Abstract
OBJECTIVES
While several factors are known to contribute to ethnic differences in pain, relatively little attention has been devoted to physiological factors. Our first aim was to examine the relationship between cortisol and pain responses during a cold-pressor task (CPT) among African American (AA) and non-Hispanic White (NHW) adults with knee osteoarthritis (OA). Our second aim was to assess the relationship between perceived racial discrimination and cortisol among AA participants.
METHODS
Participants were 91 (56 AA; 35 NHW) community-dwelling adults between the ages of 45 to 85 with knee OA based upon the American College of Rheumatology clinical criteria. Plasma cortisol was measured at 3 time points: 1) baseline, 2) prior to the CPT, and 3) 20-minutes following the CPT. Perceived racial discrimination was measured by the Experiences of Discrimination scale.
RESULTS
Using linear regression, we found a significant interaction between ethnicity and cortisol prior to the CPT with pain intensity ratings (β = −0.26; P = 0.02). Analysis of simple slopes revealed that cortisol concentrations were negatively associated with pain intensity ratings in NHW participants (β = −0.54; P = 0.001), but not in AA participants (β = −0.15; P = 0.26). Perceived racial discrimination was not related to cortisol concentrations or pain ratings.
DISCUSSION
Consistent with previous findings in young healthy adults, cold pressor pain responses are related to pre-CPT cortisol concentrations in NHW persons with knee OA but not in their AA counterparts. Additional studies are required to better understand this finding.
Keywords: Ethnicity, Cortisol, Quantitative Sensory Testing, Perceived Racial Discrimination
INTRODUCTION
Past research has consistently reported significant differences in the experience of pain between African American (AA) and non-Hispanic White (NHW) persons. Among healthy adults, AA persons demonstrate greater pain sensitivity in response to several experimental pain stimuli (i.e., quantitative sensory testing or QST) compared to NHW persons (1). Among individuals with knee osteoarthritis (OA), AA persons report greater clinical and experimental pain severity as well as greater physical and psychological disability compared to NHW persons (2–4). While several psychosocial variables have been identified that are related to ethnic differences in pain (5–7), less emphasis has been devoted to physiological factors.
Mechlin and colleagues have extensively studied the role of physiological variables in ethnic differences in pain responses among healthy persons (8–10). For example, in response to the Trier Social Stress Test, young healthy AA participants exhibited lower pre- and post-stressor cortisol concentrations compared to NHW participants (8). After stressor exposure, participants underwent QST evaluation, including the cold-pressor task (CPT). Post-Trier Social Stress Test cortisol was positively associated with pain tolerance levels during the CPT in NHW participants, but not in AA participants (8), a finding that Mechlin and colleagues replicated in a subsequent study (9). Pain ratings were not captured in either study (8, 9). Further, no investigators have determined whether similar results occur among older persons with recurrent or persistent pain, such as knee OA.
Our efforts to better understand ethnic differences in pain led us to posit that prolonged exposure to chronic stressors among AA individuals, such as perceived racial discrimination, may alter endogenous stress regulatory systems that eventually contribute to enhanced pain sensitivity (11). Indeed, studies of healthy AA persons show that perceived racial discrimination is associated with chronic stress (12), as well as flatter (less healthy) diurnal cortisol rhythms (13). Additionally, perceived racial discrimination is associated with greater body pain (14) and back pain (15), as well as greater heat pain sensitivity among AA individuals with knee OA (11). However, the relationship between perceived racial discrimination and biomarkers of stress exposure, such as cortisol, has never been specifically examined in persons with knee OA.
The aim of the present study was to determine if the relationship between cortisol and pain responses during the CPT differ as a function of ethnicity among persons with knee OA. Specifically, we hypothesized that (a) AA participants, relative to NHW participants, would produce greater pain intensity and unpleasantness ratings during the CPT (1), and (b) cortisol concentrations obtained prior to the CPT would be negatively associated with pain intensity and unpleasantness ratings in NHW participants, but not in AA participants. A second aim was to assess the relationship between perceived racial discrimination and cortisol concentrations among AA participants. We hypothesized that (c) perceived racial discrimination would be negatively associated with cortisol concentrations, and positively associated with pain intensity and unpleasantness ratings during the CPT. In exploratory analyses, we examined the relationship of cortisol reactivity (i.e., cortisol change from pre-CPT to post-CPT) with pain intensity and unpleasantness ratings and ethnicity, as well as with perceived racial discrimination among AA participants.
MATERIALS AND METHODS
Participants
The present study was part of a larger ongoing project (Understanding Pain and Limitations in Osteoarthritic Disease, UPLOAD). The UPLOAD study is a multi-site investigation that recruits participants at the University of Alabama at Birmingham (UAB) and the University of Florida (UF). Only participants recruited from UAB were used in the current study.
Participants were 91 community-dwelling adults (56 AA; 35 NHW) recruited via posted fliers, radio and print media advertisements, orthopedic clinic recruitment, and word-of-mouth referral. Inclusion criteria were as follows: 1) between 45 and 85; 2) unilateral or bilateral symptomatic knee OA based upon the American College of Rheumatology clinical criteria; and, 3) availability to complete the two-session protocol (described below). Exclusion criteria were as follows: 1) prosthetic knee replacement or other clinically significant surgery to the knee; 2) uncontrolled hypertension, heart failure, or history of acute myocardial infarction; 3) peripheral neuropathy; 4) systemic rheumatic disorders including rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia; 5) daily opioid use; 6) cognitive impairment (Mini Mental Status Exam score ≤ 22); 7) excessive anxiety regarding protocol procedures (e.g., blood draws and controlled noxious stimulation procedures); and 8) hospitalization within the preceding year for psychiatric illness. All procedures were reviewed and approved by the UAB Institutional Review Board. Participants provided informed consent and were compensated for their participation.
Procedures
Health Assessment Session (Session 1)
During session 1, all participants completed an index of cognitive capacity, the Mini Mental Status Exam. Anthropometric and health data were also obtained, including current medications, smoking status, and BMI. Additionally, participants completed several psychosocial questionnaires, including the Experiences of Discrimination scale.
Experiences of Discrimination scale
The Experiences of Discrimination scale is a validated measure of lifetime occurrences of discrimination with good internal consistency (alpha = 0.74) and re-test reliability over a two to four week period (0.70) that was designed specifically for public health research (16). The questionnaire asks, “Have you ever experienced discrimination, been prevented from doing something, or been hassled or made to feel inferior in any of the following situations because of your race, ethnicity, or color?” This question is followed by 9 response options (e.g., at school, getting hired or getting a job, bank loans, etc.). Respondents also chose from the following responses, “never,” “once,” “two or three times,” or “four or more times.” Although there are additional ways to score this questionnaire (16), the frequency of experiences was chosen to be consistent with previous studies (11, 15).
Quantitative Sensory Testing (Session 2)
QST was completed during a second visit scheduled between 1 and 4 weeks after session 1. On the day of QST, participants indicated on a 0 to 100 scale the degree of clinical pain intensity in their index (i.e., most affected) knee, where 0 represents no pain and 100 represents the most intense pain imaginable (hereafter referred to as “OA pain intensity”). During QST, a number of standard pain testing procedures were performed prior to the CPT, including: heat pain threshold and tolerance; temporal summation of heat pain; pressure pain thresholds; and temporal summation of punctate mechanical pain. In the present study, we only assessed relationships between cortisol and CPT pain responses because the CPT is more strongly related to cortisol concentrations than other experimental pain procedures (17).
Cold Pressor Task (CPT)
Each participant completed a series of hand immersions in a cold water bath (Neslab, RTE-111, Portsmouth, NH) at temperatures of 16, 12, and 8° C, with 5-minutes separating each cold water exposure. The water was maintained at ± 0.1° C and continuously recirculated to maintain a constant temperature. Participants were first instructed regarding the differences between pain intensity and pain unpleasantness. Next, they placed their hand in the cold water bath up to their wrist for as long as possible up to 60 seconds. Participants were informed they could remove their hand from the cold water at any time. Immediately after participants removed their hand, they rated pain intensity and unpleasantness using a 0 to 100 numeric rating scale. A measure of cold pain tolerance (CPTo) was also captured. Specifically, CPTo was measured as the time at which participants removed their hand from the cold water. Thus, CPTo ranged from 0 to 60 seconds.
Cortisol
At the beginning of the QST session, a nurse placed an intravenous catheter in the arm opposite the arm used for sensory testing. Plasma cortisol was measured at three different time points: baseline (time 1), prior to completing the CPT (time 2), and 20 minutes after completing the CPT (time 3). For the majority of participants (64%), cortisol at time 1 was drawn between 9:00AM and 11:00AM (range: 9:00AM – 2:00PM). There were no significant differences in cortisol at time 1 as a function of time at which blood was collected. Plasma cortisol was assayed on a TOSOH 600 II analyzer using the immunofluorescence method (TOSOH Bioscience – South San Francisco, CA). Plasma cortisol was measured in 10ul aliquots. The limit of detection was 0.2ug/dl. The estimate of inter-assay variation was 0.69% and intra-assay variation was 5.19%. Assays were performed by the UAB Center for Clinical and Translational Science (CCTS) Physiology and Metabolism core.
Data Analysis
For our outcome variable, we used verbal reports of pain (i.e., pain ratings) as opposed to non-verbal reports of pain (e.g., CPTo). Because longer durations of CPT exposure are associated with greater cortisol production (18), assessing pain ratings while controlling for CPTo is a more direct way to investigate the relationship between cortisol and pain perception during the CPT. Pain intensity ratings at 16, 12, and 8° C were highly correlated (0.7–0.9), as were pain unpleasantness ratings at 16, 12, and 8° C (0.7–0.9). Therefore, in order to reduce the total number of analyses, pain intensity and pain unpleasantness ratings were averaged in order to make a single index of pain intensity and pain unpleasantness, respectively. Thus, the present study contained two outcome variables. It is important to assess pain intensity and pain unpleasantness separately because they represent different aspects of the pain experience (sensory-discriminative verses motivational-affective, respectively), and differences between AA and NHW individuals are more consistently shown in pain unpleasantness vs. pain intensity ratings (6, 19–20).
Chi-square analyses and independent samples t-tests were performed to assess ethnic differences on demographic and clinical variables that were subsequently used as control variables. These variables were age, sex (0 = female, 1 = male), education (0 = high school or less, 1 = some college or more), smoking status (0 = not a current smoker, 1 = current smoker), corticosteroid medication use (0 = no corticosteroid medication use, 1 = current use of corticosteroid medications) and BMI. Additionally, OA pain intensity ratings obtained at the beginning of the QST session was added as a covariate to control for variations in clinical knee pain. Cohen’s f2 are presented where appropriate for tests of linear relationships, with 0.02 considered a small effect, 0.15 a medium-sized effect, and 0.35 a large effect. All data were analyzed using SPSS, version 20 (IBM; Chicago, IL).
Hypothesis 1
Linear regression models were used to assess the relationship between ethnicity and pain ratings. Unadjusted models were first employed, followed by fully adjusted models.
Hypothesis 2
A repeated measures ANOVA was used to assess differences in cortisol concentrations across time points. Independent samples t-tests were used to assess ethnic differences in cortisol concentrations. To assess the relationship between ethnicity, cortisol concentrations obtained prior to the CPT, and pain ratings, we first computed interactions terms between ethnicity and cortisol. Prior to computing interaction terms, cortisol concentrations at time 1 and time 2 were centered by subtracting respective mean values from each score. Centering is recommended when assessing interactions with continuous variables because it reduces problems with multicollinearity among interaction variables, but does not alter correlations with other variables (21). Unadjusted linear regression models were first employed (i.e., models that contained ethnicity, cortisol, and the ethnicity by cortisol interaction), followed by fully adjusted models.
Hypothesis 3
Independent samples t-tests were used to determine ethnic differences in perceived racial discrimination. Because it was assumed NHW participants would show minimal levels of perceived racial discrimination, the relationship between perceived racial discrimination and cortisol, as well as pain ratings were only evaluated in AA participants. Linear regression models were used to assess the relationship between perceived racial discrimination and cortisol, as well as between perceived racial discrimination and pain ratings. Unadjusted models were first employed, followed by fully adjusted models.
Exploratory Analyses
In exploratory analyses, we were interested in assessing cortisol change from pre-CPT to post-CPT (i.e., cortisol reactivity). Exposure to the CPT reliably increases cortisol concentrations (22–24), with maximum levels observed between 20 and 30 minutes following completion of the task (24). To obtain a measure of cortisol reactivity, a change score was computed by subtracting cortisol concentrations at time 2 from cortisol concentrations at time 3. Thus, positive values would indicate an increase in cortisol from time 2 to time 3. An independent samples t-test was used to determine ethnic differences in cortisol reactivity. Bivariate correlations were used to inspect the relationship between pain ratings and cortisol reactivity, as well as between perceived racial discrimination and cortisol reactivity among AA participants. We also assessed the interaction between ethnicity and cortisol reactivity with pain ratings using a similar approach described above.
RESULTS
Demographic Characteristics
Characteristics of the overall sample are shown in Table 1. Participants were primarily female (61.5%) with a mean age of 56.13 (±7.75). Compared to NHW participants, AA participants were younger (P < 0.001) and reported lower education (P = 0.02), as well as greater OA clinical pain intensity (P = 0.01). There were no ethnic differences in sex, smoking status, use of corticosteroid medications, or BMI.
Table 1.
Sample characteristics
| Variable | Total Sample (N = 91) |
African American (n = 56) |
Non-Hispanic White (n = 35) |
P Value |
|---|---|---|---|---|
| Age | 56.13 (7.75) | 53.73 (6.54) | 59.97 (8.08) | <0.001 |
| Sex (female) | 56 (61.5) | 35 (62.5) | 21 (60.0) | 0.81 |
| Education | ||||
| (High school or less) | 34 (37.4) | 26 (46.4) | 8 (22.9) | 0.02 |
| BMI | 32.96 (7.89) | 34.05 (8.19) | 31.20 (7.14) | 0.09 |
| Current Smoker (yes) | 18 (19.8) | 14 (25.0) | 4 (11.4) | 0.10 |
| Corticosteroid | ||||
| Medication Use (yes) | 13 (14.3) | 6 (10.7) | 7 (20.0) | 0.22 |
| OA Pain Intensitya | 11.91 (20.44) | 16.23 (23.92) | 5.00 (10.05) | 0.01 |
| Perceived Racial | ||||
| Discrimination | 8.07 (11.08) | 12.39 (12.13) | 1.07 (2.29) | <0.001 |
| Pain Intensityb | 59.01 (28.58) | 62.79 (26.97) | 52.96 (30.41) | 0.57c |
| Pain Unpleasantnessb | 61.66 (27.84) | 65.91 (25.26) | 55.05 (30.66) | 0.44c |
Note: Data presented as means (SD) or count (%); BMI = body mass index;
clinical pain intensity in the most affected knee rated on a 0 to 100 scale on the day of the QST;
pain ratings during the cold pressor task rated on a 1 to 100 scale collapsed across the three different cold water exposures;
P value after controlling for age, sex, education, BMI, smoking status, corticosteroid medication use, OA pain intensity, and cold pain tolerance.
Hypothesis 1
There were no ethnic differences in clinical pain intensity ratings (see Table 1). There was a statistical trend for AA participants to report greater pain unpleasantness ratings during the CPT in the unadjusted model (p = 0.06); however, this relationship disappeared in the fully adjusted model (P = 0.44) (see Table 1). The majority of study participants (64%) reached tolerance (i.e., 60 seconds) at all three hand immersions, and there were no ethnic differences in CPTo.
Hypothesis 2
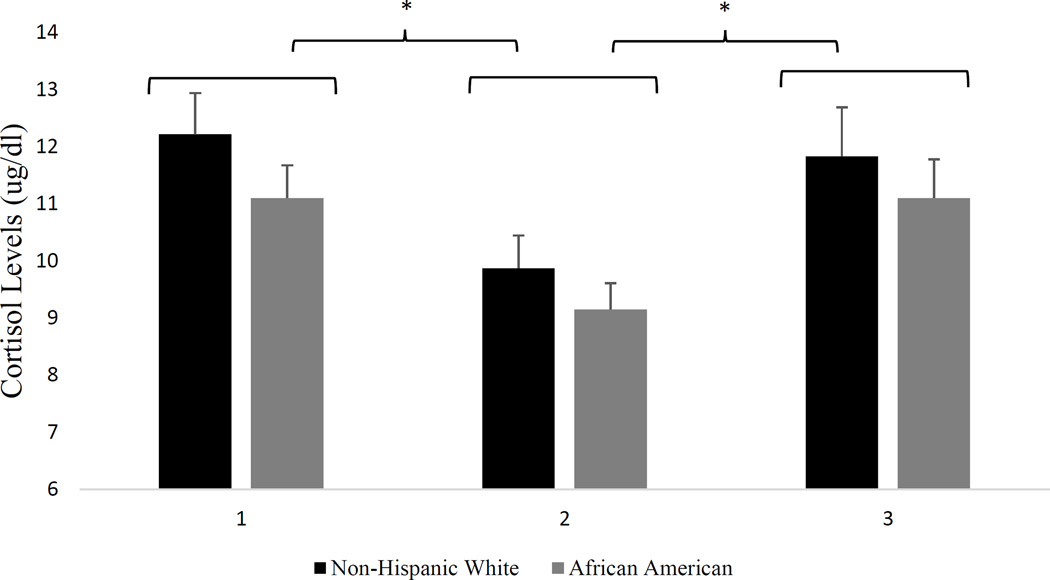
Results of the repeated measures ANOVA with a Greenhouse-Geisser correction revealed a significant main effect of cortisol (F1.4,126.08 = 14.54, P < 0.001). Post hoc tests using the Bonferroni correction revealed that cortisol concentrations significantly decreased from time 1 to time 2 (11.53 ± 4.27ug/dl vs. 9.43 ± 3.47ug/dl; P < 0.001), and significantly increased from time 2 to time 3 (9.43 ± 3.47ug/dl vs. 11.38 ± 5.06ug/dl; P < 0.001) (see Figure 1). There were no ethnic differences in cortisol concentrations at any time point.
Figure 1.
Cortisol concentrations plotted separately by ethnicity.
* (P < 0.001). Cortisol concentrations at time 1 were significantly greater than cortisol concentrations at time 2; Cortisol concentrations at time 3 were significantly greater than cortisol concentrations at time 2
Note: 1 = baseline cortisol concentrations; 2 = cortisol concentrations obtained prior to the cold pressor task; 3 = cortisol concentrations obtained 20 minutes after the cold pressor task
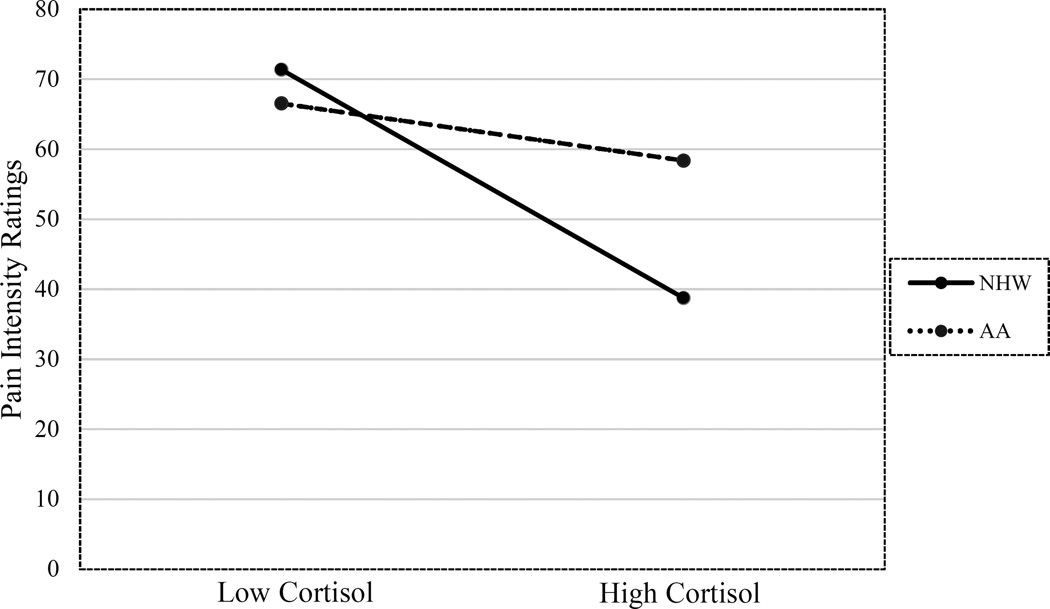
The interaction between ethnicity and cortisol was significantly related to pain intensity ratings in both unadjusted (P = 0.04) and fully adjusted models (P = 0.02) (see Table 2). Inspection of simple slopes revealed that cortisol at time 2 was inversely associated with pain intensity ratings in NHW (β = −0.54, P = 0.001; f2 = 0.39), but not AA participants (β = −0.15, P = 0.26; f2 = 0.02) (see Figure 2). Post-hoc power analysis using G*Power suggested we had 0.95 power to detect this interaction given α = 0.05, a medium effect size (f2 = 0.15), a sample size of 91, and 11 total number of predictors in the model. There were no significant interactions between ethnicity, cortisol concentrations, and pain unpleasantness ratings.
Table 2.
Linear regression analysis showing a significant interaction between ethnicity, cortisol concentrations obtained prior to the CPT, and pain intensity ratings during the CPT in persons with knee OA (N = 91).
| Variable | B (SE) | β | 95% CI | P value |
|---|---|---|---|---|
| Age | −0.06 (0.37) | −0.02 | −0.78, 0.67 | 0.88 |
| Sex | −7.65 (6.12) | −0.13 | −19.86, 4.55 | 0.22 |
| Education | −6.75 (5.59) | −0.12 | −17.88, 4.39 | 0.23 |
| Smoking Status | 6.19 (7.17) | 0.09 | −8.09, 20.47 | 0.39 |
| Corticosteroid | ||||
| Medication Use | 7.36 (7.60) | 0.09 | −7.78, 22.50 | 0.34 |
| BMI | 0.01 (0.35) | 0.00 | −0.69, 0.71 | 0.97 |
| OA Pain Intensity | 0.21 (0.14) | 0.15 | −0.06, 0.49 | 0.13 |
| CPTo | −13.86 (3.16) | −0.40 | −20.17, −7.56 | < 0.001 |
| Ethnicity | −2.83 (5.98) | −0.05 | −14.73, 9.08 | 0.64 |
| Cortisol | −0.45 (0.97) | −0.05 | −2.38, 1.48 | 0.65 |
| Ethnicity*Cortisol | −3.52 (1.51) | −0.26 | −6.52, −0.51 | 0.02 |
Note: CPT = cold-pressor task; OA = knee osteoarthritis; Sex: 0 = female; 1 = male; Education: 0 = high school or less, 1 = some college or more; Smoking Status: 0 = not a current smoker, 1 = current smoker; Corticosteroid Medication Use: 0 = no corticosteroid medication use, 1 = current use of corticosteroid medications; BMI = body mass index; CPTo = cold pain tolerance
Figure 2.
The relationship between cortisol concentrations obtained prior to the CPT and pain intensity ratings during the CPT in non-Hispanic White and African American adults with knee OA.
Note: CPT = cold-pressor task; OA = osteoarthritis; Low Cortisol = cortisol concentrations at one standard deviation below mean cortisol concentrations (− 3.47ug/dl); High Cortisol = cortisol concentrations at one standard deviation above mean cortisol concentrations (+ 3.47ug/dl).
Hypothesis 3
AA participants reported significantly greater experiences of perceived racial discrimination than NHW participants (P < 0.001) (Table 1). Perceived racial discrimination was not related to cortisol concentrations or pain ratings in unadjusted or fully adjusted analyses among AA participants.
Exploratory Analyses
There were no ethnic differences in cortisol reactivity, nor was there a significant correlation between perceived racial discrimination and cortisol reactivity among AA participants. Table 3 shows bivariate correlations between pain ratings and cortisol reactivity, as well as between pain ratings and cortisol concentrations at time 1 and time 2, collapsed across ethnic group. Cortisol reactivity (i.e., the amount of cortisol increase from time 2 to time 3) was positively correlated with pain intensity and unpleasantness ratings. Cortisol concentrations at time 1 and time 2 were negatively correlated with pain intensity and unpleasantness ratings. There were no significant interactions between ethnicity and cortisol reactivity with pain intensity or unpleasantness ratings in unadjusted or fully adjusted models.
Table 3.
Bivariate correlations between cortisol variables and pain ratings during the cold pressor task collapsed across ethnic group (N = 91).
| Cortisol Variable | ||||
|---|---|---|---|---|
| Time 1 | Time 2 | Time 3 | Cortisol Reactivity | |
| Pain Intensity | −0.26* | −0.32* | 0.04 | 0.30* |
| Pain Unpleasantness | −0.35* | −0.41** | −0.10 | 0.23* |
P< 0.05;
P< 0.001
Note: Time 1 = baseline cortisol concentrations; Time 2 = cortisol concentrations obtained prior to the cold pressor task; Time 3 = cortisol concentrations obtained 20-min after the cold pressor task; Cortisol Reactivity = cortisol concentrations at Time 3 minus cortisol concentrations at Time 2
DISCUSSION
The primary aim of the present study was to determine the relationship between ethnicity, cortisol concentrations obtained prior to the CPT, and pain responses during the CPT among persons with knee OA. Contrary to predictions, we did not find ethnic differences in pain intensity or unpleasantness ratings during the CPT, nor did we find ethnic differences in cortisol concentrations. However, cortisol concentrations obtained prior to the CPT (i.e., time 2) was inversely associated with pain intensity ratings during the CPT in NHW participants, but not in AA participants. A secondary aim was to determine the relationship of perceived racial discrimination with cortisol concentrations and pain responses during the CPT among AA participants with knee OA. Contrary to predictions, perceived racial discrimination was not related to cortisol variables or pain ratings. In exploratory analyses, we found that cortisol reactivity was positively correlated with pain ratings during the CPT across ethnic group. There were no ethnic differences in cortisol reactivity, nor was perceived racial discrimination related to cortisol reactivity among AA participants.
This is the first investigation to assess the relationship between ethnicity, cortisol, and CPT pain responses in older adults with knee OA. Mechlin and colleagues (8) previously showed that cortisol obtained after the Trier Social Stress Test was related to subsequent CPTo in healthy, young NHW participants, but not among their AA counterparts. Our finding resembles that of the Mechlin study in that cortisol obtained after exposure to a stressor (thermal, pressure, and mechanical pain testing in the present study) and before the CPT were related to pain intensity ratings in NHW but not AA participants. Our finding also is consistent with another study that showed cortisol obtained prior to the CPT was inversely associated with pain ratings during the CPT in healthy, young men (22). While these same findings were not reproduced in female participants in that study, all female participants were pre-menopausal and the fluctuation of sex-specific hormones may have influenced pain ratings (22). In contrast, all women in the present investigation were post-menopausal. The present study adds to the existing literature by showing older AA persons with knee OA similarly exhibit an absence of a relationship between cortisol concentrations and CPT pain responses.
We previously reported that perceived racial discrimination was inversely associated with heat pain tolerance levels in AA persons with knee OA (11). We hypothesized that this relationship may be related to physiological changes in neuroendocrine functioning as a consequence of chronic stress associated with perceived racial discrimination. The present study is the first to investigate the relationship between perceived racial discrimination and cortisol concentrations in the context of experimental pain testing in AA persons with knee OA. Contrary to predictions, we did not find evidence of a relationship between perceived racial discrimination and cortisol concentrations.
Several studies show that perceived racial discrimination is associated with stress in AA individuals, including augmented cardiovascular responses and increased emotional distress (25–30). However, findings are mixed. For example, acknowledging the presence of discrimination may be necessary to effectively cope and adjust to discrimination (31), and underreporting perceived racial discrimination may be related to avoidance, denial, and suppression (32), which has been linked to several negative health outcomes (33). An fMRI study found that attributing social exclusion to racial discrimination during a simulated, interactive laboratory game was associated with reduced activation in neural areas related to distress and increased activation in neural areas related to emotion regulation (34). Taken together, perceived racial discrimination is a complex construct that will require carefully designed studies in the future to delineate its effect on stress and subsequent physiological responses.
Mixed results also have been reported regarding the effects of perceived racial discrimination on diurnal cortisol rhythms in AA individuals. While Fuller-Rowell and colleagues (32) showed perceived discrimination was associated with steeper (healthier) diurnal cortisol rhythms among healthy AA persons, a more recent study suggested perceived racial discrimination was related to flatter (less healthy) diurnal cortisol rhythms among healthy AA participants (13). Similar to these studies, we assessed the frequency of perceived racial discriminatory events, and did not consider other aspects of perceived racial discrimination that may be important. For example, Tull and colleagues (35) found that perceived stress and passive coping style was associated with flatter diurnal cortisol rhythms in AA women with high levels of internalized racism (the extent to which AA persons agree with stereotypes about AA individuals), but not AA women with low levels of internalized racism. Therefore, the mixed findings in the literature and the findings in the present study do not necessarily suggest a lack of relationship between perceived racial discrimination and cortisol, but rather that the frequency of perceived discriminatory events may be less important than other aspects of racial discrimination, such as the degree that racism is internalized.
Surprisingly, we did not find ethnic differences in pain ratings during the CPT, though the results were in the anticipated direction (see Table 1). Because previous studies showing AA persons exhibit greater pain ratings than NHW persons during the CPT used relatively low temperatures (e.g., 2 – 5° C; 36, 37), it is possible the temperatures we used were not cold enough to elicit ethnic differences. However, in a recently published study from our laboratory that analyzed a larger sample of adults with knee OA, AA participants exhibited greater pain ratings during the CPT compared to NHW participants using the same CPT methodology as the present study (3). With a larger sample from our ongoing investigation, we plan to further evaluate the role of cortisol in the relationship between ethnicity and pain responses among persons with knee OA.
As shown in Table 3, cortisol concentrations obtained at baseline (time 1) and prior to the CPT (time 2) were negatively correlated with pain ratings, consistent with past findings (8, 22), while cortisol reactivity was positively correlated with pain ratings, which is also consistent with past findings (17, 38). While seemingly paradoxical, the findings of the present study may help shed light on the role of cortisol in pain perception. Whereas the perceived stress and pain experienced during the CPT may be associated with increased cortisol via HPA axis activation, circulating cortisol prior to experiencing a painful stimulus may be pain protective. In support of this latter point, Kuehl et al. (39) showed that metyrapone-induced hypocortisolism was associated with decreased pain thresholds in healthy adults, suggesting circulating cortisol levels prior to encountering a painful stimulus play a direct role in pain perception.
In the present study, all QST sessions were completed by two experimenters (one applying painful stimuli/maintaining cold-pressor temperature while the other recorded responses/operated the computer), and there were a total of four experimenters (one non-Hispanic white male, two non-Hispanic white females, and one Hispanic female). The relationship between participant ethnicity and pain ratings did not differ as a function of experimenter gender concordance/discordance (i.e., female – female vs. male – female) or experimenter ethnicity concordance/discordance (i.e., non-Hispanic white – non-Hispanic white vs. non-Hispanic white – Hispanic). We encourage investigators to continue examining this subject in future studies.
There are several limitations that should be considered when interpreting the study findings. First, our baseline measure of cortisol was significantly greater than cortisol obtained prior to the CPT (22), which may have been related to pre-test anxiety. We encourage future studies to obtain multiple cortisol samples prior to experimental pain testing to circumvent the influence of anticipatory anxiety and other factors on baseline cortisol (17). Second, in order to best understand the relationship between cortisol and pain perception in AA individuals, we encourage future research to capture both cortisol concentrations in the context of experimental pain testing and diurnal cortisol rhythms. Third, while persons using opioids on a daily basis were excluded from the present study and corticosteroid medication use was statistically controlled for, we did not control for other medications that may have affected cortisol, including antidepressants, blood pressure medications, anticonvulsants, and anxiolytics. Future studies are encouraged to consider these important factors when assessing the relationship between cortisol and pain responses in clinical populations.
The present study also has notable strengths. First, this is the only study to show ethnic differences in the relationship between cortisol and CPT pain responses in older adults with knee OA. This finding is consistent with previous findings in healthy, young adults (8, 9). Second, we were able to assess the relationship between perceived racial discrimination and cortisol among community-dwelling AA participants with knee OA. Third, we had access to a large number of control variables known to be related to cortisol responses (BMI, corticosteroid medication use, smoking status).
In summary, cortisol concentrations obtained prior to the CPT were inversely associated with pain intensity ratings during the CPT in NHW participants with knee OA, but not their AA counterparts. It is not clear why it has been consistently found that in older and younger persons with and without knee OA that cortisol is not associated with CPT pain responses in AA persons. Additional studies are required to better understand the cause of this negative finding among AA persons. Additionally, we did not find evidence for a relationship between perceived racial discrimination and cortisol concentrations. We encourage future research to capture different aspects of perceived racial discrimination in addition to the frequency of discriminatory events to better delineate the relationship between perceived racial discrimination and cortisol responses to pain.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by the National Institutes of Health/National Institute on Aging (R01AG033906), the University of Florida Clinical and Translational Science Institute (UL1TR000064, R.B.F), and the University of Alabama at Birmingham Clinical and Translational Institute (UL1TR000165). M.E.P. received support from the Agency for Healthcare Research and Quality (5 T32 HS013852-09) and the National Institute on Minority Health and Health Disparities (3 P60 MD000502-08S1).
References
- 1.Rahim-Williams B, Riley JL, Williams AK, et al. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13:522–540. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen KD, Oddone EZ, Coffman CJ, et al. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthritis Cartilage. 2010;18:160–167. doi: 10.1016/j.joca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Almeida Y, Sibille KT, Goodin BR, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 2014;66:1800–1810. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 5.Forsythe LP, Thorn B, Day M, et al. Race and sex differences in primary appraisals, catastrophizing, and experimental pain outcomes. J Pain. 2011;12:563–572. doi: 10.1016/j.jpain.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Green CR, Baker TA, Smith EM, et al. The effect of race in older adults presenting for chronic pain management: a comparative study of black and white Americans. J Pain. 2003;4:82–90. doi: 10.1054/jpai.2003.8. [DOI] [PubMed] [Google Scholar]
- 8.Mechlin MB, Maxiner W, Light KC, et al. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 9.Mechlin B, Morrow AL, Maixner W, et al. The relationship of allopregnanolone immunoreactivity and HPA-axis measures to experimental pain sensitivity: evidence for ethnic differences. Pain. 2007;131:142–152. doi: 10.1016/j.pain.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mechlin B, Heymen S, Edwards CL, et al. Ethnic differences in cardiovascular-somatosensory interactions and in the central processing of noxious stimuli. Psychophysiology. 2011;48:762–773. doi: 10.1111/j.1469-8986.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 11.Goodin BR, Pham QT, Glover TL, et al. Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychol. 2013;32:1117–1126. doi: 10.1037/a0031592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: the moderating role of racial-ethnic minority status. Psychoneuroendocrinology. 2014;50:280–288. doi: 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgess DJ, Grill J, Noorbaloochi S, et al. The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med. 2009;10:1341–1352. doi: 10.1111/j.1526-4637.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 15.Edwards RR. The association of perceived discrimination with low back pain. J Behav Med. 2008;31:379–389. doi: 10.1007/s10865-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 16.Krieger N, Smith K, Naishadham D, et al. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61:1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Goodin BR, Quinn NB, King CD, et al. Salivary cortisol and soluble tumor necrosis factor-α receptor II responses to multiple experimental modalities of acute pain. Psychophysiology. 2012;49:118–127. doi: 10.1111/j.1469-8986.2011.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bento SP, Goodin BR, Fabian LA, et al. Perceived control moderates the influence of active coping on salivary cortisol response to acute pain among women but not men. Psychoneuroendocrinology. 2010;35:944–948. doi: 10.1016/j.psyneuen.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RR, Doleys DM, Fillingim RB, et al. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Fillingin RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:345–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Tabachnick BG, Fidell LS. Using Multivariate Statistics. Fifth. Boston: Pearson Education, Inc; 2007. [Google Scholar]
- 22.al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 23.al Absi M, Peterson KL. Blood pressure but not cortisol mediates stress effects on subsequent pain perception in healthy men and women. Pain. 2003;106:285–295. doi: 10.1016/S0304-3959(03)00300-2. [DOI] [PubMed] [Google Scholar]
- 24.Goodin BR, Smith MT, Quinn NB, et al. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. 2012;91:36–41. doi: 10.1016/j.biopsycho.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeilly MD, Robinson EL, Anderson NB, et al. Effects of racist provocation and social support on cardiovascular reactivity in African American women. Int J Behav Med. 1995;2:321–338. doi: 10.1207/s15327558ijbm0204_3. [DOI] [PubMed] [Google Scholar]
- 26.Fang CY, Myers HF. The effects of racial stressors and hostility on cardiovascular reactivity in African American and Caucasian men. Health Psychol. 2001;20:64–70. doi: 10.1037//0278-6133.20.1.64. [DOI] [PubMed] [Google Scholar]
- 27.Banks KH, Kohn-Wood LP, Spencer M. An examination of the African American experience of everyday discrimination and symptoms of psychological distress. Community Ment Health. 2006;42:555–570. doi: 10.1007/s10597-006-9052-9. [DOI] [PubMed] [Google Scholar]
- 28.Brondolo E, Thompson S, Brady N, et al. The relationship of racism to appraisals and coping in a community sample. Ethn Dis. 2005;15:S5-14-9. [PubMed] [Google Scholar]
- 29.Utsey SO. Racism and the psychological well-being of African American men. Journal of African American Men. 1997;3:69–87. [Google Scholar]
- 30.Steffen PR, McNeilly M, Anderson N, et al. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosom Med. 2003;65:746–750. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- 31.Major B, Gramzow RH, McCoy SK, et al. Perceiving personal discrimination: the role of group status and legitimizing ideology. J Pers Soc Psychol. 2002;82:269–282. [PubMed] [Google Scholar]
- 32.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37:107–118. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorgensen RS, Thibodeau R. Defensive avoidance of disapproval: the relationship of a defensive style to physical health and mental health. Harv Rev Psychiatry. 2007;15:9–17. doi: 10.1080/10673220601183923. [DOI] [PubMed] [Google Scholar]
- 34.Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tull ES, Sheu YT, Butler C, et al. Relationships between perceived stress, coping behavior, and cortisol secretion in women with high and low levels of internalized racism. J Natl Med Assoc. 2005;97:206–212. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Neubert JK, Rowan JS, et al. A comparison of experimental and acute clinical pain responses in humans as pain phenotypes. J Pain. 2004;5:377–384. doi: 10.1016/j.jpain.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Rahim-Williams FB, Riley JL, III, Herrera D, Campbell C, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129:177–184. doi: 10.1016/j.pain.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gluck ME, Geliebter G, Hung J, et al. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 39.Kuehl LK, Michaux GP, Richter S, et al. Increased basal mechanical pain sensitivity but decreased perceptual wind-up in a human model of relative hypocortisolism. Pain. 2010;149:539–546. doi: 10.1016/j.pain.2010.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.