Abstract
Objectives
Smokers on chronic opioid therapy for non-cancer pain use prescription opioids at higher dosages and are at increased risk for opioid misuse and dependence relative to non-smokers. The current study aims to assess whether smoking is associated with problems and concerns with chronic opioid therapy from the perspective of the patient.
Methods
In a large sample (N = 972) of adult patients prescribed opioids for chronic noncancer pain, we examined sex-specific associations between smoking status and patient perceptions of problems and concerns with chronic opioid therapy using regression analyses, adjusting for covariates.
Results
The sample self-identified as 27% current smokers, 44% former smokers, and 29% never smokers. Current smoking (vs. never smoking) was associated with increased odds of an opioid use disorder among males and females, and higher daily opioid dose among males only. Current and former smokers reported significantly fewer problems with opioids relative to never smokers, and this was driven primarily by lower endorsement of problems that are affected by the stimulant properties of nicotine (e.g., difficulties thinking clearly, felt less alert or sleepy).
Discussion
This study contributes to an understanding of perceived problems and concerns with chronic opioid therapy among current, former, and never smokers with chronic noncancer pain. Results suggest that current and former smokers may be a difficult population to target to decrease chronic opioid therapy, given that they perceive fewer problems with prescription opioid use, despite higher odds of having an opioid use disorder (males and females) and greater opioid doses (males only).
Keywords: Prescription opioids, chronic pain, smoking, opioid misuse
INTRODUCTION
Co-occurrence of cigarette smoking and chronic pain is a critical public health concern.1, 2 Smoking is associated with increased risk of developing chronic pain,3–7 and there is a dose-response relationship between the number of cigarettes smoked per day and the likelihood of experiencing certain types of chronic pain.1 Smokers report a greater number of painful physical sites, greater pain intensity,8 and more functional impairment and long-term disability relative to non-smokers.4 Further, greater nicotine dependence is associated with more severe pain symptoms.9
Conversely, chronic pain contributes to smoking maintenance and nicotine dependence.1 Individuals with chronic pain often smoke cigarettes to provide a distraction, alleviate physical discomfort1, 10, 11 and cope with pain-related anxiety.12 Nicotine may have short-term analgesic effects, reducing sensitivity to pain and awareness of certain painful stimuli (e.g., through the activation of nicotinic acetylcholine receptors (nAChRs), hypothalamic-pituitary-adrenocortical activity, and the release of norepinephrine and serotonin).1, 4 Likewise, smoking following nicotine deprivation increases tolerance for pain, whereas nicotine withdrawal is associated with increased pain sensitivity.4
Despite the potential short-term pain inhibitory effects of nicotine, chronic exposure to tobacco smoke may sensitize pain receptors and increase pain sensitivity over time.1 Paradoxically, smokers use significantly more analgesic medications and require higher doses relative to non-smokers,1 and they are more likely than non-smokers to be on long-term opioid therapy for chronic pain.13 Further, smoking status may be an indicator of risk for problems with prescription opioids, including aberrant prescription opioid use (e.g., forging prescriptions, doctor shopping)14 and development of opioid dependence among chronic pain patients.15
Although a number of studies have focused on smokers with chronic pain,1, 4 many are limited to methadone patients, and understanding of elevated risk for opioid misuse among smokers prescribed opioids for chronic noncancer pain is limited. First, to our knowledge, no studies have examined whether smoking status is associated with patients’ perceived difficulties with chronic opioid therapy. Understanding smokers’ personal experiences with prescription opioids may illuminate psychosocial difficulties important to patients (e.g., reduced activity levels, changes in mood and cognition) that differ from their clinicians’ perceptions (e.g., abuse and misuse).16 Second, few studies have adjusted for behavioral health conditions associated with smoking and pain or distinguished former and never smokers.1 Because smoking may cause changes in the central nervous system that can persist after a person has quit smoking,4 and former smokers may differ behaviorally from non-smokers, differentiating never and former smokers when examining smoking, pain, and opioid use is important. Finally, despite evidence that female chronic pain patients are prescribed opioids more frequently,17–21 have lower levels of opioid analgesia,4, 22 utilize a lower quantity of opioids, 7 and are at decreased risk for opioid misuse and overdose23 relative to males, few studies have examined sex-specific associations between smoking status and opioid-related outcomes among chronic pain patients.
Given the current opioid epidemic and overdose deaths,24, 25 identifying factors pertinent to patients’ perceived problems and concerns with prescription opioid use is critical. The current study examines sex-specific associations between smoking status and patient-reported problems and concerns with chronic opioid therapy among patients prescribed opioids for non-cancer chronic pain, adjusting for key covariates. We hypothesized that smokers would report a greater number of perceived problems and concerns with chronic opioid therapy, and that the association between smoking status and perceived problems and concerns with chronic opioid therapy would vary by sex.
MATERIALS AND METHODS
Setting
We used data from the National Institute on Drug Abuse-funded Consortium to Study Opioid Risks and Trends study (CONSORT), which was developed to study long-term use of opioids for non-cancer pain among adults in two integrated health care systems: Group Health Cooperative in Washington State and Kaiser Permanente of Northern California (KPNC).26 The current study is limited to KPNC patients, for whom there was more detailed information on smoking behaviors. KPNC is an integrated healthcare delivery system that provides comprehensive health care services to >3.9 million members. The membership is largely employed, working and middle class, and is highly representative of the racially and socio-economically diverse northern California population. All study protocols were approved by the KPNC Institutional Review Board.
Sample Selection
Health plan members aged 21–80 were eligible if they met criteria for chronic opioid therapy for pain (filled >10 opioid prescriptions and/or received >120 day supply in the year prior to sampling, with at >190 days between the first and last opioid fill in that year; these factors predict high risk for sustained and frequent use of opioids27). The sample was limited to members with continuous KPNC enrollment for >1 year before sampling. Patients who had 2+ cancer diagnoses in automated visit records in the year prior to sampling or received a cancer diagnosis (except non-melanoma skin cancer) were excluded, as were patients using buprenorphine and methadone for opioid use disorder. PROC SURVEYSELECT was used to sample equal numbers of eligible patients within three daily dosage ranges in the 90 days before the sample selection date (1–49mg, 50–99mg, and 100+mg Morphine Equivalent Dose) to ensure patients with higher dosage levels were included despite their lower prevalence within KPNC.
Interviews were conducted from January 2008–October 2009 by experienced non-clinician survey interviewers. Potentially eligible patients were mailed a letter explaining the study and a $5 gift card pre-incentive with a toll free number to call to decline participation or contact study investigators for additional information. Interviewers obtained verbal consent and used Computer-Assisted Telephone Interview technology to complete the 45-minute interview. Participants received a $50 gift card in the mail for participating. Overall, 1,605 patients at KPNC were approached, out of which 105 were ineligible, and 972 interviews were completed, leading to a response rate of 65%. Response rates were similar across age groups and genders, and increased with higher average daily dose (58% for <50 mg. MED, 66% for 50–99 mg. MED, and 71% for 100+ mg. MED).28
Measures
Demographic Characteristics
Demographic variables included gender, age (21–43; 44–64; 65+), employment status (unemployed/unable to work for health reasons versus employed), education level, income level, body mass index (BMI), and race/ethnicity (Black, Hispanic, non-Hispanic White, Other). For analyses limited by sample size in certain strata, we collapsed the “other” race category with the Hispanic group because the two groups were most similar in their prescription opioid use and other characteristics.
Smoking Status
Individuals reported whether they were current, former, or never smokers by answering the survey question, “Are you a current smoker, an ex-smoker, or have you never smoked?”
Average Daily Opioid Dose
KPNC maintains automated pharmacy data for all covered services, including those rendered by outside providers who bill the health plan. Data on opioid prescription fills and dosage were extracted from the electronic health record (EHR), which contains one record per drug dispensed, generic drug name, strength, directions for use, quantity and date dispensed, days’ supply, prescription identification number, and national drug code. Health-plan surveys have shown that more than 90% of patients obtain their prescriptions through KPNC pharmacies.29 Average daily opioid dose was categorized: <50mg/day, 50–99mg/day, and 100+ mg/day; the daily dose was estimated as the total morphine equivalent dose (MED) during the 90 days preceding the interview, divided by ninety.28
Problems and Concerns with Opioids
Problems and concerns attributed to opioids by patients in the past two weeks were measured using the Prescribed Opioid Difficulties (PODS) Scale, which includes a problems subscale and a concerns subscale.16, 30, 31 The problems subscale includes 8 items describing interference and side effects associated with opioid use: lost interest in usual activities; trouble concentrating or remembering; felt slowed down or sedated; felt depressed, down, or anxious; side effects interfered with work, family, or social responsibilities; difficulties thinking clearly; felt less alert or sleepy while driving, operating machinery, or doing something else that required being alert, and extent of side effects severity/interference. The concerns subscale includes 7 questions that assess the degree to which the user endorses various types of worries about opioid use: felt preoccupied with opioid use; felt unable to control how much or how often used opioids; needed a higher dose to get the same effect; worried that might be dependent on or addicted to opioids; wanted to stop using or cut down on opioids; opioids have caused problems with family, friends, co-workers in past year; and family or friends thought addicted to or dependent on opioids.
Respondents answered how much they agreed with each statement (strongly disagreed, disagreed, neutral, agreed, or strongly agreed), or specified the frequency of experiencing a given event (never, rarely, sometimes, often, always or almost every day), on a scale of 0–4. The individual scores for each question contributed to each respondent’s total score on the subscale. Patient subscale scores were categorized (low, intermediate, high) based on recommended cutpoints of 0–7 (low), 8–15 (intermediate) and 16+ (high); a cutpoint of 8 was used for the medium score because this required endorsing at least two difficulties, and 16 was used for the high score because this required endorsement of at >4 difficulties.16, 30, 31
A dichotomous variable was also created to denote whether patients had experienced a given problem (strongly agree or agree = 1, else = 0; always or almost every day, or often = 1, else = 0).
Pain Intensity
Pain intensity in the past 3 months was measured using the Graded Chronic Pain Scale.32–34 Responses to three self-reported pain intensity items: pain right now, usual pain and worst pain (each ranged 0–10) were averaged resulting in a 0–10 score of overall pain.
Behavioral Health Conditions
Opioid and non-opioid substance use disorder ICD-9 diagnoses in the past three years were extracted from the EHR.
Participants were coded as having a self-reported alcohol or drug problem if they responded yes to “Have you ever had an alcohol or drug problem (except tobacco use)?” on the survey. Patients were coded as having used illegal drugs if they reported using the following substances without a medical prescription: cannabis, cocaine, amphetamine stimulants, inhalants, sedatives, hallucinogens, or opiates.
Depression was measured using the 8-item version of the Patient Health Questionnaire (PHQ), a validated and widely used self-report measure of depression.35 Scores >10 were classified as depression.36
Statistical Analysis
All statistical analyses were performed in SAS 9.3. Crude frequencies of prescription opioid use, pain perception, and demographic variables were calculated within the three strata of smoking status (never, former, current). Conditional logistic regression and linear regression were used to calculate measures of association between smoking status and opioid characteristics. PROC SURVEYLOGISTIC and PROC SURVEYREG were used to account for weights in the survey sample, as patients taking higher doses of opiates were oversampled.
For categorical outcomes, ordinal logistic regression was used to estimate associations with smoking status. Ordinal models were retained for precision if the proportional odds assumption was met, indicated by a non-significant Score test. Because the Score test is highly sensitive to sample size and often yields a significant p-value despite the presence of an inherently ordered outcome, we plotted the individual logit functions for ordered outcomes that produced a significant p-value under the Score test and assessed whether they were parallel. Parallel logits indicate that the use of an ordinal or ordered model is appropriate, despite a significant Score test. Based on the results of these diagnostics, all multinomial outcomes except race were modeled using ordinal logistic regression. In ordinal regression models, the odds ratio can be interpreted as comparing each possible combination of higher categories to the lower categories; for example, it is assumed that the same odds ratio describes the association comparing high vs. medium and low categories, as well as high and medium vs. low categories. This assumption is supported by the proportional odds assumption and parallel logits.
Analyses were stratified by sex to examine sex-specific associations between smoking and opioid use characteristics. We utilized a change-in-estimate approach to retain covariates that were meaningfully associated with the exposure and outcome, and also included covariates that are well-supported in the literature.37–39 Models were adjusted for pain intensity, BMI, race, age, depression, employment status, average daily dose, and substance use disorder, except when the given covariate was the outcome of interest. Covariates removed to maintain parsimony in the model based on the change-in-estimate approach included education level, income level, and illegal drug use.
Finally, we ran bivariate logistic regression models with individual PODs problems items as the outcome to inform the results of multivariate regression models. Statistical significance was evaluated at α = 0.05.
RESULTS
Descriptive Statistics
The sample (N = 972, 37% male, 76% non-Hispanic Caucasian) identified as 27% current, 44% former, and 29% never smokers. Other descriptive data for the sample overall and stratified by sex are presented in Table 1.
Table 1.
Demographics, behavioral characteristics, and pain and opioid characteristics by sex and smoking status
| Females Smoking Status | Males Smoking Status | Overall Smoking Status | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Variables | Current n=160 (23.9%) |
Former n=260 (42.3%) |
Never n=196 (33.8%) |
Current n=97 (23.1%) |
Former n=172 (51.1%) |
Never n=87 (25.8%) |
Current n=257 (23.6%) |
Former n=432 (45.6%) |
Never n=283 (30.9%) |
|
|
|||||||||
| Demographics | |||||||||
| Age, yrs (%) | |||||||||
| 21–44 | 19 | 18 | 25 | 32 | 17 | 20 | 24 | 18 | 24 |
| 45–64 | 65 | 50 | 52 | 57 | 58 | 60 | 61 | 54 | 55 |
| 65+ | 18 | 32 | 22 | 11 | 25 | 20 | 15 | 29 | 22 |
| Mean BMI (SD) | 29.5 (8.68) | 32.4 (9.87)* | 28.1 (6.38) | 28.5 (5.21) | 30.5 (6.89)* | 29.6 (5.90) | 28.6 (0.55) | 30.3 (0.65) | 29.2 (0.66) |
| Some college education (%) | 52 | 60 | 66 | 46* | 57 | 69 | 50* | 58 | 67 |
| Racea (%) | |||||||||
| Black | 13 | 6* | 16 | 11 | 5 | 7 | 12 | 5 | 13 |
| Hispanic | 10 | 9 | 12 | 20* | 11 | 7 | 14 | 10 | 11 |
| White (reference) | 75 | 83 | 69 | 67 | 79 | 81 | 72 | 81 | 72 |
| Other | 2 | 2 | 3 | 3 | 5 | 5 | 2 | 3 | 4 |
| Unemployedb (%) | 48* | 34 | 32 | 32 | 19 | 22 | 42* | 32 | 29 |
| Income <$50,000 (%) | 71* | 58 | 48 | 44 | 50* | 29 | 61* | 55* | 42 |
| Behavioral Characteristics | |||||||||
| Depressed (PHQ > 10) (%) | 59 | 45 | 49 | 48 | 32 | 37 | 55 | 40 | 45 |
| Non-opioid substance use disorderc (%) | 23* | 12 | 9 | 31* | 18 | 15 | 26* | 15 | 11 |
| Opioid use disorderc (%) | 13* | 7 | 5 | 13* | 4 | 4 | 13* | 6 | 5 |
| Self-reported alcohol/drug problem (%) | 33* | 22* | 11 | 45* | 35 | 27 | 37* | 27* | 16 |
| Pain and Opioid Characteristics | |||||||||
| Average pain intensityd (SD) | 6.94 (1.44) | 6.67 (1.39) | 6.83 (1.67) | 6.16 (1.40) | 6.12 (1.51) | 5.75 (1.52) | 6.66 (0.14) | 6.45 (0.09) | 6.49 (0.15) |
| Mean daily opioid dosed, mg MED (SD) | 90.6 (144) | 93.2 (153) | 79.5 (191) | 160 (325)* | 88.2 (194)* | 56.0 (86.5) | 116 (13.7)* | 91.1 (8.05) | 72.2 (7.6) |
| Daily opioid dosed, mg MED (%) | |||||||||
| 1–49 | 58 | 57 | 66 | 56* | 65 | 71 | 57* | 60 | 68 |
| 50–99 | 18 | 19 | 15 | 14* | 18 | 16 | 17* | 18 | 15 |
| 100+ | 24 | 25 | 19 | 30* | 17 | 13 | 26* | 22 | 17 |
| Opioid problems (%) | |||||||||
| Low: 0–7 | 82 | 79 | 72 | 74 | 82* | 67 | 79 | 80* | 70 |
| Medium: 8–15 | 11 | 13 | 13 | 11 | 12* | 13 | 11 | 12* | 13 |
| High: 16+ | 8 | 8 | 15 | 15 | 6.5* | 20 | 10 | 18* | 17 |
| Opioid concerns (%) | |||||||||
| Low: 0–7 | 61 | 64 | 63 | 52 | 61 | 59 | 58 | 63 | 62 |
| Medium: 8–15 | 28 | 28 | 25 | 28 | 27 | 26 | 28 | 27 | 25 |
| High: 16+ | 11 | 8 | 12 | 20 | 12 | 15 | 14 | 10 | 13 |
Notes. All percentages are weighted by survey sampling weights.
Significantly different from never smokers, p<.05;
=Un-ordered outcome modeled using polytomous logistic regression. Ordinal logistic regression used for all ordered outcomes unless otherwise specified;
= Includes those unable to work for health reasons; Does not include homemaker;
= Based on diagnoses recorded in EHR in past 3 years;
= In past 3 months.
Females
Relative to never smokers, current smokers had a lower income, were more likely to be unemployed, and more likely to have an opioid (13% vs. 5%) or non-opioid (23% vs. 9%) substance use disorder. Former smokers had a higher BMI and were less likely to be black (6% vs. 16%) than never smokers. Current (33%) and former smokers (22%) were also more likely than never smokers (11%) to endorse a history of an alcohol or drug use problem, and had slightly higher doses of opioids than never smokers (~90 vs. ~80 mg MED).
Males
Relative to never smokers, current smokers were less likely to have at least some college education, more likely to be Hispanic, more likely to have an opioid (13% vs. 4%) or non-opioid substance use disorder (31% vs. 15%), and more likely to endorse a history of an alcohol or drug use problem (45% vs. 27%). Current smokers were prescribed higher daily doses of opioids on average than never smokers (160 vs. 56 mg MED) (p’s <0.05). Relative to never smokers, former smokers had a higher BMI, lower income, higher daily dose of opioids (88 vs 56 mg MED), and were less likely to report medium or high levels (vs. low levels) of problems with opioids.
Multivariate Regression Analyses
Results examining sex-specific associations between smoking status and pain and opioid characteristics are presented in Table 2. Current smoking was associated with greater opioid doses among males (p=0.01) but not among females. For both sexes, current smoking was associated with a two-to-threefold increase in odds of an opioid use disorder, substance use disorder, or self-reported problem with alcohol or drugs. These associations were non-significant among males, likely due to power limitations. However, for both sexes, current and former smokers had significantly lower odds of self-reported problems with opioids relative to never smokers (ORs from 0.34 to 0.53, ps<.05). Smoking status was not associated with pain intensity or reported concerns with opioids.
Table 2.
Adjusted associations between smoking status and pain and opioid characteristics
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Current vs. Never Smoker | Former vs. Never Smoker | Current vs. Never Smoker | Former vs. Never Smoker | |||||
|
|
||||||||
| Adjusted β or OR (95% CI)a | P-value | Adjusted β or OR (95% CI)a | P-value | Adjusted β or OR (95% CI)a | P-value | Adjusted β or OR (95% CI)a | P-value | |
| Pain Intensity1 | −0.051 (−0.52, 0.42) | 0.83 | −0.17 (−0.61, 0.27) | 0.45 | 0.22 (−0.38, 0.82) | 0.47 | 0.31 (−0.15, 0.78) | 0.19 |
| Mean Daily Opioid Dose1 | −4.10 (−34.5, 26.3) | 0.79 | 15.2 (−12.8, 43.2) | 0.29 | 81.5 (19.0, 144.1) | 0.011 | 21.73 (−13.4, 56.8) | 0.22 |
| Problems Sub-scale2 | ||||||||
| Low: 0–7 | ||||||||
| Med: 8–15 | 0.36 (0.17, 0.75) | 0.006 | 0.53 (0.30, 0.96) | 0.035 | 0.42 (0.17, 1.02) | 0.05 | 0.34 (0.16, 0.73) | 0.006 |
| High: 16+ | ||||||||
| Concerns Sub-scale2 | ||||||||
| Low: 0–7 | ||||||||
| Med: 8–15 | 0.88 (0.48, 1.61) | 0.67 | 0.94 (0.54, 1.64) | 0.82 | 0.86 (0.41, 1.83) | 0.70 | 0.876 (0.423, 1.82) | 0.72 |
| High: 16+ | ||||||||
| Non-opioid substance use disorder | 2.47 (0.92, 6.62) | 0.07 | 1.47 (0.60, 3.57) | 0.40 | 2.27 (0.81, 6.33) | 0.12 | 1.13 (0.46, 2.80) | 0.79 |
| Opioid use disorder | 2.30 (0.998, 5.32) | 0.05 | 1.56 (0.69, 3.53) | 0.29 | 2.98 (0.85, 10.4) | 0.09 | 1.05 (0.34, 3.22) | 0.94 |
| Self-reported alcohol/drug problem | 3.57 (1.61, 4.73) | 0.002 | 2.26 (1.08, 4.73) | 0.031 | 2.15 (0.86, 5.40) | 0.10 | 1.63 (0.77, 3.47) | 0.21 |
Notes.
= Continuous variables modeled using linear regression. Logistic regression used unless otherwise specified;
= Sub-scales from the Prescribed Opioids Difficulties Scale (PODS);
= Adjusted for average pain intensity, BMI, race, age, depression, employment status, average daily dose, and substance use disorder, except when given covariate was the outcome of interest.
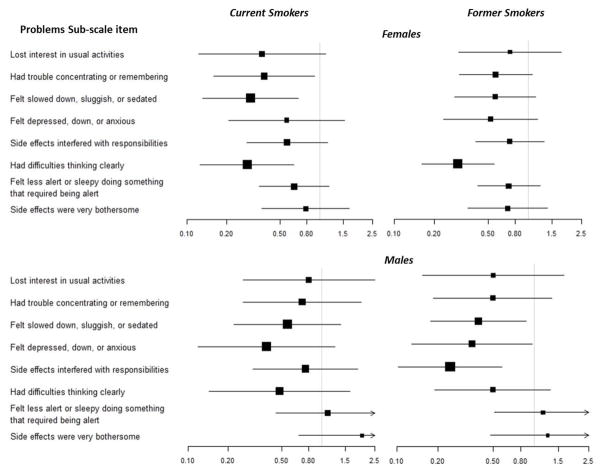
The odds of reporting specific problems from the POD problem subscale are reported in Figure 1, stratified by sex and smoking status. In general, current and former smokers had lower odds of endorsing problems with the sedating properties of opioids (e.g., difficulties thinking clearly, felt slowed down or sedated).
Figure 1.
Unadjusted odds ratios for reporting specific problems1, stratified by sex and smoking status
Notes. 1 = Problems score derived from the problems-subscale of the Prescribed Opioids Difficulties Scale. Reference group = never smokers for all analyses. Arrows on confidence bands = 95% CIs extend past visible graph.
DISCUSSION
Given the current opioid epidemic and increased interest in initiatives to reduce prescription opioid use,24, 25 identifying factors related to patients’ motivation to reduce chronic opioid use is critical. Smoking status appears to be an indicator of risk for problems with opioids, including aberrant opioid use14 and the development of opioid dependence among chronic pain patients.15 To our knowledge, this study is the first to examine whether current, former, and never smokers with chronic non-cancer pain attribute different problems and concerns to using prescription opioids.
Current smoking was associated with opioid use disorders and general substance use disorders among both males and females. Consistent with prior research,4 current and former smokers had substantially higher daily doses of prescription opioids relative to never smokers (significant only for males). This is in line with prior findings that despite the analgesic effects of nicotine, chronic cigarette smoking may result in tolerance to nicotine and reduced pain inhibition, possibly mediated by changes in the endogenous opioid system.1 However, current smoking was only associated with a large increase in opioid dose among males, suggesting that male smokers are still a high risk group for problematic opioid use who continue to use much higher doses than their non-smoking counterparts. Current smoking was not associated with greater pain intensity; perhaps smokers were already prescribed higher doses of opioids to manage increased pain sensitivity attributable to chronic smoking. Similar to prior studies of patients with chronic pain,1 current smokers were more likely than never smokers to have a substance use disorder (including opioids).
Current and former smokers were distinct from never smokers in their perceptions of problems with prescription opioids, indicating different implications by sex given key differences in dosage and behavior patterns. Current and former smokers reported significantly fewer problems with prescription opioids, even after adjusting for covariates. That the association between smoking status and perceived opioid-related problems persisted despite increased odds of an opioid use disorder (among males and females) and elevated opioid doses (among males) is interesting. Prior research on patients’ perceived problems with chronic opioid therapy indicates that patients who report fewer opioid-related problems are more likely to sustain higher-dose opioid use at one year follow-up.30 These findings suggest that current and former smokers are a difficult population to target regarding decreasing or stopping chronic opioid therapies, given their propensity for perceiving fewer drawbacks to prescription opioid therapy despite a greater likelihood of diagnosed abuse and dependence. Male chronic pain patients are at increased risk for opioid misuse relative to females,23 and males who initiate opioid use for chronic noncancer pain may be more likely than females to escalate to higher doses and die from opioid overdose.40 Thus, the low levels of reported problems with opioids among male current and former versus never smokers despite elevated opioid doses are concerning.
Whether lower reported problems with opioids among current and former smokers are attributable to under-reporting versus actually perceiving fewer problems with opioids is unclear. However, if smokers were under-reporting symptoms due to social desirability, we would also expect to see lower reported scores on the concerns subscale of the PODS which includes more stigmatized questions about control of opioid medications directly related to opioid abuse (e.g., preoccupation with opioid medication and loss of control). We found that current and former smokers were similar to never smokers in their concerns about control of opioid medication use, suggesting that opioids were well tolerated, and lower reported problems with opioids among current and former smokers were not simply due to under-reporting.
Findings highlight current smokers as at risk for opioid use disorders, but less likely to attribute problems to their use of prescription opioids. Clinicians should address the disconnect between provider-defined problems and smokers’ perceptions of opioid-related problems. Smokers’ lack of perceived problems with opioids may help explain why some studies find poorer pain treatment outcomes in smokers with chronic pain.41 For example, Hooten and colleagues found that current smokers (24%) were significantly more likely than former (10%) and never smokers (13%) to drop out of a pain rehabilitation program, although those who completed treatment were as likely as former and never smokers to successfully taper off.41 Lower endorsement of perceived problems with prescription opioids among current and former smokers appears to be driven by items that are affected by the stimulant properties of nicotine (e.g., difficulties thinking clearly, felt less alert or sleepy, felt slowed down, sluggish or sedated). Current and former smokers who attribute fewer of these negative side effects to opioids relative to never smokers may be less motivated to complete pain treatments incorporating opioid tapering. Alternatively, although smoking prevalence in the US is declining, the rate of smoking among adults with chronic pain has not declined,42 and smokers who seek treatment for chronic pain may face increasing smoking-related stigma, contributing to early dropout.
Most opioid-dependent smokers are motivated to quit smoking43 and greater pain is not associated with reduced interest in quitting smoking;7, 44 yet, quitting success rates are low in this population.45 Opioid-maintained patients have poor adherence to tobacco cessation medications,46, 47 and tobacco cessation medications may be less effective among opioid-dependent smokers.48 Physicians prescribing opioids for patients with chronic pain are well positioned to support attempts to quit smoking, as smoking cessation among patients receiving opioid treatment does not cause poorer opioid outcomes49 and there is a great opportunity to better serve patients on chronic opioid therapy by embedding tobacco cessation treatment within each patient contact. Given the complex interactions between pain, smoking and opioid medications, careful monitoring of opioid dose following smoking abstinence also is important, as painful symptoms and nicotine withdrawal may complicate short-term efforts to treat pain.4 Initial data support the efficacy of a cognitive behavioral smoking abstinence intervention for adults with chronic pain that was integrated into the treatment protocols of an outpatient multidisciplinary pain rehabilitation program.50 Additional research is needed to further develop and test efficacious, patient-centered interventions for tobacco smoking that are integrated into clinical treatment for chronic pain.
KPNC provides a well-identified population supported by rich, longitudinal data sources. KPNC’s integrated health system with a robust EHR allowed for data validation of substance use disorder history and granular information regarding opioid use and dosage. However, many outcomes of interest were self-reported and the survey did not include details on heaviness of smoking (for current smokers), duration of abstinence from cigarettes (for former smokers), or duration of time on opioids. In addition, opioid dosage information was based on data from the EHR. Given that individuals who misuse prescription opioids may escalate their use without a doctor’s permission (e.g., by obtaining additional prescriptions or purchasing opioids illegally), EHR dosage information may underestimate patients’ actual use. Further, current and former smokers may be more likely to receive an opioid use disorder diagnosis with similar or fewer abuse and dependence symptoms than never smokers (e.g., due to provider bias, being flagged as risk takers). Finally, the study was limited by a relatively small sample size of males, limiting power to assess associations in this group.
This study contributes to an understanding of perceived problems and concerns with chronic opioid therapy among current, former, and never smokers with chronic non-cancer pain. Current and former smokers attributed fewer problems to their use of prescription opioid medications relative to never smokers despite increased odds of meeting the criteria for an opioid use disorder in the past three years. Smokers with chronic pain present complex cases for healthcare providers, who should provide a collaborative framework for discussing the bi-directional relation between smoking and pain. Clinicians’ conversations with smokers on chronic opioid therapy should include a patient-centered assessment of opioid-related problems and concerns, education about coping responses other than smoking cigarettes to manage pain, advice to quit, and assistance with tobacco cessation. In addition, large discrepancies in opioid dose between male and female current smokers, despite increased risk of opioid use disorders in both groups, suggests that male and female smokers may require varied approaches to pain management to prevent future problematic opioid use. Further, providers should distinguish former and never smokers during treatment for chronic pain, as both current and former smokers may benefit disproportionately from psychoeducation about the relation between prescription opioids and problems with mood and cognition, and the provision of tools to help them recognize and monitor psychosocial difficulties attributed to chronic opioid therapy. Given the significant negative health consequences of smoking and opioid addiction and the dearth of research on smokers’ experiences with prescription opioids, additional studies are needed. Of specific interest is whether and how smoking interacts with opioid medications to produce fewer perceived negative side effects among current and former smokers, despite their elevated risk for opioid use disorders.
Supplementary Material
Footnotes
For Submission to Clinical Journal of Pain
References
- 1.Ditre JW, Brandon TH, Zale EL, et al. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137(6):1065–93. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zvolensky MJ, McMillan K, Gonzalez A, et al. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tob Res. 2009;11(12):1407–14. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hestbaek L, Leboeuf-Yde C, Kyvik KO. Are lifestyle-factors in adolescence predictors for adult low back pain? A cross-sectional and prospective study of young twins. BMC Musculoskelet Disord. 2006;7:27. doi: 10.1186/1471-2474-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 2010;113(4):977–92. doi: 10.1097/ALN.0b013e3181ebdaf9. [DOI] [PubMed] [Google Scholar]
- 5.Scott SC, Goldberg MS, Mayo NE, et al. The association between cigarette smoking and back pain in adults. Spine (Phila Pa 1976) 1999;24(11):1090–8. doi: 10.1097/00007632-199906010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Palmer KT, Syddall H, Cooper C, et al. Smoking and musculoskeletal disorders: findings from a British national survey. Ann Rheum Dis. 2003;62(1):33–6. doi: 10.1136/ard.62.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooten WM, Townsend CO, Bruce BK, et al. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain Med. 2009;10(2):347–55. doi: 10.1111/j.1526-4637.2008.00494.x. [DOI] [PubMed] [Google Scholar]
- 8.John U, Hanke M, Meyer C, et al. Tobacco smoking in relation to pain in a national general population survey. Prev Med. 2006;43(6):477–81. doi: 10.1016/j.ypmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Weingarten TN, Moeschler SM, Ptaszynski AE, et al. An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician. 2008;11(5):643–53. [PubMed] [Google Scholar]
- 10.Hooten WM, Shi Y, Gazelka HM, et al. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–9. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooten WM, Vickers KS, Shi Y, et al. Smoking cessation and chronic pain: patient and pain medicine physician attitudes. Pain Pract. 2011;11(6):552–63. doi: 10.1111/j.1533-2500.2011.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ditre JW, Zale EL, Kosiba JD, et al. A pilot study of pain-related anxiety and smoking-dependence motives among persons with chronic pain. Exp Clin Psychopharmacol. 2013;21(6):443–9. doi: 10.1037/a0034174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–75. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michna E, Ross EL, Hynes WL, et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28(3):250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Fishbain DA, Cole B, Lewis JE, et al. Is smoking associated with alcohol-drug dependence in patients with pain and chronic pain patients? An evidence-based structured review. Pain Med. 2012;13(9):1212–26. doi: 10.1111/j.1526-4637.2012.01446.x. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan MD, Von Korff M, Banta-Green C, et al. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain. 2010;149(2):345–53. doi: 10.1016/j.pain.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoni-Wastila L, Ritter G, Strickler G. Gender and other factors associated with the nonmedical use of abusable prescription drugs. Subst Use Misuse. 2004;39(1):1–23. doi: 10.1081/ja-120027764. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MD, Edlund MJ, Steffick D, et al. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119(1–3):95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Parsells Kelly J, Cook SF, Kaufman DW, et al. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507–13. doi: 10.1016/j.pain.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 20.McCabe SE, Teter CJ, Boyd CJ. Illicit use of prescription pain medication among college students. Drug Alcohol Depend. 2005;77(1):37–47. doi: 10.1016/j.drugalcdep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhong W, Maradit-Kremers H, St Sauver JL, et al. Age and sex patterns of drug prescribing in a defined American population. Mayo Clin Proc. 2013;88(7):697–707. doi: 10.1016/j.mayocp.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97(5):1464–8. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 23.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24(6):497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 24.Park LS, Hernandez-Ramirez RU, Silverberg MJ, et al. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS. 2016;30(2):273–91. doi: 10.1097/QAD.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Korff M, Kolodny A, Deyo RA, et al. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325–8. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Korff M, Balderson BH, Saunders K, et al. A trial of an activating intervention for chronic back pain in primary care and physical therapy settings. Pain. 2005;113(3):323–30. doi: 10.1016/j.pain.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Saunders KW, Von Korff M, Campbell CI, et al. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain. 2012;13(3):266–75. doi: 10.1016/j.jpain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selby JV, Smith DH, Johnson ES, et al. Kaiser Permanente Medical Care Program. In: Strom BL, editor. Pharmacoepidemiology. New York: Wiley; 2005. pp. 241–59. [Google Scholar]
- 30.Thielke SM, Turner JA, Shortreed SM, et al. Do patient-perceived pros and cons of opioids predict sustained higher-dose use? Clin J Pain. 2014;30(2):93–101. doi: 10.1097/AJP.0b013e31828e361b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banta-Green CJ, Von Korff M, Sullivan MD, et al. The prescribed opioids difficulties scale: a patient-centered assessment of problems and concerns. Clin J Pain. 2010;26(6):489–97. doi: 10.1097/AJP.0b013e3181e103d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunders KW, Von Korff M, Campbell CI, et al. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J Pain. 2012;13(3):266–75. doi: 10.1016/j.jpain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Korff M, Dworkin SF, Le Resche L. Graded chronic pain status: an epidemiologic evaluation. Pain. 1990;40(3):279–91. doi: 10.1016/0304-3959(90)91125-3. [DOI] [PubMed] [Google Scholar]
- 34.Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain. 1992;50(2):133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–59. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders. 2009;114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease C and Prevention. Vital signs: current cigarette smoking among adults aged >/=18 years with mental illness - United States, 2009–2011. MMWR Morb Mortal Wkly Rep. 2013;62(5):81–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Jamal A, Homa DM, O’Connor E, et al. Current cigarette smoking among adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–40. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 39.Substance Abuse and Mental Health Services Administration and Center for Behavioral Health Statistics and Quality. The NSDUH Report: Adults with mental illness or substance use disorder account for 40 percent of all cigarettes smoked. Rockville, MD: Substance Abuse and Mental Health Services Administration; Mar 20, 2013. [Google Scholar]
- 40.Kaplovitch E, Gomes T, Camacho X, et al. Sex Differences in Dose Escalation and Overdose Death during Chronic Opioid Therapy: A Population-Based Cohort Study. PLoS One. 2015;10(8):e0134550. doi: 10.1371/journal.pone.0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooten WM, Townsend CO, Bruce BK, et al. The effects of smoking status on opioid tapering among patients with chronic pain. Anesth Analg. 2009;108(1):308–15. doi: 10.1213/ane.0b013e31818c7b99. [DOI] [PubMed] [Google Scholar]
- 42.Orhurhu VJ, Pittelkow TP, Hooten WM. Prevalence of smoking in adults with chronic pain. Tob Induc Dis. 2015;13(1):17. doi: 10.1186/s12971-015-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahvi S, Richter K, Li X, et al. Cigarette smoking and interest in quitting in methadone maintenance patients. Addict Behav. 2006;31(11):2127–34. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Hahn EJ, Rayens MK, Kirsh KL, et al. Brief report: pain and readiness to quit smoking cigarettes. Nicotine Tob Res. 2006;8(3):473–80. doi: 10.1080/14622200600670355. [DOI] [PubMed] [Google Scholar]
- 45.Richter KP, Gibson CA, Ahluwalia JS, et al. Tobacco use and quit attempts among methadone maintenance clients. Am J Public Health. 2001;91(2):296–9. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter KP, McCool RM, Catley D, et al. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. J Addict Dis. 2005;24(4):79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- 47.Stein MD, Caviness CM, Kurth ME, et al. Varenicline for smoking cessation among methadone-maintained smokers: a randomized clinical trial. Drug Alcohol Depend. 2013;133(2):486–93. doi: 10.1016/j.drugalcdep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller ME, Sigmon SC. Are pharmacotherapies ineffective in opioid-dependent smokers? Reflections on the scientific literature and future directions. Nicotine Tob Res. 2015;17(8):955–9. doi: 10.1093/ntr/ntv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn KE, Sigmon SC, Reimann E, et al. Effects of smoking cessation on illicit drug use among opioid maintenance patients: a pilot study. J Drug Issues. 2009;39(2):313–328. doi: 10.1177/002204260903900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooten WM, Townsend CO, Hays JT, et al. A cognitive behavioral smoking abstinence intervention for adults with chronic pain: a randomized controlled pilot trial. Addict Behav. 2014;39(3):593–9. doi: 10.1016/j.addbeh.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.