Abstract
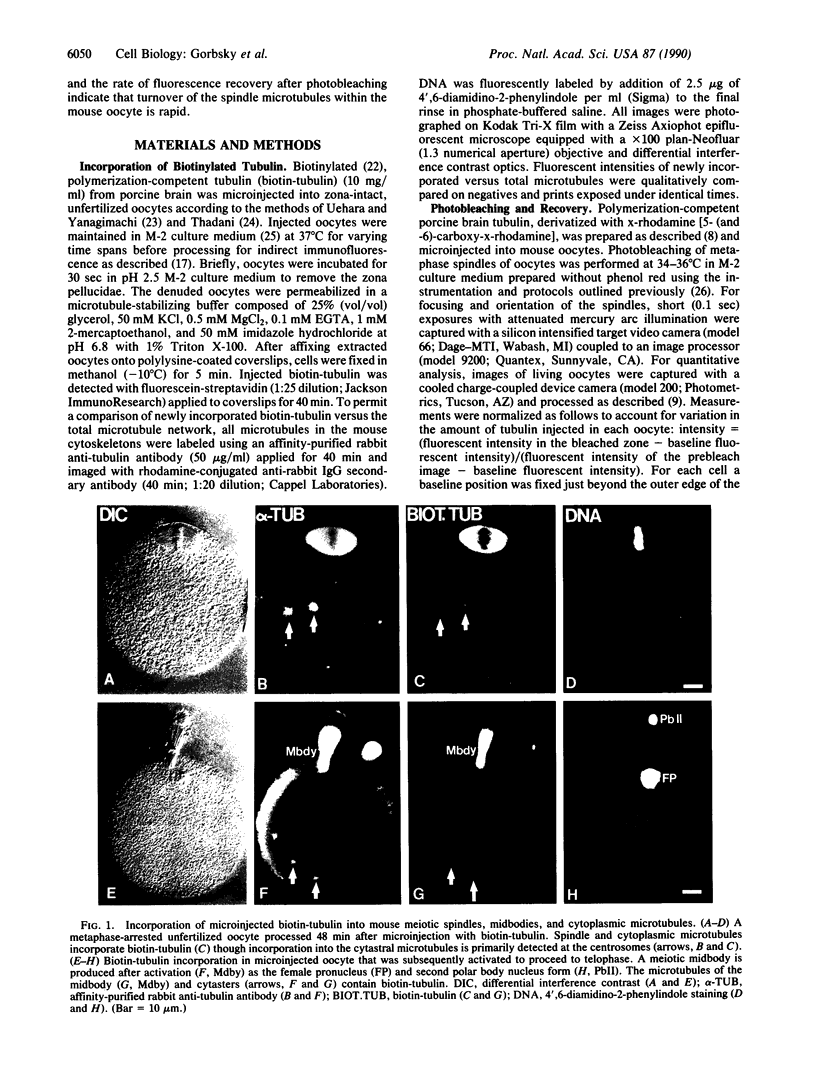
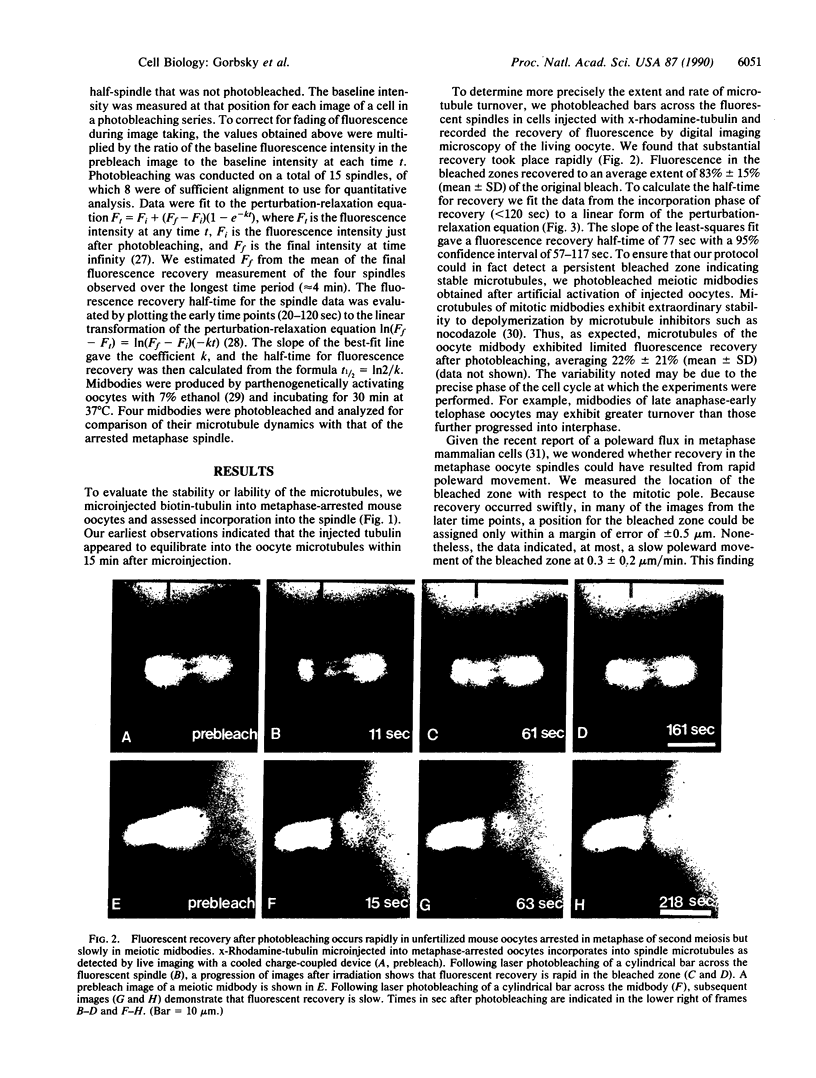
After ovulation mammalian oocytes arrest in second meiotic metaphase. We asked whether the microtubules that comprise the meiotic spindle of mouse oocytes were stable or were undergoing rapid cycles of assembly and disassembly. Porcine brain tubulin, derivatized with biotin or x-rhodamine [5- (and -6)-carboxy-x-rhodamine], was microinjected into living oocytes. Biotinylated tubulin incorporated into the meiotic spindle to apparent equilibrium within 15 min. To assess quantitatively the rates of disassembly and assembly of the microtubules, small domains within the spindles of oocytes injected with x-rhodamine-tubulin were photobleached and their recovery was analyzed by digital imaging microscopy. Fluorescence recovery in the spindles was rapid and extensive, plateauing to an average of 83% at 4 min. The calculated half-time for turnover of the spindle microtubules was 77 sec. In contrast, fluorescence recovery of the spindle midbodies in telophase oocytes was much more limited, averaging approximately 22% at 4 min. These data indicate that most microtubules within the arrested metaphase spindle of the mouse oocyte undergo rapid cycles of assembly and disassembly. Microtubules of the telophase midbody are more stable.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassimeris L., Pryer N. K., Salmon E. D. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson K. S. Parthenogenetic activation of mouse oocytes in vitro with ethanol and benzyl alcohol. J Exp Zool. 1983 May;226(2):311–314. doi: 10.1002/jez.1402260217. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., Aerts F., De Mey J. Microtubule dynamics during the cell cycle: the effects of taxol and nocodazole on the microtubule system of Pt K2 cells at different stages of the mitotic cycle. Int Rev Cytol. 1986;101:215–274. doi: 10.1016/s0074-7696(08)60250-8. [DOI] [PubMed] [Google Scholar]
- Fulton B. P., Whittingham D. G. Activation of mammalian oocytes by intracellular injection of calcium. Nature. 1978 May 11;273(5658):149–151. doi: 10.1038/273149a0. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J., Borisy G. G. Microtubules of the kinetochore fiber turn over in metaphase but not in anaphase. J Cell Biol. 1989 Aug;109(2):653–662. doi: 10.1083/jcb.109.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G. J., Sammak P. J., Borisy G. G. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J Cell Biol. 1987 Jan;104(1):9–18. doi: 10.1083/jcb.104.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi Y., Toriyama M., Sakai H., Hiramoto Y. Redistribution of fluorescently labeled tubulin in the mitotic apparatus of sand dollar eggs and the effects of taxol. Cell Struct Funct. 1987 Feb;12(1):43–52. doi: 10.1247/csf.12.43. [DOI] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Inoué S., Ritter H., Jr Dynamics of mitotic spindle organization and function. Soc Gen Physiol Ser. 1975;30:3–30. [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Kirschner M. W. Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol. 1985 Sep;101(3):755–765. doi: 10.1083/jcb.101.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989 Aug;109(2):637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Paules R. S., Buccione R., Moschel R. C., Vande Woude G. F., Eppig J. J. Mouse Mos protooncogene product is present and functions during oogenesis. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5395–5399. doi: 10.1073/pnas.86.14.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L., Davison E. A., Jensen L. C., Cassimeris L., Salmon E. D. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J Cell Biol. 1986 Aug;103(2):581–591. doi: 10.1083/jcb.103.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma. 1981;84(1):145–158. doi: 10.1007/BF00293368. [DOI] [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Leslie R. J., Saxton W. M., Karow M. L., McIntosh J. R. Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol. 1984 Dec;99(6):2165–2174. doi: 10.1083/jcb.99.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E. D. Spindle microtubules: thermodynamics of in vivo assembly and role in chromosome movement. Ann N Y Acad Sci. 1975 Jun 30;253:383–406. doi: 10.1111/j.1749-6632.1975.tb19216.x. [DOI] [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Sammak P. J., Gorbsky G. J., Borisy G. G. Microtubule dynamics in vivo: a test of mechanisms of turnover. J Cell Biol. 1987 Mar;104(3):395–405. doi: 10.1083/jcb.104.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. M., Stemple D. L., Leslie R. J., Salmon E. D., Zavortink M., McIntosh J. R. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984 Dec;99(6):2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatten G., Simerly C., Asai D. J., Szöke E., Cooke P., Schatten H. Acetylated alpha-tubulin in microtubules during mouse fertilization and early development. Dev Biol. 1988 Nov;130(1):74–86. doi: 10.1016/0012-1606(88)90415-0. [DOI] [PubMed] [Google Scholar]
- Schatten G., Simerly C., Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4152–4156. doi: 10.1073/pnas.82.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986 Mar;102(3):1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. New features of microtubule behaviour observed in vivo. Nature. 1988 Jul 28;334(6180):356–359. doi: 10.1038/334356a0. [DOI] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R. P. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci. 1972 Sep;11(2):521–541. doi: 10.1242/jcs.11.2.521. [DOI] [PubMed] [Google Scholar]
- Uehara T., Yanagimachi R. Behavior of nuclei of testicular, caput and cauda epididymal spermatozoa injected into hamster eggs. Biol Reprod. 1977 Apr;16(3):315–321. doi: 10.1095/biolreprod16.3.315. [DOI] [PubMed] [Google Scholar]
- Wadsworth P., Salmon E. D. Analysis of the treadmilling model during metaphase of mitosis using fluorescence redistribution after photobleaching. J Cell Biol. 1986 Mar;102(3):1032–1038. doi: 10.1083/jcb.102.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P., Salmon E. D. Microtubule dynamics in mitotic spindles of living cells. Ann N Y Acad Sci. 1986;466:580–592. doi: 10.1111/j.1749-6632.1986.tb38434.x. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]
- Webster D. R., Borisy G. G. Microtubules are acetylated in domains that turn over slowly. J Cell Sci. 1989 Jan;92(Pt 1):57–65. doi: 10.1242/jcs.92.1.57. [DOI] [PubMed] [Google Scholar]
- de Pennart H., Houliston E., Maro B. Post-translational modifications of tubulin and the dynamics of microtubules in mouse oocytes and zygotes. Biol Cell. 1988;64(3):375–378. doi: 10.1016/0248-4900(88)90012-3. [DOI] [PubMed] [Google Scholar]