Summary
It is estimated that 13.000 to 22.000 individuals suffer from inhalational burns each year in the United States alone. Despite these high numbers, inhalational burns remain a major challenge to otolaryngologists. In this paper, a review of literature is presented in order to provide otolaryngologists with a systematic approach to patients with inhalational burns to optimize treatment, cost, morbidity and, most importantly, mortality. For this purpose, a broad PubMed search was conducted. The available literature was found to highlight the importance of airway management in terms of the timing of intubation, method of intubation, trachea-esophageal (TE) fistula formation and TE rupture. It also emphasizes the importance of carbon monoxide intoxication and prompt correction. Drugs such as heparin sulfate, N-acetylcysteine and albuterol have been proven to help in the treatment of patients with inhalational burns, and more research is currently underway with the purpose of developing chelating drugs that scavenge the toxic substances in the smoke before they can damage the airway.
Keywords: burn, airway, inhalational
Abstract
On estime que 13 000 à 22 000 personnes présentent chaque année, aux États-Unis, une inhalation de fumées. Cette pathologie représente un défi majeur pour les ORL. Cette revue de littérature a pour but de proposer aux ORL une approche systématisée de la prise en charge de cette pathologie afin d’en optimiser le traitement pour diminuer mortalité, morbidité et coûts. Les articles trouvés dans PubMed insistent sur le délai d’intubation et sa technique, les intoxications au CO, les complications trachéales (ruptures) et trachéo-oesophagiennes (fistule). L’héparine, la N-acétyl cystéine et l’albutérol ont montré leur intérêt en traitement d’appoint. Les recherches sur les chélateurs permettant de diminuer la toxicité des fumées sont en cours.
Introduction
To this day, inhalational burns remain a major challenge to otolaryngologists worldwide, and yet there is no consensus on a proper, systematic, evidence-based approach to treat patients in this acute setting. It has been shown that inhalational injury, in addition to total burn surface area and age, is one of the three most important predictors of mortality subsequent to a burn injury.1 The major sources of inhalational injury are industrial or domestic accidents, fires, and intentional release of respirable toxicants in the setting of wars or terrorist acts.2 It is estimated that 13.000 to 22.000 individuals suffer from inhalational burns each year in the United States alone.3 Twenty to 30% of burn patients who are hospitalized are found to have inhalational injury, and in a study of 1447 burn patients, it was estimated that around 30% of burn victims with smoke inhalation die, compared to only 2% of burn patients without inhalation injury.4 In an inhalational burn, there is direct thermal injury to the airway, and the lung parenchyma is affected as a result of a chemical insult by the reagents found in smoke.4 Respiratory failure, a known complication of inhalational injury, requires ventilator support as well as extended hospital stay in many cases.1 Inhalation of smoke also leads to the absorption of many toxins in the blood, including carbon monoxide and cyanide, thereby causing the entire body to be affected, and making inhalational injury a systemic insult.5 The exact mechanisms underlying inhalational burns is yet to be fully understood, since an inhalational burn is a systemic insult with multiple variables contributing to the final outcome of the patient. 3,6
It is now well known that rapid diagnosis and treatment are key when it comes to inhalational burns, as acute complications, which sometimes go unnoticed, are the reason behind long term sequels2 and the high mortality rate seen with this type of injury. Over the last three decades, survival rates of patients with burn injuries have steadily increased due to new treatment modalities, as well as a decrease in the severity of burns.7 Studies to elucidate the systemic repercussions of inhalational injury have suggested specific antidotes, in addition to the general life-sustaining measures usually used. 2,5 Despite these advances, the literature still lacks sufficient material to guide the otolaryngologist in how to manage the airway of a patient with an inhalational burn. In this paper, the authors’ aim is to present a review of the literature to provide otolaryngologists with a systematic approach to patients with inhalational burns in order to optimize treatment, cost, morbidity and, most importantly, mortality.
Methodology
To fulfil the aforementioned goals, a PubMed search was conducted. In order to maximize the number of articles, the search was conducted using synonyms of any keyword that was incorporated in the search. The search string created was as follows: “(Airway burns OR Airway edema OR Airway inhalational injury OR Fume inhalation OR Inhalational burns OR Inhalational injury OR Smoke inhalation OR Smoke inhalation injury OR Oropharyngeal burns OR Pharyngeal burns OR Ventilation burns) and (ENT OR Ear nose throat OR Laryngology OR Otolaryngology OR Otorhinolaryngology) OR (Inhalational burns)”. “Inhalational burns” was added at the end of the search with the Boolean operator “OR” in order to include any article that is out of the scope of otolaryngology, but may prove to be useful in the treatment of patients with inhalational burns. The search yielded a total of 549 articles. There were no exclusion criteria. The only inclusion criterion required the article to address at least one of the aspects of airway treatment in an inhalational injury, even if the former is only partially addressed. A total of 24 articles were reviewed after refining the search.
Findings
As in any emergency, securing the airway in patients with inhalational injuries is of primary and utmost importance. When dealing with inhalational burns, airway complications can be divided into mechanical airway complications and physiological airway complications.
Mechanical airway complications
The human airway has an adequate heat dissipating quality due to the highly functional heat counter-exchange mechanisms present in the upper airway. Thus, it is very rare to find thermal injury at or below the level of the vocal cords. 3,8 Thereby, most patients who suffer from a smoke inhalation injury will not require intubation due to the absence of vocal cord edema. However, it is of utmost importance to identify the patients with smoke inhalation injury who will require intubation.9
Initial assessment and determining the need for intubation.
In the initial assessment of a patient, determining whether the patient needed to undergo laryngoscopy used to be a matter of controversy and physician preference,3 until a study conducted by Madnani et al. found that the presence of soot in the oral cavity, as well as facial or body burns, warrant a fiberoptic laryngoscopy, since patients with these findings are at a higher risk of developing laryngeal edema.9 Close physical examination of patients with inhalational injury can reveal signs of smoke inhalation, including facial burns, perioral burns and singed nasal hairs. This warrants laryngoscopy, and evidence of significant edema, blisters or ulcerations should lead to consideration for intubation to stabilize the airway.2 It was also found that the classical symptoms of inhalational injury, namely stridor, drooling, hoarseness and dysphasia were not associated with the need for intubation.9 It should be noted that the absence of significant burns in the oropharynx does not rule out involvement of structures at or below the supraglottic level.10 In some instances, chest examination, chest x-ray and blood gas analysis are normal. Flexible bronchoscopy can reveal large airway injury in asymptomatic patients in which lab tests and imaging are within range, which is why a study by Bai et al. suggested that evaluation of the airway by bronchoscopy should be incorporated in routine clinical practice.1 Edema of the oral mucosa and/or the trachea can develop within 0.5 hours of the time of injury, and can progress to mucosal necrosis within 12-24 hours.10 However, clinically significant injuries usually manifest three to four days following exposure.1 Supraglottic injury, swelling and resulting obstruction of the airway occur more commonly in children due to the smaller size of the trachea, and relatively large epiglottis.10 When a child first presents following inhalational injury, it is more difficult to appreciate signs of impending airway obstruction. Clinicians should therefore have high clinical suspicion and recognize the signs of impending airway obstruction in a child, in order to avoid rapid progression and deterioration.11
Considering the patient’s airway may have concurrent edema, it is highly recommended that the physician use less than the average, safe intracuff pressure when performing an intubation on patients in need.12 The risk for fistula formation was shown to be higher in patients on whom the usual intracuff pressure was used when intubating. In addition to using a lower than average intracuff pressure, the endotracheal tube should be left uncut, since swelling in the 48 hours post injury may cause the end of the tube to regress into the oropharynx.8 In such a case, re-intubation will be warranted but is unlikely to be successful due to the massive edema of the upper airway.
Determining the need for tracheostomy.
Early tracheostomy is not advised as it has been shown that this procedure does not improve outcome in burn patients.3 It was actually found to increase the incidence of respiratory tract infection and superimposed infections. Some evidence suggests that in patients with anterior neck burns who require tracheostomy, excision of the burned tissue with skin grafting a week prior to the tracheostomy will lead to lower risk of wound and respiratory infections.2 In addition, Prater et al. found that the number of airway complications due to tracheostomy was not less than the number of complications subsequent to endotracheal intubation.13 In the latter study, the number of cases of subglottic stenosis due to endotracheal intubation and the number of cases of tracheomalacia due to tracheostomy were identical. However, it is recommended that patients with vocal cord damage undergo a tracheostomy in order to prevent any further damage to the vocal cords and airway.14
Management of tracheal complications.
Complications resulting from the severely injured airway of a patient with inhalational injury include tracheal stenosis, tracheo-esophageal fistula formation, and rupture of the trachea. It was shown that the success rate of airway reconstruction in patients suffering inhalational burns is identical to the success rate of airway reconstruction in other groups of patients.15
The high temperature of the smoke in patients with inhalational burns can cause necrosis of part of the trachea, and could lead to the formation of a tracheo-esophageal fistula. In an intubated patient, increased secretions, pneumonia and evidence of aspiration of gastric contents are the most important signs of a trachea-esophageal fistula. When a patient is extubated, coughing after swallowing is most suggestive of a tracheaesophageal fistula.16 Physicians should have a high suspicion of a trachea-esophageal fistula when these signs are witnessed. In almost all cases of traumatic tracheoesophageal fistula formation, surgical closure of the fistula is required. Many approaches have been identified to this end, including pedicle muscle flaps, free microvascular flaps, in addition to other methods. Almost all of the literature has shown preference for a collar approach, with closure of the fistula, and an end-toend anastomosis of the trachea. This method has good results and a low rate of recurrence.17
In addition to the possibility of fistula formation, the trachea is weakened and may sometimes rupture. Though tracheal rupture is a rare complication, it is life threatening, which is the reason behind it being mentioned in many of the articles reviewed. Early symptoms of tracheal rupture include subcutaneous emphysema of the head, neck and chest, in addition to pneumomediastinum, tracheal bleeding, respiratory insufficiency, tension pneumothorax and a sudden increase in ventilation pressures. Late symptoms of tracheal rupture include stridor, asphyxia, chest pain, mediastinitis, pneumonia, ARDS and even sepsis. It is advised that injuries smaller than 4 cm be treated by fixing the positioning of the tube, and administering antibiotics. On the other hand, injuries more than 4 cm will require surgical intervention. In cases where conservative management does not lead to improvement, one should resort to surgery to treat the tracheal rupture. Conservative management is usually the choice for children, as well as for elderly patients with multiple medical co-morbidities.
In the case of tracheal rupture where immediate surgical intervention is indicated, two approaches have been described. One approach is a transverse cervical incision. The other is a right thoracic approach through the fifth intercostal space. Both techniques are equally useful, and have good success rates with non-significant differences for the post-operative results. If, for some reason, first intention closure is not an option, the otolaryngologist can resort to closing the defect using a pedicle or free flap. One of the main disadvantages of closing with a flap is that a close follow-up is required, as re-operation may be warranted in case stenosis develops. Stenosis at the suture area can occur at any time, even years after the operation.17
Management of delayed airway obstruction.
If patients with inhalational injury have sustained burns to the face, neck or upper chest, care must be taken in following them up as they are at risk of developing contractures that could compress the airway. This patient population can present with airway obstruction weeks or even months after the insult. Respiratory symptoms suggestive of airway obstruction, such as stridor, may occur in these patients. It may be extremely difficult to perform endotracheal intubation in patients with cervical contractures, and these patients usually do not tolerate decannulation. In such cases, bronchoscopy and direct laryngoscopy become crucial to assess the degree of airway obstruction. The Cormack and Lehane classification is usually used to describe the patency of the airway, as seen on direct laryngoscopy. A grade I view indicates that the glottis can be seen on direct laryngoscopy. A grade II view indicates that only the posterior portion of the glottis is visualized. A grade III view indicates that only the epiglottis can be visualized, whereas in a grade IV view neither the glottis, nor the epiglottis can be visualized. The higher the Cormack and Lehane classification, the more obstructed the airway, and the more difficult endotracheal intubation or successful decannulation will be. All burn patients that develop delayed symptoms of airway obstruction should undergo direct laryngoscopy and bronchoscopy to assess airway patency. In addition, direct laryngoscopy and bronchoscopy should be considered in patients with delayed airway obstruction at the time of tracheotomy, so as to have a baseline to compare later results to.18
Physiological airway complications
As mentioned earlier, it is rather rare to find an inhalation burn patient with glottic edema, but if this complication is present, physicians usually use aerosolised adrenaline or steroids as well as head elevation in addition to endotracheal intubation. Unfortunately, there are no studies to prove that either the use of aerosolized adrenaline or steroids, or head elevation, have any benefit in patients with glottic edema.8
Management of patients on mechanical ventilation.
In the case of an intubated patient, it is very important to reassess the need for intubation on a regular basis in the intent of avoiding prolonged intubation and its dreadful side effects, which include pneumonia, necrotizing tracheobronchitis, bronchopulmonary dysplasia, and others.19 A study by Miller et al. has shown that the use of nebulized heparin sulfate, N-acetylcysteine and albuterol sulfate significantly improved survival in patients requiring mechanical ventilation. This improved survival was thought to be due to the following mechanisms of action: the inhibition of airway clot formation by heparin, mucolysis achieved by N-acetylcysteine, and bronchodilation subsequent to albuterol administration. The effects of these drugs lead to a decrease in the time spent by the patient on mechanical ventilation, in addition to a reduction in morbidity and mortality, and in the cost of medical care.19
Management of carbon monoxide toxicity.
One of the most dangerous aspects of a fire is the production of carbon monoxide gas. The latter constitutes the primary cause of mortality from a fire.8 Carbon monoxide gas is a hazard because of it being colourless, odourless and tasteless. In addition, hemoglobin’s affinity to carbon monoxide is 200 times more than hemoglobin’s affinity to oxygen.3 Once carbon monoxide binds to hemoglobin, the red blood cell is unable to undergo proper gas exchange. This toxic gas also inhibits the binding of oxygen to cytochrome oxidase, thus making cellular respiration less effective.8 Carbon monoxide intoxication can be suspected when a patient is found to be lethargic, confused or obtunded. In such cases, treatment should be initiated promptly with 100% oxygen, using a nonrebreather mask. In the scenario where the patient is intubated, hyperventilation with 100% oxygen is recommended. Whether or not hyperbaric oxygen should be used remains controversial.3 A study by Benson et al. suggested that for carboxyhemoglobin levels below 30%, treatment should be initiated with 100% oxygen, whereas in patients with severely elevated carboxyhemoglobin levels or in a coma, hyperbaric oxygen can be considered.2 In all cases of carbon monoxide intoxication, arterial blood gases should be withdrawn to follow up the patient.
Management of cyanide toxicity.
Recent evidence has shown that cyanide toxicity has a role to play in mortality following inhalation injury. Cyanide is released in fires subsequent to the combustion of acrylic, rubber and plastic materials. It binds cytochrome oxidase in the electron transport chain and thereby inhibits aerobic respiration of cells. Early signs of cyanide toxicity include hypertension, palpitations, tachycardia, tachypnea, anxiety, nausea, dizziness and headache, all of which are caused by activation of the sympathetic system. Another sign of cyanide intoxication is a smell of bitter almonds on the patient’s breath. Blood cyanide concentrations of 0.5 – 1 mg/L are considered toxic and levels that reach 2.5 – 3 mg/L and are not promptly treated could lead to death. However, blood cyanide levels are not routinely measured and the diagnosis is most often made clinically. A few studies have shown a correlation between elevated lactate levels and severity of cyanide intoxication. In addition to supportive treatment with high-flow oxygen, repeated monitoring of vital signs, mechanical ventilation and correction of metabolic acidosis, a study published in 2014 by MacLennan et al. showed that hydroxycobalamin is an adequate first-line antidote for cyanide toxicity in terms of safety, effectiveness and onset of action. Its side effects are very mild, and include hypotension, bradycardia, headache and discoloration of skin and urine. Sodium thiosulphate was also found to be beneficial, but its slow onset of action limits its use as a single agent.5 Chen et al. suggested the use of inhaled amyl nitrite and/or injection sodium nitrite as an antidote for cyanide toxicity, in addition to the usual supportive treatment. There is no data to support the use of hyperbaric oxygen in patients with cyanide intoxication.2 Clinical signs of cyanide toxicity are similar to those of carbon monoxide toxicity; however, the risk of treatment for cyanide toxicity in a patient who only has carbon monoxide toxicity is believed to outweigh the benefit of early treatment of cyanide intoxication. The presence of a consistent history, in addition to changes in neurological status and increased lactate levels in the blood, is sufficient to start treatment for cyanide toxicity.2,5
Management of bronchospasm and ARDS.
More often than not, patients with inhalational lung injury have bronchospasm due to the irritants found in smoke. When faced with bronchospasm, nebulised bronchodilators such as beta2-agonists are the agents of choice. Another important aspect of beta2-agonists is their anti-inflammatory effect.8 In an animal model exposed to inhalational injury, beta2-agonists were found to cause a significant reduction in the amount of lung edema when compared to the control. It is speculated that this effect is achieved through a reduction in pulmonary vascular permeability to proteins.20 It should be noted that the use of beta2-agonists is to be avoided in patients with a known history of arrhythmias.
Acute lung injury/ARDS are major determinants of mortality in patients who suffer from an inhalational smoke injury. In fact, around 20% of patients with inhalational injury will go on to develop ARDS.19 These patients present with a picture of bilateral lung edema and arterial hypoxemia, in the absence of left atrial hypertension.20 In patients with ARDS subsequent to inhalational injury, low tidal mechanical lung protective ventilation can be of help, and early extracorporeal life support was found to be very beneficial. A case has been reported in the literature where a 22-year-old patient developed ARDS 48 hours after sustaining an inhalation injury.21 The patient was started early on extracorporeal life support, thus providing gas exchange while allowing his lungs to heal. He eventually recovered and was discharged home on room air.
Another case report described a 24-year-old patient with inhalational burn who developed ARDS 4 days prior to admission. He was treated with N-acetylcysteine, nebulized heparin and nebulized epoprostenol. He gradually improved and was ultimately extubated and discharged on room air.22
Discussion
Guidelines are still lacking when it comes to the management of a patient with inhalational burns. In the acute setting, studies suggest that the use of laryngoscopy could help the physician make a decision on the need for intubation, and one should not draw conclusions based solely on an intact upper airway on physical examination.1,2,9 Assessing the airway with laryngoscopy is important in all patients post inhalational burn and should be recommended, as the procedure is of short duration and minimally invasive, causes minimal patient discomfort, and could help guide airway management as well as prevent the development of complications later on.
When it comes to children, it has been found that acute signs of impending airway obstruction are usually not flagrant, and one should have a higher level of suspicion than in adults.11 There is, however, a lack of studies to give clear recommendations on the management of the acute airway in children with inhalational burns. One should keep in mind that management is not necessarily the same in children and adults, as the airway of children is smaller and more likely to suffer from edema. It is recommended that both children and adults with inhalational burns be observed in the acute setting for prompt treatment of any complications that might arise, mainly airway edema or obstruction, leading to respiratory distress.
Patients who develop ARDS post inhalational burn do not usually survive due to very extensive respiratory injury. However, cases have been reported where the use of ECMO, mucolytics and bronchodilators led to a good prognosis and positive outcome.19,21 One could consider these drugs as potential treatment after further studies have been made to prove their efficacy in patients with inhalational injuries.
It is interesting to note that patients with diabetes mellitus are less likely to develop ARDS than the general population. It is thought that leptin resistance is responsible for this phenomenon.23 Further studies are needed to understand how this protective effect works and to apply the findings to all inhalational burn victims.
The effect of carbon monoxide toxicity and cyanide toxicity has already been studied, and treatment methods have been suggested.2,5 One should however consider that these are not the only toxic gases inhaled by patients exposed to fires.2 The presence of other gases released by various burning materials could also potentially lead to systemic damage after absorption in the circulation. Studying what these gases are and how to manage their toxicity could lead to a further decrease in morbidity and mortality due to inhalational burns.
Studies are currently focusing on the development of chelating substances that can be nebulised into the respiratory tract and deactivate the toxins present in the airway due to inhalational burns. The idea is analogous to activated charcoal used in toxic ingestions. These substances should have nonspecific effects so that they can scavenge a wide range of possible toxic molecules that may be produced in the fire.8 Such products are available now for emergent eye or skin chemical splashes24 but have not yet been made available for smoke inhalation.
Conclusion
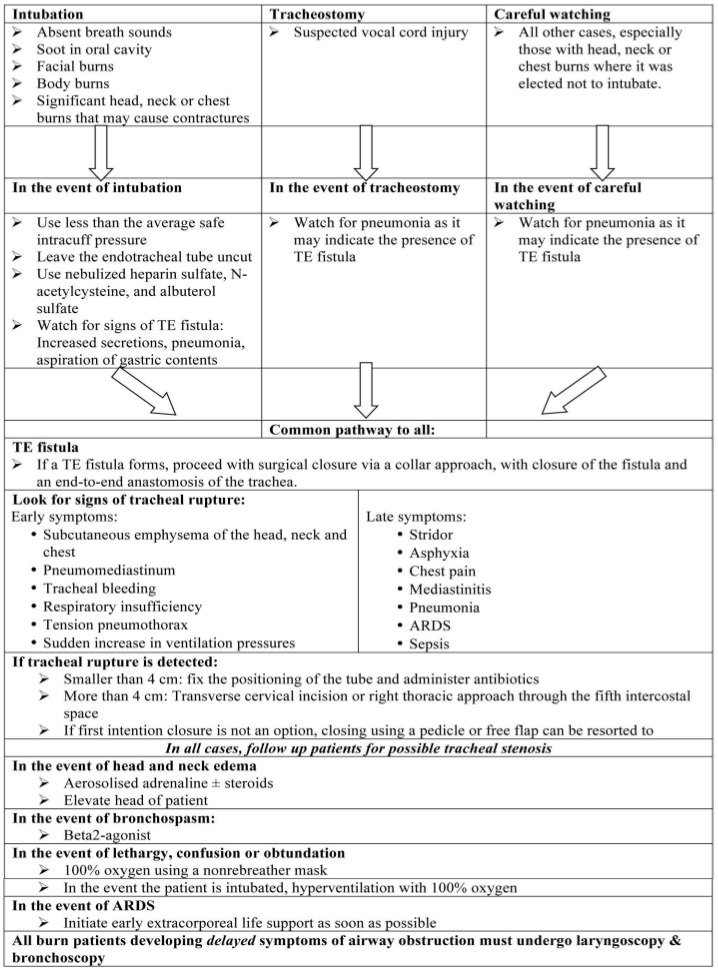
Inhalational burns remain a major challenge to otolaryngologists and the treating medical team. There is a paucity of research conducted in this field. The available literature highlights the importance of airway management in terms of timing of intubation, method of intubation, tracheo-esophageal fistula formation, trachea-esophageal rupture and airway obstruction as some potential complications. Carbon monoxide intoxication remains a hazard and should be treated promptly. Drugs such as heparin sulfate, N-acetylcysteine and albuterol have been proven to greatly help in the treatment of patients with inhalational burns. More research is underway to develop chelating drugs that can scavenge the toxic materials in smoke before they damage the airway. The use of clinical sense and team work, careful overall assessment of the patient, appropriate lab work and fiberoptic endoscopy are essential to decide on the best management technique to protect the airway and avoid complications (Table I).
Table I. Pathway of treatment.
Acknowledgments
Conflict of interest.The authors declare that they have no conflict of interest related to this article.
References
- 1.Bai C, Huang H, Yao X. Application of flexible bronchoscopy in inhalation lung injury. Diagnostic Pathology. 2013;8:174. doi: 10.1186/1746-1596-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tze-Ming Benson Chen Toxic Inhalational Exposures. J Intensive Care Med. 2013;28(6):323–333. doi: 10.1177/0885066611432541. [DOI] [PubMed] [Google Scholar]
- 3.Sicoutris H. Fire and smoke injuries. Crit Care Nurs Clin N Am. 2006;18:403–417. doi: 10.1016/j.ccell.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Smith DL, Cairns BA, Ramadan F, Dalston FS. Effect of inhalation injury, burn size, and age on mortality: a study of 1447 consecutive burn patients. J Trauma. 1994;37:655–659. doi: 10.1097/00005373-199410000-00021. [DOI] [PubMed] [Google Scholar]
- 5.MacLennan L, Moiemen N. Management of cyanide toxicity in patients with burns. Burns. 2015;41:18–24. doi: 10.1016/j.burns.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Murtaza B, Sharif MA, Tamimy MS, Dar MF. Clinico-pathological profile and outcome of inhalational burns. J Coll Physicians Surg Pak. 2009;10:609–613. doi: 10.2009/JCPSP.609613. [DOI] [PubMed] [Google Scholar]
- 7.Colohan SM. Predicting prognosis in thermal burns with associated inhalational injury: a systematic review of prognostic factors in adult burn victims. J Burn Care Res. 2010;31(4):529–539. doi: 10.1097/BCR.0b013e3181e4d680. [DOI] [PubMed] [Google Scholar]
- 8.Toon MH, Maybauer MO, Greenwood JE. Management of acute smoke inhalation injury. Crit Care Resusc. 2010;12:53–61. [PubMed] [Google Scholar]
- 9.Madnani DD, Steele NP, de Vries E. Factors that predict the need for intubation in patients with smoke inhalation injury. Ear Nose Throat J. 2006;85:278–280. [PubMed] [Google Scholar]
- 10.Kudchadkar SR, Hamrick JT, Mai CL. The heat is on… Thermal epiglottitis as a late presentation of airway steam injury. J Emerg Med. 2014;46:e43–e46. doi: 10.1016/j.jemermed.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Hyland E, D’Cruz R, Menon S, Chan Q. Airway compromise in children with anterior neck burns: Beware the scalded child. J Paediatr Child Health. 2015;51:976–981. doi: 10.1111/jpc.12912. [DOI] [PubMed] [Google Scholar]
- 12.Beckenham E. Acquired tracheo-esophageal fistula in the pediatric population. International Journal of Pediatric Otorhinolaryngology. 1998;44:109–113. doi: 10.1016/s0165-5876(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 13.Prater ME, Deskin RW. Bronchoscopy and laryngoscopy findings as indications for tracheotomy in the burned child. Arch Otolaryngol Head Neck Surg. 1998;124:1115–1117. doi: 10.1001/archotol.124.10.1115. [DOI] [PubMed] [Google Scholar]
- 14.Maybauer DM. Treatment strategies for acute smoke inhalation injury [German] Anaesthesist. 2006;55:980–988. doi: 10.1007/s00101-006-1050-3. [DOI] [PubMed] [Google Scholar]
- 15.White DR, Preciado DA, Stamper B. Airway reconstruction in pediatric burn patients. Otolaryngology–Head and Neck Surgery. 2005;133:362–365. doi: 10.1016/j.otohns.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003;13:271–289. doi: 10.1016/s1052-3359(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 17.Seidl RO. Tracheal rupture in burns - a retrospective study. Burns. 2008;34:525–530. doi: 10.1016/j.burns.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Rose AS, Gore MR, Hultman CS, Cairns BA. Contracture related airway obstruction (CRAO) treated successfully with incisional release. International Journal of Pediatric Otorhinolaryngology. 2011;75:286–288. doi: 10.1016/j.ijporl.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Miller AC. Influence of nebulized unfractionated heparin and Nacetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30:249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- 20.Wiener-Kronish JP. Beta-2–agonist treatment as a potential therapy for acute inhalational lung injury. Crit Care Med. 2006;34:1841–1842. doi: 10.1097/01.CCM.0000220050.03102.ED. [DOI] [PubMed] [Google Scholar]
- 21.Nelson J, Cairns B, Charles A. Early extracorporeal life support as rescue therapy for severe acute respiratory distress syndrome after inhalation injury. J Burn Care Res. 2009;30(6):1035–1038. doi: 10.1097/BCR.0b013e3181bfb7fd. [DOI] [PubMed] [Google Scholar]
- 22.Dube KM, Ditch KL, Hills L. Use of nebulized heparin, nebulized NAcetylcysteine, and nebulized epoprostenol in patient with smoke inhalational injury and acute respiratory distress syndrome. Journal of Pharmacy Practice. 2016;29(6):1–5. doi: 10.1177/0897190016663071. [DOI] [PubMed] [Google Scholar]
- 23.Maybauer MO, Maybauer DM, Herndon DN. Incidence and outcomes of acute lung injury. N Engl J Med. 2006;354:416–417. [PubMed] [Google Scholar]
- 24.Hall AH. Diphoterine for emergent eye/skin chemical splash decontamination: a review. Vet Hum Toxicol. 2002;44:228–231. [PubMed] [Google Scholar]


