Abstract
Acoustic startle response (ASR) modification procedures, especially prepulse inhibition (PPI), are increasingly used as behavioral measures of auditory processing and sensorimotor gating in rodents due to their perceived ease of implementation and short testing times. In practice, ASR and PPI procedures are extremely variable across animals, experimental setups, and studies, and the interpretation of results is subject to numerous caveats and confounding influences. We review considerations for modification of the ASR using acoustic stimuli, and we compare the sensitivity of PPI procedures to more traditional operant psychoacoustic techniques. We also discuss non-auditory variables that must be considered. We conclude that ASR and PPI measures cannot substitute for traditional operant techniques due to their low sensitivity. Additionally, a substantial amount of pilot testing must be performed to properly optimize an ASR modification experiment, negating any time benefit over operant conditioning. Nevertheless, there are some circumstances where ASR measures may be the only option for assessing auditory behavior, such as when testing mouse strains with early-onset hearing loss or learning impairments.
Keywords: acoustic startle, prepulse inhibition, prepulse facilitation, animal psychoacoustics, operant conditioning
1. Introduction
The acoustic startle response (ASR) consists of a rapid contraction of facial and skeletal muscles in mammalian and non-mammalian species in response to an abrupt, intense acoustic stimulus. The magnitude of the ASR can be modulated by a number of background and prestimulus conditions, as well as by fear conditioning, attentional modulation, and behavioral state (Koch 1999; Li et al. 2009). Commonly used to screen for sensorimotor gating deficits and drug effects in models of neuropsychological disorders (Swerdlow et al. 2001, 2016; Geyer et al. 2002) and to evaluate the effects of genes on sensorimotor behavior (Plappert and Pilz 2001), ASR modification procedures have gained popularity as behavioral measures of hearing function in rodents due to their perceived ease of implementation. For instance, studies have identified temporal, spatial, and hearing in noise processing deficits in mutant strains (Lauer and May 2011; Jalabi et al. 2014; Truong et al. 2014; Altschuler et al. 2015; Karcz et al. 2015; Tziridis et al. 2016; Ison et al. 2017), abnormal responsivity after chronic or acute noise exposure (Lauer and May 2011; Hickox and Liberman 2014; Salloum et al. 2014; Longenecker et al. 2016), abnormal reactivity to sounds in mouse models of early-onset hereditary hearing loss, fragile X syndrome, and Alzheimer’s disease (Chen and Toth 2001; McGuire et al. 2015; O’Leary et al. 2017), auditory processing deficits in mild traumatic brain injury (Amanipour et al. 2016), and hormonal effects on auditory processing (Charitidi et al. 2012). Other studies have used ASR modification procedures as behavioral readouts during physiological manipulationand development of neurons in the auditory pathway (Weible et al. 2014a, b; Aizenberg et al. 2015; Moyer et al. 2015).
The most common form of ASR modification is prepulse inhibition (PPI), where a brief sound pulse or a change in an ongoing sound that does not itself elicit an ASR is presented prior to a startle-eliciting stimulus (SES). Under some conditions, the prepulse can actually facilitate the ASR, an effect called prepulse facilitation (PPF) or augmentation. Presumably, any measurement of the ASR involves a combination of facilitating and inhibiting processes.
In this review, we discuss the various ways to modify the ASR, including the caveats and interactions of non-auditory factors that should be taken into account whether studying auditory processing or the general neurophysiological underpinnings of sensorimotor gating using acoustic stimuli. We also review comparisons between ASR modification behavioral measures and perceptual measures obtained using traditional operant conditioning techniques. It should be understood that ASR modification may not be a measure of auditory perception, per se. Perception infers the active reception of a signal by an organism and is shaped by attention, experience, motivation, and expectation. An animal is not required to attend to or make decisions about a stimulus in ASR modification tasks, and in fact ASR modification works even in sleeping infants (Hoffman et al. 1985). We do not review the growing literature that uses a gap-PPI paradigm to attempt to measure tinnitus in rodent models. The reader is referred to previous accounts (Hayes et al. 2014, Lobarinas et al. 2014; Galazyuk and Hebert 2015; Brozoski and Bauer 2015) for discussions of gap-PPI tinnitus screening.
2. Acoustic startle circuits
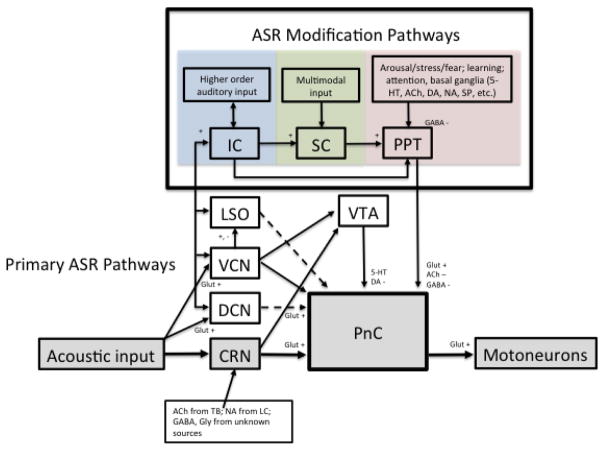
The primary ASR circuit and its known modulatory inputs are summarized in Figure 1. It should be noted that there is some debate about the involvement of various nuclei in the primary ASR pathway, as well as which nuclei are important for producing long- or short-latency effects. Our intent is not to provide an exhaustive review, but rather to highlight the major findings. The reader is referred to Koch and Schnitzler (1997), Koch (1999), Fendt et al. (2001), and Li et al. (2009) for a thorough treatment of the known pathways and discrepancies across studies. The purpose of describing these pathways in the present paper is to highlight the complex routes through which various neural systems can influence the ASR.
Figure 1.
Summary of primary and modulatory acoustic startle response (ASR) circuits with presumed or confirmed primary neurotransmitters. See abbreviation list.
The “fast” acoustic startle circuit begins with cochlear root neurons (CRNs) that are excited by acoustic input from the auditory nerve produced by loud sounds (Lingenhöhl and Friauf 1992; Harrison and Warr 1962; Brown et al. 1988; Osen et al. 1991; Gómez-Nieto et al. 2014). Auditory nerve inputs to the CRNs are organized in a cochleotopic manner, such that basal cochlear regions innervate CRN somata or proximal dendrites, and middle and apical cochlear regions sparsely innervate CRN dendrites (Brown et al. 1988; López et al. 1993; Gómez-Nieto et al. 2014). This innervation pattern indicates that high frequency cochlear regions dominate the auditory input to CRNs. CRNs also receive numerous synaptic inputs whose morphology indicates non-cochlear origins (López et al. 1993; Merchán et al 1988). The CRNs send converging excitatory inputs to the caudal pontine reticular nucleus (PnC) that in turn activates motoneurons (Nodal and López 2003; Szabo and Hazafi 1965; Hammond 1973; Leitner et al. 1980; Davis et al. 1982; Koch et al. 1992; Krase et al. 1993; Wu et al. 1988; Lingenhöhl and Friauf 1992). The ASR may also be elicited by acoustic input to the ventral and dorsal cochlear nuclei (VCN and DCN, respectively) that project to the PnC, and by the lateral superior olive (LSO) via the VCN (Davis et al. 1982; Kandler and Herbert 1991; Lingenhöhl and Friauf 1992, 1994). Both the VCN and CRNs project to the ventral tegmental area (VTA) that also projects to the PnC (Yeomans and Frankland 1996; Kandler and Herbert 1991). Thus, activation of the VTA may directly influence the ASR (Spiera & Davis 1988; Yeomans and Frankland 1996; but see Lee at al. 1996; Miserendino and Davis 1993). Activation of the PnC neurons occurs via fast glutamatergic synapses (Schmid et al. 2003; Steidl et al. 2004).
The ASR amplitude can be modulated by preceding or background sounds, stimuli from other sensory modalities, and behavioral state. These pathways are complex, and almost certainly there are connections to the primary ASR circuit and within the modulating pathways that have yet to be discovered. Only a general accounting of these pathways will be described in this review.
Acoustic prestimuli are the most relevant modulators for the purposes of most auditory neuroscientists. In addition to direct contributions of caudal auditory brainstem nuclei to the ASR, complex ascending and descending auditory pathways in the brainstem, midbrain, and cortex process various aspects of acoustic prestimuli in ways that must influence the ASR. Again, these connections are simplified in Figure 1. The pathways involved in sensory processing of a given prestimulus must depend on stimulus parameters such as frequency spectrum, amplitude modulation, intensity, and time between the prestimulus and the startle-eliciting stimulus (SES). The exact mechanisms producing the inhibitory effects on the ASR, termed prepulse inhibition (PPI), are not completely understood. Acoustic prepulses inhibit activity in the mouse PnC (Carlson and Willott 1998). The PPI behavioral process requires the inferior colliculus (IC) (Leitner and Cohen 1985). The IC inputs may in turn target the superior colliculus (SC) before being relayed to the PPT (Koch and Schnitzler 1997), or may bypass the SC altogether. Inhibition of the PnC may occur through activation of an inhibitory cholinergic pathway from the PPT to the PnC (Bosch and Schmid 2006, 2008). Muscarinic nicotinic acetylcholine receptors appear to produce the PPI effects for interstimulus intervals of 100 to 1000ms (Fendt and Koch 1999; Jones and Shannon 2000a,b; Ukai et al. 2004; Yeomans et al. 2006). There is also evidence for GABAergic inhibition of PnC in response to prestimuli (Koch et al. 2000; Yeomans et al. 2010).
Non-acoustic stimuli can inhibit the ASR as well. Multimodal (e.g. visual, tacile) inputs may contribute to ASR modification via pathways connecting to the SC, which in turn projects to the PnC via the PPT (Koch and Schnitzler 1997; Yeomans et al. 2002). Lesions of the SC induce PPI deficits of about 45% (Fendt et al. 1994; Fendt and Yeomans 2001). The PnC also receives inputs from stress, arousal, fear, attention, and learning pathways via the PPT. These pathways are quite complex and involve numerous neurotransmitter and neuropeptide systems in the basal ganglia, prefrontal cortex, hippocampus, amygdala, nucleus accumbens, locus coeruleus, and other areas (Koch and Schnitzler 1997). The effects of behavioral state, learning, and attention are briefly reviewed in a later section of this review.
Descending inputs may also directly modulate CRNs. Bilateral cholinergic projections from the trapezoid body area to the CRNs have been identified in rats (Gómez-Nieto 2008a). CRN dendrites also receive GABAergic and glycinergic inputs of unknown origin and noradrenergic inputs from the locus coeruleus (Osen et al. 1990; Gómez-Nieto et al. 2008b; Hormigo et al. 2014). In addition to anatomical evidence for modulatory inputs from multiple sources, there is physiological evidence that short-latency (<20ms) PPI may occur at the level of the CRN (Gómez-Nieto et al. chapter & 2013).
The take-away message from the ASR and PPI circuit complexities is that there are many pathways for affecting the response. Care should be taken when screening mutants with affected brain areas and neurotransmitter systems. Exposure to conditions that affect stress, alertness, and other aspects of behavioral state may also produce profound and potentially unwanted effects on the ASR and PPI. What appears superficially to be a simple behavioral task is in fact quite complex in terms of the underlying neurocircuitry and number of potential modulatory influences, both in the context of auditory processing and general sensorimotor gating studies. It is advisable to screen animals for hearing loss using auditory brainstem response audiometry (Henry 1979; Parhap et al. 2001) prior to implementation of behavioral testing that involves acoustic stimuli, whether the purpose of the experiment is to assess auditory processing or sensorimotor gating.
3. Typical acoustic startle modification experiments
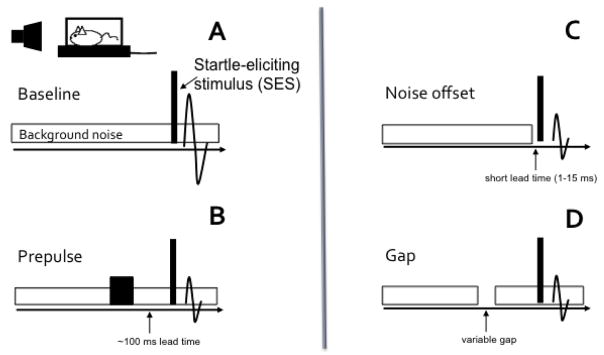
Basic ASR modification procedures have been reviewed in detail elsewhere (Hoffman and Ison 1980; Ison and Hoffman 1983; Ison 2001; Carlson and Willott 2001). The most commonly used form of ASR modification tests is the PPI using brief tones pulses or noise-bursts presented approximately 100ms prior to an SES (Figure 2A, 2B). This type of test is performed in quiet or in background noise. The SES is typically a brief, loud broadband noise with a rapid onset and offset, but tones have also been used to elicit startle. The prepulse is typically a short tone or noise presented at a level that is below the ASR-eliciting threshold (Figure 2B). In addition, the possibility exists that an animal’s responsiveness to different SES frequencies may change with age (Willott and Carlson 1995; Ison et al. 2007). Common variations on the traditional prepulse stimulus paradigm are depicted in Figure 2C–2D. The effects of a silent gap produced by an abrupt noise offset at very short lead times (~1–15ms) or a noise offset and onset presented at longer lead times (~50–100ms) have been used to assess temporal processing in mice (Ison et al. 1998; Ison and Allen 2003).
Figure 2.
Typical ASR modification stimulus paradigms. A) Background sounds such as noise can potentiate or inhibit the response to a startle-eliciting stimulus (SES), depending on the background sound level. B) Traditional experiments present noise or tone non-startling prepulses ~2–100 ms before a loud, brief SES. Short lead times (<20 ms) between the prepulse and SES result in facilitation of the ASR. Longer lead times inhibit the ASR. These stimuli are often presented in the presence of low level background noise to mask extraneous sounds. C) Offsets and onsets of otherwise continuous background noise can be used to assess temporal processing. The offset-only conditions are typically presented with very short lead times between the noise offset and the SES to inhibit the ASR. D) Silent gaps that include a noise offset followed by an onset are typically presented at longer lead times. The amount of gap-induced inhibition of the ASR varies the duration of the gap, the decay time, the noise bandwidth, and the lead time between the gap and the SES.
The ASR amplitude (e.g. as determined from peak-to-peak or RMS measurements of the waveform produced by a force transducer) in prepulse conditions relative to baseline conditions without a prepulse is typically reported rather than raw ASR amplitudes to account for potentially large inter-individual variability in absolute ASR amplitude. ASR amplitude measured with a force transducer, for example, varies with the weight of the animal subject. However, the effect of the weight can be reduced by normalizing for an individual’s mass as is possible by ballistic modeling (Grimsley et al. 2015). The reduction of the ASR amplitude due to the prepulse may be directly represented by the ASR amplitude (Ison et al. 1997) or expressed as percent inhibition relative to the SES alone response (Paylor and Crawley 1997), more general as the proportion of the baseline (Willott et al. 2003), or by some form of response value derived from signal-detection theory (d’, the standardized effect size measure Cohen’s d, e.g., Ison et al. 2005, or the area A’ under the ROC curve and the da value derived from this area, e.g., Behrens and Klump 2015). Average ASR values for specific stimulus conditions either were calculated on the basis of response waveforms in all recorded startle trials or from a selection of the recorded wavefoms that aims at excluding trials with movement artifacts (e.g., see Grimsley et al. 2015). Different threshold criteria have been applied. Some studies have used a fixed reduction of the ASR (e.g., 20% PPI) as the threshold criterion, whereas others have applied criteria being related to the mean and the variance of the ASR (e.g., Longenecker et al. 2016–95% confidence interval around the mean; Allen and Ison 2010 statistical significant difference [p<0.05] between the mean ASR with and without a prepulse). A much more conservative threshold criterion that allows a comparison of the results of different procedures relates to d’. A threshold defined as the value for the prepulse feature that corresponds to a d’ of 1.0 for the ASR difference from baseline usually results in higher thresholds than thresholds derived from statistical significance (Behrens and Klump 2015).
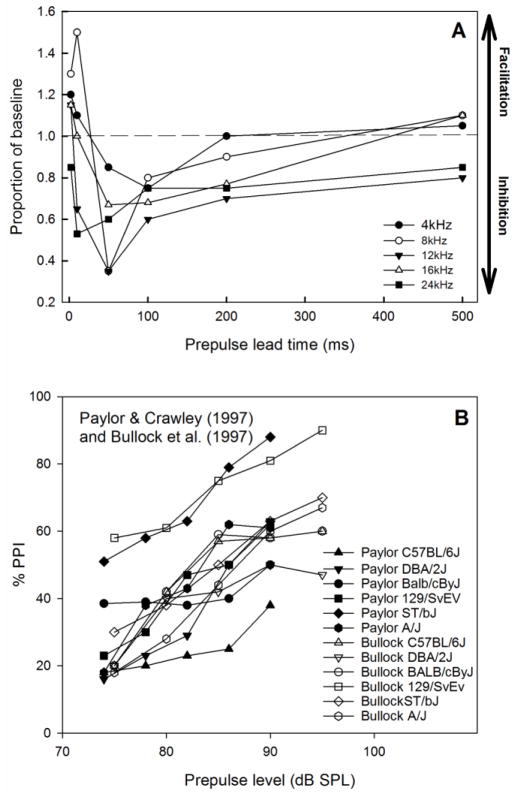
The frequency of the prepulse and the lead time between the prepulse and SES can have major effects on the amount of PPI observed (Figure 3A). At very short lead times, prepulse facilitation (PPF) may be observed, such that the ASR amplitude is actually larger in prepulse compared to baseline conditions. The level of the prepulse also affects the amount of PPI, such that increasing prepulse stimulus intensity results in greater inhibition (Figure 3B); however, the amount of PPI increase varies across strains and studies (e.g., Paylor and Crawley 1997; Bullock et al. 1997).
Figure 3.
Lead time, frequency, and level effects on ASR. A) Tone prepulses presented at very short lead times (<10ms) produce prepulse facilitation (PPF). Longer lead times (50–200ms) produce prepulse inhibition (PPI). Lead times of >200ms produce mixed effects. Data replotted from Willott and Carlson (1995), CBA/CaJ strain. B) Effects of prepulse level on PPI. The amount of PPI increases as prepulse level increases, shown here for strains tested using SR-lab systems and similar stimulus conditions in two reports. Data replotted from Paylor and Crawley (1997) and Bullock et al. (1997).
Systematic evaluations of lead time, frequency, and level effects on PPI are only available for a few strains. The frequency difference between the prepulse and background sound may also affect PPI strength, with increased PPI observed for larger frequency differences (Basavaraj and Yan 2012). The presence of, level, and frequency of a background sound also differentially affect the ASR amplitude (Ison 2001; Carlson and Willott 2001), and there may be complex interactions between background, prepulse, and SES characteristics. These interactions likely vary with strain and age (Carlson and Willott 2001). It is important to take these factors into consideration when interpreting PPI differences between control and experimental groups. A substantial amount of pilot testing may be required to properly optimize an experiment to produce maximum ASR modification.
4. Strain, sex, age, and developmental effects
Differences in ASR and ASR modification have been reported to vary with strain, age, development, and sex. Possible interactions of these factors must be considered when designing and interpreting ASR modification experiments. The effects of these factors on modification of the ASR also provide interesting opportunities for investigation.
4.1 Strain
Considerable strain differences in ASR and PPI effects have been reported in the literature. Most studies comparing ASR and PPI in different strains use a traditional tone or noise prepulse stimulus paradigm with a limited number of conditions. In general, the strength of PPI and relative ASR amplitude varies substantially across studies. This is not surprising given the variety of equipment, stimulus parameters, housing environments, handling procedures, ages, sex ratio of males and females), and other factors that vary across different laboratories. Figure 4A depicts correlations in percent PPI between 7 mouse strains tested in two different laboratories using different experimental apparatuses and stimulus conditions (Willott et al. 1994; Paylor and Crawley 1997). The relationship is very weak, suggesting that specific patterns of strain differences may be particular to a study. Figure 4B shows that a weak relationship in percent PPI also occurs between two studies testing 6 strains using similar apparatuses and stimulus parameters (Paylor and Crawley 1997; Bullock et al. 1997). PPI data from two identical strains tested in two different laboratories using similar apparatuses and stimulus parameters show stronger correlations are shown in Figures 4C (C57BL6) and 4D (DBA/2J). The responses in DBA/2J mice are more similar in the two studies than the data from the C57BL6, but the reasons for the discrepancies are unknown. Strain differences in ASR and PPI may also interact with age. Age-related strain differences in ASR and PPI are discussed in the section 4.3 on Age & Development.
Figure 4.
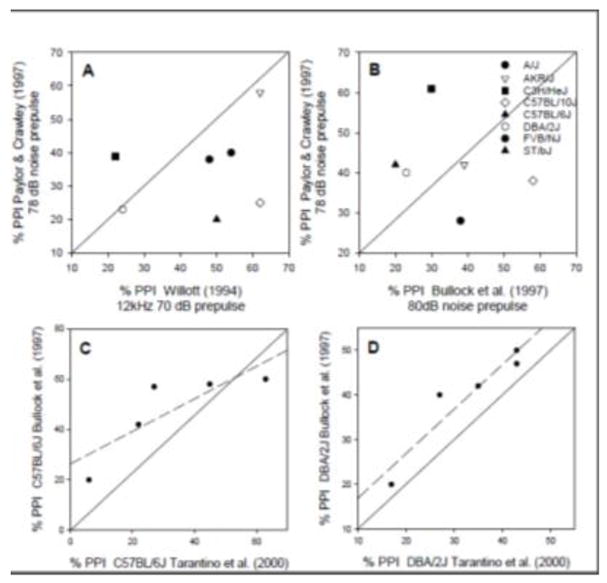
Strain differences in PPI across laboratories. (A) Comparison of seven strains tested using different experimental conditions and apparatuses in two laboratories. (B) Comparison of six strains tested using somewhat similar stimulus conditions and similar apparatuses in two laboratories. Symbols are the same in 4 A and B. (C) Comparison of PPI in C57BL/6J mice tested using very similar stimulus conditions and apparatuses in two laboratories. (D) Comparison of PPI in DBA/2J mice tested using very similar stimulus conditions and apparatuses in two laboratories. Data replotted from: A-Willott et al. (1994), A, B-Paylor and Crawley 1997, B, C, D-Bullock et al. (1997), C, D Tarantino et al. (2000).
Despite the variability across studies, some consistent trends emerge (Bullock et al. 1997; Paylor and Crawley 1997; Tarantino et al. 2000; Willott et al. 2003). ASR amplitudes increase with increasing stimulus intensity, and most strains do not show an ASR for stimuli below 80–90 dB SPL. DBA/2J mice show very small maximum ASR amplitudes, whereas BALBcByJ and BUB/BnJ mice show very large ASRs. C57BL/6J mice tend to have intermediate ASR strength. The DBA/2J and C57BL/10J strains show relatively weak maximum PPI, whereas 129/SvEv mice show strong PPI. ASR and PPI strength in other strains, including the “normal-hearing” CBA strains, tends to vary from study to study (Bullock et al. 1997; Paylor and Crawley 1997; Tarantino et al. 2000; Willott et al. 2003). It is possible that these strains are more susceptible to extra-experimental effects such as housing environment and inadvertent fear potentiation (see section 5).
A handful of studies have attempted to use PPI strain differences to investigate the genetic basis for sensorimotor gating. Considering the large number of neuronal circuits that modulate the ASR, it is not surprising that susceptibility to PPI is a polygenic trait (Geyer et al. 2002). The majority of studies have focused on mutant strains with relevance to neuropsychiatric disorders (Powell et al. 2011), and the genetic basis for differences in PPI strength in the absence of a neuropsychiatric phenotype are still essentially unknown. Several studies have identified quantitative trait loci that are thought to be related to PPI strength in mice. These include areas on chromosomes 2, 3, 4, 5, 7, 10, 11, 12, 16, and 17 (Joober et al. 2002; Petryshen et al. 2005; Leussis et al. 2009; Samocha et al. 2010; Loos et al. 2012). Takata et al. (2010) found that Uqcr10 and Nipsnap1 located on chromosome11qA1 are candidate genes for PPI. Large-scale efforts to describe the effects of genetic mutations on phenotypes in mice such as the International Mouse Phenotyping Consortium and the Knockout Mouse Project will yield additional data on the genetics of ASR and PPI. One caveat is that hearing status may interact with PPI in mutant strains.
4.2 Sex
Effects of sex and estrous cycle on ASR and PPI have been reviewed in detail by Ison and Allen (2007). Males typically show larger ASRs, but these effects may be due to differences in weight or muscle strength (Plappert et al. 2005). Sex effects on PPI in mice are often reported to be non-significant in most strains (Willott et al. 2003; Logue et al. 1997; Tarantino et al. 2000). The exceptions typically occur when large sample sizes are used. Ison and Allen (2007) reported that males showed increased PPI by gaps in broadband noise compared to pre-menopausal, but not post-menopausal females. Similarly, Ralph et al. (2001) found increased PPI by noise prepulses in young adult males compared to females. These results are similar to what has been found for humans and rats (Lehmann et al. 1999; Swerdlow et al. 1993). In contrast to other studies, Willott et al. (2003) showed stronger PPI and ASR in females of some strains. Discrepancies across studies and the failure to find significant effects may be attributable to age, strain, and stimulus interactions as well as sample sizes and extraneous influences.
The effects of estrous cycle on ASR and PPI in mice are similarly ambiguous. Plappert et al. (2005) found no effect of estrous stage on PPI measured in two strains of mice. Similarly, Adams et al. (2008) found no effect of estrous cycle on PPI in rats. However, studies in rats and humans have found varying PPI during different estrous or menstrual cycle phases (Koch 1998; Swerdlow et al. 1999). Artificial manipulation of estrogen levels has significant effects on PPI in mice (Charitidi et al. 2012), so it remains possible that future studies using larger sample sizes and more strains might identify differences in PPI over the course of the estrous cycle in mice.
4.3 Age and development
The age at which ASR and PPI testing occurs is an extremely important variable to consider in addition to the strain, sex, and experimental history of the animals to be tested. Very little is known about the pre-weaning development of the ASR and PPI in mice, but one study showed that the frequency of responses to higher frequency and lower intensity stimuli increases from postnatal days 12 to 17 in C57BL/6J mice (Shnerson and Willott 1980). The strength of PPI increases over a similar time period in rats (Parisi and Ison 1979). The ASR amplitude shows further increases in some mouse strains between 1 and 2–6 months of age and then decreases with age as depicted in Figure 5 (Ison et al. 1997; Ison et al. 1998; Willott and Turner 1999; Yun et al. 2006). Age-related changes in individual baseline ASR amplitudes are not correlated with PPI strength in CBA/J mice tested up to 8 months of age (Ison et al. 1997), but the relationship has not been directly measured in other strains. CBA/CaJ and CBA/CaJxC57BL/6J hybrids maintain strong PPI to noise offsets despite decreasing ASR amplitudes after 6 months of age. Age-related change in PPI strength measured in several studies is summarized in Figure 6.
Figure 5.
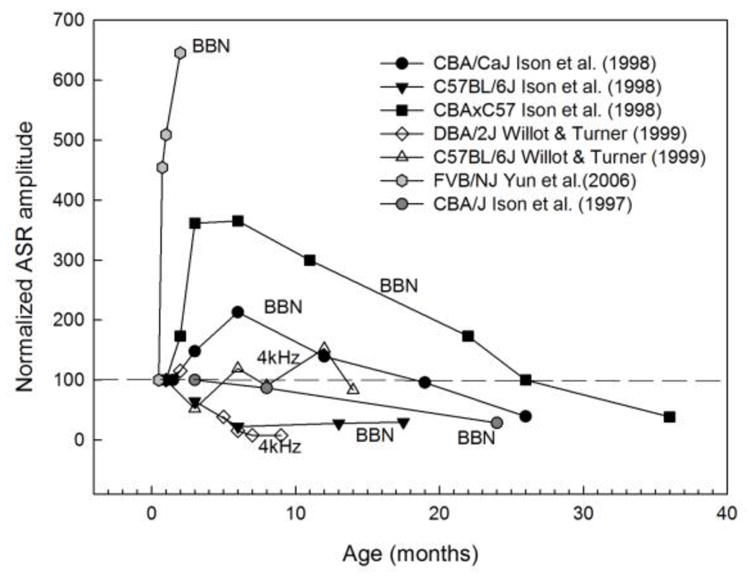
Age effects on the ASR amplitude in different strains. The startle-eliciting stimulus varied across studies and are denoted for each curve. ASR is normalized to baseline at 1 month of age because different units were reported across studies. Data replotted from: Ison et al. (1998), Willott and Turner (1999), Yun et al. (2006), Ison et al. (1997).
Figure 6.
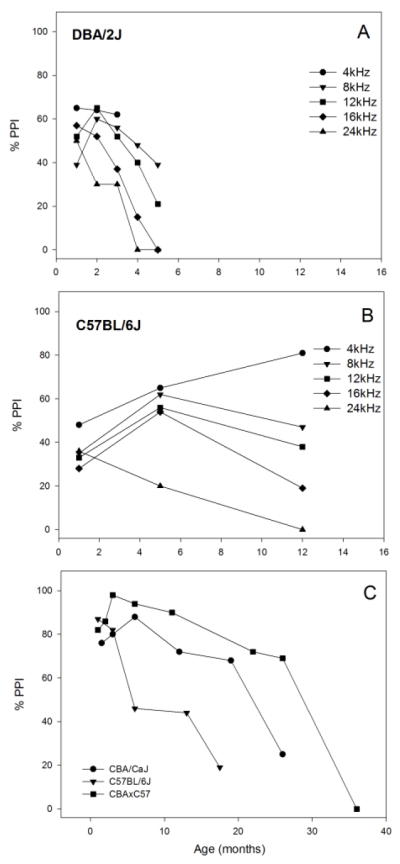
Tone PPI changes differently and depends on prepulse frequency with age in two strains of mice with early-onset age-related hearing loss: A) DBA/2J, and B) C57BL/6J. C) Noise offset PPI also changes differently in three strains with different progressions of age-related hearing loss. Data replotted from Willott et al. (1994) and Ison et al. (1998).
DBA/2J mice with early-onset cochlear degeneration show decreasing ASRs in response to 4 kHz tone stimuli from one month of age onward (Willott et al. 1984; Willott and Turner 1999). PPI strength generally declines after 1 month of age in DBA/2J mice, but sometimes shows a modest increase at 8 and 12 kHz between 1 and 2 months of age as depicted in Figure 6A (Willott et al. 1994; Turner and Willott 1998; Willott and Turner 1999). The rapid decrease in both ASR and PPI strength in DBA/2J mice are most likely explained by the rapid progressive hearing loss that renders the ASR and PPI stimuli less audible in this strain (Ralls 1967; Erway et al. 1993; McGuire et al. 2015).
Age-related changes in PPI strength depend on the prestimulus conditions in C57BL/6J mice. Tone pulse PPI remains robust except for high frequencies (Figure 6B) (Willott et al. 1994; Willott and Turner 1999), but noise-offset PPI declines rapidly after one month of age in this strain (Figure 6C) (Ison et al. 1998). CBA/CaJ and CBAxC57 hybrid mice also show age-related decline in noise offset PPI, but at different timecourses (Figure 6C) (Ison et al. 1998). Presumably, the accelerated age-related reduction in PPI after noise offset is related to the reduced audibility of the high frequency components of the noise due to high frequency hearing loss (Ison and Allen 2007; Mikaelian 1979). C57BL/6J mice develop increased ASR amplitudes for low frequency tone stimuli at about 6 months of age. This effect has been interpreted as a behavioral correlate of central auditory hyperactivity (Ison and Allen 2007). Thus, tone prepulses may remain salient for some time despite the onset of hearing loss in this strain.
5. Non-associative and associative learning effects
PPI studies require that responses need to be recorded in a relatively large number of trials (usually more than 100 trials, often a number of sessions with 100 trials each are combined in data collection). Such an extended testing requires taking into account the experience-dependent changes of the ASR and PPI over time, e.g., by presenting the different prepulses in random sequence in blocks including all conditions and repeating these blocks. The potential effects of learning should be considered when interpreting the results of ASR and PPI studies. Additionally, variables affecting learning in ASR and PPI tests may be of interest to the experimenter.
Both non-associative learning effects such as habituation and sensitization and associative effects such as fear potentiation have been observed. Habituation can have two effects. First, the ASR response can decrease with repeated presentation of the startle stimulus within a session and in sequential sessions, as shown in Figure 7A and B (Bullock et al. 1997; Pilz et al. 2004; Plappert and Pilz 2005; Simons-Weidenmaier et al. 2006). Often, the first block of trials in a session is discarded since during that initial time period in a session the most habituation is observed. There are several caveats to the habituation phenomenon. The BALB/C strain has also been reported to show short term sensitization (reported as averages of 5-trial blocks; Plappert and Pilz 2002). Both long- and short-term habituation of the ASR can depend on age and sex interactions (Plappert et al. 2005). Additionally, some strains show sensitization of the ASR with repeated testing in sequential sessions (Typlt et al. 2013a; Plappert and Pilz 2002).
Figure 7.
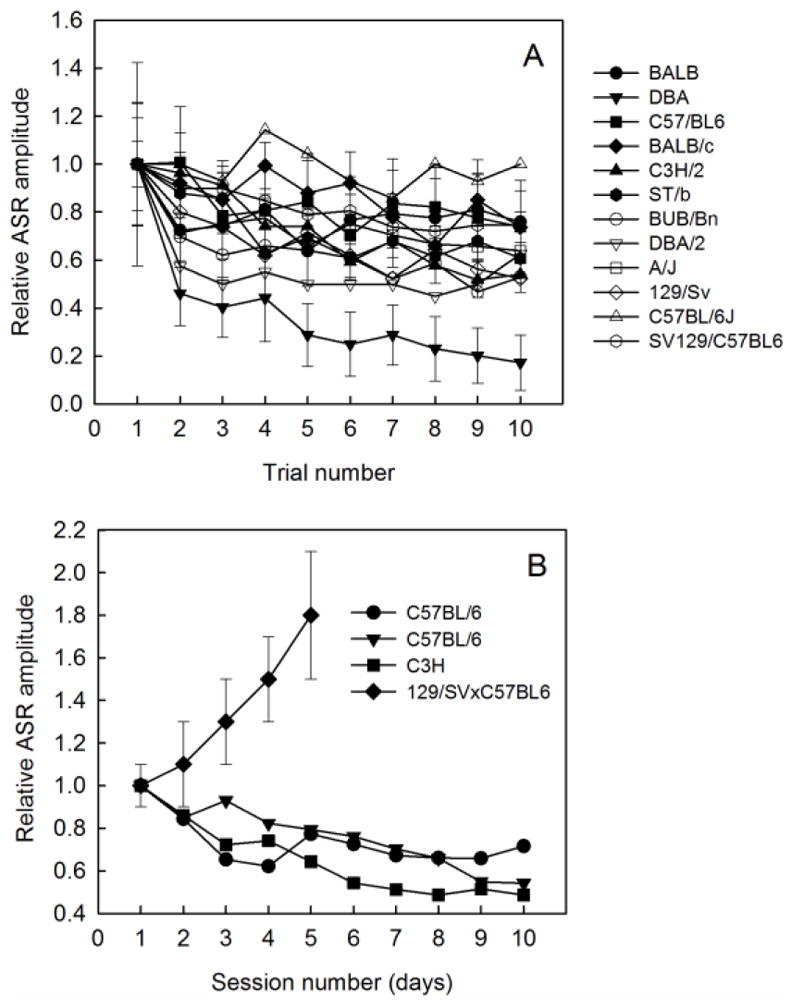
A) Short term habituation across the first ten trials within a test session varies across strains. Data from Bullock et al. 1997; Pilz et al. 2004; Simons-Weidenmaier 2006; Typlt et al. 2013a. B) Long term habituation across test days also varies with strain. Data from Plappert and Pilz (2005); Pilz et al. (2004); Typlt et al. 2013b.
The PPI response shows a different pattern. With continuing habituation of the ASR the PPI response in mice has shown to be enlarged, and the PPI increases over a series of sessions when testing the same individual mice (Plappert et al. 2006; Typlt et al. 2013b). However, PPI induced by gaps in noise increases with experience in rats, and some individual animals actually show PPF in response to short gaps that shifts to PPI with experience (Friedman et al. 2004; Swetter et al. 2010).
PPF has also been shown to change over time. However, independent processes that hint at associative learning have been attributed to eliciting the change of PPI and PPF over time (Plappert et al. 2006). Interestingly, the amount of gap-induced PPF early in the course of testing predicts stronger PPI later in the course of testing in rats (Swetter et al., 2010), indicating that these processes may not be completely orthogonal. An understanding of the mechanisms underlying PPF and how findings of PPF should be interpreted with respect to auditory processing awaits further investigation.
Another associative learning mechanism that has been demonstrated to modulate the ASR is fear potentiation of the response. If the prepulse is temporally coupled with an aversive stimulus for a number of times, increased ASR amplitude and reduced PPI have been observed (Davis et al. 1993, Falls et al. 1997, Heldt et al. 2000; McCaughran et al. 2000; Dirks et al. 2001; Balogh et al. 2002; Rose et al. 2008; Li et al. 2009). It is a common observation that ASR, PPI, and how the modification of the response changes over time differs between mouse strains and individuals of the same strain (e.g., Bullock et al. 1997; Pilz et al. 2004; Simons-Weidenmaier et al. 2006; Typlt et al. 2013a Yee et al. 2005, and see section 4.1 Strain). In some strains a relatively large change is observed (e.g., C3H, DBA, 129/Svev) while in others it is not so prominent (e.g. C57BL/6). Thus, in addition to randomizing the different prepulse conditions to counteract reduction of the ASR and PPI response over time, studies should also take the genetic background into account if aiming at obtaining sensitive, and reliable perceptual threshold measurements with the PPI method that may require a larger number of trials.
Though fear-potentiated ASR has primarily been used in the study of anxiety (Gerwitz and Radke 2016), the possibility of unintended fear potentiation occurring in auditory processing experiments must be taken into account. Interestingly, unconditioned anxiogenic compounds alone do not increase fear-potentiated ASR, highlighting the importance of the learned association between the SES and the aversive stimulus (Risbrough et al. 2005). Aversive stimuli such as handling, restraint, injections, and other anxiogenic stimuli may come to be associated with the ASR stimuli during routine testing (Longenencker and Galazyuk 2012).
6. Other considerations
There are several other important factors to consider when designing and interpreting ASR and PPI experiments, such as whether the sound is presented to one or both ears, housing environment, and handling and husbandry effects. On one hand, manipulation of these factors may provide important information about auditory processing. On the other hand, lack of control or reporting of these factors may introduce unintended effects into a study.
6.1 Monaural vs. binaural stimulation
Counterintuitively, prepulses presented monaurally produce stronger PPI than binaural prepulses of equal level in humans, possibly due to spatial attention factors (Marsh et al. 1976; Hoffman and Stitt 1980; Hackley and Graham 1987; Kumari et al. 2015). However, binaural presentation of prepulses where the level of the stimulus presented to one ear is substantially greater (~40dB) than the level presented to the other ear reduces the monaural advantage in humans (Hoffman and Stitt 1980). Separating binaural stimuli in time up to about 15 ms also reduces the monaural advantage (Ison and Pinckney 1990). It should be noted that these experiments were performed using a cutaneous stimulus to elicit an eyeblink reflex, a non-auditory stimulus, and these effects have not been explored in rodents. Reduced ASR amplitudes after monaural acoustic trauma have been demonstrated in both rats and mice, and unilateral conductive hearing loss produced similar effects (Lobarinas et al. 2013; Longenecker et al. 2016; Karsz et al. 2015). However, different patterns of startle behavior were observed with a tactile startle-eliciting stimulus (Lobarinas et al. 2013). The binaural firing rate of PnC giant neurons was found to be greater for binaural than for monaural conditions (Gómez-Nieto et al. 2014).
6.2 Housing environment, handling, and socialization effects
Many environmental and experiential variables outside of the PPI testing context have the potential to affect ASR and PPI. Variables such as maternal separation, rearing, and other social effects may influence the expression of ASR and PPI (Francis et al. 2003; Millstein et al. 2006; Rose et al. 2008). Social isolation produces PPI deficits in at least four strains of mice (C57BL6/J and 129T2-Varty et al. 2006; ddY-Sakaue et al. 2003; C57BL/6-Pietropaolo et al. 2008; C57BL6/J-Martin and Brown 2010). There also may be circadian effects on ASR and PPI strength, though circadian fluctuations are not consistent across studies (Chabot and Taylor 1991; Frankland and Ralph 1995; Adams et al. 2008). Finally, individually ventilated cage systems can increase ASR amplitude in C3H mice and enhance fear-potentiated startle in C57BL/6J mice, possibly because of stress (Kallnik et al. 2007). Hsbandry and housing variables may affect fear and anxiety levels that alter ASR and PPI reactivity, or they may operate via more subtle neurosensory mechanisms.
Other effects that have not received considerable attention in the literature, but that may nonetheless affect ASR and PPI, include: stress experienced during shipping (Landi et al. 1982); handling by the experimenter and caretakers (Van Boegaert et al. 2005; Hurst and West 2010); ambient noise fluctuations in the housing area (Lauer et al. 2009; Lauer and May 2011); lighting, temperature, humidity, room configuration, and other conditions in the housing and testing environments (Crabbe et al. 1999; Wahlsten et al. 2003; Lewejohann et al. 2006); odors encountered during experimentation (from the test room, experimenter, other animals, etc.; Yang and Crawley 2009); access to and type of enrichment (Würbel 2001). All of these influences may interact with strain, age, and sex, but few systematic studies have been completed in mice. The experimenter is encouraged to consider these variables when designing PPI experiments, and details such as housing conditions and time of day tested should be described in the Methods. Changes to these conditions during the course of a study should be avoided as much as possible. Even when test conditions are standardized across laboratories, there can be substantial differences for some behavioral phenotyping outcomes in mice (Crabbe et al. 1999; Wahlsten et al. 2003; Lewejohann et al. 2006). Additionally, repeated testing of mice as part of a behavioral phenotyping battery can affect task performance (Võikar et al. 2003).
7. Essentials for a well designed PPI experiment
For conducting an acoustic PPI experiment, a number of factors have to be taken into account (for an extended review of technical issues with a focus on tinnitus assessment, see Longenecker and Galazyuk 2012). Many of these factors can be found in published protocols (e.g., Geyer and Swerdlow 1998; Valsamis and Schmid 2011), but most available protocols do not address all of the important parameters to consider when assessment of auditory function is the primary goal of the experiment. The following recommendations are based on the many studies cited in this review and Ison (2001). As is required for any testing of auditory function, the setup must allow a sufficient control of the acoustic parameters. Background noise (e.g., from the apparatus or ventilation in the animal facility) and sound reflecting surfaces in the booth and setup used for testing provide the major obstacles to a sufficient control of the acoustic stimulus parameters. A sound-attenuating booth that diminishes the noise from the room to an overall broadband noise level of less than 30 dB SPL(C) provides for a good reduction of background noise. Sound-absorbing foam with a structured surface and a thickness of about 7–10 cm resulting in an absorption coefficient of 0.95 (i.e., 95% of the sound is absorbed) covering the inside of the booth with a volume of about 0.15 m3 will reduce the reflections from the walls to provide a reverberation time T60 (i.e., the time period over which the sound level in the booth drops by 60 dB when the sound is switched off) of less than 20 ms. Such a short reverberation time is necessary to preserve the temporal structure of the envelope of a prepulse signal being presented to the animal (e.g., if temporal gaps in noise are used as the prepulse) as well as assuring a fast onset and offset of the SES. Absorbing surfaces also insure that the signal broadcast by the loudspeaker is not degraded (e.g., spectrally modified, see Longenecker and Galazyuk 2012) and a correction of the loudspeaker’s transfer function by signal processing algorithms is effective. Spectral composition of the SES may by itself affect the ASR in the mouse, with spectrally simple tones being less potent response elicitors than spectrally rich noise stimuli (e.g., Stoddart et al. 2008). This may relate to the frequency dependent variation of hearing threshold and temporal summation. It is also desirable to design the container holding the animal in a way that makes it acoustically transparent and does not have extended reflecting surfaces, e.g., a wire cage mounted with thin metal rods onto a base plate covered with sound absorbing foam. The loudspeaker, driving amplifier and the sound card of the computer should be suitable for presenting the frequency range of the stimuli. Often, separate loudspeakers are used for presenting the prepulse and the startle stimuli, since the former should be optimized to assure a high fidelity of the broadcast signal and the latter should be suitable to present the brief startle sounds with a sufficiently high sound level.
Two types of transducers are commonly used for determining the ASR. The first type is an accelerometer that is attached to the cage hosting the subject. This type of transducer can readily be calibrated and, due to the calibration, the value of the ASR can be compared across setups (which is important, if experiments are run in parallel in different setups to collect a large sample of results). The disadvantage is the cost, since calibrated accelerometers are expensive and require costly amplifiers to condition the signals for A/D conversion for further processing by a computer. The second type of transducer being commonly used is a piezo transducer on which one of the metal rods of the cage of the animal or a pointed extension of the base plate of the cage rests. An operational amplifier conditions the output of the piezo plate to make it suitable for further processing by the computer. Since this type of device does not rely on calibrated components, an absolute calibration of the piezo device is rather difficult.
However, it often is sufficient to assure the same sensitivity of different setups without knowing the absolute value of the acceleration of the startle cage. Such a relative adjustment of the sensitivity of different setups is possible by mimicking the startle response of an animal by dropping a weight in a reproducible way onto the base of the cage. This relative adjustment allows comparing the results from different setups. Recently, Grimsley et al. (2015) applied a ballistic model to normalize the effects of the weight of different individuals. Often, the output signal from the transducer is band-pass filtered (e.g., Cassella and Davis 1986) to enhance the signal-to-noise ratio in favor of the frequencies representing the movement of the cage. These technical provisions make the measurements of the ASR quite reproducible. However, there are some issues rooted in the animal’s behavior that also must be taken care of to allow for reliable measurement of the ASR. The ASR can be modulated by other activities of the animal or by its level of anxiety. To prevent the animal from engaging in other activities, the cages hosting the subjects are typically made as small as possible (e.g., to not allow a mouse to raise and groom), which may actually stress the animal. Another way to reduce the effects of large body movements on ASR measurements is to monitor the animal’s baseline movement levels and delay trials until the animal is calm, meaning that movement is below a certain criterion level, for a specified period prior to presenting the prepulse and startle stimuli (e.g., Lauer and May 2011; McGuire et al. 2015). Furthermore, subjects are tested for an extended time period and the results from the initial part of the experimental session are discarded to work with a well-habituated subject to reduce the effects of initially large ASR amplitudes (typically, the first 10 ASR responses are discarded, for a general discussion of effects of habituation see section 5). Since PPI is commonly recorded as the relative reduction of the ASR, it can be affected by habituation. Such influences should be counteracted by presenting the different variants of the prepulse in random order with blocks of trials that are repeated throughout the experimental session. Considerable differences have been observed between different mouse strains regarding the time course and persistence of habituation or the occurrence of sensitization (see section 5). Large and persistent habituation or sensitization can limit the number of trials in which data from an individual can be collected (Longenecker and Galazyuk 2012).
For achieving a sound PPI effect, the temporal pattern of the acoustic background and prepulse stimuli and the relative time offset between the presentation of the prepulse and the startle eliciting stimulus must be optimized to produce maximum PPI (see section 3). If the prepulse is presented in a quiet background, the level of excitation that it provides to the cochlea will determine its efficacy rather than other features of the sound. Therefore, a large (e.g., 500 ms) silent interval between background and prepulse stimuli will preclude a sensitive PPI response that is related to cue differences between background and prepulse stimuli. If such cues are to be compared, it is better to present a continuous stream of background stimuli with abutting sounds in which the rare prepulse differing in the cue of interest is embedded (e.g., Behrens and Klump 2016). That way, it is the cue difference rather than the onset of the prepulse after a silent interval that drives the PPI response. Roving of stimulus features such as random level variation has been used in psychoacoustic studies to suppress the relevance of the feature for eliciting the response (e.g., Behrens and Klump 2016). It is not clear how much the roving will affect the PPI response that is due to the stimulus feature of interest that is not roved, and large amounts of roving of stimulus features may result in background stimuli operating as a substitute of a prepulse, thus, reducing the reference startle amplitude being elicited without a prepulse. As is outlined above (section 3), short intervals between prepulse onset and startle stimulus onset (e.g. 10 ms) may lead to PPF rather than PPI and the PPI effect may decay if the startle stimulus is presented after a long time period (e.g., 500 ms). Therefore, in many studies a delay between prepulse and startle stimulus of between 50 and 100 ms has been applied.
There have been conflicting results regarding the optimal ASR strength for maximizing PPI. In some studies (e.g., Sandner and Canal 2007, Plappert et al. 2004), it is suggested that the PPI effect is independent of the ASR response level while other studies demonstrate that PPI both depends on the level and on the type of startle stimulus (Stoddart et al. 2008; Csomor et al. 2008). Investigators should carefully consider the effects of baseline ASR reactivity when interpreting their results to avoid incorrect interpretations of group differences in PPI and PPF. In general, the relative amount of PPI (% reduction of the ASR) for the same prepulse does not vary much if the startle stimulus is a brief broadband noise signal and the sound pressure level is sufficiently high. Thus, a commonly used SES being generally suitable that is likely to result in a robust PPI is a 20–40ms broadband-noise pulse of between 100 and 115 dB peak sound pressure level.
Finally, different methods have been applied for analyzing the electrical waveform representing the ASR. Often, a sensitive recording device with the startle cage comes with the disadvantage that the startle response of the animal elicits an underdamped oscillation of the recording apparatus. The first peak, the difference between the first positive and negative peaks, the rms value of the startle waveform, or fits of a theoretical waveform to the measured waveform have been used to represent the value of the startle response (Grimsley et al. 2015). These different values are highly correlated, and any of these values can be used to represent the strength of the startle response. While the first two values require that peaks of the waveform can be identified that must be sufficiently high above the background noise, the rms estimation and curve fitting can easily be performed automatically given that the ASR is of sufficient amplitude well above the background noise (e.g., Grimsley et al. 2015). The startle amplitude values obtained by the same stimulus with or without a prepulse have been compared using various methods (e.g., by evaluating the % PPI, an ANOVA of the % PPI or ASR values, or a signal-detection theory (SDT) analysis, see Longenecker and Galazyuk 2012; Behrens and Klump 2015, 2016). Either ANOVA methods or SDT methods will provide for an objective estimate of the PPI effect in startle and are both suited. However, only the SDT methods will provide a measure that is both independent of the trial number (provided more than 10 to 20 trials per prepulse condition are analyzed) and can easily be compared across studies using other methods than the ASR.
8. Comparison between acoustic startle modification and operant conditioning
It is often argued that PPI procedures provide for a time-efficient testing since no prior training is required. Young and Fechter (1983) used a prepulse inhibition paradigm to estimate the audiogram of young adult Long-Evans rats. Short prepulse tones at a range of intensities were presented 100ms prior to a 115 dB startle-eliciting noise burst. This interstimulus interval was selected as the optimal interval for eliciting PPI based on previous studies and pilot experiments in the authors’ laboratory (these initial pilot studies should be factored into the time needed to estimate a threshold). Each subject was tested for 14 days for a total of 11,200 trials per subject (approximately 1000 trials per stimulus frequency plus control trials). Thresholds were measured for nine frequencies ranging from 2.5 to 40 kHz. Threshold criterion was set as the prepulse intensity that produced 15% inhibition. PPI thresholds were approximately 10–20 dB higher than thresholds obtained using operant conditioning in albino and hooded Norway rats (Kelly and Masterton 1977; Heffner et al. 1994). This threshold difference is akin to the typical discrepancy between auditory brainstem response and operant thresholds in mammals without hearing loss. Sensitivity indices were not provided and cannot be calculated from the data included in the paper.
Longenecker and colleagues (2016) determined the audiogram of CBA/CaJ mice using PPI procedures. By shortening the inter-trial interval to between 4 and 6s, they managed to collect the data for an audiogram ranging from 4 to 31.5 kHz in about 3 hours (39 trials for each combination of 6 frequencies and 8 amplitudes plus 468 startle only trials). Using a smaller number of trials than in the rat study was only possible by applying a pattern matching data analysis approach that allowed discriminating startle-related waveforms from waveforms resulting from other movements of the animal that were recorded from the transducer. The threshold estimates in the study by Longenecker et al. (2016) were based on a statistical criterion that generally results in lower thresholds than those obtained with a criterion derived from signal-detection theory (see Behrens and Klump 2015). PPI based thresholds for 20-ms tones reported by Longenecker et al. (2016) on average were between 8 to 21 dB. Thresholds determined using operant conditioning in the same strain with 2000-ms tones and a more conservative threshold criterion derived from signal-detection theory (d’ = 1.5) range from −2.8 (SD=4.59) to 8.36 (2.96) dB in the range of best hearing (8 to 24kHz; Radziwon et al. 2009). This comparison suggests that the operant-conditioning procedures may result in lower audibility thresholds if the same threshold criterion would be applied. Mice with substantial auditory nerve fiber loss due to unilateral ouabain treatment show a loss of the ASR, but can still detect an 8kHz tone when tested using and operant procedure (Chambers et al. 2016). Thus, PPI and ASR audiometry may underestimate behavioral audibility in mice. Furthermore, an audiometric threshold can be obtained from a trained animal using a sensitive operant-conditioning procedure in approximately three to four test sessions consisting of ~50 to 100 trials each (Klump et al. 1995; Klink et al. 2006; Behrens and Klump 2015). Initial training usually can be limited to 20 hours which renders operant conditioning procedures efficient despite the investment of time prior to threshold testing.
Also with respect to discrimination tasks in the mouse, the sensitivity of the PPI methods has been found to be inferior to discrimination performance measured with operant conditioning. Data for comparing both procedures are available for discrimination of level increments (intensity difference limen, IDL, see Figure 8A and Behrens & Klump 2015), and for discriminating the spatial position of a sound source (minimum audible angle, MAA, see Figure 8B and Behrens & Klump 2016), and gap detection (Figure 8C, Ison et al 2005; Radziwon et al. 2009). In every case, the sensitivity measure obtained with a signal-detection theory approach for discriminating the cues is much lower if applying PPI rather than operant conditioning. Thus, if similar signal-detection threshold criteria are used (e.g., d’ = 1), thresholds will be considerably higher if measured with PPI than with operant conditioning, and in some parameter settings in PPI experiments no discrimination threshold could be determined since the sensitivity did not reach a value close to 1.
Figure 8.
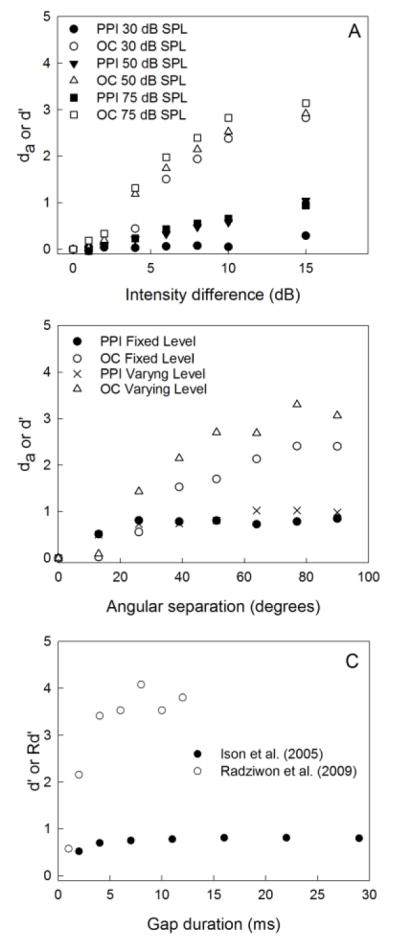
Comparing operant conditioning and PPI sensitivity using signal-detection measures. A) Discrimination of intensity increments. Data from Behrens and Klump (2015). B) Sound location discrimination; Data from Behrens and Klump (2016); C) Gap detection. Data replotted from Ison et al. (2005); Radziwon et al. (2009).
For example, in the IDL measurements PPI provided values of 17 dB or higher while operant conditioning provided values of between 3.5 and 5 dB (Behrens & Klump 2015). For the MAA measurements, the general pattern was similar to that observed for the IDL revealing more than twofold higher thresholds with PPI than with operant conditioning, and in the case of narrowband noise, no MAA could be obtained with a threshold criterion of d’=1 (Behrens and Klump 2016). Ison and colleagues have performed a number of detailed studies manipulating various temporal and spectral parameters of gaps in otherwise continuous noise (Ison et al. 2002, 2005; Ison and Allen 2003). Ison et al. (2002) report detection thresholds of 2.46 (SD=0.27) for gaps in broadband noise for CBA/CaJ x C57BL/6J F1 hybrid mice. In this case, threshold was defined as the minimum gap duration at which the ASR was inhibited by at least 50% of maximum inhibition. This threshold is very close to thresholds for detecting gaps in broadband noise measured using an operant procedure in CBA/CaJ mice (mean=2.2 ms, SD=1.0; threshold defined as d’=1.5; Radziwon et al. 2009). Sensitivity of gap detection assessed using PPI and operant methods is shown in Figure 7. Ison et al. (2005) calculated a measure of sensitivity Rd’ that is closely related to the d’ value from signal detection theory. Unfortunately, Rd’ did not reach 1.0 for any gap duration tested, and the upper limit of Rd’ (mean 1.43, SEM 0.08) was determined by the background activity level. Thus, the sensitivity of the gap PPI paradigm is again lower than the sensitivity of the operant paradigm where performance on suprathreshold gap durations exceeded d’=3.0, and thresholds corresponding to a specific d’ criterion may not be attainable using these procedures. Ison et al. (2005) also reported varying levels of inhibition of the ASR for gaps in noise with varying spectral bandwidth. Gaps with lower frequency spectra produced less inhibition. Lower frequency stimuli also decreased the detectability of gaps in CBA/CaJ mice tested in the operant Radziwon et al. (2009) study.
In summary, PPI procedures are commonly much less sensitive than operant conditioning procedures in reporting perceptual differences between sounds and a failure to observe a “perceptual” difference in a PPI procedure does not imply that the cue values characterizing the prepulse cannot be discriminated by the mouse. Rather, the particular stimulus conditions may not be sufficient to produce strong PPI.
9. Why use PPI?
In light of the issues raised in the previous sections, the reader might wonder why PPI should be used at all as a measure of auditory behavior. It is perhaps incorrect to consider PPI a measure of perception, per se. The term perception implies a conscious behavioral response to a sound or changes in sound. Typical PPI experiments are thought to reflect mainly pre-attentive processes, and conventional PPI using a discrete prepulse burst and also noise offset PPI do not even require the auditory cortex, although PPI using gaps or intensity increments does (Ison et al. 2003). Some PPI tests may even work when the animal is sedated (Ison et al. 1998). PPI is best considered more generally as a behavioral response to changes in an acoustic or multimodal stimulus input whereby the effects of brainstem and higher order processing may be tested. PPI does not apparently serve as an interchangeable substitute for traditional operant conditioning psychoacoustic methods in most situations. However, there are some conditions under which the use of PPI might be the preferable option for evaluating auditory behavioral changes in mouse models.
Obviously, ASR and PPI measures should be used if the goal is to map the neural substrates of the startle and startle-modification circuits. When testing auditory behavior in mouse strains with early-onset, rapidly progressing hearing loss or very young animals (e. g., Lauer and May 2011; McGuire et al. 2015), PPI may be the only behavioral assay that produces measureable data. It is very difficult to train and test extremely young mice given time constraints and uncertain consequences of water or food deprivation required for motivating the subject. It could be similarly difficult to train mice with rapidly deteriorating hearing that begins at an early age if the animal experiences profound deafness before thresholds can be obtained.
Mutant strains with learning or attentional deficits may prove to be impossible subjects for operant experiments. Strains with severe vestibular or motor control phenotypes such as circling, waltzing, shaking, or quivering may not be physically capable of performing complex motor tasks involved in some operant conditioning paradigms. In these mice, PPI tests may provide a means to obtain behavioral hearing data; however, these strains may also be deaf or have motor deficits that prevent the expression of a startle response. Conversely, hearing-impaired strains should be avoided in studies aimed at investigating the effects of genetic and environmental factors on sensorimotor gating. One potential solution to these issues is to use a tactile startle-eliciting stimulus as an alternative to an acoustic startle-eliciting stimulus.
In other cases, the experimenters may wish to purposely exclude the effect of training (e.g., Lauer and May, 2011). Training and testing using traditional operant conditioning techniques requires repeated exposure to stimuli over many trials and successive days, and testing on repetitive, routine task could preclude identification of auditory processing abnormalities since the animal is presented with increased opportunities to compensate with time and practice. However, as described in an earlier section, PPI is also subject to the effects of learning and experience with repeated testing.
When considering using PPI in the situations described above, one must take into consideration that extensive pilot work must be completed to optimize stimulus parameters for a particular strain and test paradigm. Conditions have been optimized for only a handful of stimulus paradigms and strains (for example, temporal processing papers by Ison and colleagues 1998, 2002, 2003, 2005, 2007). Investigations of sensorimotor gating in mouse models of neurological and neuropsychological dysfunction should take care to consider a strain’s hearing status prior to implementing ASR testing procedures. If the animal cannot hear the stimuli or sounds are not processed normally in the brain, the behavioral output may produce a false positive indication of sensorimotor gating deficits. Experimenters are encouraged consulting with a seasoned behaviorist who has expertise in rodent auditory phenotyping procedures.
Highlights.
We review acoustic startle modification for behavioral auditory phenotyping, and we compare the sensitivity of startle modification procedures to more traditional psychoacoustic techniques
Numerous non-auditory variables must be taken into account, including strain, sex, age, environment, housing conditions, and influence of non-auditory inputs to the startle circuit
Acoustic startle modification procedures show low sensitivity compared to operant conditioning techniques
Acoustic startle modification is useful when testing mice with early-onset hearing loss or with learning impairments
Acknowledgments
Funding: This work was supported by grants from the National Institute for Deafness and Other Communication Disorders DC012352, DC009353, and DC00521; the David M. Rubenstein Fund for Hearing Research; and the Deutsche Forschungsgemeinschaft EXC 1077 “Hearing4all.” We thank Michael Koch for his comments on a previous version of the manuscript.
Abbreviations
Behavioral terms
- ASR
Acoustic startle response
- IPI
Inter-pulse interval
- PPF
Prepulse facilitation
- PPI
Prepulse inhibition
- SES
Startle-eliciting stimulus
Anatomical Areas
- CRN
Cochlear root neurons
- DCN
Dorsal cochlear nucleus
- IC
Inferior colliculus
- LC
Locus coeruleus
- LSO
Lateral superior olive
- PnC
Caudal pontine reticular nucleus
- PPT
Pedunculopontine tegmental nucleus
- SC
Superior colliculus
- TB
Trapezoid body
- VCN
Ventral cochlear nucleus
- VTA
Ventral tegmental area
Neurotransmitters
- 5-HT
Serotonin
- ACh
Acetylcholine
- DA
Dopamine
- GABA
Gamma-aminobutyric acid
- Glut
Glutamate
- Gly
Glycine
- NA
Noradrenaline
- SP
Substance P
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AL, Hudson A, Ryan CL, Doucette TA. Effects of estrous stage and time of day on prepulse inhibition in female rats. Journal of neuroscience methods. 2008 Aug 30;173(2):295–8. doi: 10.1016/j.jneumeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Aizenberg M, Mwilambwe-Tshilobo L, Briguglio JJ, Natan RG, Geffen MN. Bidirectional regulation of innate and learned behaviors that rely on frequency discrimination by cortical inhibitory neurons. PLoS Biol. 2015 Dec 2;13(12):e1002308. doi: 10.1371/journal.pbio.1002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PD, Ison JR. Sensitivity of the mouse to changes in azimuthal sound location: angular separation, spectral composition, and sound level. Behavioral neuroscience. 2010 Apr;124(2):265–277. doi: 10.1037/a0018913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler RA, Dolan DF, Halsey K, Kanicki A, Deng N, Martin C, Eberle J, Kohrman DC, Miller RA, Schacht J. Age-related changes in auditory nerve–inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice. Neuroscience. 2015 Apr 30;292:22–33. doi: 10.1016/j.neuroscience.2015.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanipour R, Cresoe S, Borlongan C, Frisina R, Walton J. Effects of Mild Traumatic Brain Injury on Auditory Function in a Mouse Model. Biomedical Engineering Conference (SBEC), 2016 32nd Southern; 2016 Mar 11; IEEE; pp. 13–14. [DOI] [PubMed] [Google Scholar]
- Barsz K, Ison JR, Snell KB, Walton JP. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiology of aging. 2002 Aug 31;23(4):565–78. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Basavaraj S, Yan J. Prepulse inhibition of acoustic startle reflex as a function of the frequency difference between prepulse and background sounds in mice. PloS one. 2012 Sep 11;7(9):e45123. doi: 10.1371/journal.pone.0045123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens D, Klump GM. Comparison of the sensitivity of prepulse inhibition of the startle reflex and operant conditioning in an auditory intensity difference limen paradigm. Hearing research. 2015 Mar 31;321:35–44. doi: 10.1016/j.heares.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Behrens D, Klump GM. Comparison of mouse minimum audible angle determined in prepulse inhibition and operant conditioning procedures. Hearing research. 2016 Mar 31;333:167–78. doi: 10.1016/j.heares.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Bosch D, Schmid S. Activation of muscarinic cholinergic receptors inhibits giant neurones in the caudal pontine reticular nucleus. European Journal of Neuroscience. 2006 Oct 1;24(7):1967–75. doi: 10.1111/j.1460-9568.2006.05085.x. [DOI] [PubMed] [Google Scholar]
- Bosch D, Schmid S. Cholinergic mechanism underlying prepulse inhibition of the startle response in rats. Neuroscience. 2008 Jul 31;155(1):326–35. doi: 10.1016/j.neuroscience.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. Journal of Comparative Neurology. 1988 Dec 22;278(4):581–90. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. Animal models of tinnitus. Hearing research. 2015 Nov 10;338:88–97. doi: 10.1016/j.heares.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Bullock AE, Slobe BS, Vázquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behavioral neuroscience. 1997 Dec;111(6):1353. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Carlson S, Willott JF. Caudal pontine reticular formation of C57BL/6J mice: responses to startle stimuli, inhibition by tones, and plasticity. Journal of neurophysiology. 1998 May 1;79(5):2603–14. doi: 10.1152/jn.1998.79.5.2603. [DOI] [PubMed] [Google Scholar]
- Carlson S, Willott JF. Modulation of the acoustic startle response by background sound in C57BL.6J mice. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; 2001. pp. 83–95. [Google Scholar]
- Cassella JV, Davis M. The design and calibration of a startle measurement system. Physiology & Behavior. 1986 Dec 31;36(2):377–83. doi: 10.1016/0031-9384(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Chabot CC, Taylor DH. Circadian modulation of the rat acoustic startle response. Behavioral neuroscience. 1992 Oct;106(5):846. doi: 10.1037//0735-7044.106.5.846. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron. 2016 Feb 17;89(4):867–79. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Canlon B. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 2012 Jul 9;153(9):4412–21. doi: 10.1210/en.2012-1416. [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001 Apr 4;103(4):1043–50. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999 Jun 4;284(5420):1670–2. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behavioral neuroscience. 2008 Aug;122(4):885. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- Davis MI, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. The journal of neuroscience. 1982 Jun 1;2(6):791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A, de Jongh R, Groenink L, van der Gugten J, Hijzen TH, Olivier B. Footshock-induced sensitization of the acoustic startle response in two strains of mice. Behavioural brain research. 2001 Aug 27;123(1):17–21. doi: 10.1016/s0166-4328(01)00193-0. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hearing research. 1993 Feb 1;65(1–2):125–32. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner JG, Willott JF. Fear-potentiated startle in two strains of inbred mice. Behavioral neuroscience. 1997 Aug;111(4):855. [PubMed] [Google Scholar]
- Fendt M, Koch M. Cholinergic modulation of the acoustic startle response in the caudal pontine reticular nucleus of the rat. European journal of pharmacology. 1999 Apr 9;370(2):101–7. doi: 10.1016/s0014-2999(99)00156-9. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001 Jul 1;156(2–3):216–24. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Sensorimotor gating deficit after lesions of the superior colliculus. Neuroreport. 1994 Sep 8;5(14):1725–8. doi: 10.1097/00001756-199409080-00009. [DOI] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nature neuroscience. 2003 May 1;6(5):445–6. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ralph MR. Circadian modulation in the rat acoustic startle circuit. Behavioral neuroscience. 1995 Feb;109(1):43. doi: 10.1037//0735-7044.109.1.43. [DOI] [PubMed] [Google Scholar]
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experience-related improvements in gap detection in the rat. Developmental brain research. 2004 Sep 17;152(2):83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Galazyuk A, Hébert S. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Frontiers in neurology. 2015 Apr 28;6:88. doi: 10.3389/fneur.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Radke AK. InAnimal Models of Behavior Genetics. Springer; New York: 2016. Potentiation of the Startle Reflex as a Behavioral Measure of Anxiety; pp. 333–357. [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Current protocols in neuroscience. 1998:8–7. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Mcllwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Molecular psychiatry. 2002 doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Gómez-Nieto R, Rubio ME, López DE. Cholinergic input from the ventral nucleus of the trapezoid body to cochlear root neurons in rats. Journal of Comparative Neurology. 2008a Jan 20;506(3):452–68. doi: 10.1002/cne.21554. [DOI] [PubMed] [Google Scholar]
- Gómez-Nieto R, Horta-Junior JA, Castellano O, Herrero-Turrión MJ, Rubio ME, López DE. Neurochemistry of the afferents to the rat cochlear root nucleus: possible synaptic modulation of the acoustic startle. Neuroscience. 2008b Jun 12;154(1):51–64. doi: 10.1016/j.neuroscience.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Nieto R, José de Anchieta C, Castellano O, Millian-Morell L, Rubio ME, López DE. Origin and function of short-latency inputs to the neural substrates underlying the acoustic startle reflex. Frontiers in neuroscience. 2014:8. doi: 10.3389/fnins.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley CA, Longenecker RJ, Rosen MJ, Young JW, Grimsley JM, Galazyuk AV. An improved approach to separating startle data from noise. Journal of neuroscience methods. 2015 Sep 30;253:206–17. doi: 10.1016/j.jneumeth.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackley SA, Graham FK. Effects of attending selectively to the spatial position of reflex-eliciting and reflex-modulating stimuli. Journal of Experimental Psychology: Human Perception and Performance. 1987 Aug;13(3):411. doi: 10.1037//0096-1523.13.3.411. [DOI] [PubMed] [Google Scholar]
- Harrison JM, Warr WB. A study of the cochlear nuclei and ascending auditory pathways of the medulla. Journal of Comparative Neurology. 1962 Dec 1;119(3):341–79. doi: 10.1002/cne.901190306. [DOI] [PubMed] [Google Scholar]
- Hayes SH, Radziwon KE, Stolzberg DJ, Salvi RJ. Behavioral models of tinnitus and hyperacusis in animals. Frontiers in neurology. 2014 Sep 17;5:179. doi: 10.3389/fneur.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing research. 1994 Mar 1;73(2):244–7. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Heldt S, Sundin V, Willott JF, Falls WA. Posttraining lesions of the amygdala interfere with fear-potentiated startle to both visual and auditory conditioned stimuli in C57BL/6J mice. Behavioral neuroscience. 2000 Aug;114(4):749. [PubMed] [Google Scholar]
- Henry KR. Auditory brainstem volume-conducted responses: origins in the laboratory mouse. Ear and Hearing. 1979 Mar 1;4(5):173–8. [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? Journal of Neurophysiology. 2014 Feb 1;111(3):552–64. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Stitt CL. Inhibition of the glabella reflex by monaural and binaural stimulation. Journal of Experimental Psychology: Human Perception and Performance. 1980 Nov;6(4):769. doi: 10.1037//0096-1523.6.4.769. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Cohen ME, English LM. Reflex modification by acoustic signals in newborn infants and in adults. Journal of experimental child psychology. 1985 Jun 1;39(3):562–79. doi: 10.1016/0022-0965(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological review. 1980 Mar;87(2):175. [PubMed] [Google Scholar]
- Hormigo S, Gómez-Nieto R, Castellano O, Herrero-Turrión MJ, López DE. The noradrenergic projection from the locus coeruleus to the cochlear root neurons in rats. Brain Structure and Function. 2015 May 1;220(3):1477–96. doi: 10.1007/s00429-014-0739-3. [DOI] [PubMed] [Google Scholar]
- Hurst JL, West RS. Taming anxiety in laboratory mice. Nature Methods. 2010 Oct 1;7(10):825–6. doi: 10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- Ison JR. The acoustic startle response: Reflex elicitation and reflex modification by preliminary stimuli. In: Willott JF, editor. Handbook of mouse auditory research: from behavior to molecular biology. CRC Press; 2001. pp. 59–82. [Google Scholar]
- Ison JR, Allen P. A diminished rate of “physiological decay” at noise offset contributes to age-related changes in temporal acuity in the CBA mouse model of presbycusis. The Journal of the Acoustical Society of America. 2003 Jul 1;114(1):522–8. doi: 10.1121/1.1577553. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD. Pre-but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behavioural brain research. 2007 Dec 28;185(2):76–81. doi: 10.1016/j.bbr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychological Bulletin. 1983 Jul;94(1):3. [PubMed] [Google Scholar]
- Ison JR, Pinckney LA. Inhibition of the cutaneous eyeblink reflex by unilateral and bilateral acoustic input: the persistence of contralateral antagonism in auditory processing. Perception & psychophysics. 1990 Jul 1;47(4):337–41. doi: 10.3758/bf03210873. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, Oertel D. Deleting the HCN1 Subunit of Hyperpolarization-Activated Ion Channels in Mice Impairs Acoustic Startle Reflexes, Gap Detection, and Spatial Localization. Journal of the Association for Research in Otolaryngology. doi: 10.1007/s10162-016-0610-8. available online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE. Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. Journal of the Association for Research in Otolaryngology. 2007 Dec 1;8(4):539–50. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: a study of the acoustic startle reflex and its inhibition by brief decrements in noise level. The Journal of the Acoustical Society of America. 1998 Sep 1;104(3):1696–704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, Rivoli PJ, Moore JT. The behavioral response of mice to gaps in noise depends on its spectral components and its bandwidth. The Journal of the Acoustical Society of America. 2005 Jun 1;117(6):3944–51. doi: 10.1121/1.1904387. [DOI] [PubMed] [Google Scholar]
- Ison JR, Bowen GP, Pak J, Gutierrez E. Changes in the strength of prepulse inhibition with variation in the startle baseline associated with individual differences and with old age in rats and mice. Psychobiology. 1997 Sep 1;25(3):266–74. [Google Scholar]
- Jalabi W, Kopp-Scheinpflug C, Allen PD, Schiavon E, DiGiacomo RR, Forsythe ID, Maricich SM. Sound localization ability and glycinergic innervation of the superior olivary complex persist after genetic deletion of the medial nucleus of the trapezoid body. Journal of Neuroscience. 2013 Sep 18;33(38):15044–9. doi: 10.1523/JNEUROSCI.2604-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Shannon HE. Effects of scopolamine in comparison with apomorphine and phencyclidine on prepulse inhibition in rats. European journal of pharmacology. 2000a Mar 10;391(1):105–12. doi: 10.1016/s0014-2999(00)00055-8. [DOI] [PubMed] [Google Scholar]
- Jones CK, Shannon HE. Muscarinic cholinergic modulation of prepulse inhibition of the acoustic startle reflex. Journal of Pharmacology and Experimental Therapeutics. 2000b Sep 1;294(3):1017–23. [PubMed] [Google Scholar]
- Joober R, Zarate JM, Rouleau GA, Skamene E, Boksa P. Provisional mapping of quantitative trait loci modulating the acoustic startle response and prepulse inhibition of acoustic startle. Neuropsychopharmacology. 2002 Nov 1;27(5):765–81. doi: 10.1016/S0893-133X(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Kallnik M, Elvert R, Ehrhardt N, Kissling D, Mahabir E, Welzl G, Faus-Kessler T, de Angelis MH, Wurst W, Schmidt J, Hölter SM. Impact of IVC housing on emotionality and fear learning in male C3HeB/FeJ and C57BL/6J mice. Mammalian Genome. 2007 Mar 1;18(3):173–86. doi: 10.1007/s00335-007-9002-z. [DOI] [PubMed] [Google Scholar]
- Kandler K, Herbert H. Auditory projections from the cochlear nucleus to pontine and mesencephalic reticular nuclei in the rat. Brain research. 1991 Oct 25;562(2):230–42. doi: 10.1016/0006-8993(91)90626-7. [DOI] [PubMed] [Google Scholar]
- Karcz A, Allen PD, Walton J, Ison JR, Kopp-Scheinpflug C. Auditory deficits of Kcna1 deletion are similar to those of a monaural hearing impairment. Hearing research. 2015 Mar 31;321:45–51. doi: 10.1016/j.heares.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JB, Masterton B. Auditory sensitivity of the albino rat. Journal of comparative and physiological psychology. 1977 Aug;91(4):930. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]
- Klink KB, Bendig G, Klump GM. Operant methods for mouse psychoacoustics. Behavior research methods. 2006 Feb 1;38(1):1–7. doi: 10.3758/bf03192744. [DOI] [PubMed] [Google Scholar]
- Klump GM, Dooling RJ, Fay RR, Stebbins W, editors. Methods in comparative psychoacoustics. Birkhäuser; 1995. [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiology & behavior. 1998 Jul 31;64(5):625–8. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Progress in neurobiology. 1999 Oct 31;59(2):107–28. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats—circuits mediating evocation, inhibition and potentiation. Behavioural brain research. 1997 Dec 31;89(1):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Koch M, Lingenhöhl K, Pilz PK. Loss of the acoustic startle response following neurotoxic lesions of the caudal pontine reticular formation: possible role of giant neurons. Neuroscience. 1992 Aug 31;49(3):617–25. doi: 10.1016/0306-4522(92)90231-p. [DOI] [PubMed] [Google Scholar]
- Koch M, Fendt M, Kretschmer BD. Role of the substantia nigra pars reticulata in sensorimotor gating, measured by prepulse inhibition of startle in rats. Behavioural brain research. 2000 Dec 20;117(1):153–62. doi: 10.1016/s0166-4328(00)00299-0. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Sumich AL, Sharma T. Startle gating in antipsychotic-naive first episode schizophrenia patients: one ear is better than two. Psychiatry research. 2007 May 30;151(1):21–8. doi: 10.1016/j.psychres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Krase W, Koch M, Schnitzler HU. Glutamate antagonists in the reticular formation reduce the acoustic startle response. Neuroreport. 1993 Jan 1;4(1):13–6. doi: 10.1097/00001756-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Landi MS, Kreider JW, Lang CM, Bullock LP. Effects of shipping on the immune function in mice. American journal of veterinary research. 1982 Sep;43(9):1654–7. [PubMed] [Google Scholar]
- Lauer AM, May BJ. The medial olivocochlear system attenuates the developmental impact of early noise exposure. Journal of the Association for Research in Otolaryngology. 2011 Jun 1;12(3):329–43. doi: 10.1007/s10162-011-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, May BJ, Hao ZJ, Watson J. Analysis of environmental sound levels in modern rodent housing rooms. Lab animal. 2009 May;38(5):154–60. doi: 10.1038/laban0509-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, López DE, Meloni EG, Davis M. A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. The Journal of neuroscience. 1996 Jun 1;16(11):3775–89. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behavioural Brain Research. 1999 Oct 31;104(1):113–7. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Cohen ME. Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiology & behavior. 1985 Jan 31;34(1):65–70. doi: 10.1016/0031-9384(85)90079-4. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Powers AS, Hoffman HS. The neural substrate of the startle response. Physiology & Behavior. 1980 Aug 1;25(2):291–7. doi: 10.1016/0031-9384(80)90219-x. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Frayne ML, Saito M, Berry EM, Aldinger KA, Rockwell GN, Hammer RP, Jr, Baskin-Hill AE, Singer JB, Nadeau JH, Sklar P. Genomic survey of prepulse inhibition in mouse chromosome substitution strains. Genes, Brain and Behavior. 2009 Nov 1;8(8):806–16. doi: 10.1111/j.1601-183X.2009.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Görtz N, Schachner M, Sachser N. Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes, Brain and Behavior. 2006 Feb 1;5(1):64–72. doi: 10.1111/j.1601-183X.2005.00140.x. [DOI] [PubMed] [Google Scholar]
- Li L, Du Y, Li N, Wu X, Wu Y. Top–down modulation of prepulse inhibition of the startle reflex in humans and rats. Neuroscience & Biobehavioral Reviews. 2009 Sep 30;33(8):1157–67. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? The Journal of Neuroscience. 1994 Mar 1;14(3):1176–94. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingenhohl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? The Journal of Neuroscience. 1994 Mar 1;14(3):1176–94. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL. The gap-startle paradigm for tinnitus screening in animal models: limitations and optimization. Hearing research. 2013 Jan 31;295:150–60. doi: 10.1016/j.heares.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F 1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997 Aug 11;80(4):1075–86. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain research. 2012 Nov 16;1485:54–62. doi: 10.1016/j.brainres.2012.02.067. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Pattij T, Smit AB, Spijker S. Independent genetic loci for sensorimotor gating and attentional performance in BXD recombinant inbred strains. Genes, Brain and Behavior. 2012 Mar 1;11(2):147–56. doi: 10.1111/j.1601-183X.2011.00754.x. [DOI] [PubMed] [Google Scholar]
- López DE, Merchán MA, Bajo VM, Saldaña E. The Mammalian Cochlear Nuclei. Springer; US: 1993. The cochlear root neurons in the rat, mouse and gerbil; pp. 291–301. [Google Scholar]
- Marsh RR, Hoffman HS, Stitt CL. Eyeblink inhibition by monaural and binaural stimulation: One ear is better than two. Science. 1976 Apr 23;192(4237):390–1. doi: 10.1126/science.1257776. [DOI] [PubMed] [Google Scholar]
- Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behavioural brain research. 2010 Feb 11;207(1):196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- McCaughran JA, Bell J, Hitzemann RJ. Fear–potentiated startle response in mice: genetic analysis of the C57BL/6J and DBA/2J intercross. Pharmacology Biochemistry and Behavior. 2000 Feb 29;65(2):301–12. doi: 10.1016/s0091-3057(99)00216-6. [DOI] [PubMed] [Google Scholar]
- McGuire B, Fiorillo B, Ryugo DK, Lauer AM. Auditory nerve synapses persist in ventral cochlear nucleus long after loss of acoustic input in mice with early-onset progressive hearing loss. Brain research. 2015 Apr 24;1605:22–30. doi: 10.1016/j.brainres.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchán MA, Collia F, López DE, Saldana E. Morphology of cochlear root neurons in the rat. Journal of neurocytology. 1988 Oct 1;17(5):711–25. doi: 10.1007/BF01260998. [DOI] [PubMed] [Google Scholar]
- Mikaelian DO. Development and degeneration of hearing in the C57/b16 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses. The Laryngoscope. 1979 Jan 1;89(1):1–5. doi: 10.1288/00005537-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes, Brain and Behavior. 2006 Jun 1;5(4):346–54. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Davis M. NMDA and non-NMDA antagonists infused into the nucleus reticularis pontis caudalis depress the acoustic startle reflex. Brain research. 1993 Oct 1;623(2):215–22. doi: 10.1016/0006-8993(93)91430-z. [DOI] [PubMed] [Google Scholar]
- Moyer CE, Erickson SL, Fish KN, Thiels E, Penzes P, Sweet RA. Developmental trajectories of auditory cortex synaptic structures and gap-prepulse inhibition of acoustic startle between early adolescence and young adulthood in mice. Cerebral Cortex. 2015 Mar 10; doi: 10.1093/cercor/bhv040. bhv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, López DE. Direct input from cochlear root neurons to pontine reticulospinal neurons in albino rat. Journal of Comparative Neurology. 2003 May 19;460(1):80–93. doi: 10.1002/cne.10656. [DOI] [PubMed] [Google Scholar]
- O'Leary TP, Shin S, Fertan E, Dingle RN, Almuklass A, Gunn RK, Yu Z, Wang J, Brown RE. Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer's disease. Genes, Brain and Behavior. 2017 Jan 1; doi: 10.1111/gbb.12370. [DOI] [PubMed] [Google Scholar]
- Osen KK, López DE, Slyngstad TA, Ottersen OP, Storm-Mathisen J. GABA-like and glycine-like immunoreactivities of the cochlear root nucleus in rat. Journal of neurocytology. 1991 Jan 1;20(1):17–25. doi: 10.1007/BF01187131. [DOI] [PubMed] [Google Scholar]
- Parham K, Sun XM, Kim DO. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. CRC Press; New York: 2001. Noninvasive assessment of auditory function in mice: auditory brainstem response and distortion product otoacoustic emissions; pp. 37–58. [Google Scholar]
- Parisi T, Ison JR. Development of the acoustic startle response in the rat: ontogenetic changes in the magnitude of inhibition by prepulse stimulation. Developmental psychobiology. 1979 May 1;12(3):219–30. doi: 10.1002/dev.420120305. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology. 1997 Jul 1;132(2):169–80. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Kirby A, Hammer RP, Purcell S, O'leary SB, Singer JB, Hill AE, Nadeau JH, Daly MJ, Sklar P. Two quantitative trait loci for prepulse inhibition of startle identified on mouse chromosome 16 using chromosome substitution strains. Genetics. 2005 Dec 1;171(4):1895–904. doi: 10.1534/genetics.105.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo S, Singer P, Feldon J, Yee BK. The postweaning social isolation in C57BL/6 mice: preferential vulnerability in the male sex. Psychopharmacology. 2008 May 1;197(4):613–28. doi: 10.1007/s00213-008-1081-3. [DOI] [PubMed] [Google Scholar]
- Pilz PK, Carl TD, Plappert CF. Habituation of the acoustic and the tactile startle responses in mice: two independent sensory processes. Behavioral neuroscience. 2004 Oct;118(5):975. doi: 10.1037/0735-7044.118.5.975. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK. The acoustic startle response as an effective model for elucidating the effect of genes on the neural mechanism of behavior in mice. Behavioural brain research. 2001 Nov 8;125(1):183–8. doi: 10.1016/s0166-4328(01)00299-6. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK. Difference in anxiety and sensitization of the acoustic startle response between the two inbred mouse strains BALB/cAN and DBA/2N. Genes, Brain and Behavior. 2002 Aug 1;1(3):178–86. doi: 10.1034/j.1601-183x.2002.10306.x. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK. Long-term habituation of the startle response in mice evoked by acoustic and tactile stimuli. Behavioural brain research. 2005 Jul 30;162(2):307–10. doi: 10.1016/j.bbr.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Rodenbücher AM, Pilz PK. Effects of sex and estrous cycle on modulation of the acoustic startle response in mice. Physiology & behavior. 2005 Mar 31;84(4):585–94. doi: 10.1016/j.physbeh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK, Schnitzler HU. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behavioural brain research. 2004 Jul 9;152(2):403–12. doi: 10.1016/j.bbr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Kuhn S, Schnitzler HU, Pilz PK. Experience increases the prepulse inhibition of the acoustic startle response in mice. Behavioral neuroscience. 2006 Feb;120(1):16. doi: 10.1037/0735-7044.120.1.16. [DOI] [PubMed] [Google Scholar]
- Powell SB, Weber M, Geyer MA. Behavioral Neurogenetics. Springer; Berlin Heidelberg: 2011. Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders; pp. 251–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralls K. Auditory sensitivity in mice: Peromyscus and Mus musculus. Animal Behaviour. 1967 Jan 31;15(1):123–8. doi: 10.1016/s0003-3472(67)80022-8. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Paulus MP, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits and perseverative motor patterns in dopamine transporter knock-out mice: differential effects of D1 and D2 receptor antagonists. The Journal of neuroscience. 2001 Jan 1;21(1):305–13. doi: 10.1523/JNEUROSCI.21-01-00305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon KE, June KM, Stolzberg DJ, Xu-Friedman MA, Salvi RJ, Dent ML. Behaviorally measured audiograms and gap detection thresholds in CBA/CaJ mice. Journal of Comparative Physiology A. 2009 Oct 1;195(10):961–9. doi: 10.1007/s00359-009-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA. Anxiogenic treatments do not increase fear-potentiated startle in mice. Biological psychiatry. 2005 Jan 1;57(1):33–43. doi: 10.1016/j.biopsych.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Rose C, Röhl FW, Hanke J, Schwegler H, Yilmazer-Hanke DM. Maternal and genetic effects on the acoustic startle reflex and its sensitization in C3H/HeN, DBA/2JHd and NMRI mice following blastocyst transfer. Behavior genetics. 2008 Nov 1;38(6):596–611. doi: 10.1007/s10519-008-9222-3. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology. 2003 Oct 1;170(1):73–9. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Salloum RH, Yurosko C, Santiago L, Sandridge SA, Kaltenbach JA. Induction of enhanced acoustic startle response by noise exposure: dependence on exposure conditions and testing parameters and possible relevance to hyperacusis. PloS one. 2014 Oct 31;9(10):e111747. doi: 10.1371/journal.pone.0111747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samocha KE, Lim JE, Cheng R, Sokoloff G, Palmer AA. Fine mapping of QTL for prepulse inhibition in LG/J and SM/J mice using F2 and advanced intercross lines. Genes, Brain and Behavior. 2010 Oct 1;9(7):759–67. doi: 10.1111/j.1601-183X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandner G, Canal NM. Relationship between PPI and baseline startle response. Cognitive neurodynamics. 2007 Mar 1;1(1):27–37. doi: 10.1007/s11571-006-9008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S, Simons NS, Schnitzler HU. Cellular mechanisms of the trigeminally evoked startle response. European Journal of Neuroscience. 2003 Apr 1;17(7):1438–44. doi: 10.1046/j.1460-9568.2003.02565.x. [DOI] [PubMed] [Google Scholar]
- Shnerson A, Willott JF. Ontogeny of the acoustic startle response in C57BL/6J mouse pups. Journal of comparative and physiological psychology. 1980 Feb;94(1):36. doi: 10.1037/h0077648. [DOI] [PubMed] [Google Scholar]
- Simons-Weidenmaier NS, Weber M, Plappert CF, Pilz PK, Schmid S. Synaptic depression and short-term habituation are located in the sensory part of the mammalian startle pathway. BMC neuroscience. 2006 May 9;7(1):1. doi: 10.1186/1471-2202-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiera RF, Davis M. Excitatory amino acid antagonists depress acoustic startle after infusion into the ventral nucleus of the lateral lemniscus or paralemniscal zone. Brain research. 1988 Mar 29;445(1):130–6. doi: 10.1016/0006-8993(88)91081-5. [DOI] [PubMed] [Google Scholar]
- Steidl S, Faerman P, Li L, Yeomans JS. Kynurenate in the pontine reticular formation inhibits acoustic and trigeminal nucleus-evoked startle, but not vestibular nucleus-evoked startle. Neuroscience. 2004 Dec 31;126(1):127–36. doi: 10.1016/j.neuroscience.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Stoddart CW, Noonan J, Martin-Iverson MT. Stimulus quality affects expression of the acoustic startle response and prepulse inhibition in mice. Behavioral neuroscience. 2008 Jun;122(3):516. doi: 10.1037/0735-7044.122.3.516. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Geyer MA, Braff D. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001 Jul 1;156(2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. Journal of Psychopharmacology. 2016 Nov 1;30(11):1072–81. doi: 10.1177/0269881116661075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Auerbach P, Monroe SM, Hartston H, Geyer MA, Braff DL. Men are more inhibited than women by weak prepulses. Biological psychiatry. 1993 Aug 15;34(4):253–60. doi: 10.1016/0006-3223(93)90079-s. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Hartman PL, Sprock J, Auerbach PP, Cadenhead K, Perry W, Braff DL. Sex differences in sensorimotor gating of the human startle reflex: all smoke? Psychopharmacology. 1999 Sep 1;146(2):228–32. doi: 10.1007/s002130051111. [DOI] [PubMed] [Google Scholar]
- Swetter BJ, Fitch RH, Markus EJ. Age-related decline in auditory plasticity: experience dependent changes in gap detection as measured by prepulse inhibition in young and aged rats. Behavioral neuroscience. 2010 Jun;124(3):370. doi: 10.1037/a0019519. [DOI] [PubMed] [Google Scholar]
- Szabo I, Hazafi K. Elicitability of the acoustic startle reaction after brain stem lesions. Acta Physiol Acad Sci Hung. 1965 Jan 1;27:155–65. [Google Scholar]
- Takata A, Kakiuchi C, Ishiwata M, Kanba S, Kato T. Behavioral and gene expression analyses in heterozygous XBP1 knockout mice: possible contribution of chromosome 11qA1 locus to prepulse inhibition. Neuroscience research. 2010 Nov 30;68(3):250–5. doi: 10.1016/j.neures.2010.07.2042. [DOI] [PubMed] [Google Scholar]
- Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mammalian Genome. 2000 Jul 1;11(7):555–64. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- Turner JG, Willott JF. Exposure to an augmented acoustic environment alters auditory function in hearing-impaired DBA/2J mice. Hearing research. 1998 Apr 30;118(1):101–13. doi: 10.1016/s0378-5955(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Truong DT, Che A, Rendall AR, Szalkowski CE, LoTurco JJ, Galaburda AM, Holly Fitch R. Mutation of Dcdc2 in mice leads to impairments in auditory processing and memory ability. Genes, Brain and Behavior. 2014 Nov 1;13(8):802–11. doi: 10.1111/gbb.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typlt M, Mirkowski M, Azzopardi E, Ruth P, Pilz PK, Schmid S. Habituation of reflexive and motivated behavior in mice with deficient BK channel function. Frontiers in integrative neuroscience. 2013a Nov 19;7:79. doi: 10.3389/fnint.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typlt M, Mirkowski M, Azzopardi E, Ruettiger L, Ruth P, Schmid S. Mice with deficient BK channel function show impaired prepulse inhibition and spatial learning, but normal working and spatial reference memory. PloS one. 2013b Nov 26;8(11):e81270. doi: 10.1371/journal.pone.0081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziridis K, Buerbank S, Eulenburg V, Dlugaiczyk J, Schulze H. Deficit in acoustic signal-in-noise detection in glycine receptor α3 subunit knockout mice. EuropeanJournal of Neuroscience. 2016 Nov 1; doi: 10.1111/ejn.13489. Available online ahead of print. [DOI] [PubMed] [Google Scholar]
- Ukai M, Okuda A, Mamiya T. Effects of anticholinergic drugs selective for muscarinic receptor subtypes on prepulse inhibition in mice. European journal of pharmacology. 2004 May 25;492(2):183–7. doi: 10.1016/j.ejphar.2004.03.066. [DOI] [PubMed] [Google Scholar]
- Valsamis B, Schmid S. Habituation and prepulse inhibition of acoustic startle in rodents. JoVE (Journal of Visualized Experiments) 2011 Sep;1(55):e3446. doi: 10.3791/3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, Van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes, Brain and Behavior. 2006 Mar 1;5(2):139–49. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behavioural brain research. 2006 Apr 25;169(1):162–7. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes, Brain and Behavior. 2004 Feb 1;3(1):27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R. Different data from different labs: lessons from studies of gene–environment interaction. Journal of neurobiology. 2003 Jan 1;54(1):283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Weible AP, Liu C, Niell CM, Wehr M. Auditory cortex is required for fear potentiation of gap detection. Journal of Neuroscience. 2014a Nov 12;34(46):15437–45. doi: 10.1523/JNEUROSCI.3408-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, Moore AK, Liu C, DeBlander L, Wu H, Kentros C, Wehr M. Perceptual gap detection is mediated by gap termination responses in auditory cortex. Current Biology. 2014b Jul 7;24(13):1447–55. doi: 10.1016/j.cub.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würbel H. Ideal homes? Housing effects on rodent brain and behaviour. Trends in neurosciences. 2001 Apr 1;24(4):207–11. doi: 10.1016/s0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- Willott JF, Carlson S. Modification of the acoustic startle response in hearing-impaired C57BL/6J mice: Prepulse augmentation and prolongation of prepulse inhibition. Behavioral neuroscience. 1995 Jun;109(3):396. doi: 10.1037//0735-7044.109.3.396. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hearing research. 1999 Sep 30;135(1):78–88. doi: 10.1016/s0378-5955(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Willott JF, Kulig J, Satterfield T. The acoustic startle response in DBA/2 and C57BL/6 mice: relationship to auditory neuronal response properties and hearing impairment. Hearing research. 1984 Nov 1;16(2):161–7. doi: 10.1016/0378-5955(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O'Steen J, Johnson KR, Bogue MA, Gagnon L. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behavioral neuroscience. 2003 Aug;117(4):716. doi: 10.1037/0735-7044.117.4.716. [DOI] [PubMed] [Google Scholar]
- Wu MF, Suzuki SS, Siegel JM. Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle. Brain research. 1988 Aug 9;457(2):399–406. doi: 10.1016/0006-8993(88)90716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Current protocols in neuroscience. 2009 Jul;:8–24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Chang T, Pietropaolo S, Feldon J. The expression of prepulse inhibition of the acoustic startle reflex as a function of three pulse stimulus intensities, three prepulse stimulus intensities, and three levels of startle responsiveness in C57BL6/J mice. Behavioural brain research. 2005 Sep 8;163(2):265–76. doi: 10.1016/j.bbr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain research reviews. 1995 Nov 30;21(3):301–14. doi: 10.1016/0165-0173(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Lee J, Yeomans MH, Steidl S, Li L. Midbrain pathways for prepulse inhibition and startle activation in rat. Neuroscience. 2006 Nov 3;142(4):921–9. doi: 10.1016/j.neuroscience.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Bosch D, Alves N, Daros A, Ure RJ, Schmid S. GABA receptors and prepulse inhibition of acoustic startle in mice and rats. European Journal of Neuroscience. 2010 Jun 1;31(11):2053–61. doi: 10.1111/j.1460-9568.2010.07236.x. [DOI] [PubMed] [Google Scholar]
- Young JS, Fechter LD. Reflex inhibition procedures for animal audiometry: A technique for assessing ototoxicity. The Journal of the Acoustical Society of America. 1983 May 1;73(5):1686–93. doi: 10.1121/1.389391. [DOI] [PubMed] [Google Scholar]
- Yun SW, Platholi J, Flaherty MS, Fu W, Kottmann AH, Toth M. Fmrp is required for the establishment of the startle response during the critical period of auditory development. Brain research. 2006 Sep 19;1110(1):159–65. doi: 10.1016/j.brainres.2006.06.086. [DOI] [PubMed] [Google Scholar]