized cantilever (PEMC) sensors increase sensitivity and selectivity. PEMC sensors can detect resonance in the range of 1 MHz with mass change sensitivity of 1–300 µg/Hz. [22]. A DNA aptamer functionalized acoustic wave sensing platform was also fabricated using quartz substrate to detect cocaine. Such type of sensor is known as electromagnetic piezoelectric acoustic sensor [23].
Fig. 6.
Piezoelectric based DNA sensor for detection of pathogen
Electrochemical Transducers
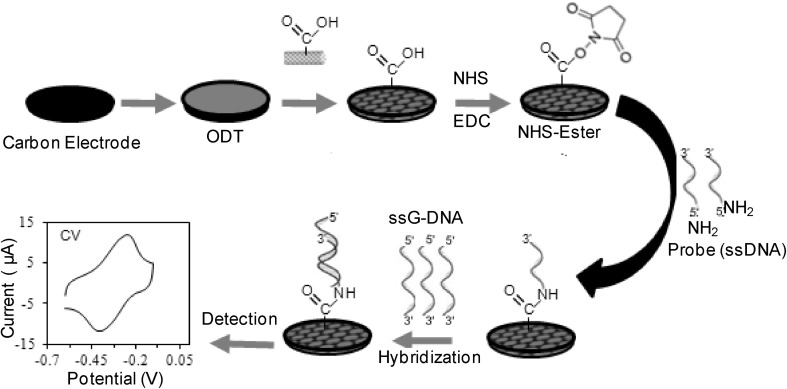
Electrochemical transducers have been prevalent from several decades and may be categorized into potentiometric, impedimetric and amperometric sensors. General functioning and signal transduction of electrochemical sensors are analyzed based on change in surface architectures that connect the sensing element to the biological sample at the nanoscale (Fig. 7). Carbon nanotubes, nanoparticles (gold, silver and others) and QDs have been used as templates for signal enhancement. Additionally, linkers consisting of polymers of aniline, pyrrole, thiophenes and phenazines tend to amplify the signal transduction of DNA sensors for the detection of hybridization events [24, 32]. The schematic presentation of electrochemical DNA sensor using modified carbon electrode with octadecane thiol (ODT) and carboxylated multiwalled carbon nanotubes (c-MWCNT) to attach amino labeled ssDNA probe through 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) chemistry for hybridization with target DNA (Fig. 7).
Fig. 7.
Schematic presentation of electrochemical DNA sensor
Detection of Pathogens
Over the years many sensors have been designed for organism specific detection.
E. coli
E. coli O157 (cytotoxic pathogen) because of its ability to induce acute renal failure within short time frame, different transducers have been utilized to generate biosensors against this dreaded bacterium [25]. The quantitative detection of Enterobacteriaceae bacteria was mediated by integrating exonuclease III with an electrochemical sensor platform. Exonuclease digests the target/capture DNA to initiate next hybridization and cleavage cycle on sensor surface. The sensor showed sensitivity in the range of 0.01 pM–1.0 nM DNA which provides a platform for biothreat environmental monitoring and detection of food safety [26]. An allosteric molecular beacon (aMB) based E-sensor against E. coli possessed stable hairpin structure which blocked the binding capability of the complimentary streptavidin (SA) aptamer. Incubation with target DNA resulted in binding of SA aptamer to SA-HRP (streptavidin–horseradish peroxidase), which in turn reduced tetra methyl benzidine (TMB) and eliciting an electrochemical signal. Sensitivity of the E-sensors had a linear range of 10−9–10−6 g/ml DNA [3].
Another species specific DNA biosensor was constructed using interdigitated aluminium electrode coated with TiO2 nanoparticles. Duplex formation with complementary target was quantified with the sensor and detection limit was 1.0 × 10−13 M DNA. The sensor was found stable up to 6 months with 99% of its original sensitivity [27]. Gene specific electrochemical biosensor was also designed using capture probe against rfbE gene, specific to E. coli O157. Screen-printed nano gold electrodes (nanoAu/SPE) were customized with thiol-capped ssDNA (capture probe). Competition for hybridization occurs between the target gene and reporter DNA-tagged with hexaammineruthenium(III) chloride-encapsulated liposomes. Detection of free Ru[NH3]3+6 was monitored by square wave voltammetry, generated sigmoidal dose–response curve. Ultra trace amount of 0.75 µmol rfbE DNA was measured with liposome competitive assay.
Bundle forming pilus (BFP) protein enables the colonization of enteropathogenic E. coli (EPEC) on the epithelial cells and thus facilitate the spread of infection. bfpA encodes BFP and thus has become an important target for identification of EPEC in samples. The amplified biotinylated PCR product linked with electrode and sandwiched with biotinylated probe connected to enzyme linked streptavidin. Electrochemical signals enabled detection of 10−13–10−9 M DNA of EPEC. Another marker for EPEC is the eaeA gene, encoding protein enabling attachment to the epithelial cells. The eaeA biomarker was used as template for construction of surface enhanced fluorescence with a linear range from 10−17 to 10−11 M DNA with detection limit 3.33 × 10−18 M DNA whereas, QCM biosensor showed sensitivity of 2.0 × 103 colony forming unit/ml [28].
Similarly, 16 s-rDNA based sensor was designed which could distinctly discriminate between E. coli and Lactobacillus reuteri DNA. The sensor response was linear vs. log concentration in the range 10−12–10−7 M for E. coli with a detection limit of 6.5 × 10−13 M DNA. This sensor was projected to be utilized for an in-field bacteria detection. A piezoelectric-excited millimeter cantilever (PEMC) sensor was also fabricated for detection of stx2 (shiga toxin homologue) present in E. coli. The successful hybridization was resulted in detectable resonant frequency in PEMC sensor indicating the presence of stx2. [29, 30].
Neisseria meningitidis
Another morbid bacterium N. meningitidis possessing the potential to cause epidemics and if not detected timely causing death. Hence, detection of this obligate human pathogen is of paramount importance. Over the years several biomarkers specific for N. meningitidis have been recognized [31]. Some out of these markers were used as target DNA for development of human brain meningitis sensors. Cyclic voltammetry (CV) and impedance spectroscopy (EIS) were studied using zinc oxide (ZnO) nanoparticles on platinized silicon substrate. The fabricated sensor can detect 5–240 ng μl−1 complimentary target ssDNA of ctrA gene. Similarly, a SPR biosensor employing Kretschmann configuration presented a higher sensitivity of 0.03 ng μl−1 with lower limit of detection of 5 ng μl−1. The sensor showed higher specificity and longer shelf life. Another sensor, using Carbon/1-octadecanethiol/carboxylated multiwalled carbon nanotubes (carbon modified electrode) had sensitivity of 1.35 × 102–3.44 × 104 pM of single-stranded genomic DNA (ssG-DNA) which can detect N. meningitidis presence only in 10 min. The improved sensitivity and LOD was attributed to an efficient immobilization of probe and good electrical conductivity of cMWCNT. Similarly, several other electrochemical biosensors were developed for efficient detection of human brain meningitis directly from cerebrospinal fluids of the patient samples with good sensitivity, specificity and lower limit of detections [32, 33].
Streptococcus pyogenes
S. pyogenes is a group A streptococcus (GAS) bacteria which infects the human throat and causes pharyngitis at initial stages. If it remains untreated, it tends to cause progressive rheumatic heart disease. Gene specific probe based DNA sensor was designed to detect the concentration of the infecting bacterium using non invasive mechanism [34]. 5′-carboxyl modified ssDNA probe was immobilized on gold-mercaptopropionic acid-polyethylenimine composite electrode and electrochemical response was measured after hybridization with ssG-DNA obtained from throat swabs using CV, differential pulse voltammetry (DPV) and EIS. The sensitivity of the sensor was 110.25 μA cm−2 ng−1 with DPV and the lower limit of detection was 10 pg per 6 μl within a time frame of 30 min. Singh et al. in continuation, further increased the sensitivity by using label free single-walled carbon nanotubes. Single stranded DNA (ssDNA) probe was covalently immobilized using carbodiimide based reaction. S. pyogenes genosensor thus developed, displayed linear response to G-DNA from 10−4 ng ml−1 with a limit of detection of 0.16 ng ml−1.
Salmonella typhi
Typhoid fever, if kept untreated is fatal in nature and hence point-of-care detection of S. typhi is mandatory. Researchers have developed nanocomposite (graphene oxide-chitosan) platform for electrochemical DNA biosensor having a detection range of 10 fM–50 nM and LOD 10 fM in 60 s hybridization. Higher sensitivity and better response time is attributed to use of chitosan proving as an improved conducive platform. Additionally it may detect target in serum with LOD of 100 fM at 25 °C. Another, electrochemical sensor was developed using biocompatible gold nanoaggregates-indium tin oxide electrode and methylene blue as redox indicator. The sensor showed detection range 24 fM–4 µM at 25 °C and worked equally well in blood and urine samples [35, 36].
Flagellin gene (fla) of S. typhi was also used as a target for a pencil graphite electrode fixated with ssDNA probe. Presence of the infectious organisms was analyzed by CV [37]. Another group has assembled a SPR based DNA hybridization biosensor immobilized with ssDNA probe complementary to conserved Vi capsular protein of Salmonella enteric Serovars typhi. The biosensor works at room temperature up to 40 hybridization cycles and differentiate between complementary and mismatched sequences [38].
Mycobacterium tuberculosis
Tuberculosis has re-emerged globally as an important health concern, due to the emergence of multi drug resistant and extensively drug resistant strains. Polypyrrole (PPy) and polyamidoamine (PAMAM) dendrimers on MWCNTs/ferrocene conjugate was used for recognition of rpoB gene of M. tuberculosis [39]. Electrochemical signal was generated on identification of target DNA with a LOD of 0.3 fM, indicating development of high performing sensor. Another group working on similar concept formulated a microfluidic-multiplexed platform integrated electrochemical sensors shoed detection limit 0.1 fM–1.0 pM [40]. Rifampicin resistant M. tuberculosis strains were electrochemically detected using hybridization of immobilized probes with PCR products of different strains of mycobacterium. However, biosensors based detection of M. tuberculosis is difficult (variations in strains) due to making specific probe for universal detection of M. tuberculosis pathogen.
Conclusions
An immense potential is presented by BioMEMS (biomedical microelectromechanical systems) for development of point-of-care diagnostics. Utility of biosensors have presented itself with numerous advantages such as lower cost and complexity, nanoscale sample volumes, easy portability and large-scale multiplexing. Further advances in this technology would enable control of epidemics and hence reinforced healthcare precautions.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kalia VC, Kumar R, Kumar P, Koul S. A genome-wide profiling strategy as an aid for searching unique identification biomarkers for Streptococcus. Indian J Microbiol. 2016;56:46–58. doi: 10.1007/s12088-015-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koul S, Kumar P, Kalia VC. A unique genome wide approach to search novel markers for rapid identification of bacterial pathogens. J Mol Genet Med. 2015;9:194. doi: 10.4172/1747-0862.1000194. [DOI] [Google Scholar]
- 3.Jung LS, Nelson KE, Campbell CT, Stayton PS, Yee SS, Pérez-Luna V, López GP. Surface plasmon resonance measurement of binding and dissociation of wild-type and mutant streptavidin on mixed biotin-containing alkylthiolate monolayers. Sens Actuators B Chem. 1999;54:137–144. doi: 10.1016/S0925-4005(98)00332-3. [DOI] [Google Scholar]
- 4.Hossain MB, Rana MM. DNA hybridization detection based on resonance frequency readout in graphene on Au SPR biosensor. J Sens. 2016;501:6070742. [Google Scholar]
- 5.Caucheteur C, Guo T, Albert J. Review of plasmonic fiber optic biochemical sensors: improving the limit of detection. Anal Bioanal Chem. 2015;407:3883–3897. doi: 10.1007/s00216-014-8411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vo-Dinh T, editor. Biomedical photonics handbook: biomedical diagnostics. Boca Raton: CRC Press; 2014. [Google Scholar]
- 7.Green M, Gilhooly NS, Abedeen S, Scott DJ, Dillingham MS, Soultanas P. Engineering a reagentless biosensor for single-stranded DNA to measure real-time helicase activity in Bacillus. Biosens Bioelectron. 2014;61:579–586. doi: 10.1016/j.bios.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Stakenborg T, Cheng Y, Colle F, Steylaerts T, Jans K, Van Dorpe P, Lagae L. Label-free genosensor based on immobilized DNA hairpins on gold surface. Biosens Bioelectron. 2011;26:3121–3126. doi: 10.1016/j.bios.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Ning Y, Gao Q, Zhang X, Wei K, Chen L. A graphene oxide–based sensing platform for the determination of methicillin-resistant Staphylococcus aureus based on strand-displacement polymerization recycling and synchronous fluorescent signal amplification. J Biomol Screen. 2016;21:851–857. doi: 10.1177/1087057116653564. [DOI] [PubMed] [Google Scholar]
- 10.Borsa BA, Tuna BG, Hernandez FJ, Hernandez LI, Bayramoglu G, Arica MY, Ozalp VC. Staphylococcus aureus detection in blood samples by silica nanoparticle-oligonucleotides conjugates. Biosens Bioelectron. 2016;15:27–32. doi: 10.1016/j.bios.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Dworak L, Matylitsky VV, Ren T, Basché T, Wachtveitl J. Acceptor concentration dependence of förster resonance energy transfer dynamics in dye–quantum dot complexes. J Phys Chem C. 2014;118:4396–4402. doi: 10.1021/jp409807x. [DOI] [Google Scholar]
- 12.Peng ZA, Peng X. Formation of high-quality CdTe, CdSe and CdS nanocrystals using CdO as precursor. J Am Chem Soc. 2001;123:183–184. doi: 10.1021/ja003633m. [DOI] [PubMed] [Google Scholar]
- 13.Pan Z, Mora-Seró I, Shen Q, Zhang H, Li Y, Zhao K, Wang J, Zhong X, Bisquert J. High-efficiency green quantum dot solar cells. J Am Chem Soc. 2014;136:9203–9210. doi: 10.1021/ja504310w. [DOI] [PubMed] [Google Scholar]
- 14.Holzinger M, Le Goff A, Cosnier S. Nanomaterials for biosensing applications: a review. Front Chem. 2014;27:2–63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghrera AS, Pandey CM, Ali MA, Malhotra BD. Quantum dot-based microfluidic biosensor for cancer detection. Appl Phys Lett. 2015;106:193–703. doi: 10.1063/1.4921203. [DOI] [Google Scholar]
- 16.Stobiecka M, Chałupa A. Biosensors based on molecular beacons. Chem Pap. 2015;69:62–76. doi: 10.1515/chempap-2015-0026. [DOI] [Google Scholar]
- 17.Xu Y, Yang L, Ye X, He P, Fang Y. Impedance-based DNA biosensor employing molecular beacon DNA as probe and thionine as charge neutralizer. Electroanalysis. 2006;18:873–881. doi: 10.1002/elan.200503465. [DOI] [Google Scholar]
- 18.Horejsh D, Martini F, Poccia F, Ippolito G, Di Caro A, Capobianchi MR. A molecular beacon, bead-based assay for the detection of nucleic acids by flow cytometry. Nucleic Acids Res. 2005;33:e13. doi: 10.1093/nar/gni015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CH, Chae JI, Ko HY, Kim S. Molecular beacon-based micro RNA biosensor for imaging EPC-treated cellular therapy of ischemia. J Mol Imaging Dyn. 2013;3:113. [Google Scholar]
- 20.Gerasimova YV, Kolpashchikov DM. Enzyme-assisted target recycling (EATR) for nucleic acid detection. Chem Soc Rev. 2014;43:6405–6438. doi: 10.1039/C4CS00083H. [DOI] [PubMed] [Google Scholar]
- 21.Dong H, Ma J, Wang J, Wu ZS, Sinko PJ, Jia LA. Biofunctional molecular beacon for detecting single base mutations in cancer cells. Mol Ther Nucleic Acids. 2016;5:e302. doi: 10.1038/mtna.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DM, Kang SH, Kim S, Seung W, Tsege EL, Kim SW, Kim HK, Hong SW, Hwang YH. Preparation of ZnO Nanorod/Graphene/ZnO Nanorod epitaxial double hetero structure for piezoelectrical nanogenerator by using preheating hydrothermal. J Vis Exp. 2016;107:e53491. doi: 10.3791/53491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neves MA, Blaszykowski C, Bokhari S, Thompson M. Ultra-high frequency piezoelectric aptasensor for the label-free detection of cocaine. Biosens Bioelectron. 2015;72:383–392. doi: 10.1016/j.bios.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Walcarius A. Mesoporous materials-based electrochemical sensors. Electroanalysis. 2015;27:1303–1340. doi: 10.1002/elan.201400628. [DOI] [Google Scholar]
- 25.Zainudin N, Hairul AR, Yusoff MM, Tan LL, Chong KF. Impedimetric graphene-based biosensor for the detection of Escherichia coli DNA. Anal Methods. 2014;6:7935–7941. doi: 10.1039/C4AY01836B. [DOI] [Google Scholar]
- 26.Liao WC, Ho JA. Attomole DNA electrochemical sensor for the detection of Escherichia coli O157. Anal Chem. 2009;81:2470–2476. doi: 10.1021/ac8020517. [DOI] [PubMed] [Google Scholar]
- 27.Nadzirah S, Azizah N, Hashim U, Gopinath SCB, Kashif M. Titanium dioxide nanoparticle-based interdigitated electrodes: a novel current to voltage DNA biosensor recognizes E. coli O157:H7. PLoS One. 2015;10:e0139766. doi: 10.1371/journal.pone.0139766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Luo C, Zhong L, Nie S, Cheng W, Zhao D, Ding S. Sensitive detection of enteropathogenic E. coli using a bfpA gene-based electrochemical sensor. Microchim Acta. 2013;180:1233–1240. doi: 10.1007/s00604-013-1061-0. [DOI] [Google Scholar]
- 29.Sun J, Ji J, Sun Y, Abdalhai MH, Zhang Y, Sun X. DNA biosensor based on fluorescence detection of E. coli O157: H7 by Au@Ag nanorods. Biosens Bioelectron. 2015;70:239–245. doi: 10.1016/j.bios.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Rijal K, Mutharasan R. A method for DNA-based detection of E. coli O157: H7 in a proteinous background using piezoelectric-excited cantilever sensors. Analyst. 2013;138:2943–2950. doi: 10.1039/c3an36814a. [DOI] [PubMed] [Google Scholar]
- 31.Briassoulis G, Galani A. Prognostic markers of pediatric eningococcal sepsis. Expert Rev Anti Infect. 2014;12:1017–1020. doi: 10.1586/14787210.2014.945431. [DOI] [PubMed] [Google Scholar]
- 32.Dash SK, Sharma M, Kumar A, Khare S, Kumar A. A carbon composite biomaterial based electrochemical DNA nanosensor for human brain meningitis. J Solid State Electrochem. 2014;8:2647–2659. doi: 10.1007/s10008-014-2525-9. [DOI] [Google Scholar]
- 33.Dash SK, Sharma M, Khare S, Kumar A. rmpM genosensor for detection of human brain meningitis in cerebrospinal fluid. Appl Biochem Biotechnol. 2013;171:198–208. doi: 10.1007/s12010-013-0339-3. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Kumar A, Khare S, Mulchandani A. Single-walled carbon nanotubes based chemiresistive genosensor for label-free detection of human rheumatic heart disease. Appl Phys Lett. 2014;105:213–701. [Google Scholar]
- 35.Singh A, Choudhary M, Singh MP, Verma HN, Singh SP, Arora K. DNA functionalized direct electro-deposited gold nanoaggregates for efficient detection of Salmonella typhi. Bioelectro Chem. 2015;105:7–15. doi: 10.1016/j.bioelechem.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Lawal AT. Synthesis and utilization of graphene for fabrication of electrochemical sensors. Talanta. 2015;31:424–443. doi: 10.1016/j.talanta.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Hartati YW, Wyantuti S, Firdaus ML, Auliany N, Surbakti R, Gaffar S. A rapid and sensitive diagnosis of typhoid fever based on nested PCR-voltammetric DNA biosensor using flagellin gene fragment. Indones J Chem. 2016;16:87–91. doi: 10.22146/ijc.21182. [DOI] [Google Scholar]
- 38.Singh A, Verma HN, Arora K. Surface plasmon resonance based label-free detection of Salmonella using DNA self assembly. Appl Biochem Biotechnol. 2015;175:1330–1343. doi: 10.1007/s12010-014-1319-y. [DOI] [PubMed] [Google Scholar]
- 39.Miodek A, Mejri N, Gomgnimbou M, Sola C, Korri-Youssoufi H. E-DNA sensor of Mycobacterium tuberculosis based on electrochemical assembly of nanomaterials (MWCNTs/PPy/PAMAM) Anal Chem. 2015;87:9257–9264. doi: 10.1021/acs.analchem.5b01761. [DOI] [PubMed] [Google Scholar]
- 40.Zribi B, Roy E, Pallandre A, Chebil S, Koubaa M, Mejri N, Gomez HM, Sola C, Korri-Youssoufi H, Haghiri-Gosnet AM. A microfluidic electrochemical biosensor based on multiwall carbon nanotube/ferrocene for genomic DNA detection of Mycobacterium tuberculosis in clinical isolates. Biomicrofluidics. 2016;10:014115. doi: 10.1063/1.4940887. [DOI] [PMC free article] [PubMed] [Google Scholar]