Abstract
Deficient self-regulation plays a key role in the etiology of early onset disruptive behavior disorders and signals risk for chronic psychopathology. However, to date, there has been no research comparing preschool children with and without high levels of disruptive behavior using Event Related Potentials (ERPs) associated with specific self-regulation sub-processes. We examined 15 preschool children with high levels of disruptive behavior (35% female) and 20 peers with low disruptive behavior (50% female) who completed a Go/No-go task that provided emotionally valenced feedback. We tested whether 4 ERP components: the Error Related Negativity, the Error Positivity, the Feedback Related Negativity, and the No-go N2, differed in preschool children with and without high levels of disruptive behavior. Preschoolers with high levels of disruptive behavior showed less differentiation between the Error Positivity and corresponding waveforms following correct responses at posterior sites. Preschoolers with high and low disruptive behavior also showed differences in Go/No-go N2 waveform amplitudes across electrodes. These findings suggest that preschool children with high levels of disruptive behavior may show abnormal brain activity during certain self-regulation sub-processes, informing potential advances in conceptualizing and treating early disruptive behavior.
Keywords: Preschool Children, Disruptive Behavior, Self-Regulation Event Related Potential
Problematic disruptive behavior, including aggression, hostility, defiance, non-compliance, and violating the rights of others, are common reasons preschool children are referred for clinical services (Egger & Angold, 2006; Nock, Kazdin, Hiripi, & Kessler, 2007). Unlike typical peers, preschoolers with severe disruptive behavior are likely to remain impaired as they move into later developmental stages (Keenan et al., 2011), and have an increased risk for future peer problems, school failure, substance abuse, and juvenile delinquency (Lahey et al., 1999; Olson et al., 2011; Shaw, Gilliom, Ingoldsby, & Nagin, 2003). To elucidate the etiology of this clinical pathway, researchers have investigated deficient self-regulation processes that appear to be driving disruptive behavior, such as poor inhibitory control and feedback processing (Martel, Gremillion, Roberts, von Eye, & Nigg, 2010; Willoughby, Kupersmidt, Voegler-Lee, & Bryant, 2011). These investigations have typically measured self-regulation deficits at the overt behavior level (Barkley, 1997; Ponitz, McClelland, Matthews, & Morrison, 2009). In contrast, little is known as to whether immediate, discrete, neural events occurring at the onset of different regulatory demands are abnormal in preschool children with high levels of disruptive behavior. fMRI work has shown that school-age children and adolescents with high disruptive behavior show myriad structural and functional abnormalities related to self-regulation, such as less anterior cingulate cortex (ACC) activation during executive function (Noordermeer, Luman, & Oosterlaan, 2016). Event Related Potentials (ERPs), electroencephalographic (EEG) waveforms time-locked to specific cognitive or emotional events (Luck, 2014), may significantly advance our understanding of dysregulated processes that underpin early disruptive behavior. In early childhood, high disruptive behavior may be associated with abnormal neuro-electrical waveforms at the onset of specific self-regulation demands, representing potential endophenotypes of future risk (Euser, Evans, Greaves-Lord, Huizink, & Franken, 2013; Loo, Lenartowicz, & Makeig, 2016). However, to our knowledge, there are no studies comparing self-regulation-related ERPs in preschoolers with and without high levels of disruptive behavior. The goal of the present study was to determine whether preschool-age children with high levels of disruptive behavior, compared to peers, showed abnormal ERPs associated with distinct aspects of self-regulation: The No-go N2, the error-related negativity (ERN) and error positivity (Pe), and the feedback related negativity (FRN) components.
The ERN, Pe, FRN, and No-go N2 Components
Error Related Negativity and Error Positivity
When children and adults make a commission error, such as during a Go/No-go task, the mistake is associated with a negative voltage deflection, occurring −20–100ms around the commission error onset, with a fronto-central distribution, known as the error related negativity (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993; Gehring, Liu, Orr, & Carp, 2012). The ERN has been hypothesized to reflect error or conflict detection (Carter et al., 1998; Coles, Scheffers, & Holroyd, 2001), learning and performance modification (Holroyd & Coles, 2002), and/or the negative affect triggered by making an error (Luu, Tucker, Derryberry, Reed, & Poulsen, 2003). The ERN typically precedes a positive voltage deflection known as the error positivity, or Pe, approximately 200–400ms after commission error onset (Falkenstein, Hoormann, & Hohnsbein, 1999; Overbeek, Nieuwenhuis, & Ridderinkhof, 2005). Across studies of adult samples, the maximum amplitude of the Pe has been reported at frontal, fronto-central, and parietal locations (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005). Initially framed as a component related to conscious processing or evaluation of errors after they have occurred (Nieuwenhuis, Yeung, Van Den Wildenberg, & Ridderinkhof, 2003) recent research suggests that the Pe may also be associated with processing post-error negative affect (Tops, Koole, & Wijers, 2013) and is associated with a greater autonomic nervous system response to errors (Wessel, Danielmeier, & Ullsperger, 2011). Arbel and Donchin (2009) found that the positive deflection following the ERN comprises an early fronto-central component and a later central-parietal component, and found that only the so-called late Pe was associated with error saliency. For an in depth review of the multiple explanations of the underlying function of the ERN and Pe, see Gehring et al., 2012.
Preschool and school-age children with significant disruptive behavior have been underrepresented in the ERN and Pe literatures (Tamnes, Walhovd, Torstveit, Sells, & Fjell, 2013). Stieben and colleagues (2007) found that 8–12 year old children with disruptive behavior problems had significantly smaller ERN amplitudes compared to typical peers, but did not examine group differences in the Pe. To our knowledge, few studies have examined the Pe in samples including children under 5 years (Grammer, Carrasco, Gehring, & Morrison, 2014; Kim et al., 2016) and no study has yet examined the Pe in preschoolers with psychopathology.
Feedback Related Negativity
The Feedback Related Negativity component is a negative-polarity deflection occurring, in healthy adults, at approximately 250ms at fronto-central electrodes after the presentation of a valenced outcome, such as after making a choice in a gambling task (Gehring & Willoughby, 2002; Hajcak, Moser, Holroyd, & Simons, 2007; Holroyd & Coles, 2002). Like the ERN and Pe, the functional significance of the FRN is unclear, particularly in early childhood. Studies of the FRN response often employ gambling, or gambling-like paradigms, where the subject cannot anticipate what the outcome is going to be (e.g., Gehring & Willoughby, 2002). These studies consistently have revealed, in adults, larger amplitude FRN waveforms for “bad” (either loss or non-win) outcomes than “good” (win) outcomes (Gehring & Willoughby 2002; Hajcak, Holroyd, Moser, & Simons, 2007). More recently, however, researchers have found that the FRN may be more associated with feedback stimuli unexpectedness rather than valence (Ferdinand, Mecklinger, Kray, & Gehring, 2012). As such, the FRN may reflect the degree to which expectations have been violated (Bismark, Hajcak, Whitworth, & Allen, 2013). The FRN may also underlie learning (Holroyd & Coles, 2002), or relate to subjective stress and reward sensitivity (Banis, Geerligs, & Lorist, 2014; Bress & Hajcak, 2013).
To date, few researchers have investigated the FRN in children under 5 years of age, providing a limited and mixed picture of this component in early childhood. Mai and colleagues (2011) used a prize-guessing task to examine the FRN in 4- and 5-year-old children, and found no difference in amplitude between positive and negative outcomes. Roos, Pears, Bruce, Kim, and Fisher (2014) examined the FRN in a performance-based flanker task in a sample of maltreated preschool children, and found larger FRN amplitudes for negative outcomes, but only in children who were also high in impulsivity. This finding suggests a correspondence between the FRN and clinical symptoms in the preschool years, yet, to the best of our knowledge, no investigators have compared the FRN in preschoolers with and without high levels of disruptive behavior.
No-go N2
The No-go N2 is a negative-going deflection occurring, in adults, between 200 and 400ms after presentation of a No-go stimulus (Eimer, 1993; Falkenstein et al., 1999; Jodo & Kayama, 1992) and observed at fronto-central electrodes (Folstein & Van Petten, 2008). The No-go N2 is thought to reflect the cognitive control required to inhibit a response (e.g., Bruin and Wijers, 2002) or the conflict between competing responses (executive versus inhibition) (Nieuwenhuis, Yueng, van den Wildenberg, and Ridderinkhof, 2003). As reviewed by Folstein and Van Petten (2008), there is empirical evidence supporting both views.
Research on the No-go N2 in pediatric clinical populations has largely focused on older children with attention deficit-hyperactivity disorder (ADHD), which is comorbid with but not in and of itself a disruptive behavior disorder (American Psychiatric Association, 2013). Wiersema, Van Der Meere, Roeyers, Van, and Baeyens (2006) found that 7–13 year olds with ADHD had smaller No-go N2 amplitudes than non-disordered peers. However, No-go N2 differences between groups disappeared when controlling for disruptive behavior, suggesting a blunted No-go N2 may play an important role in middle-childhood disruptive behavior. To our knowledge, only one other research team has examined the No-go N2 in children recruited based on disruptive behavior (as opposed to ADHD) symptom criteria. The aforementioned study by Stieben and colleagues (2007) found that No-go N2 amplitudes were significantly smaller in clinically-referred children compared to peers. To our knowledge, whether a blunted No-go N2 indicates high disruptive behavior in the preschool years has not been investigated.
The Present Study and Hypotheses
Our goal was to determine whether preschool children with high disruptive behavior showed abnormal ERPs at the onset of different self-regulation challenges compared to low-risk peers, and identify which ERPs most clearly discriminated these two groups. We used an adapted Go/No-go task that allowed us to simultaneously measure multiple ERP components: the No-go N2, the ERN and Pe, and the FRN. Preschoolers with severe disruptive behavior are more likely to exhibit impairment in executive function, response to positive and negative feedback, and emotion regulation (Barkley et al., 1997; Martel et al., 2010). We therefore hypothesized, consistent with ERP investigations in older children (e.g., Stieben et al., 2007), that the ERN, Pe, and No-go N2 would be blunted in preschoolers with high levels of disruptive behavior compared to peers with low levels of disruptive behavior. Given the dearth of research on the FRN in the development of psychopathology, and the myriad theories on the function of the FRN component, we had an exploratory goal to test if FRN amplitudes differed between preschoolers with high and low levels of disruptive behavior.
Methods
Participants
We recruited two groups of preschool children: those with high versus low levels of disruptive behavior. All children participated in a larger study on the development of emotion regulation, and were included in the sample described below if they assented to participate in the ERP portion of the study. Parents of all prospective subjects completed a short screening interview with a laboratory member to determine if their child was eligible to participate. Children were screened for, and excluded, if parents endorsed any of the following criteria: diagnosis of mental retardation, autism spectrum disorder, psychosis, significant medical condition, epilepsy, or history of head injury resulting in loss of consciousness.
High disruptive behavior group
Twenty children aged 3 to 5 years (M = 59 months, SD = 8.76 months; 35% female) comprised the group of preschoolers with high levels of disruptive behavior. Of these, 12 children were recruited from three outpatient mental health clinics affiliated with a large local university. Families seeking treatment at these clinics for their child’s disruptive behavior, per their intake evaluation, were recruited via flyers or contacted directly by study team members around the time of their initial appointment. An additional 8 children were recruited from online or paper advertisements targeting preschool children with disruptive or defiant behavior. Based on the screening interview, children were included in the high-disruptive behavior group if their parents endorsed their child’s disruptive behavior as “significant or concerning”, reported that their child was being treated at a private practice clinic, or if they stated that the child’s disruptive behavior was noticeable in both home and school settings (e.g., if their teacher expressed concerns). All children were right handed or ambidextrous. Based on maternal report, 62.1% of the children were identified by their mothers as Caucasian, 20.7% identified as African American, and 17.2% as mixed-race. Children whose parents endorsed disruptive behavior comorbid with other behavior problems, such as mood or anxiety problems, were included in the study if they otherwise endorsed concerns about disruptive behavior. One child had been taking stimulant medication per parent report but abstained 24 hours before coming into the lab. Data were lost for one child in the high disruptive behavior group due to a technical failure. Furthermore, a subset of children in the high disruptive behavior group did not generate enough artifact-free trials, with the number of excluded children varying by component. Within the high disruptive behavior group we examined ERP data on 14 children for No-go N2 analyses, 14 children for ERN and Pe analyses, and 11 children for FRN analyses.
Low disruptive behavior group
Thirty typically developing preschool-age children (M = 57.9 months, SD = 7.8 months; 50% female) were recruited from area preschools via community flyers and web advertisements. Based on maternal report, 75% of the sample identified as Caucasian, 13.8% as mixed-race, 5.6% as African American, and 5.6% as Asian. All were right handed or identified as ambidextrous. During the screening interview, parents were screened to ensure they, as well as the child’s teacher or pediatrician (per parent report), did not have concerns about their child’s mood or behavior problems. ERP data were lost for 3 children in the low disruptive behavior group due to a technical failure, 1 child only completed a single block, and 1 child had a rapid-response pattern and made too many errors. Finally, some children in the low disruptive behavior group generated too many artifact-corrupted trials, with the exact number varying by component. Within the low disruptive behavior group, we analyzed data on 16 children for the No-go N2, 18 for the ERN and Pe, and 14 for the FRN.
Socio-demographic characteristics of both samples are shown in Table 1. The high and low disruptive behavior group did not differ in terms of age, gender, income, or ethnicity. Preschoolers low in disruptive behavior had mothers who went significantly further in school compared to peers with high disruptive behavior.
Table 1.
Socio-demographic Characteristics of Preschool Children with High and Low levels of Disruptive Behavior
| Characteristic | High Disruptive Behavior (n = 15) M (SD) |
Low Disruptive Behavior (n = 20) M (SD) |
t/χ2(df) |
|---|---|---|---|
| Age (months) | 59.68(8.5) | 59.86(6.4) | ns |
| Gender (% male) | 64% | 33% | ns |
| Ethnicity (% Caucasian) | 64% | 77% | ns |
| Income | 9.61(3.8) | 7.93(3.3) | ns |
| Mother Age (years) | 34.57(5.8) | 35.24(4.7) | ns |
| Mother Education | 4.86(1.4) | 6.00(1.4) | 2.24(29)* |
| Father Age (years) | 36.79(7.6) | 39.88(8.9) | ns |
| Father Education | 5.00(2.0) | 5.75(1.4) | ns |
| Marital Status (% married) | 83% | 81% | ns |
| WPPSI-IV Vocab. score | 20 | 21 | |
| CBCL ODD T-score | 63.15(7.5) | 53 | −4.5(27)*** |
| CBCL ADHD T-score | 57.00(6.2) | 51.31(2.1) | −3.4(27)** |
| CBCL Affective T-score | 53.92(3.9) | 54.00(5.3) | ns |
| CBCL Anxiety T-score | 53.54(4.7) | 51.50(3.2) | ns |
Note.
p<.05,
p<.01,
p<.001.
WPPSI-IV Vocab. Score = Wechsler Preschool and Primary Scale of Intelligence, 4th Edition, Vocabulary Subtest.
Procedure
Go/No-go task with performance-based feedback
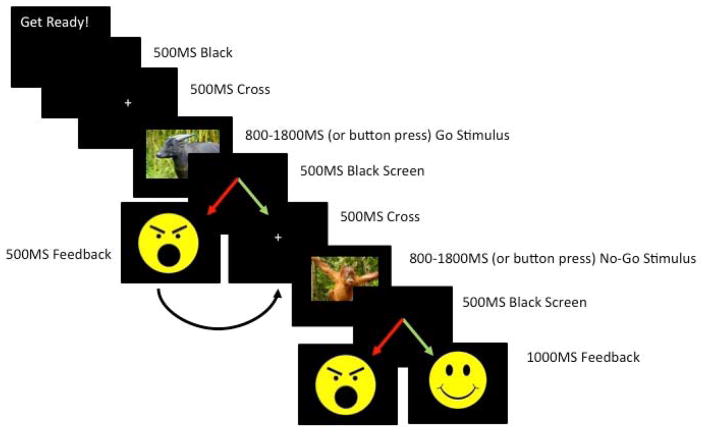
Participants completed a child-friendly Go/No-go task originally developed by McDermott, Henderson, Degnan, and Fox (2014), and expanded by Grammer and colleagues (2014). We further adapted the task to provide performance-based, emotionally-valenced feedback to examine multiple ERP components including the FRN. Children were told they were going to play a computer game called “Zookeepers” to win a toy. The task had 8 trial blocks; each block consisted of 40 trials. Children were instructed to click a button each time they saw an animal picture (Go stimuli), with the exception of an orangutan picture (No-go stimuli) (See Figure 1). The premise of the game was that all the animals had escaped from the zoo and the child had to press a button each time an animal was presented on the screen to put them back in a cage. Children were told that the orangutans were “special helpers” who lived in the Zookeeper’s house and therefore should not be put in a cage. Children completed two practice blocks, the first without No-go stimuli and the second with No-go stimuli, to ensure they understood the task. Children were awarded special animal stickers after each trial block, regardless of performance, to increase motivation. Research assistants were trained to praise children on their speed, regardless of their performance, rather than their accuracy to ensure children made enough errors for analyses.
Figure 1.
Flow chart of the emotion-inducing Go/No-go task. Response contingencies represented with arrows. Red arrows denote incorrect responses and green arrows denote correct responses.
No-go stimuli were randomly presented 25% of the time. As shown in Figure 1, for every correct No-go trial (child correctly abstained from hitting the button), the child was presented with a happy face after 500ms of black screen. For every incorrect No-go trial (child accidentally hit the button), the child was presented with an unhappy face after 500ms of black screen. This 500ms gap was designed to separate ERN and FRN waveforms. Happy and unhappy faces were designed to be as similar as possible in terms of their size, color saturation, percentage of yellow to black pixels, and position on the screen to ensure these stimulus characteristics did not influence waveforms. Happy and unhappy faces were not linked to any reward. Children were told before playing the game that happy faces meant the orangutan was glad the child had correctly abstained from putting them in a cage, and “grumpy” faces meant the orangutan was unhappy the child had put them in a cage where they didn’t belong. Children also saw the same unhappy face if they did not respond to go stimuli in a sufficient amount of time. Children were told that these “grumpy” faces meant they needed to click faster. The purpose of presenting negative feedback on unresponsive Go trials was to prevent children from trying to avoid No-go errors by engaging in a non-response strategy. We did not examine waveforms related to omission error feedback in the present study.
As noted by (Shiels & Hawk, 2010), Olvet and Hajcak (2008), and others, clinical-control group differences in ERPs, specifically the ERN, may be due to differences in the number of errors made during the task rather than differences in the neural generator of the component itself, as fewer errors are associated with a larger amplitude ERN. In the present study, a dynamic algorithm ensured all children, regardless of their inhibitory ability, would make approximately the same number of errors, and thus encounter the same number of emotion-eliciting events across the task. Specifically, the duration of Go and No-go stimuli decreased by 100ms increments each time children completed a successful No-go trial. Similarly, Go and No-go stimulus duration increased by 100ms increments each time children made an error on a No-Go trial. Adjustments in duration were capped such that Go and No-go stimuli could be no faster than 800 ms and no slower than 1800 ms. Speed adjustments did not affect the duration of black screen, fixation cross, or feedback presentation.
Prior to EEG recording, children were seated in a comfortable chair and engaged in rapport building with research assistants. This included reading a picture book together about animals in a zoo, and administrating an assent script in child-friendly language. Parents were allowed to remain in the room with their child during both the assent and EEG recording. EEG data were recorded using a 128 channel Geodesic Sensor Net (EGI; Tucker, 1993). Impedance for all electrodes was kept below 50 KΩ, and all recordings were referenced to the vertex, Cz. The EEG signal was amplified using a 0.01–100 Hz bandpass and digitized at 500 Hz. The electro-oculogram (EOG) was monitored with 6 electrodes placed bilaterally in the external canthi (Ch 128 and 125), supraorbital (Ch 25 and 8) and infraorbital (Ch 127 and 126) regions. Recording in every channel was vertex-referenced. Data were recorded and processed using Net Station 4.4 (EGI software).
Once acquired, an Elliptical infinite impulse response (IIR) filter was used for highpass filtering and a Kaiser finite impulse response (FIR) filter was used for lowpass filtering, with a 2Hz roll-off. EEG data were filtered between 0.1 and 20Hz. The continuous EEG was segmented into epochs for components of interest. Go and No-go N2 segments began 200 ms before the onset of the stimulus and ended 1200 ms after. Segments comprising the ERN, CRN, Pe, and Pc began 400 ms before the onset of the response and ended 800 ms after the response. FRN segments began 200 ms before the onset of feedback presentation and ended 1200 ms after. Segmented files were scanned for artifacts with the Artifact Detection NS tool for excessive muscle activities, eye blinks and eye movements. Segments were marked as an artifact if activity exceeded pre-defined thresholds for eye movement (55 μV), blinks (100 μV), and bad channels (200μV). Following this automated process, segments were manually inspected. Segments with more than 10 bad channels were excluded from analyses. In segments fewer than 10 bad channels, and no movement artifacts, spherical spline interpolation was used to replace bad channels using values from neighboring channels. Subjects with at least 5 good segments in each trial were included in analyses, consistent with other pediatric ERP studies that have used a lower cutoff compared to the adult ERP literature (Grammer et al., 2014; Roos et al., 2015). There were no significant differences between groups in the number of artifact-free trials for any condition.
Artifact-free No-go N2, ERN, Pe and FRN segments were averaged separately and then re-referenced against the average of all channels. A baseline correction was performed with baselines specified as follows: −200 to −100ms prior to response onset for ERN and CRN, Pe and Pc segments, −100 to 0ms for FRN (positive and negative feedback) segments, and −100ms to 0ms prior to stimulus onset for Go and No-go N2 segments. Based on initial visual inspection of the grand averaged ERPs, and in accordance with previously published reports (Espinet, Anderson, & Zelazo, 2012; Grammer et al., 2014; Mai et al., 2011), we defined ERP component windows of interest as the following: The ERN was defined as the average amplitude between −25 and 50ms aligned with response onset. The Pe was defined as the average amplitude between 200 and 500ms after the response onset. The FRN was defined as the average amplitude between 350 and 450ms after the feedback onset (but note additional adjustments made below). The No-go N2 was defined as the average amplitude between 200 and 570ms after stimulus onset. Furthermore, we made manual adjustments for a small number of children who showed peak amplitudes of these components outside, but within 50ms of, these predefined windows of interest. Manual adjustments were made by shifting, not extending, the window of interest by 25 or 50ms. These small adjustments were made to better capture the ERN for three low disruptive behavior preschoolers, and two high disruptive behavior preschoolers, the Pe for one low disruptive behavior preschooler, and the FRN for three low disruptive behavior preschoolers and one high disruptive behavior preschooler.
Group Comparisons of Behavior Problems and Verbal Intelligence
Parents rated their child’s behavior using the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000). As shown in Table 1, high disruptive behavior preschoolers had significantly higher Oppositional Defiant Disorder and Attention Deficit Hyperactivity Disorder T-scores compared to low-risk peers. Among high disruptive behavior preschoolers, Oppositional Defiant Disorder T-scores fell in the borderline clinical range, whereas ADHD, Affective, and Anxiety T-scores fell within the typical range. Moreover, Affective and Anxiety T-scores did not differ between groups. Across the entire sample, CBCL Oppositional Defiant Disorder and Attention Deficit Hyperactivity Disorder T-scores were highly correlated, r(32) = .842, p < .001. Children completed the Wechsler Preschool and Primary Scale of Intelligence- Fourth Edition (WPPSI-IV) (Wechsler, 2012) Vocabulary subtest to assess verbal intelligence. There were no differences in WPPSI-IV vocabulary scores between groups.
Statistical Analyses
Independent sample t-tests were used to test for differences in task accuracy and reaction time between groups. For each ERP component of interest (ERN, Pe, FRN, and No-go N2), we first examined the visual characteristics of the waveforms for high- and low-disruptive behavior groups at central-midline 10–10 sites: Fz, FCz, Cz, and Pz. The four sites were chosen a priori to be consistent with how adult and child studies commonly display and analyze the ERN, Pe, FRN, and No-go N2 (Overbeek et al., 2005, Folstein & Van Petten, 2008). We also examined how voltages for each condition, and differences between conditions, were distributed across the entire scalp for each group. Next, we conducted repeated measures ANOVAs with condition and electrode as within subject factors and group as a between subject factor. An alpha level of .05 was used for all statistical tests. It is not currently the universal standard in ERP research to correct for multiple comparisons (for examples see Buss, Dennis, Brooker, & Sippel, 2011; Roos et al., 2014; Stieben et al., 2007; and Wiersema et al., 2006) with some researchers arguing for caution in situations where alpha correction might reduce the sensitivity needed to evaluate hypotheses (Burwell, Malone, & Iocono, 2016). Further, other psychophysiological methodologists have argued that conventional multiple comparison corrections are not appropriate for ERP data in cases where different waveforms are derived from the same task and are thus dependent on each other (Causeur, Chu, Hsieh, & Sheu, 2012). Nonetheless, we employed the False Discovery Rate (FDR) correction (Benjamini & Hochberg, 1995) to provide a more conservative estimate of effects. However, to balance risk of Type I and Type II errors (Wasserstein & Lazar, 2016), we also report both uncorrected p-values and effect sizes for all significant findings. The Greenhouse Geisser correction was used to correct for sphericity violations when needed.
Results
Group Comparisons of Go/No-go Task Performance
Accuracy and reaction time data for each group are shown in Table 2. Preschoolers with high and low levels of disruptive behavior did not differ in accuracy or reaction time for Go or No-go trials. The average duration for Go and No-go trials was significantly different between groups such that the task slowed down significantly for high disruptive behavior preschool children in response to their No-go performance. Therefore, differences found in the ERP waveforms between groups were not due to a significant difference in the number of errors made.
Table 2.
Adapted Go/No-go Task Behavioral Data for Preschool Children with High and Low Levels of Disruptive Behavior
| High Disruptive Behavior (n = 15) M (SD) |
Low Disruptive Behavior (n = 20) M (SD) |
t(df) | |
|---|---|---|---|
| Go Trial Reaction Time (ms) | 599(61) | 589(40) | ns |
| No Go Trial Reaction Time (ms) | 482(64) | 469(62) | ns |
| Go Accuracy (% Correct) | 81% (12) | 82%(8) | ns |
| No-go Accuracy (% Correct) | 73%(14) | 69%(8) | ns |
| Stimuli Duration (ms) | 887(65) | 951(111) | 2.26(36)* |
| Number of usable, artifact-free trials | |||
| CRN | 71(33) | 79(26) | ns |
| ERN/Pe | 12(6) | 13(7) | ns |
| FRN, positive outcome | 20(6) | 29(8) | ns |
| FRN, negative outcome | 9(5) | 10(4) | ns |
| No-go N2 | 15(8) | 20(9) | ns |
| N2, Go trials | 69(39) | 88(36) | ns |
Note.
p<.05
ERP Waveforms
Results of repeated measures ANOVAs for the ERN, Pe, FRN, and No-go N2 are presented in Table 3.
Table 3.
Results of the repeated measures ANOVAs with F-values (df), p-values, and effect sizes for the ERN, Pe, FRN, and No go N2, including between subjects factor Group and within subjects factors Condition and Electrode.
| ERN/CRN
|
Pe/Pc
|
FRN
|
Go/No go N2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | F | p | ηp2 | |
| Group | 3.30 (1, 30) | 0.08 | - | .173 (1, 30) | 0.70 | - | 1.72 (1, 23) | 0.20 | - | 0.92 (1, 28) | 0.35 | - |
| Condition | 51.28 (1, 30) | <0.001 | 0.63 | 3.22 (1, 30) | 0.08 | - | 29.77 (1, 23) | <0.001 | 0.56 | 0.23 (1, 28) | 0.64 | - |
| Electrode | 2.26 (3, 90) | 0.09 | 0.07 | 35.01 (3, 90) | <0.001 | 0.54 | 7.07 (2, 32.2) | 0.002 | 0.24 | 37.59 (3, 84) | <0.001 | 0.57 |
| G*C | 0.00 (1, 30) | 0.97 | - | 0.03 (1, 30) | 0.86 | - | 5.44 (1, 23) | 0.47 | - | 0.60 (1, 28) | 0.45 | - |
| G*E | 0.27 (3, 90) | 0.86 | - | 4.82 (3, 90) | 0.004 | 0.14 | 0.19 (1.4, 32.2) | 0.83 | - | 3.64 (3, 84) | 0.02 | 0.12 |
| C*E | 4.82 (3, 90) | 0.004 | 0.14 | 25.43 (3, 90) | <0.001 | 0.46 | 1.69 (1.2, 28.4) | 0.20 | - | 1.08 (3, 84) | 0.36 | - |
| G*C*E | 0.33 (3, 90) | 0.81 | - | 1.58 (3, 90) | 0.20 | - | 1.14 (1.2, 28.4) | 0.33 | - | 0.73 (3, 84) | 0.54 | - |
Note. Significant statistics indicated in bold.
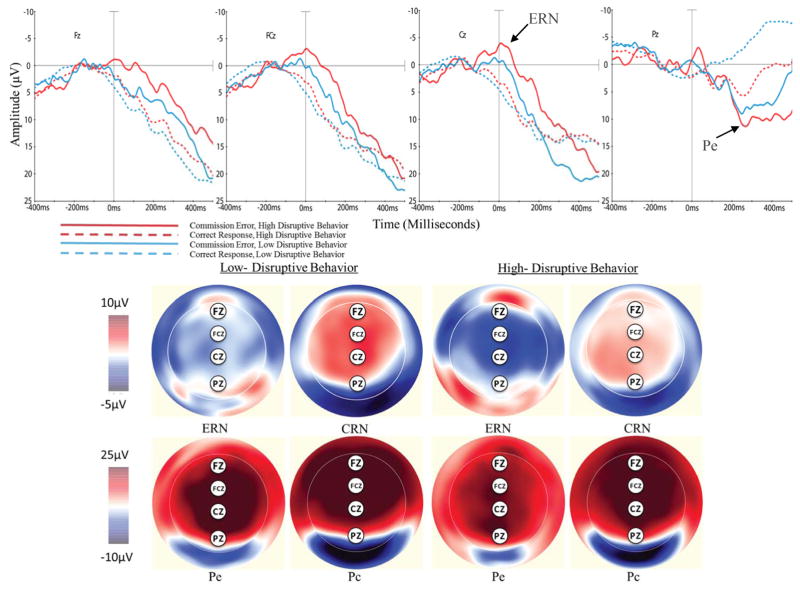
ERN and Pe
ERN
Response-locked waveforms showing the ERN and Pe components at midline electrode sites, and scalp distribution maps, can be seen in Figure 2. High- and low-disruptive behavior preschoolers showed a negative deflection around the time of error commission that was larger in magnitude (more negative) compared to corresponding correct trials (CRN), most robustly seen at FCz and Cz. A 2 (condition: ERN, CRN) x 4 (electrode: Fz, FCZ, Cz, Pz) x 2 (group) ANOVA revealed a main effect of condition such that ERN amplitudes were significantly larger than CRN amplitudes. There was also a significant condition by electrode interaction such that ERN-CRN differences were larger at FCz and Cz than at Fz and Pz. High-disruptive behavior preschoolers showed larger ERN amplitudes and smaller CRN amplitudes than low disruptive behavior peers, however the main effect of group was non-significant. The main effect of condition and the condition by electrode interaction survived after FDR correction for multiple comparisons.
Figure 2.
Response locked waveforms and scalp distributions following correct Go and incorrect No-go trials for preschool children with high and low levels of disruptive behavior.
Pe
In addition, both groups showed a positive deflection between 200–500ms that was larger for error trials than correct trials at FCz, Cz, and Pz. A 2 (condition: Pe, Pc) x 4 (electrode: Fz, Fcz, Cz, Pz) x 2 (group) ANOVA did not reveal significant group or condition main effects. There was a main effect of electrode suggesting greater overall positivity at posterior scalp sites across error and correct trials. There was also a significant group by electrode and a condition by electrode interaction. Paired-sample t-tests revealed Pe amplitudes were significantly larger than corresponding Pc amplitudes at Pz for low-disruptive behavior preschoolers, t(17) = 3.37, p = .004, uncorrected, d = 1.63, a trend that was non-significant for high-disruptive behavior peers, t(13) = 2.15, p = .051, uncorrected. An independent samples t-test revealed that Pc amplitudes at Pz were smaller (less positive) for low disruptive behavior preschoolers than high disruptive behavior peers, t(30) = −2.53, p = .02, uncorrected, d = .92. Further, for both groups, Pe amplitudes were significantly smaller (less positive) than Pc amplitudes at Fz (low-disruptive behavior: t(17) = −3.81, p = .001, uncorrected, d = 1.85; high-disruptive behavior: t(13) = −3.09, p = .01, uncorrected, d = 1.71) and FCz (low-disruptive behavior: t(17) = −2.68, p = .02, uncorrected, d = 1.30; high-disruptive behavior: t(13) = −2.54, p = .02, uncorrected, d = 1.41. All significant effects survived after FDR correction for multiple comparisons.
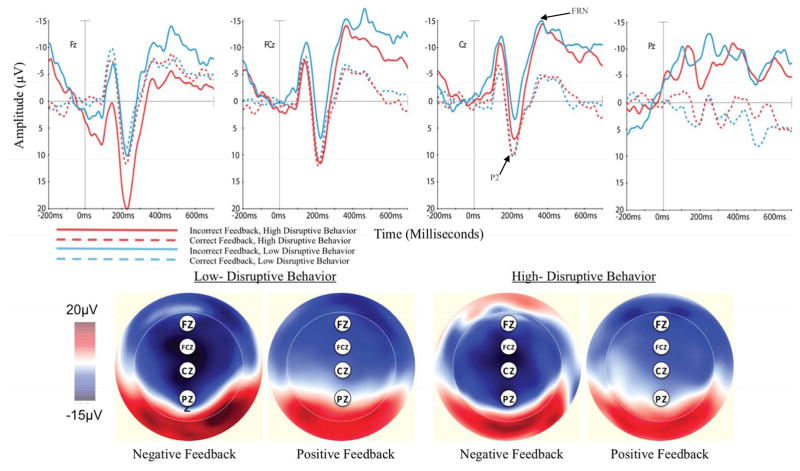
FRN
As shown in Figures 3, visualization of the waveforms showed a negative deflection in both groups, beginning around 375ms post-feedback and continuing through later in the epoch, at Fz, FCz, and Cz, and a larger deflection for negative feedback than positive feedback. Here, we refer to the negative-going activity as the FRN, recognizing that it does not return in the positive-voltage direction as is more typical of the FRN and does not resemble the typical FRN seen in older children and adults (e.g., Crowley et al., 2013). Further complicating interpretation, the waveforms also show differences in the positive and negative feedback conditions prior to the 350–450ms window of interest, with the exception of site FCz. These differences prevented us from simply comparing the average amplitude between 350–450ms. Therefore, for each condition, we calculated the peak-to-peak difference between the FRN and the preceding P2. P2 peaks were defined as the maximum (most positive) amplitude between 200 and 250ms, and FRN peaks were defined as the minimum (most negative) amplitude between 350 and 450ms. In addition, a notable difference in amplitude between positive and negative feedback waveforms, particularly at sites Fz and Pz, was observed prior to the −100 to 0ms baseline correction window. Given the task design, differences in amplitude by condition before the baseline may reflect differences in activity prior to stimulus onset. At Pz, the lack of an apparent P2 and FRN prevented us from calculating a peak-to-peak difference, and thus we did not include Pz in the ANOVA model.
Figure 3.
Response locked negative and positive feedback waveforms and scalp distributions for correct and incorrect No-go trials for preschool children with high and low levels of disruptive behavior.
A 2 (condition: positive, negative feedback) x 3 (electrode: Fz, FCz, Cz) x 2 (group) ANOVA revealed a main effect of feedback type such that negative feedback was associated with larger amplitudes (more negative) than positive feedback. There was also a main effect of electrode, suggesting greater negativity at frontal scalp sites across positive and negative feedback trials. There was no main effect of group and interaction terms were non-significant. The main effects of condition and electrode survived after FDR correction for multiple comparisons.
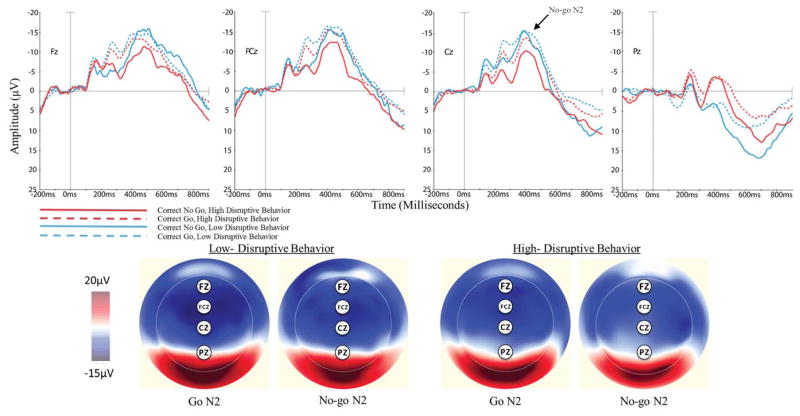
No-go N2
Visualization of No-go N2 waveforms (see Figure 4) revealed that both groups showed a negative deflection between 200 and 500ms for correct Go and No-go trials at Fz, FCz, and Cz. Furthermore, each of these waveforms showed two distinct peaks in the window of interest. A 2 (condition: Go, No-go) x 4 (electrode: Fz, FCz, Cz, Pz) x 2 (group) ANOVA revealed a main effect of electrode, suggesting less negativity at posterior scalp sites across Go and No-go trials. There was no main effect of condition or group; however, there was a significant electrode by group interaction. Examination of the interaction revealed that Go/No-go N2 estimated marginal means were smaller (less negative) for high disruptive preschoolers than low disruptive preschoolers at Fz, Fcz, and Cz, consistent with our hypothesis, and larger (more negative) at Pz. Numerically, group differences in average amplitude were larger for No-go N2 waveforms than Go N2 waveforms, with the largest No-go N2 difference appearing at Cz. However, non-significant group by condition and group by electrode by condition interactions prevented us from testing whether No-go N2 waveforms significantly differed by group at specific electrodes. After FDR correction for multiple comparisons, the main effect of electrode survived and the electrode by group interaction survived at the p = .08 threshold but not the p = .05 threshold.
Figure 4.
Waveform and scalp distributions following correct Go and No-go trials for preschool children with high and low levels of disruptive behavior.
Discussion
In the present study, preschool children between the ages of 3 to 5 years with high or low levels of disruptive behavior completed a child-friendly, ERP compatible Go/No-go task adapted to provide performance-based feedback. Children with both high and low disruptive behavior generated waveforms following responses and feedback consistent with the ERN, Pe, FRN, and No-go N2 in terms of waveform onsets and valences, and magnitude differences by condition. We found evidence that the Pe and Go/No-go N2 components discriminated preschool children with high levels of disruptive behavior from peers with low levels of disruptive behavior. Specifically, whereas preschoolers with low disruptive behavior showed larger (more positive) Pe amplitudes than Pc amplitudes at electrode Pz, the difference was non-significant in peers with high disruptive behavior. This difference appeared to be driven by Pc amplitudes that were larger (more positive) at Pz in preschoolers with high disruptive behavior. In addition, Go/No-go N2 analyses revealed a group by electrode interaction showing high disruptive behavior preschoolers had smaller Go/No-go N2 waveforms at frontal midline sites and larger waveforms at Pz than low disruptive peers. However, we were unable to further unpack this interaction to test if No-go N2 waveforms specifically were significantly blunted in high disruptive preschoolers. There were no differences in the ERN and FRN components between groups. These findings have potentially important implications for how we conceptualize the etiology of early, clinically significant disruptive behavior and how future prevention and interventions might take a more targeted approach.
Compared to peers with high disruptive behavior, the Pe generated by preschoolers with low disruptive behavior showed greater discrimination between Pe and Pc waveforms that more closely resembled reports of this component in older children and adults. A robust Pe-Pc difference has been consistently reported in samples of older, healthy children (Grammer et al., 2014) and adults (Arbel & Donchin, 2009), suggesting high disruptive preschoolers in the present study may have exhibited more immature waveforms linked to error saliency processing. Evidence that preschoolers with high disruptive behavior also showed different Go/No-go N2 waveform amplitudes extends limited research on disruptive behavior in middle childhood reporting blunted No-go N2 waveforms (Stieben et al., 2007). Specifically, the present study suggests that electrode differences in Go/No-go N2 waveforms may be detectable and linked to behavior problems very early in the lifespan. Collectively, these findings indicate that an abnormal Pe and differences in Go/No-go N2 waveform amplitudes may be potential endophenotypes signaling developing psychopathology and risk for myriad negative outcomes. The burgeoning interest in ERPs as clinical endophenotypes of psychiatric risk has, to date, largely focused on the ERN and risk for anxiety, depression, and substance abuse in older populations, as opposed to early onset externalizing psychopathology (Euser et al., 2013; Iacono & Malone, 2011; Loo et al., 2016). More recent studies showed that the ERN, Pe, and No-go N2 are rank stable over a 1 to 2 year period in child and adolescent populations (Burwell, Malone, & Iacono, 2016; Meyer, Bress, & Proudfit, 2014). Our findings warrant future research testing whether the Pe and Go/No-go N2 are rank stable from early to middle childhood and forecast clinical outcomes.
Furthermore, our finding that preschoolers with high and low disruptive behavior showed no differences in the ERN, CRN, or FRN, suggests a rethinking of how early self-regulation deficits contribute to significant disruptive behavior. When measured as behavioral performance or parent ratings, children with high disruptive behavior exhibit myriad Executive Function deficits and difficulty managing negative emotion (Barkley, 1997; Cole, Hall, & Radzioch, 2009; Nigg, Blaskey, Huang-Pollock, & Rappley, 2002). Moreover, the anterior cingulate cortex (ACC) and overlapping portions of the medial frontal cortex (MFC) are hypothesized to generate each of the four components we examined in the present study (Botvinick, Cohen, & Carter, 2004; Ferdinand et al., 2012; Nieuwenhuis et al., 2003). With this literature in mind, our findings suggest caution in assuming early disruptive behavior problems result from global self-regulation deficits and underlying neural dysfunction. We found evidence that most ERPs generated by the adapted Go/No-go task were not sensitive to level of disruptive behavior, indicating some self-regulation sub-processes may be intact in preschoolers with high disruptive behavior, at least in certain contexts. Alternatively, it is possible certain self-regulation sub-processes, such as those underlying the Pe and Go/No-go N2, disproportionately contribute to individual differences in early disruptive behavior. Processing the emotion associated with making a mistake, and discerning when to make or inhibit a response, are challenges preschool children experience daily and learn to master across early childhood (Posner & Rothbart, 2000). Neural activity while processing error-related emotional salience and producing and/or inhibit responses appropriately, at the instance these challenges occur, may be particularly salient markers of early onset disruptive behavior problems. Clinically, these findings suggest that self-regulation-based targets for reducing early disruptive behavior may be quite specific. For example, interventions designed to reduce preschool disruptive behavior by improving self-regulation often target a range of skills (e.g., Bodrova & Leong, 2007). Pinpointing self-regulation sub-processes that make the largest contribution to early disruptive behavior may eventually lead to more efficacious treatments.
Strengths and Limitations
By using a task capable of measuring multiple ERPs, the present study is, to our knowledge, the first to detect ERPs indicative of high disruptive behavior in children as young as three years. Although these findings potentially advance our understanding of self-regulation deficits and early disruptive behavior, limitations must be acknowledged. While the sample size for each group was consistent with other ERP studies of young children with behavior problems (Carrasco et al., 2013; Kuhl, Coffey-Corina, Padden, & Dawson, 2005; Stieben et al., 2007), larger samples may have yielded more robust differences between groups. Specifically, teasing apart whether the Go/No-go N2 finding is due to Go or No-go N2 waveforms is a critical future direction that will require testing with larger samples. Further, it appeared preschool children with high disruptive behaviors had larger ERN amplitudes and smaller CRN amplitudes than peers with low disruptive behavior, but the difference was a non-significant trend (p = .08). It is possible this non-finding represents a Type II error that would have been detected with a larger sample. Another limitation of the study was that the adapted Go/No-go task was not designed to examine the No-go P3 component, which occurs 300 to 600ms after stimulus onset and has been associated with self-regulation (Falkenstein et al., 1999; Polich, 2007). Specifically, we observed a later developing No-go N2 (similar to Espinet, Anderson, and Zelazo’s 2012 investigation of the N2 in preschoolers), and a post-N2 positive deflection that did not develop into a peak due, perhaps in part, to the onset of feedback stimulus. Finally, we identified waveforms consistent with the ERN, Pe, FRN, and No-go N2, but observed some morphological differences with descriptions of these components in the adult literature. ERP studies in adults have consistently shown that No-go N2 amplitudes are larger than Go N2 amplitudes, which was not the case in the present study, and that the adult FRN occurs earlier, and at more anterior sites, than the FRN reported here. Some literature suggests these inconsistencies may be developmental differences between early childhood and adulthood. Buss and colleagues (2011), in a study of non-clinical preschoolers, similarly did not find N2 differences following congruent and incongruent trials of a flanker task. Mai and colleagues’ (2011) study of typical preschool children found a later occurring FRN that was most robust at FCz and Cz, which is highly similar to what we report here. However, because there are so few studies of these ERPs in early childhood, we cannot rule out whether morphological differences between preschoolers and adults are due to development or adapting tasks to make them early-childhood friendly. For example, unlike gambling tasks used to examine the FRN in adults, preschoolers in the present study could hypothetically have anticipated whether feedback would be good or bad (Holroyd & Coles, 2002).
Future Directions and Conclusions
The present study represents an exciting first step toward using ERPs to elucidate the etiology of clinically significant disruptive behavior early in the lifespan. In the present study, we allowed comorbid symptoms to vary in order to measure an ecologically valid group of disruptive preschool children, similar to those typically presenting in clinics (Egger & Angold, 2006). Studies designed to test whether these ERP differences are moderated by specific symptom profiles (e.g., aggression versus defiance; with and without ADHD), or severity levels, may lead to major strides in our understanding of the development of psychopathology. In addition, given ERPs like the Pe and Go/No-go N2 are theorized to relate to multiple functions (Overbeek et al., 2005; Folstein & Van Petten, 2008), studies employing different paradigms may be able to clarify the precise meaning of the abnormal Pe and Go/No-go N2 observed in preschool children with high disruptive behavior. Relatedly, ERPs may represent immediate neural events in unfolding and complex cascading processes that eventually include higher order skills and processes (Hajcak et al., 2007; Zelazo & Cunningham, 2007). Follow-up multi-modal work, such as linking ERPs to other physiological measures and overt behaviors, may explicate how brain activation at the onset of a self-regulation challenge triggers processes at different levels of analysis, resulting in maladaptive behaviors like defiance and aggression. Thus, identifying specific self-regulation-related ERPs that are biomarkers of early disruptive behaviors represents a potentially novel avenue for investigating how psychopathology emerges across development.
Acknowledgments
This research was funded by intramural grants administered through the University of Michigan Rackham Graduate School, the University of Michigan Department of Psychology, and the University of Michigan’s Mary Rackham Institute. We would like to thank the children and families that participated in this research study. We would also like to thank the numerous research assistants, in particular Julia Feldman, who made this research possible.
Conflict of Interest: All authors of this manuscript declare no conflict of interest.
Footnotes
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Arbel Y, Donchin E. Parsing the componential structure of post-error ERPs: A principal component analysis of ERPs following errors. Psychophysiology. 2009;46:1179–1189. doi: 10.1111/j.1469-8986.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Banis S, Geerligs L, Lorist MM. Acute stress modulates feedback processing in men and women: differential effects on the feedback-related negativity and theta and beta power. PloS one. 2014;9:e95690. doi: 10.1371/journal.pone.0095690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bismark AW, Hajcak G, Whitworth NM, Allen JJ. The role of outcome expectations in the generation of the feedback-related negativity. Psychophysiology. 2013;50:125–133. doi: 10.1111/j.1469-8986.2012.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrova E, Leong DJ. Tools of the mind. Pearson Columbus; OH: 2007. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bress JN, Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50:610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Bruin K, Wijers A. Inhibition, response mode, and stimulus probability: a comparative event-related potential study. Clinical Neurophysiology. 2002;113:1172–1182. doi: 10.1016/s1388-2457(02)00141-4. [DOI] [PubMed] [Google Scholar]
- Burwell SJ, Malone SM, Iacono WG. One-year developmental stability and covariance among oddball, novelty, go/no-go, and flanker event-related potentials in adolescence: A monozygotic twin study. Psychophysiology. 2016 doi: 10.1111/psyp.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Dennis TA, Brooker RJ, Sippel LM. An ERP study of conflict monitoring in 4–8-year old children: Associations with temperament. Developmental Cognitive Neuroscience. 2011;1:131–140. doi: 10.1016/j.dcn.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Harbin SM, Nienhuis JK, Fitzgerald KD, Gehring WJ, Hanna GL. Increased error-related brain activity in youth with obsessive-compulsive disorder and unaffected siblings. Depression and Anxiety. 2013;30:39–46. doi: 10.1002/da.22035. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Causeur D, Chu MC, Hsieh S, Sheu CF. A factor-adjusted multiple testing procedure for ERP data analysis. Behavior Research Methods. 2012;44(3):635–643. doi: 10.3758/s13428-012-0230-0. [DOI] [PubMed] [Google Scholar]
- Cole PM, Hall SE, Radzioch AM. Emotional dysregulation and the development of serious misconduct. In: Sameroff AJ, Olson SL, editors. Biopsychosocial regulatory processes in the development of childhood behavioral problems. New York: Cambridge University Press; 2009. pp. 186–211. [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Hommer RE, South M, Molfese PJ, Fearon R, Mayes LC. A Developmental Study of the Feedback-Related Negativity From 10–17 Years: Age and Sex Effects for Reward Versus Non-Reward. Developmental Neuropsychology. 2013;38:595–612. doi: 10.1080/87565641.2012.694512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry. 2006;47:313–337. doi: 10.1111/j.1469-7610.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Espinet SD, Anderson JE, Zelazo PD. N2 amplitude as a neural marker of executive function in young children: an ERP study of children who switch versus perseverate on the dimensional change card sort. Developmental Cognitive Neuroscience. 2012;2:S49–S58. doi: 10.1016/j.dcn.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser AS, Evans BE, Greaves-Lord K, Huizink AC, Franken IH. Diminished error-related brain activity as a promising endophenotype for substance-use disorders: evidence from high-risk offspring. Addiction Biology. 2013;18:970–984. doi: 10.1111/adb.12002. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Ferdinand NK, Mecklinger A, Kray J, Gehring WJ. The processing of unexpected positive response outcomes in the mediofrontal cortex. The Journal of Neuroscience. 2012;32:12087–12092. doi: 10.1523/JNEUROSCI.1410-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Liu Y, Orr J, Carp J. The error-related negativity (ERN/Ne) In: Luck SJ, Kappenman ES, editors. Oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 231–291. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Grammer JK, Carrasco M, Gehring WJ, Morrison FJ. Age-related changes in error processing in young children: A school-based investigation. Developmental Cognitive Neuroscience. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology. 2007;44:905–912. doi: 10.1111/j.1469-8986.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM. Developmental endophenotypes: Indexing genetic risk for substance abuse with the P300 brain event-related potential. Child Development Perspectives. 2011;5:239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Keenan K, Boeldt D, Chen D, Coyne C, Donald R, Duax J, … Danis B. Predictive validity of DSM-IV oppositional defiant and conduct disorders in clinically referred preschoolers. Journal of Child Psychology and Psychiatry. 2011;52:47–55. doi: 10.1111/j.1469-7610.2010.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Grammer JK, Marulis LM, Carrasco M, Morrison FJ, Gehring WJ. Early math and reading achievement are associated with the error positivity. Developmental Cognitive Neuroscience. 2016 doi: 10.1016/j.dcn.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Goodman SH, Waldman ID, Bird H, Canino G, Jensen P, … Applegate B. Relation of age of onset to the type and severity of child and adolescent conduct problems. Journal of Abnormal Child Psychology. 1999;27:247–260. doi: 10.1023/a:1022661224769. [DOI] [PubMed] [Google Scholar]
- Loo SK, Lenartowicz A, Makeig S. Research review: Use of EEG biomarkers in child psychiatry research–current state and future directions. Journal of Child Psychology and Psychiatry. 2016;57:4–17. doi: 10.1111/jcpp.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge: MIT press; 2014. [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Mai X, Tardif T, Doan SN, Liu C, Gehring WJ, Luo YJ. Brain activity elicited by positive and negative feedback in preschool-aged children. PloS one. 2011;6:e18774. doi: 10.1371/journal.pone.0018774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Gremillion M, Roberts B, von Eye A, Nigg JT. The structure of childhood disruptive behaviors. Psychological Assessment. 2010;22:816. doi: 10.1037/a0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J, Henderson H, Degnan K, Fox N. Behavioral inhibition and inhibitory control: Independent and interactive effects on socio-emotional behavior in young children 2014 [Google Scholar]
- Meyer A, Bress JN, Proudfit GH. Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology. 2014;51:602–610. doi: 10.1111/psyp.12208. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Lifetime prevalence, correlates, and persistence of oppositional defiant disorder: results from the National Comorbidity Survey Replication. Journal of Child Psychology and Psychiatry. 2007;48:703–713. doi: 10.1111/j.1469-7610.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- Noordermeer SD, Luman M, Oosterlaan J. A Systematic Review and Meta-analysis of Neuroimaging in Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) Taking Attention-Deficit Hyperactivity Disorder (ADHD) Into Account. Neuropsychology Eeview. 2016;26:44–72. doi: 10.1007/s11065-015-9315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SL, Tardif TZ, Miller A, Felt B, Grabell AS, Kessler D, … Hirabayashi H. Inhibitory control and harsh discipline as predictors of externalizing problems in young children: a comparative study of US, Chinese, and Japanese preschoolers. Journal of Abnormal Child Psychology. 2011;39:1163–1175. doi: 10.1007/s10802-011-9531-5. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek TJ, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponitz CC, McClelland MM, Matthews J, Morrison FJ. A structured observation of behavioral self-regulation and its contribution to kindergarten outcomes. Developmental Psychology. 2009;45:605–619. doi: 10.1037/a0015365. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Roos LE, Pears K, Bruce J, Kim HK, Fisher PA. Impulsivity and the association between the feedback-related negativity and performance on an inhibitory control task in young at-risk children. Psychophysiology. 2014:704–713. doi: 10.1111/psyp.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Gilliom M, Ingoldsby EM, Nagin DS. Trajectories leading to school-age conduct problems. Developmental Psychology. 2003;39:189–200. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW. Self-regulation in ADHD: The role of error processing. Clinical Psychology Review. 2010;30:951–961. doi: 10.1016/j.cpr.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieben J, Lewis MD, Granic I, Zelazo PD, Segalowitz S, Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455–480. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Torstveit M, Sells VT, Fjell AM. Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Developmental Cognitive Neuroscience. 2013;6:1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Koole SL, Wijers AA. The Pe of perfectionism: Concern over mistakes predicts the amplitude of a late frontal error positivity. Journal of Psychophysiology. 2013;27:84–94. [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. The American Statistician 2016 [Google Scholar]
- Wechsler D. Technical and interpretative manual: WPPSI-IV. NY: Pearson Inc; 2012. [Google Scholar]
- Wessel JR, Danielmeier C, Ullsperger M. Error awareness revisited: accumulation of multimodal evidence from central and autonomic nervous systems. Journal of Cognitive Neuroscience. 2011;23:3021–3036. doi: 10.1162/jocn.2011.21635. [DOI] [PubMed] [Google Scholar]
- Wiersema R, Van Der Meere J, Roeyers H, Van C, Baeyens D. Event rate and event-related potentials in ADHD. Journal of Child Psychology and Psychiatry. 2006;47:560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- Willoughby M, Kupersmidt J, Voegler-Lee M, Bryant D. Contributions of hot and cool self-regulation to preschool disruptive behavior and academic achievement. Developmental Neuropsychology. 2011;36:162–180. doi: 10.1080/87565641.2010.549980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Cunningham WA. Executive function: Mechanisms underlying emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 135–158. [Google Scholar]