Abstract
Neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease currently affect tens of millions of people worldwide. Unfortunately, as the world’s population ages, the incidence of many of these diseases will continue to rise and is expected to more than double by 2050. Despite significant research and a growing understanding of disease pathogenesis, only a handful of therapies are currently available and all of them provide only transient benefits. Thus, there is an urgent need to develop novel disease-modifying therapies to prevent the development or slow the progression of these debilitating disorders. A growing number of pre-clinical studies have suggested that transplantation of neural stem cells (NSCs) could offer a promising new therapeutic approach for neurodegeneration. While much of the initial excitement about this strategy focused on the use of NSCs to replace degenerating neurons, more recent studies have implicated NSC-mediated changes in neurotrophins as a major mechanism of therapeutic efficacy. In this mini-review we will discuss recent work that examines the ability of NSCs to provide trophic support to disease-effected neuronal populations and synapses in models of neurodegeneration. We will then also discuss some of key challenges that remain before NSC-based therapies for neurodegenerative diseases can be translated toward potential clinical testing.
Introduction
The potential of cell transplantation as a therapy for neurodegenerative disorders was first examined nearly three decades ago with landmark studies of fetal mesencephalic tissue transplantation in Parkinson’s disease (PD) patients (Lindvall et al., 1988). Although these initial studies produced variable results, the field’s interest in this approach continued to grow and increasingly focused on the regenerative potential of neural stem cells (NSCs) in the hope that they could provide a renewable and more precise source of cells for transplantation. Building upon preclinical findings from PD models, researchers began to also investigate NSC transplantation for other neurodegenerative conditions including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD). As a result the field’s understanding of NSC transplantation and the mechanisms by which they influence these disorders has grown immensely. Yet significant challenges remain regarding if and how these promising preclinical findings can be translated into successful clinical trials.
Are NSCs a viable therapeutic approach for neurodegenerative disorders?
Researchers initially hypothesized that NSCs could likely only be practically developed as a therapy for disorders that involve relatively focal neural degeneration. For example, PD that is characterized primarily by a loss of dopaminergic neurons within the Substantia Nigra Pars Compacta represented an excellent candidate disease in which to develop a neuronal replacement paradigm (Lotharius and Brundin, 2002, Martino and Pluchino, 2006). While the development of this approach continues to advance, the concept of neuronal replacement has proven to be far more complex that initially expected. For example, transplanted stem cells need to survive within the adult brain and migrate from a small initial injection site to populate the target region. They then need to differentiate with high fidelity into the appropriate neuronal subtype, such as dopaminergic neurons. Perhaps most challenging of all, these transplanted cells then need to project to the appropriate target neurons and form appropriate synaptic connections. In the PD field this has led most studies to instead pursue transplantation of cells into the striatum, the target of dopaminergic innervation, rather than the substantia nigra where the disease-affected neurons normally reside. In the case of other neurodegenerative disease that exhibit more widespread pathology and loss of varying neuronal subtypes in multiple brain regions, such as AD, this kind of neuronal replacement paradigm becomes even more challenging (Davies and Maloney, 1976, Davies et al., 1980, Hardy et al., 1987, Hyman et al., 1987, Lowe et al., 1990). How then can NSCs be a reasonable therapy for these more complex neurodegenerative disorders?
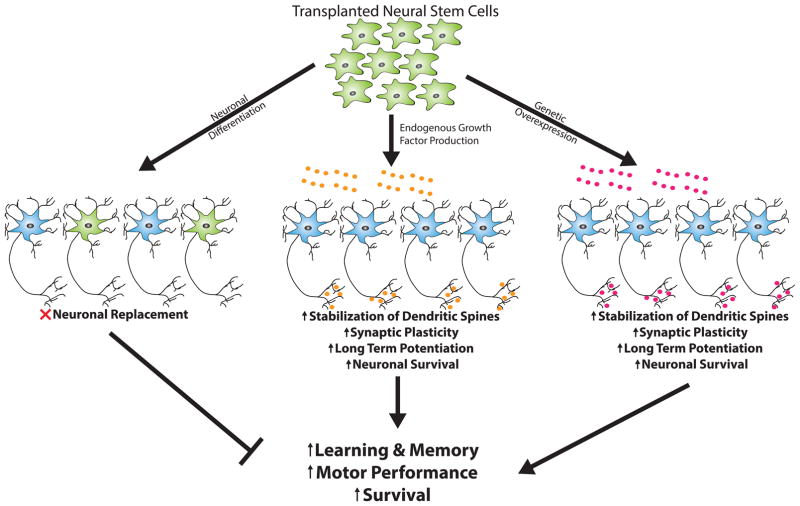
As we will discuss, recent studies have demonstrated that some of the most profound recovery following NSC transplantation in preclinical models is likely mediated via modulation of neurotrophic systems. Indeed a growing number of studies have found that differentiation of NSCs into supportive glial subtypes such as astrocytes that can produce key growth factors to influence synaptic plasticity and neuronal function and regulate brain vascularization and energetics may play an important role in functional recovery (Figure 1).
Figure 1. Potential mechanisms of neurotrophic support provided by transplanted neural stem cells (NSCs).
Initially many attempted to use NSCs for neuronal replacement, theorizing that following neuronal differentiation (neuron; green) cells would integrate with host neuronal circuitry (blue) to replace neurons that have died during the course of AD, PD, or other neurodegenerative disorders. However, work towards this goal has proven challenging and results are mixed. Recent research has instead demonstrated that endogenous secretion (center, orange) or genetic overexpression (right, purple) of neurotrophic factors (BDNF, GDNF, IGF-1, NGF) may provide an alternative and perhaps more promising approach for NSC-based therapy. Transplanted NSCs that secrete neurotrophins are capable of increasing synaptic plasticity, enhancing long-term potentiation, and promoting neuronal survival that together lead to improved cognitive and motor performance. (Figure adapted from Marsh & Blurton-Jones, 2015).
Neurotrophic Factors in CNS disorders & Potential Therapeutic Approaches
Beginning with the discovery of the first neurotrophic factor, nerve growth factor (NGF), more than half a century ago researchers have identified approximately 50 such factors that can influence the growth, survival, and homeostatic functions of cells within the central and peripheral nervous systems (Levi-Montalcini and Hamburger, 1951, Nagahara and Tuszynski, 2011). Among the most prominently studied neurotrophic factors in the CNS are NGF, brain-derived neurotrophic factor (BDNF), glial cell line derived neurotrophic factor (GDNF), and insulin-like growth factor 1 (IGF-1). These neurotrophins and their receptors are highly expressed in both the human and rodent CNS (Adem et al., 1989, Kawamoto et al., 2000, Lu et al., 1989, Tang et al., 2010) and research has demonstrated critical roles for these factors in the growth and stabilization of dendritic spines, synaptic plasticity, long-term potentiation, survival of neurons and glia, and therefore unsurprisingly learning and memory (for review see (Vicario-Abejón et al., 2002, Arancio and Chao, 2007, Park and Poo, 2013, Russo et al., 2005, Tuszynski and Gage, 1995).
The commonality of neuronal and synaptic loss, cognitive impairment, and inflammation across many of the most prevalent neurodegenerative disorders has led researchers to speculate that neurotrophic-based therapies might be successful in treating these debilitating disorders (Lu et al., 2013, Marsh and Blurton-Jones, 2015, Nagahara and Tuszynski, 2011). However, many of these neurotrophic factors do not effectively cross the blood brain barrier if administered peripherally and direct injection either of neurotrophic peptides or overexpression via gene therapy have thus far exhibited significant drawbacks and challenges (Marks et al., 2010, Warren Olanow et al., 2015). As NSCs produce a variety of neurotrophic factors and can migrate extensively, many groups have instead begun to investigate their use as a potential delivery vehicle for neurotrophins (Blurton-Jones et al., 2009, Ebert et al., 2010, Kamei et al., 2007, Lu et al., 2003, Wu et al., 2016, Zimmermann et al., 2016). In this mini-review we will provide an overview of some of the key studies that have examined the role of neurotrophins in NSC-mediated functional improvements and discuss remaining challenges to clinical translation.
NSC Transplantation for Alzheimer’s Disease
Alzheimer’s disease is the most common cause of age-related neurodegeneration, affecting over 5 million people in the U.S. alone (Alzheimer’s Association, 2016). AD is characterized by a progressive loss of memory and cognition that leads to an inability to perform daily activities and eventually death (Alzheimer’s Association, 2016). Despite its prevalence and initial discovery over 100 years ago, there are still no disease modifying therapies for Alzheimer’s disease and currently approved drugs provide only mild symptomatic relief without significantly altering the course of the disease (Birks, 2006, Russ and Morling, 2012, Tan et al., 2014). Pathologically, AD is characterized by widespread synaptic and neuronal loss, inflammation, and two hallmark protein aggregates known as plaques, composed of beta-amyloid (Aβ) and neurofibrillary tangles composed of hyperphosphorylated tau (Masliah et al., 2001, Selkoe, 2001, Terry et al., 1991, Wyss-Coray and Rogers, 2012). The prevailing theory of AD is that amyloid aggregation as a result of overproduction or impaired clearance initiates a pathological cascade of events including tau hyperphosphorylation, inflammatory gliosis, and synaptic and neuronal loss (Beyreuther and Masters, 1991, Hardy and Allsop, 1991, Selkoe, 1991, Hardy and Higgins, 1992, Hardy and Selkoe, 2002, Hardy, 2009, Selkoe and Hardy, 2016)
Although AD pathology is widespread throughout the brain, certain regions important for learning and memory such as the hippocampus, entorhinal cortex, and basal forebrain are more profoundly affected than others. Importantly, decreased neurotrophin expression and impaired axonal transport of neurotrophins have been observed in AD patients and animal models and likely play a significant role in synaptic and neuronal loss (Arancio and Chao, 2007, Hock et al., 2000, Poon et al., 2011, Rivera et al., 2005, Salehi et al., 2006). Therefore restoration of these lost neurotrophins is an appealing therapeutic target especially given the well-established roles of neurotrophins in synaptic plasticity, neuronal health, and learning and memory.
Our group was one of the first to demonstrate the potential benefits of NSCs in delivering neurotrophic support in an AD mouse model (Blurton-Jones et al., 2009). Using 3xTg-AD mice which develops both amyloid and tau pathology in addition to synaptic deficits and impaired cognition (Oddo et al., 2003) we found that bilateral transplantation of mouse NSCs into the hippocampus of aged 3xTg-AD mice rescued hippocampal dependent learning and memory. We further demonstrated that NSC transplantation improved hippocampal synaptic density, providing a potential structural basis for improved cognition. Interestingly, levels of amyloid and tau were unchanged following NSC transplantation, suggesting that NSC transplantation likely targets downstream aspects of AD neuronal dysfunction but also implying that this approach might provide benefits in patients with substantial existing pathology. Given that NSCs had previously been shown to produce high levels of neurotrophins (Kamei et al., 2007, Lu et al., 2003) and that neurotrophins can dramatically influence both cognition and synaptic plasticity (Arancio and Chao, 2007), we hypothesized that NSC-induced changes in neurotrophin signaling might underlie the observed effects. In support of this, we found that NSCs produced high levels of BDNF in vitro and that brain levels of BDNF protein were also elevated following NSC transplantation. Furthermore, BDNF production was necessary for the behavioral and synaptic response observed, as treatment of AD mice with NSCs in which BDNF production had been reduced via shRNA-mediated knockdown prevented cognitive benefits and reduced the effects on synaptic density. In a subsequent study, Zhang et al., (Zhang et al., 2014) found consistent results using a different model of AD. In this report, they again found that NSC transplantation had no effect on Aβ pathology but nevertheless rescued both cognitive deficits and synaptic deficits in long-term potentiation (LTP). As in our own study, this group similarly found that NSC-transplanted animals exhibited significant increases in BDNF but also further extended their analysis to demonstrate a corresponding increase in the high-affinity BDNF receptor; TrkB (Soppet et al., 1991, Tang et al., 2010).
Utilizing a mouse model of tauopathy which develops accumulation of hyperphosphorylated tau and neuronal loss similar to that observed in AD, another group found similar benefits of NSC transplantation (Hampton et al., 2010). This group demonstrated that NSC transplantation could reduced neuronal loss in the cortex compared to vehicle treatment and that the majority of NSCs differentiated into astrocytes, indicating that neuronal differentiation and replacement was likely not required for recovery. To further confirm the effect they observed was due to astrocytic differentiation and not neuronal replacement the experiments were repeated using astrocyte transplantation and again neuronal loss was reduced. Examination of mRNA levels of several key neurotrophic factors found that while BDNF and NGF mRNA were unchanged in this study, GDNF mRNA was increased greater than 4 fold compared to vehicle following NSC transplantation, suggesting this other key neurotrophin might play an important role in the preservation of neurons in tau transgenic mice.
Additional evidence supporting the use of NSCs to produce neurotrophins and overcome AD pathology has been observed in vitro in a study that demonstrated that NSCs modified to produced high levels of either BDNF, GDNF, or IGF-1 were capable of rescuing amyloid induced neuronal toxicity and enhancing cholinergic function (Kitiyanant et al., 2012). Taken together these in vivo and in vitro results have begun to lay the groundwork for NSC-based therapies for AD aimed at restoring lost neurotrophin signaling in areas of the brain highly affected by disease pathology. However, further work including the testing of human NSCs in appropriate mouse models of AD will clearly be needed before these findings can be translated toward a clinical trial. Likely a significant challenge will be to identify clinical grade NSCs that produce high levels of these neurotrophins or to alternatively define safe methods to genetically modify NSCs to produce them.
Neurotrophins and Parkinson’s Disease
Parkinson’s disease is a debilitating neurodegenerative disorder that is characterized by impairment in motor function. Patients often exhibit hallmark motor phenotypes consisting of tremors, slowed or excessive rigidity of movement, and difficulty initiating movements (Lotharius and Brundin, 2002). Unfortunately, up to 70% of PD patients also go on to develop Parkinson’s disease dementia (PDD) during the course of the disease (Dubois and Pillon, 1997, Marsh and Blurton-Jones, 2012). While therapies for PD such as LevaDOPA are far more effective than those currently available for AD, there remain no disease-modifying therapies for PD and the effectiveness of L-DOPA therapy typically diminishes with time and leads to increasingly severe side effects (Aarts et al., 2014, Bonelli et al., 2004, Robbins and Cools, 2014). Pathologically, PD is characterized by a loss of dopamine (DA) neurons within the substantia nigra, a midbrain nucleus that projects to the striatum and plays an integral role in the initiation and control of movement. As a result, the striatum exhibits a dramatic loss in DA innervation. In most cases, PD is further characterized by the accumulation of a hallmark protein aggregates known as Lewy bodies that are composed of the presynaptic protein α-synuclein which aggregates into insoluble intraneuronal inclusions (Lotharius and Brundin, 2002).
Due to the relatively focal nature of neuron loss that occurs in PD this disease was thought to be an ideal candidate for stem cell therapy with the goal of using NSCs to replace dead and dying dopaminergic neurons. Using a similar strategy, human clinical trials began nearly 30 years ago with the transplantation of fetal mesencephalic tissue grafts (Lindvall et al., 1988). However, to date double-blind placebo-controlled trials of this approach have not yet been successful (Lindvall, 2013, Morizane et al., 2008). Yet the failure of these earlier clinical trials does not necessarily indicate that this approach cannot be refined to eventually become successful. For example, the field has recognized that variability in the cellular make up of fetal tissue grafts likely contributed to highly varied outcomes in these earlier trials (Lindvall, 2013). Indeed researchers continue to refine this approach using more purified populations of fetal dopaminergic neurons, improved delivery approaches, and more rigorous trial designs such as those being tested in the ongoing TransEuro trial (NCT01898390) (Evans et al., 2012). Another potential approach that is showing considerable promise in preclinical trials is the use of induced pluripotent stem cells (iPSCs) to produce homogenous transplantable dopaminergic precursors (Hallett et al., 2015). While future clinical studies will determine whether neuronal replacement is an optimal approach for treating PD, this therapy may not address the underlying initial causes of the disease. In addition, some research suggests that transplanted cells in PD and proteinopathies could potentially succumb to the same disease process (Cicchetti et al., 2014, Chu and Kordower, 2010). A comprehensive review of neuronal replacement strategies for PD and other disorders is beyond the scope of this mini-review and several existing reviews provide an excellent assessment of this approach and current clinical perspectives (Barker et al., 2013, Lindvall, 2015). Instead, we focus specifically on those studies that have examined the potential role of NSC-derived neurotrophins in stem cell mediated functional recovery.
Similar to AD, Parkinson’s disease is also characterized by a loss of neurotrophic trophic factor support within critical brain regions. In PD patients loss of both BDNF and GDNF has been reported within the substantia nigra (Chauhan et al., 2001, Mogi et al., 1999) and critically, both BDNF and GDNF, have been implicated in the development, survival, and function of the midbrain dopamine neurons which degenerate in PD (Hyman et al., 1991, Lapchak et al., 1997, Lin et al., 1993).
Given the lack of conclusive positive results from previous transplants of DA precursors in PD patients some have attempted to refine the technique by co-transplanting DA neurons with NSCs overexpressing neurotrophic factors in order to enhance graft survival and function. One initial study found that co-transplantation of GDNF overexpressing NSCs with primary DA neurons in a rat 6-hydroxydopamine lesion model of PD resulted in significantly increased survival of co-transplanted neurons (Ostenfeld et al., 2002). However, despite this increase in neuronal survival the authors did not observe any change in the behavioral phenotype of the animals. In this study the authors attributed the lack of motor recovery to the fact that the GDNF overexpression may have become down-regulated after long-term engraftment.
Several other studies have examined the potential for NSCs overexpressing GDNF or other neurotrophins transplanted alone into animal models of PD might yield an effective therapy. One study used a similar lesion model of PD and found that mouse NSCs overexpressing GDNF could prevent the degeneration of DA neurons in the substantia nigra and reduced behavioral deficits (Akerud et al., 2001). In an effort to translate these early efforts towards clinical trials a series of studies led by Clive Svendsen and colleagues have also examined whether fetal-derived human NSCs modified to overexpress various neurotrophins are capable of protecting DA neurons and rescuing motor deficits. In one such study this group found that transplantation of either IGF-1 or GDNF overexpressing human NSCs was sufficient to rescue motor phenotypes in a rat lesion model of PD (Ebert et al., 2008). Furthermore, both IGF-1 and GDNF expressing NSC transplants resulted in reduced endogenous DA neuron loss in the substantia nigra. Interestingly, NSCs modified with either growth factor resulted in equivalent behavioral recovery and neuron survival despite the fact that significantly more NSCs modified to express IGF-1 survived compared to GDNF-expressing NSCs. Additionally, only GDNF expressing NSCs resulted in sparing or recovery of DA neuron fibers in the striatum surrounding the cells, indicating the mechanisms by which GDNF influence behavior may be more amendable to therapeutic development than IGF. In a second study utilizing only the GDNF expressing cells in a non-human primate model of PD, the authors found that transplantation in animals with more mild pathology resulted in small improvements in disease severity and such transplants were associated with increased dopaminergic fibers surrounding the grafts (Emborg et al., 2008).
Taken together, evidence from animal models of dopamine cell death certainly supports the potential use of neurotrophin-producing NSCs for PD. However, none of these models recapitulate the α-synuclein pathology found in most human patients that likely plays an important role in the development of PD. Encouragingly, a recent report from our laboratory in an animal model of synucleinopathy further supports the potential use of NSCs to deliver neurotrophins for PD and other synucleinopathies. In this study we found that transplantation of BDNF-producing mouse NSCs can improve both motor and cognitive deficits in a transgenic model of synucleinopathy and that BDNF production was necessary for these improvements (Goldberg et al., 2015). While this particular animal model more closely models the related disorder dementia with Lewy bodies (DLB) than PD, these results nevertheless suggest that NSC-based neurotrophic support can effective improve nigrostriatal based deficits in motor function in the presence of established synuclein pathology.
Huntington’s Disease
Huntington’s disease is a dominantly-inherited progressive neurodegenerative disorder that results in impairments in both cognitive and motor functions that eventually lead to death (Albin and Tagle, 1995). HD is caused by an expansion of a triplet repeat DNA sequence within the Huntington gene that leads to the production of a mutant protein containing an enlarged and pathogenic polyglutamine tract (Albin and Tagle, 1995, Maucksch et al., 2013, Group, 1993). HD symptoms typically begin around age 35–40 and progress continuously, eventually leading to death 15–20 years after symptomatic onset. Pathologically, HD patients exhibit neuronal and synaptic loss that effects the medium spiny GABAergic interneurons of the striatum but as the disease progresses also leads to degeneration in cortical and other brain regions (Albin and Tagle, 1995, Maucksch et al., 2013, Group, 1993).
Given the initial region-specific degeneration that occurs in HD researchers have speculated that NSC transplantation might provide a viable therapeutic approach (Peschanski et al., 1995). There have even been fetal tissue transplants performed in human HD patients similar to those examined in PD (Bachoud-Lévi et al., 2000, Cicchetti et al., 2009, Freeman et al., 2000, Keene et al., 2007). However, the results of these clinical studies have again been mixed (Cicchetti et al., 2009), suggesting again that neuronal replacement paradigms remain extremely challenging. As with PD and AD, neurotrophic factor expression is significantly reduced in HD patient brains (Zuccato et al., 2001, Zuccato and Cattaneo, 2007). Furthermore, neurotrophic factors have been found to be critical for the survival of striatal neurons in a neurotoxin model of HD (Pérez-Navarro et al., 1996).
Similar to strategies tested in PD models, several groups have overexpressed neurotrophins in NSC lines prior to transplantation into animal models of HD. One such study compared the ability of NSCs overexpressing either NGF or BDNF to rescue striatal neuron degeneration in a quinolinic acid lesion model of HD (Martínez-Serrano and Björklund, 1996). This study found that NGF expressing NSCs resulted in rescue of medium spiny interneurons and cholinergic striatal interneurons, and also reduced signs of inflammation. Interestingly, BDNF overexpressing NSCs had little to no effect on any of these endpoints in this study. Reports from another group using a transgenic model of HD found similar results utilizing GDNF overexpressing NSCs (Ebert et al., 2010). The authors of this study found that not only did GDNF overexpressing NSCs rescue striatal neuronal loss but they also prevented a decline in motor function compared to unmodified NSCs. Another recent study examined the effects of BDNF-overexpressing NSCs compared to unmodified NSCs in both the quinolinic acid model of HD as well as two transgenic models of the disease (Zimmermann et al., 2016). However, this study only found the BDNF-overexpressing NSCs provided greater benefits compared to NSCs alone in the quinolinic acid model but not in the transgenic models where both types of NSCs provided modest benefits. The authors hypothesized that this may be due to differences in survival of NSCs in the different models as cell survival was decreased in both of the transgenic models. The impact of model on cell survival is an important characteristic that should be carefully monitored going forward in preclinical testing of HD models. In efforts to move NSC transplantation towards further clinical trials for HD groups have also utilized human NSCs in rodent models of HD. One group led by Dr. Jeffrey Kordower, found that in a lesion model of HD, human NSCs that had been pre-treated with ciliary neurotrophic factor (CNTF) prior to transplantation led to significantly greater improvements in motor performance compared to those receiving normally cultured NSCs (McBride et al., 2004). Additionally, only NSCs pretreated with CNTF protected striatal neurons from death. While the authors did not examine the production of neurotrophic factors in vivo they speculated that enhanced neurotrophic support many partially underlie the observed effects. As HD is inherited in an autosomal dominant fashion this disease provides a unique opportunity to begin therapies prior to disease onset in patients carrying the disease mutation. One study that modeled this potential preventative paradigm examined the efficacy of transplanting human NSCs in rats prior to performing a striatal lesion compared to transplantation after lesioning (Ryu et al., 2004). When animals received hNSC transplants prior to lesioning the authors found that the rats exhibited improved motor performance and reduced neuronal loss. However, when transplants were performed after the lesion no rescue of the motor phenotype or neuronal loss was observed. Surviving hNSCs were found to express BDNF in vivo and in vitro analyses demonstrated that hNSCs were producing and secreting BDNF even in an undifferentiated state. Thus the authors concluded that the neuroprotective activity of BDNF in these experiments was critical.
Taken together the region specificity of neuronal loss in HD along with the ability to predict disease prior to clinical onset, suggests that NSC-mediated delivery of neurotrophic factors could provide a promising potential therapy for HD.
Remaining Challenges
The studies discussed in this mini-review support the conclusion that NSC-mediated delivery of neurotrophins, either through endogenous production or overexpression, could provide a viable approach to treat many neurodegenerative disorders. However, there remain important questions and limitations that need to be addressed before this approach can be translated toward clinical trials. One of the greatest challenges when attempting to integrate the work of many different groups into the rationale for a clinical trial, is the large variation in methodology and cells utilized. This challenge is by no means unique to the stem cell field but is exacerbated by the complexity of stem cell therapies and the many inherent differences between varying stem cell lines. Indeed, it is becoming increasingly clear that no two NSC lines are completely equivalent. Even when derived from similar source material using similar methods we have found disparate differences in NSC phenotype and behavior (Ager et al., 2015, Marsh et al., in press). It follows that identifying the optimal NSC line for clinical translation may be extremely challenging and that clinical grade NSC lines intended for trials need to be individually tested for efficacy and safety in appropriate animal models prior to translation. Quite surprisingly, current food and drug administration (FDA) guidelines do not require this.
NSCs genetically modified to overexpress different neurotrophins also present significant challenges for translation. One of the positive aspects of stem cells compared to viral gene therapy is the ability of cells to potentially migrate and integrate into the brain and respond to the endogenous CNS environment as needed. For example, many neurotrophins are secreted in an activity-dependent manner, thus endogenous production of neurotrophins by NSCs or cells derived from them could allow for more physiologic production and release of neurotrophins. In contrast, when cells are modified to constitutively overexpress neurotrophic factors, excess neurotrophins could be produced which could lead to detrimental side effects.
In order to translate many of the preclinical studies with murine NSCs towards clinical trials there is a need to test human NSCs in appropriate preclinical models first. The use of human cells brings up two issues that need to be considered when interpreting the results of studies with human cells. First, is the need for immune suppression when performing xenotransplantation in order to achieve stable engraftment (For review see (Anderson et al., 2011). Most studies including those detailed in the current review utilize calcineurin inhibitors such as tacrolimus (FK506) or Cyclosporine-A to suppress the immune system and permit hNSC engraftment. Unfortunately, we and others have found that these paradigms achieve only partial <30% engraftment of transplanted cells and can also lead to considerable toxicity when administered chronically (Ager et al., 2015, Anderson et al., 2011, Mollison et al., 1998). In several animal models of neurodegeneration including models of AD, PD, and HD these compounds have also been shown to modulate important aspects of disease pathology, greatly complicating interpretation (Cavallucci et al., 2013, Hong et al., 2010, Kitamura et al., 1994, Rozkalne et al., 2011, Yoshiyama et al., 2007).
Finally, there is very little data directly comparing functional differences between embryonic-derived, fetal-derived, or induced pluripotent stem cell (iPSC) derived NSCs. Unpublished data from our laboratory suggests that fetal-derived NSCs exhibit significantly greater migratory capacity following transplantation than embryonic- or iPSC-derived NSCs. Furthermore, all of the studies presented in this review with human cells utilized fetal derived cells. In addition to the ethical and legal complications of using fetal tissue there are likely scientific complications as well. For instance very little has been done to compare the functional differences of fetal NSCs lines derived in identical fashion but from different tissue sources.
Conclusions
Taken together the studies discussed in this review have begun to provide insight into the efficacy of NSC transplantation as a potential therapy for a variety of neurodegenerative disorders. Many studies of NSC transplantation initially focused on replacement of dead or dying cells, however, a growing body of work has now shown that NSC-mediated regulation of neurotrophic support likely provides many additional and important benefits. While there are still many challenges and questions that remain, the work detailed in this review offers hope that NSC transplantation may one day provide a true disease modifying therapy for this devastating group of neurodegenerative disorders.
Acknowledgments
S.E.M. and M.B-J. have received research funding from NIH RF1AG048099 (M.B-J.), NIH P50 AG016573 (M.B-J.), the Alzheimer’s Association BFG-14-317000 (M.B-J.), California Institute for Regenerative Medicine (CIRM) RT3-07893 (M.B-J.), NIA T32 AG00096-30 (S.E.M.), and NINDS T32 NS082174-01 (S.E.M.).
Footnotes
Author Contributions:
S.E.M and M.B-J. wrote the paper.
Conflicts of Interest:
No conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, et al. Greater striatal responses to medication in Parkinson’s disease are associated with better task-switching but worse reward performance. Neuropsychologia. 2014;62:390–397. doi: 10.1016/j.neuropsychologia.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Adem A, et al. Insulin-like growth factor 1 (IGF-1) receptors in the human brain: quantitative autoradiographic localization. Brain Res. 1989;503:299–303. doi: 10.1016/0006-8993(89)91678-8. [DOI] [PubMed] [Google Scholar]
- Ager RR, et al. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer’s disease and neuronal loss. Hippocampus. 2015;25:813–826. doi: 10.1002/hipo.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerud P, et al. Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson’s disease. J Neurosci. 2001;21:8108–8118. doi: 10.1523/JNEUROSCI.21-20-08108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Tagle DA. Genetics and molecular biology of Huntington’s disease. Trends Neurosci. 1995;18:11–14. doi: 10.1016/0166-2236(95)93943-r. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, et al. Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient. Regen Med. 2011;6:367–406. doi: 10.2217/rme.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Bachoud-Lévi AC, et al. Motor and cognitive improvements in patients with Huntington’s disease after neural transplantation. Lancet. 2000;356:1975–1979. doi: 10.1016/s0140-6736(00)03310-9. [DOI] [PubMed] [Google Scholar]
- Barker RA, et al. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- Beyreuther K, Masters CL. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991;1:241–251. doi: 10.1111/j.1750-3639.1991.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli SB, et al. L-dopa responsiveness in dementia with Lewy bodies, Parkinson disease with and without dementia. Neurology. 2004;63:376–378. doi: 10.1212/01.wnl.0000130194.84594.96. [DOI] [PubMed] [Google Scholar]
- Cavallucci V, et al. Calcineurin inhibition rescues early synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuromolecular Med. 2013;15:541–548. doi: 10.1007/s12017-013-8241-2. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J Chem Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci. 2010;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, et al. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann Neurol. 2014;76:31–42. doi: 10.1002/ana.24174. [DOI] [PubMed] [Google Scholar]
- Cicchetti F, et al. Neural transplants in patients with Huntington’s disease undergo disease-like neuronal degeneration. Proc Natl Acad Sci U S A. 2009;106:12483–12488. doi: 10.1073/pnas.0904239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980;288:279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Ebert AD, et al. Ex vivo delivery of GDNF maintains motor function and prevents neuronal loss in a transgenic mouse model of Huntington’s disease. Exp Neurol. 2010;224:155–162. doi: 10.1016/j.expneurol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Ebert AD, et al. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Emborg ME, et al. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 2008;17:383–395. [PubMed] [Google Scholar]
- Evans JR, Mason SL, Barker RA. Current status of clinical trials of neural transplantation in Parkinson’s disease. Prog Brain Res. 2012;200:169–198. doi: 10.1016/B978-0-444-59575-1.00008-9. [DOI] [PubMed] [Google Scholar]
- Freeman TB, et al. Transplanted fetal striatum in Huntington’s disease: phenotypic development and lack of pathology. Proc Natl Acad Sci U S A. 2000;97:13877–13882. doi: 10.1073/pnas.97.25.13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg NR, et al. Neural Stem Cells Rescue Cognitive and Motor Dysfunction in a Transgenic Model of Dementia with Lewy Bodies through a BDNF-Dependent Mechanism. Stem Cell Reports. 2015;5:791–804. doi: 10.1016/j.stemcr.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, et al. Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease. Cell Stem Cell. 2015;16:269–274. doi: 10.1016/j.stem.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton DW, et al. Cell-mediated neuroprotection in a mouse model of human tauopathy. J Neurosci. 2010;30:9973–9983. doi: 10.1523/JNEUROSCI.0834-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hardy J, et al. Region-specific loss of glutamate innervation in Alzheimer’s disease. Neurosci Lett. 1987;73:77–80. doi: 10.1016/0304-3940(87)90034-6. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hock C, et al. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- Hong HS, et al. FK506 reduces amyloid plaque burden and induces MMP-9 in AbetaPP/PS1 double transgenic mice. J Alzheimers Dis. 2010;22:97–105. doi: 10.3233/JAD-2010-100261. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR. Alzheimer’s disease: glutamate depletion in the hippocampal perforant pathway zone. Ann Neurol. 1987;22:37–40. doi: 10.1002/ana.410220110. [DOI] [PubMed] [Google Scholar]
- Hyman C, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Kamei N, et al. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine (Phila Pa 1976) 2007;32:1272–1278. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- Kawamoto Y, et al. Immunohistochemical localization of glial cell line-derived neurotrophic factor in the human central nervous system. Neuroscience. 2000;100:701–712. doi: 10.1016/s0306-4522(00)00326-2. [DOI] [PubMed] [Google Scholar]
- Keene CD, et al. Neural transplantation in Huntington disease: long-term grafts in two patients. Neurology. 2007;68:2093–2098. doi: 10.1212/01.wnl.0000264504.14301.f5. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, et al. Suppressive effect of FK-506, a novel immunosuppressant, against MPTP-induced dopamine depletion in the striatum of young C57BL/6 mice. J Neuroimmunol. 1994;50:221–224. doi: 10.1016/0165-5728(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kitiyanant N, et al. BDNF-, IGF-1- and GDNF-secreting human neural progenitor cells rescue amyloid β-induced toxicity in cultured rat septal neurons. Neurochem Res. 2012;37:143–152. doi: 10.1007/s11064-011-0592-1. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, et al. Pharmacological activities of glial cell line-derived neurotrophic factor (GDNF): preclinical development and application to the treatment of Parkinson’s disease. Exp Neurol. 1997;145:309–321. doi: 10.1006/exnr.1997.6501. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116:321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- Lin LF, et al. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Developing dopaminergic cell therapy for Parkinson’s disease--give up or move forward. Mov Disord. 2013;28:268–273. doi: 10.1002/mds.25378. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Treatment of Parkinson’s disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140370. doi: 10.1098/rstb.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, et al. Fetal dopamine-rich mesencephalic grafts in Parkinson’s disease. Lancet. 1988;2:1483–1484. doi: 10.1016/s0140-6736(88)90950-6. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Lowe SL, et al. Ante mortem cerebral amino acid concentrations indicate selective degeneration of glutamate-enriched neurons in Alzheimer’s disease. Neuroscience. 1990;38:571–577. doi: 10.1016/0306-4522(90)90051-5. [DOI] [PubMed] [Google Scholar]
- Lu B, et al. Expression of NGF and NGF receptor mRNAs in the developing brain: evidence for local delivery and action of NGF. Exp Neurol. 1989;104:191–199. doi: 10.1016/0014-4886(89)90029-0. [DOI] [PubMed] [Google Scholar]
- Lu B, et al. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Lu P, et al. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Experimental Neurology. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Marks WJ, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Marsh SE, Blurton-Jones M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimers Res Ther. 2012;4:11. doi: 10.1186/alzrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh SE, Blurton-Jones M. Prospects of neural stem cell therapy for Alzheimer’s disease. In: Shetty AK, editor. Neural Stem Cells in Health and Disease. World Scientific Publishing Company; Singapore: 2015. pp. 439–465. [Google Scholar]
- Marsh SE, et al. HuCNS-SC human neural stem cells fail to terminally differentiate, form ectopic ventricular clusters, and provide no cognitive benefits in an immune-deficient transgenic model of Alzheimer’s disease. Stem Cell Reports. doi: 10.1016/j.stemcr.2016.12.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Serrano A, Björklund A. Protection of the neostriatum against excitotoxic damage by neurotrophin-producing, genetically modified neural stem cells. J Neurosci. 1996;16:4604–4616. doi: 10.1523/JNEUROSCI.16-15-04604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- Masliah E, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- Maucksch C, et al. Stem cell-based therapy for Huntington’s disease. J Cell Biochem. 2013;114:754–763. doi: 10.1002/jcb.24432. [DOI] [PubMed] [Google Scholar]
- McBride JL, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comp Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- Mogi M, et al. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–48. doi: 10.1016/s0304-3940(99)00463-2. [DOI] [PubMed] [Google Scholar]
- Mollison KW, et al. Nephrotoxicity studies of the immunosuppressants tacrolimus (FK506) and ascomycin in rat models. Toxicology. 1998;125:169–181. doi: 10.1016/s0300-483x(97)00167-4. [DOI] [PubMed] [Google Scholar]
- Morizane A, Li JY, Brundin P. From bench to bed: the potential of stem cells for the treatment of Parkinson’s disease. Cell Tissue Res. 2008;331:323–336. doi: 10.1007/s00441-007-0541-0. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Oddo S, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, et al. Neurospheres modified to produce glial cell line-derived neurotrophic factor increase the survival of transplanted dopamine neurons. J Neurosci Res. 2002;69:955–965. doi: 10.1002/jnr.10396. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Pérez-Navarro E, et al. Glial cell line-derived neurotrophic factor protects striatal calbindin-immunoreactive neurons from excitotoxic damage. Neuroscience. 1996;75:345–352. doi: 10.1016/0306-4522(96)00336-3. [DOI] [PubMed] [Google Scholar]
- Peschanski M, Cesaro P, Hantraye P. Rationale for intrastriatal grafting of striatal neuroblasts in patients with Huntington’s disease. Neuroscience. 1995;68:273–285. doi: 10.1016/0306-4522(95)00162-c. [DOI] [PubMed] [Google Scholar]
- Poon WW, et al. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging. 2011;32:821–833. doi: 10.1016/j.neurobiolaging.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera EJ, et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cools R. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov Disord. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- Rozkalne A, Hyman BT, Spires-Jones TL. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011;41:650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132. doi: 10.1002/14651858.CD009132.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo VC, et al. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- Ryu JK, et al. Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease. Neurobiol Dis. 2004;16:68–77. doi: 10.1016/j.nbd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Salehi A, et al. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016 doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppet D, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Tan CC, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41:615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- Tang S, Machaalani R, Waters KA. Immunolocalization of pro- and mature-brain derived neurotrophic factor (BDNF) and receptor TrkB in the human brainstem and hippocampus. Brain Res. 2010;1354:1–14. doi: 10.1016/j.brainres.2010.07.051. [DOI] [PubMed] [Google Scholar]
- Terry RD, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Group THDCR. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Gage FH. Maintaining the neuronal phenotype after injury in the adult CNS. Neurotrophic factors, axonal growth substrates, and gene therapy. Mol Neurobiol. 1995;10:151–167. doi: 10.1007/BF02740673. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejón C, et al. Role of neurotrophins in central synapse formation and stabilization. Nat Rev Neurosci. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- Warren Olanow C, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann Neurol. 2015;78:248–257. doi: 10.1002/ana.24436. [DOI] [PubMed] [Google Scholar]
- Wu CC, et al. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci Rep. 2016;6:27358. doi: 10.1038/srep27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang W, et al. Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer’s disease. Mol Neurobiol. 2014;50:423–437. doi: 10.1007/s12035-014-8640-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann T, et al. ESC-Derived BDNF-Overexpressing Neural Progenitors Differentially Promote Recovery in Huntington’s Disease Models by Enhanced Striatal Differentiation. Stem Cell Reports. 2016;7:693–706. doi: 10.1016/j.stemcr.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Zuccato C, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]