Abstract
Psychobehavioral intervention is an effective treatment of Internet Addiction, including Internet gaming disorder (IGD). However, the neural mechanisms underlying its efficacy remain unclear. Cortical-ventral striatum (VS) circuitry is a common target of psychobehavioral Interventions in drug addiction, and cortical-VS dysfunction has been reported in IGD, hence, the primary aim of study was to investigate how the VS circuitry responds to psychobehavioral Interventions in IGD. In a cross-sectional study, we examined resting-state functional connectivity (rsFC) of the VS in 74 IGD subjects (IGDs) and 41 healthy controls (HCs). In a follow-up Craving Behavioral Intervention (CBI) study, of the 74 IGD subjects, 20 IGD subjects received CBI (CBI+) and 16 IGD subjects did not (CBI-). All participants were scanned twice with similar time interval to assess the effects of CBI. IGD subjects showed greater rsFC of the VS to left inferior parietal lobule (lIPL), right inferior frontal gyrus (rIFG) and left middle frontal gyrus (lMFG), in positive association with the severity of IGD. Moreover, compared to CBI-, CBI+ showed significantly greater decrease in VS-lIPL connectivity, along with amelioration in addiction severity following the intervention. These findings demonstrated that functional connectivity between VS and lIPL, each presumably mediating gaming craving and attentional bias, may be a potential biomarker of the efficacy of psychobehavioral intervention. These results also suggested that noninvasive techniques such as transcranial magnetic or direct current stimulation targeting the VS-IPL circuitry may be used in the treatment of Internet gaming disorders.
Keywords: Internet gaming disorder, ventral striatum, craving behavioral intervention, resting-state functional connectivity, inferior parietal lobule, attentional bias
INTRODUCTION
Internet gaming disorder (IGD) is defined as an inability to control excessive Internet game playing, with notable functional impairment in academic, social, and occupational settings (Ha et al., 2006). Although yet to be firmly established as a diagnosis, IGD has been found to share similar neuropsychological (i.e., development of euphoria, craving, and tolerance) and pathogenetic processes with substance use disorders as well as pathological gambling (Grant et al., 2010; Ko et al., 2009a; Ko et al., 2013), and has been included in the section of the DSM-5 on disorders deserving further studies (American Psychiatric Association 2013). As a behavioral addiction and relatively free from the confounding effects of substance use, IGD offers an ideal model to study the neural mechanisms of addiction (Cho et al., 2014). Meta-analyses showed that psychobehavioral intervention is an effective treatment for Internet (Winkler et al., 2013) and online gaming addiction (Lemos et al., 2014). However, the neural mechanisms underlying the efficacy of psychobehavioral Interventions in IGD remain unclear.
Craving is critical to the development and maintenance of addiction (Tiffany & Wray, 2012), and may serve as a mediator explaining how treatment works on reducing addictive behaviors (Witkiewitz et al., 2011). The cortical-VS circuitry, a significant component of the “craving pathways” (Volkow et al., 2004), responds to the acute reinforcing effects of online gaming and the memory of the conditioned responses (Ko et al., 2013). Altered cortical-VS connectivity has been reported not only for IGD (Hong et al., 2015; Kühn & Gallinat, 2015; Lin et al., 2015a; Lorenz et al., 2013) but also in individuals with drug addiction (Forbes et al., 2014; Schmidt et al., 2015; Wilcox et al., 2011) and pathological gambling (Gelskov et al., 2016; Peters et al., 2013; van Holst et al., 2014). For instance, pathological computer gamers demonstrated higher functional connectivity between frontal cortical regions and ventral striatum, implicating a link between craving and cognitive control (Lorenz et al., 2013).
Changes in cortical striatal functions have been observed following behavioral interventions in individual with drug or behavioral addiction. A previous study reported a change of striatal activity following family therapy in individuals with IGD (Han et al., 2012). Multiple studies of the neural mechanisms of psychobehavioral Interventions for drug addiction also suggested the cortical-ventral striatum circuitry is a major target of cognitive therapy (Konova et al., 2013), cue-exposure based extinction training (Vollstädt-Klein et al., 2011), and mindfulness-oriented recovery enhancement (Garland et al., 2014). Thus, one may posit that the VS circuitry may be compromised in IGD and remediation of this circuit function underlies the therapeutic efficacy of psychobehavioral Interventions.
Resting-state functional connectivity (rsFC) identifies networks of brain regions exhibiting synchronous fluctuations in inherent activity (Fox & Raichle, 2007). Recent work has employed rsFC analyses to characterize cerebral connectivity of sub-cortical nuclei (Chiang-shan et al., 2014). Importantly, rsFC analysis has shown validity in probing altered functional integration of cortico-striatal circuitry in Internet addiction disorder (Hong et al., 2015; Lin et al., 2015a).
Here, we developed a psychobehavioral intervention (Craving Behavioral Intervention or CBI), aimed at reducing craving and based on the craving framework of boundary conditions (McCarthy et al., 2010). In the latter conceptual framework craving occurs in response to drug, drug-related cues, and negative emotional states (Curtin et al., 2006); and fulfillment of psychological needs through Internet use may lead to a habitual desire for continued Internet use (Suler, 1999). Our goals were to evaluate whether and how rsFC of the VS is altered in IGD and how the altered patterns of connectivity respond to the CBI.
MATERIALS AND METHODS
Participants, clinical assessments
This study was part of a larger study aiming to develop and evaluate efficient intervention for IGD, please see the flow chart in Supplementary Material Figure S1 for study procedures. In this paper we focused on the fMRI data in relation to CBI. It was conducted under a protocol approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University (BNU). All participants signed written informed consent prior to the study and were financially compensated for their participation.
The study was posted on our lab website or advertised via flyers at local universities. Given the higher prevalence of IGD in men than in women (Ko et al., 2009b), a total of 701 male college students were screened through online questionnaires and telephone interviews. The IGD subjects met the following criteria: a score of the Chinese Internet Addiction Scale (CIAS; Chen et al., 2003) was higher than 67 (Ko et al., 2009b); more than half of the time spent online was on games (Lin et al., 2015b); and the time spent on Internet gaming per week was not less than 14 hours (with at least 2 hours spent on Internet gaming every day), as assessed by a semi-structured interview (Zhang et al., 2015). The HC met the following criteria: CIAS ≤ 60 and never having spent more than 2 hours per week on Internet gaming (Zhang et al., 2015).
Additional criteria for all of the participants included: 18–30 years of age; right-handed only; eligibility for MRI as assessed using the BNU imaging center for brain research screening form; a score ≤ 6 on the Fagerstrom Test for Nicotine Dependence (FTND; Fagerstrom, 1978); a score ≤ 9 on the Alcohol Use Disorder Identification Test (AUDIT-C; Bush et al., 1998); no history of other psychiatric or neurological illness, no current or previous use of illegal substances or gambling, and currently not taking any psychotropic medications.
Cross-sectional study
Seventy-six IGD subjects and 41 gender, age, and education matched HCs participated in functional magnetic resonance imaging (fMRI) in the first, cross-section study. Two IGD subjects had excessive head motion and were excluded from analyses, so the final dataset contained 74 IGD subjects and 41 HC, who were matched on gender, age, and education. Participants were also evaluated with the Beck Anxiety Inventory (BAI; Beck et al., 1988), Beck Depression Inventory (BDI; Beck et al., 1961), Barratt Impulsiveness Scale-version 11 (BIS-11; Barratt & Pritchard, 1985) and subjective craving of Internet (gaming), an 8-item Likert scale adapted from the Questionnaire of Smoking Urges (QSU-brief; Cox et al., 2001).
Follow-up Craving Behavioral Intervention (CBI) study
Forty-four of the 74 IGD subjects participated in the follow-up intervention study voluntarily and opted whether to receive the intervention or not, among whom 25 agreed to receive CBI therapy (CBI+) and 19 did not (CBI-). Individuals in both CBI+ and CBI- were specifically instructed that they do not contact each other throughout the intervention. The CBI is a behavioral intervention program developed to reduce craving for internet gaming, based on theories of the craving framework of boundary conditions (McCarthy et al., 2010) and the fulfillment of psychological needs for Internet use (Suler, 1999).
In the CBI+ group, participants were grouped by 8 to 9 subjects to receive six sessions of CBI, conducted weekly with each session lasting for 2.5 hours. The topic of each session respectively was: 1) perceiving subjective craving and mindfulness training for gaming-cue-induced craving and tension; 2) recognizing and testing irrational beliefs regarding craving; 3) detecting craving and training in mindfulness to relieve craving-related negative emotions; 4) training on coping with cravings and altering participants’ fulfillment of psychological needs; 5) time management and skills training to cope with craving; 6) reviewing, practicing, and implementing skills. In addition, mindfulness training was administrated for about 20 minutes at the end of each session, and self-administered whenever they experienced craving beyond the intervention as an assignment. The intervention as employed in our previous study (Zhang et al., 2016) is described in detail in the Supplementary Material.
Following the intervention (or no intervention), both CBI+ and CBI- were scanned again. Five CBI+ and 3 CBI- subjects were excluded from data analyses because of excessive head motion (translation > 3.0 mm or rotation > 3°), so the final dataset contained 20 subjects in CBI+ and 16 subjects in CBI-. There were no differences in IGD severity (CIAS: F(2,71)) = 1.43, p = .247, partial η2 = .04; Time spent on Internet gaming: F(2,71)) = .77, p = .469, partial η2 = .02) or rsFC of VS (no voxels survived whole-brain search) between CBI+ and CBI- and those who did not participate in follow-up CBI study.
Image acquisition
MR imaging was conducted with a Siemens Trio 3-Tesla scanner (Siemens, Erlangen, Germany). The resting-state functional imaging data comprised 200 continuous echo-planar imaging (EPI) whole-brain functional volumes: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; slice number = 33; field of view (FOV) = 200× 200 mm; matrix size = 64 × 64; voxel size = 3.1× 3.1 × 3.5 mm3; gap = 0.7 mm. The subjects were instructed to keep their eyes open looking at a black screen, remain motionless, stay awake, and not to think of anything in particular.
Preprocessing
Data were preprocessed using DPARSF version 3.0 (Yan & Zang, 2010; http://rfmri.org/DPARSF). To allow the magnetization to approach a dynamic equilibrium and participants to get used to scanning noise, the first 10 volumes were discarded. Slice time correction was applied to the EPI data. Participants who had a head motion exceeding 3.0 mm in translation or 3° in rotation were excluded. Further, we used Fristion’s 24-parameter model (Yan et al., 2013) to reduce the confounds of head motion. We also covaried signals from the cerebrospinal fluid and white matter to reduce the effect of physiological artifacts. EPI data were normalized to the Montreal Neurological Institute (MNI) space, smoothed with a spatial filter of 4 mm full width at half maximum (FWHM) Gaussian kernel, and band-pass filtered (0.01–0.08 Hz).
Seed region: VS and rsFC
To investigate the rsFC of VS, we used WFU-PickAtlas (Maldjian et al., 2003) Tool V3.0.4 to define a bilateral spherical ROI of 3.5mm in radius: VS (±9,9,-8, Figure1(A)), corresponding to the inferior VS (VSi) on the basis of a large-scale meta-analysis of striatal connectivity, as reported by Di Martino et al. (2008). RsFC was assessed using REST version 1.8 (Song et al., 2011; http://restfmri.net/forum/REST_V1.8), by computing whole brain correlations with the mean time series of the VS seed. Voxel-wise correlation coefficients were converted to z-scores via Fisher’s r-to-z transform.
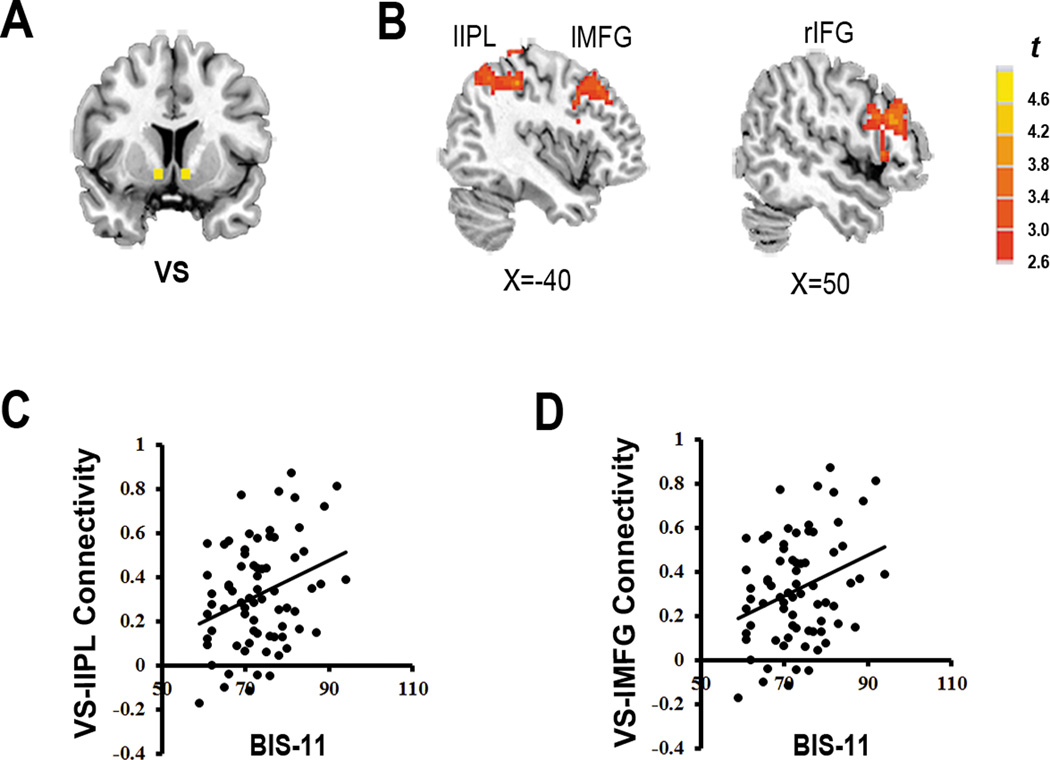
Figure 1.
Group differences in resting-state functional connectivity (rsFC) of the ventral striatum (VS) (pcorrected<.01), and the correlation with score of impulsivity in IGD. (A) VS seed in a coronal section (with MNI coordinates ±9, 9, −8). (B) The IGD subjects had significantly higher VS connectivity with three brain regions: rIFG (right inferior frontal gyrus), lMFG (left middle frontal gyrus), and lIPL (left inferior parietal lobule). Clusters are color-coded based on uncorrected P-values. IGD: individuals with Internet gaming disorder. (C) Scatter plot depicting positive correlation between score of BIS-11 and rsFC of VS with lIPL and (D) lMFG in IGD. BIS-11: Barratt Impulsiveness Scale-version 11; VS- lIPL Connectivity: the strength of rsFC between VS and lIPL; VS-lMFG Connectivity: the strength of rsFC between VS and lMFG. Lines represent the best-fit regressions.
Statistical Analyses
We performed voxel-wise two sample t-tests on the Z-score maps to compute the group difference map, with correction for multiple comparison by means of a Monte Carlo simulation using REST version 1.8 (Song et al., 2011; http://restfmri.net/forum/REST_V1.8) in which the smoothing kernel was estimated based on the t map. We reported the results at height threshold of P< .01 and cluster P< .01 (cluster size > 244 voxels), equivalently a family-wise-error rate of 1% across the whole brain. The brain regions that showed altered connectivity to the VS were identified as regions of interest (ROIs) to extract the magnitude of altered functional connectivity. Pearson correlations were performed between the magnitude of connectivity of the ROIs to the VS and CIAS/craving/BIS-11 score/time spent on Internet gaming per week across groups or within IGD/HC.
To examine the effect of CBI on clinical assessments and the potential neural markers of IGD, we conducted a 2 (intervention: CBI+ /CBI-) by 2 (session: pretest/posttest) repeated measures analysis of variance on the score of craving, CIAS and the time spent on Internet gaming, and on the altered rsFC, separately. Simple effect analyses were conducted following the identification of significant interactions.
RESULTS
Participant characteristics
Compared to HC, IGD individuals had significantly higher score on CIAS, craving, anxiety, depression, BIS-11, and more time spent on Internet gaming (p’s ≤ .001; Table 1), and higher proportion of cigarette user (p = .029; Table 1). There were no significant differences between CBI+ and CBI- on any of the clinical assessments (Table 1). The head motion was not significantly different between IGD subjects and HCs, or between CBI+ and CBI- (Table 1).
Table 1.
Demographic characteristics and clinical assessments of 74 IGD subjects vs. 41 HC and 20 CBI+s vs. 16 CBI-s
| IGD (n = 74) | HC (n = 41) | t113(p) | CBI+ (n = 20) | CBI- (n = 16) | t34(p) | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (yr) | 22.28 (1.98) | 23.02 (2.09) | −1.89 (.062) | 21.80 (1.70) | 22.38 (1.71) | −1.01 (.322) |
| Education (yr) | 15.74 (1.84) | 16.32 (1.71) | −1.64 (.104) | 15.90 (1.41) | 15.44 (1.83) | .86 (.397) |
| CIAS | 78.46 (8.40) | 43.49 (9.64) | 20.27 (<.001) | 79.70 (6.55) | 75.38 (6.90) | 1.92 (.063) |
| Craving | 35.65 (8.49) | 13.29 (5.88) | 16.59 (<.001) | 38.80(6.93) | 38.00(7.27) | .34 (.738) |
| BAI | 5.42 (5.43) | 2.61 (3.26) | 3.47 (.001) | 3.65 (3.59) | 7.88 (7.58) | −2.05 (.053) |
| BDI | 8.78 (5.54) | 2.85 (3.64) | 6.91 (<.001) | 9.15 (6.05) | 10.44 (4.26) | −.75 (.460) |
| BIS- 11(72–34) | 73.21 (8.03) | 62.27 (7.47) | 7.02 (<.001) | 72.85 (8.44) | 69.63 (6.95) | 1.23 (.227) |
| Time spent on Internet gaming per week (hr/week) |
25.07 (10.27) | .68 (.72)n | 1036.00 (<.001)a | 28.58 (10.62) | 25.31 (7.87) | 1.02 (.313) |
| Number of drinkers | 57 (3.30±1.95) | 29 (2.41±1.30) | .55 (.457)b | 16 (3.44±2.00) | 10 (2.40±1.26) | 1.36 (.244)b |
| Number of smokers | 8 (2.38±1.77) | 0 | 4.76 (.029)b | 2 (2.00±.00) | 0 | 1.69 (.193)b |
| Mean FD Power | .13 (.06) | .12 (.05) | 1.55 (.125) | .15 (.08) | .13 (.05) | .92 (.364) |
Abbreviations: SD, standard deviation; IGD subjects, Internet gaming disorder subjects; HCs, healthy controls; CBI+s: Internet gaming disorder subjects with CBI; CBI-s: Internet gaming disorder subjects without CBI; CIAS, Chen Internet addition scale; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BIS-11, Barratt Impulsiveness Scale-version 11; Mean FD Power: the mean value of frame-wise displacement (FD) of Power.
Mann-Whitney U Test
Chi- square test
data of 14 HCs were included; other HCs never played Internet games.
Difference in rsFC of VS across whole brain: IGD vs. HC
Compared to HC, IGD individuals showed a significantly higher rsFC of VS to the left inferior parietal Lobule (lIPL), right inferior frontal gyrus (rIFG) and left middle frontal gyrus (lMFG) (Table 2). To examine whether the identified clusters remained independent of the head motion, we applied the scrubbing 0.5 on frame-wise displacement (FD) of Power (FD Power; Power et al., 2012; Power et al., 2014), and the results remained similar (Supplementary Material Table S1). The smoking status (smoker vs. nonsmoker, smoker=1, nonsmoker=0) or anxiety and depression (BAI/BDI scores) were also covaried in separate analyses, and the main results stood (Supplementary Material Table S2 and Table S3), suggesting that difference in smoking or anxiety and depression cannot fully explain the group difference in rsFC of VS.
Table 2.
Increased resting-state functional connectivity (rsFC) of ventral striatum (VS) in IGD as compared with HC
| Region | Hemisphere | BA | x | y | z | t | Cluster size |
|---|---|---|---|---|---|---|---|
| IFG/ MFG | R | 45/46 | 42 | 30 | 30 | 4.49 | 379 |
| MFG/ IFG | L | 44 | −48 | 6 | 27 | 3.94 | 264 |
| IPL/SPL | L | 7/40 | −30 | −69 | 60 | 4.97 | 478 |
Coordinates are reported in MNI space. Cluster size indicates the number of contiguous voxels (3* 3* 3 mm3). BA: Brodmann area; IFG: IFG: inferior frontal gyrus, MFG: middle frontal gyrus, IPL: inferior parietal lobule, SPL: superior parietal lobule.
Correlation between clinical assessments and altered rsFC
Across groups, score of CIAS, craving and BIS-11 were both positively correlated with the rsFC between VS and rIFG/lIPL/lMFG (Supplementary Material Table S4). In the IGD group, only BIS-11 was positively correlated with the ROI-wise rsFC between VS and lIPL/lMFG (r=.26, p= .03; r = .31, p = .01; Figure 1 (C) and 1 (D)).
The effect of intervention on clinical assessments
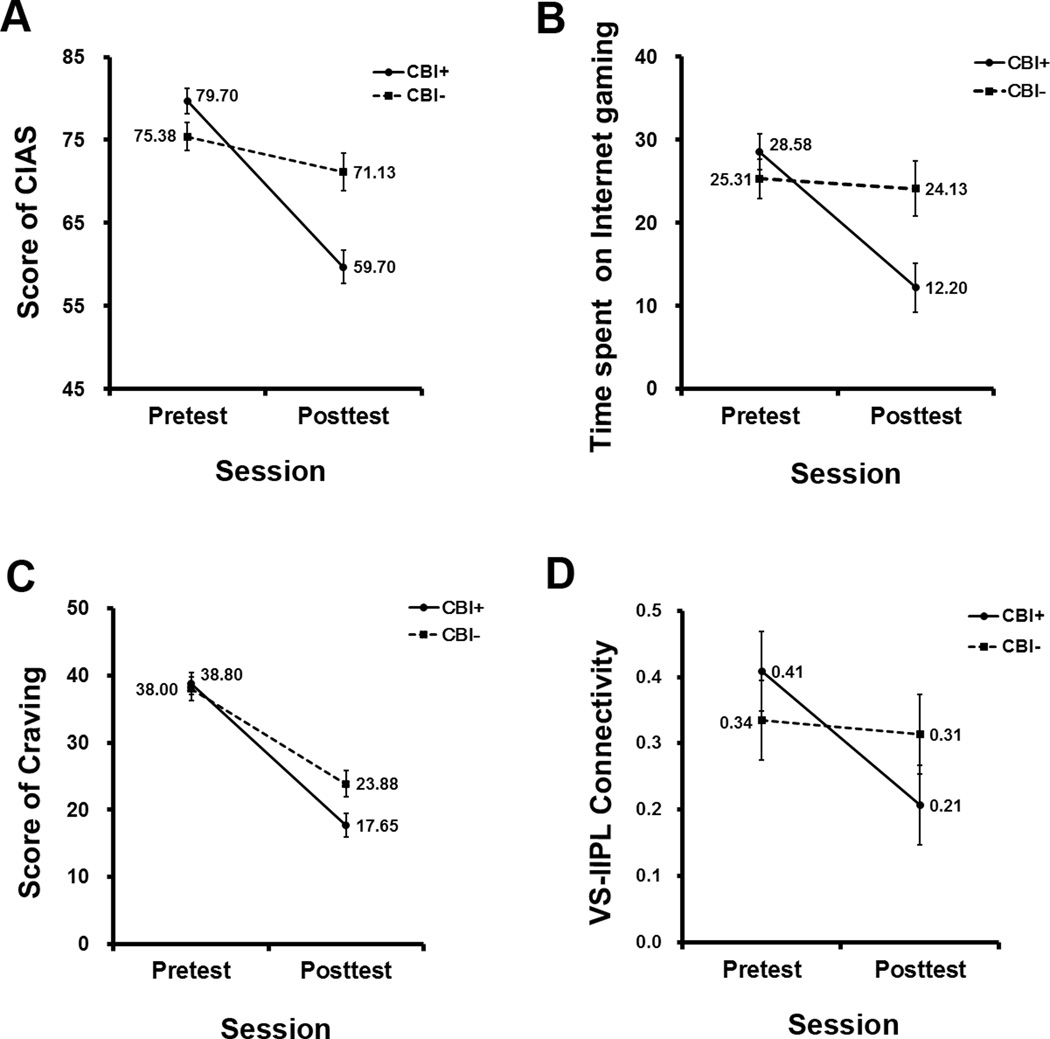
There were significant interactions between intervention (CBI+ vs. CBI-) and session (pretest vs. posttest) on score of CIAS and the time spent on Internet gaming (F(1,34) = 26.60, p < .001, partial η2 = .43; F(1,34) = 7.07, p = .012, partial η2 = .17). Simple effect analyses (Figure 2 (A–B), Supplementary Material Table S5) suggested that CBI+ showed significant decrease in CIAS score and the time spent on Internet gaming in posttest versus pretest (F(1,34) = 96.49, p< .001, partial η2 = .74; F(1,34) = 18.49, p< .001, partial η2 = .35), whereas CBI- showed no changes (F(1,34) = 3.49, p = .071, partial η2 = .09; F(1,34) = .08, p = .782, partial η2 = .00). There also was a marginally significant interaction on score of craving (F(1,34) =3.94, p =.055, partial η2 = .10); the score of craving decreased in both CBI+ and CBI- (F(1,34) =80.38, p < .001, partial η2 = .70; F(1,34) = 28.68, p < .001, partial η2 = .46), but the decrease in CBI+ was marginally significant larger than in CBI- (Figure 2 (C); Supplementary Material Table S5).
Figure 2.
Repeated measures ANOVA on CIAS score, the time spent on Internet gaming, craving score and resting-state functional connectivity (rsFC) between ventral striatum (VS) and left inferior parietal Lobule (lIPL) Cluster. (A) A significant interaction between intervention (CBI+/CBI-) and session (pretest/posttest) on score of CIAS; CIAS: Chen Internet addition scale; (B) A significant interaction between intervention (CBI+/CBI-) and session (pretest/posttest) on the time spent on Internet gaming; (C) A marginally significant interaction between intervention (CBI+/CBI-) and session (pretest/posttest) on score of craving; (D) A significant interaction between intervention (CBI+/CBI-) and session (pretest/posttest) on VS-lIPL Connectivity, VS-lIPL Connectivity: the strength of rsFC between VS and lIPL. CBI+: Internet gaming disorder subjects with CBI; CBI-: Internet gaming disorder subjects without CBI. Black circles: the mean value of CBI+ in pretest/posttest; black square: the mean value of CBI- in pretest/posttest; Error bars indicated the standard errors of the mean.
The effect of intervention on rsFC of VS
In pretest, CBI+ and CBI- did not differ in VS-lIPL/rIFG/lMFG connectivity (F(1,34) = .79, p = .382; F(1,34) = .56, p = .461; F(1,34) = .35, p = .556). There was a significant interaction between session and intervention on rsFC between VS and lIPL (F(1,34) = 4.95, p = .033, partial η2 = .13). Simple effect analyses (Figure 2 (D), Supplementary Material Table S5) suggested that CBI+ showed significant decrease in VS-lIPL connectivity in posttest compared with pretest (F(1,34) = 13.89, p = .001, partial η2 = .29), whereas CBI- showed no changes (F(1,34) = .12, p = .730, partial η2 = .00). The lIPL clusters, survived when controlling for head motion or covarying for smoking or anxiety and depression in separate models (Supplementary Material Table S1–3), were also identified as regions of interest (ROIs) to compute their functional connectivities to VS, and then to examine the effect of CBI using repeated ANOVA. The results also showed significant interactions between session and intervention on these VS-lIPL connectivities (scrubbing FD Power ≥ 0.5: F(1,34) = 4.30, p = .046, partial η2 = .11; covarying for smoking: F(1,34) = 5.57, p = .024, partial η2 = .14; covarying for anxiety and depression (F(1,33) = 4.23, p = .047, partial η2 = .11; Supplementary Material Table S6). Although the rsFC between VS and lMFG showed a similar trend with VS-lIPL connectivity (Supplementary Material Table S5, Supplementary Material Figure S2 (A)), the interaction between session and intervention on VS-lMFG connectivity was marginally significant (F(1,33) = 3.75, p = .061, partial η2 = .09). While there was no significant interaction on rsFC between VS and rIFG (F(1,34) = .03, p = .866, partial η2 = .00), because the strength of VS-rIFG decreased both in CBI+ and CBI- (Supplementary Material Table S5, Supplementary Material Figure S2 (B)).
DISCUSSION
To the best of our knowledge, the current study was the first to investigate the effect of psychobehavioral intervention on rsFC of the VS in individuals with IGD. The IGD group had a significant stronger rsFC between VS and lIPL/rIFG/lMFG, and the strength of VS-lIPL connectivity reduced significantly after CBI.
In the cross-sectional study, IGD group showed greater rsFC between VS and lIPL/rIFG/lMFG, namely higher synchronous fluctuations in inherent activity among these regions, These clusters also stood when controlling for the head motion, smoking or anxiety and depression separately, demonstrating that altered cortico-striatal connectivity can distinguish IGD from HC. Previous work showed that direct stimulation of the VS could elicite craving (Ko et al., 2013) and modulate reinforcement learning and decision-making (Belin et al., 2009). The inferior parietal lobule (IPL) is involved in self-referential processing (Buckner et al., 2008) and attentional bias towards salient stimuli including rewards and drug cues (Claus et al., 2013), which could precipitate craving and drug seeking (McBride et al., 2006). The greater rsFC between VS and lIPL was thus consistent with reward-related responses including “craving for games” in IGD, as reported by Claus et al. (2013). Further, the positive correlations between VS-lIPL connectivity and the score of CIAS, craving and BIS-11 were in accord with a correlation between nicotine dependence and cue-induced activation of lIPL in cigarette smokers (Yalachkov et al., 2013).
The inferior frontal gyrus (IFG) and middle frontal gyrus (MFG) integrate affective information from the VS (Skinner & Aubin, 2010) and maintain a link of sensory inputs to memories to generate goal-directed actions (Bonson et al., 2002). Thus, the higher rsFC between VS and rIFG/lMFG may support a link between reward anticipation and motivation to “play games” in IGD (Ko et al., 2009a). The latter findings are in accord with increased rsFC strength in striatal-frontal circuitry in positive correlations with impulsivity, craving score and cocaine use in pathological computer game players, pathological gamblers and cocaine users (Hu et al., 2015; Koehler et al., 2013; Lorenz et al., 2013).
In the follow-up intervention study, we investigated the effect of CBI on clinical outcomes and the strengths of rsFC between VS and lIPL/rIFG/lMFG. We found that CBI+ showed a greater decrease in CIAS score and the time spent on Internet gaming, consistent with earlier work of psychobehavioral intervention in Internet addiction (Lemos et al., 2014; Winkler et al., 2013). As for the interaction of intervention (CBI+/CBI-) and session (pretest/posttest) on craving score, it was only marginally significant. Of note, there were 24 additional participants who were involved in the CBI intervention but did not receive fMRI scans, and in this larger sample a significant interaction on craving score was found for CBI intervention (F(1,58) = 5.84, p = .019, partial η2 = .09). Moreover, with the potential outlier - subject 122 - removed (Supplementary Material Figure S3), the CBI+ decreased significantly in craving (F(1,33) = 6.60, p = .015, partial η2 = .17; Supplementary Material Figure S4, Table S7).
There was a significant interaction of intervention (CBI+/CBI-) and session (pretest/posttest) on rsFC between VS and lIPL: the strength of VS - lIPL rsFC in CBI+ but not CBI- was reduced, suggesting that the altered connectivity was partially reversible with Interventions. In support, mindfulness training has shown efficacy in decreasing craving (Bowen et al., 2009) and cue-related attentional bias (Garland et al., 2012), with VS and IPL activity remediated by psychobehavioral interventions in drug addicts (Garland et al., 2014; Konova et al., 2013; Vollstädt-Klein et al., 2011). Further, striatal connectivity was discovered to be associated with cocaine relapse and impulsive decision making (Contreras-Rodríguez et al., 2015; Schmaal et al., 2014). The effect of CBI also remained significant on the rsFC between VS and lIPL cluster which was identified when controlling for the head motion, smoking or anxiety and depression separately. Thus, VS-lIPL connectivity may be a stable biomarker for the efficacy of psychobehavioral Interventions in IGD. On the other hand, VS-rIFG/lMFG, especially the VS-rIFG connectivity, which decreased significantly in both CBI+ and CBI-, may not represent specific makers of psychobehavioral Interventions.
To demonstrate the regional specificity of the current findings, we repeated the same analyses for the supplementary motor area (SMA), a motor area not typically implicated in craving, from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) as a control region. No group differences in cross sectional study or significant interaction between session and intervention in the follow-up study were found. Besides, to investigate the effect of threshold on the findings, we repeated the same analyses with the threshold of voxel level p < .005 combined with cluster level p < .05, and the threshold of voxel level p < .001 combined with cluster level p < .05. The main group differences (Supplementary Material Table S8–9) and CBI effect both remained significant (the VS-lIPL connectivity was significantly decreased in CBI+ group but not in CBI- group; Supplementary Material Table S10), although the interaction was only marginally significant (voxel level p < .005; F(1,34) = 4.01, p = .053, partial η2 = .11) and not significant (voxel level p < .001; F(1,34) = 2.07, p = .160, partial η2 = .06). Thus, although the findings appeared to be largely reliable, more work is needed to verify these findings.
Given the important role in gaming craving and attentional bias of VS and IPL (Claus et al., 2013; Ko et al., 2009a), these results can be explained in terms of previous theories of addiction. For example, the incentive sensitization theory of addiction held that long-term drug use could change the Nucleus Accumbens (NAcc)-related functions and increase the sensitivity to the drug-related stimuli (attentional bias), precipitating memory bias and positive expectancies and leading to “wanting” (craving) and drug-seeking (Franken, 2003). In addition, cognitive-behavioral model of pathological Internet use considered maladaptive cognition (such as “they thought they could only do well in and realize their values through Internet gaming”) and reinforcement of Internet experience as key factors to Internet addiction (Davis, 2001). Considered with these theories, individuals with IGD have increased motivation and attentional bias to gaming and gaming related cues, as manifested in the altered connectivity linking reward processing (VS) and attentional bias (IPL). psychobehavioral Interventions may reduce attentional bias and craving through changes in the rsFC between VS and lIPL.
Limitations
There were several limitations to consider in this study. First, we recruited only male participants, so further studies with female participants are needed to confirm and/or extend the current results. Second, although the main findings stood when smoking status was accounted for, one cannot rule out the potential impact of cigarette and alcohol use on the current results. Third, because IGDs participated in the follow-up CBI study (CBI+ /CBI-) voluntarily, we could not exclude the effects of motivation to reduce Internet gaming as a confound to the current findings. This along with other clinical characteristics that are known to impact VS functions need to be examined in more detail in future studies of IGD.
Implications
The current study has implications for both assessment and treatment of IGD. Motivation and attentional bias play important roles in the development and maintenance of IGD, and behavioral interventions targeting these factors are likely to be effective in the treatment of IGD. In particular, psychobehavioral intervention that focus on reducing craving and altering the fulfillment of psychological needs can be successful in reducing attentional bias and relapse. The VS-IPL connectivity is a potential biomarker of the efficacy of psychobehavioral intervention. One may speculate that noninvasive techniques such as transcranial magnetic stimulation and transcranial direct current stimulation to target this circuitry may be used in the treatment of Internet gaming and other behavioral addictions.
Supplementary Material
Acknowledgments
We thank all subjects for participating in our study. This work was supported by the National Natural Science Foundation of China (No. 31170990, No. 81100992), and Project of Humanities and Social Sciences from Ministry of Education in China (No.15YJA190010), the Fundamental Research Funds for the Central Universities (No.2015KJJCA13), and an NIH grant (No. K02DA026990).
Footnotes
Further, the authors declare no conflict of interest.
AUTHORS CONTRIBUTIONS
JTZ, and XYF were responsible for the study concept and design. LL, CCX, JL, LJW, BL, and YWY contributed to the data acquisition. SSM assisted with data analysis and drafted the manuscript. JTZ, CSRL, and XYF provided critical revision of the manuscript. All authors critically reviewed the content and approved final version for publication.
References
- Association, A. P. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Barratt ES, Pritchard W. Impulsiveness Subtraits - Augmenting Reducing of Visual Erps and Information-Processing. Personality and Individual Differences. 1985;6:R11–R11. [Google Scholar]
- Beck AT, Brown G, Epstein N, Steer RA. An Inventory for Measuring Clinical Anxiety - Psychometric Properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An Inventory for Measuring Depression. Archives of General Psychiatry. 1961;4 doi: 10.1001/archpsyc.1961.01710120031004. 561-&. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behavioural brain research. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Clifasefi S, Garner M, Douglass A, Larimer ME. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Substance Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of internal medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Chen S, Weng L, Su Y, Wu H, Yang P. Development of a Chinese Internet addiction scale and its psychometric study. Chinese Journal of Psychology. 2003;45:279–294. [Google Scholar]
- Chiang-shan RL, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L. Resting state functional connectivity of the basal nucleus of Meynert in humans: In comparison to the ventral striatum and the effects of age. NeuroImage. 2014;97:321–332. doi: 10.1016/j.neuroimage.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Kwon M, Choi J-H, Lee S-K, Choi JS, Choi S-W, Kim D-J. Development of the Internet addiction scale based on the Internet Gaming Disorder criteria suggested in DSM-5. Addictive behaviors. 2014;39:1361–1366. doi: 10.1016/j.addbeh.2014.01.020. [DOI] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Rodríguez O, Albein-Urios N, Perales JC, Martínez-Gonzalez JM, Vilar-López R, Fernández-Serrano MJ, Lozano-Rojas O, Verdejo-García A. Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction. 2015;110:1953–1962. doi: 10.1111/add.13076. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and Explicit Drug Motivational Processes: A Model of Boundary Conditions. Thousand Oaks, CA: Sage Publications, Inc.; 2006. [Google Scholar]
- Davis RA. A cognitive-behavioral model of pathological Internet use. Computers in human behavior. 2001;17:187–195. [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly A, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring Degree of Physical-Dependence to Tobacco Smoking with Reference to Individualization of Treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R. Prefrontal Response and Frontostriatal Functional Connectivity to Monetary Reward in Abstinent Alcohol-Dependent Young Adults. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0094640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Gaylord S, Chanon VW, Howard MO. Mindfulness is inversely associated with alcohol attentional bias among recovering alcohol-dependent adults. Cognitive therapy and research. 2012;36:441–450. doi: 10.1007/s10608-011-9378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. Journal of behavioral medicine. 2014;38:327–336. doi: 10.1007/s10865-014-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelskov SV, Madsen KH, Ramsoy TZ, Siebner HR. Aberrant neural signatures of decision-making: Pathological gamblers display cortico-striatal hypersensitivity to extreme gambles. NeuroImage. 2016;128:342–352. doi: 10.1016/j.neuroimage.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to Behavioral Addictions. American Journal of Drug and Alcohol Abuse. 2010;36:233–241. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JH, Yoo HJ, Cho IH, Chin B, Shin D, Kim JH. Psychiatric comorbidity assessed in Korean children and adolescents who screen positive for Internet addiction. The Journal of clinical psychiatry. 2006;67:1,478–1, 826. doi: 10.4088/jcp.v67n0517. [DOI] [PubMed] [Google Scholar]
- Han DH, Kim SM, Lee YS, Renshaw PF. The effect of family therapy on the changes in the severity of on-line game play and brain activity in adolescents with on-line game addiction. Psychiatry Research: Neuroimaging. 2012;202:126–131. doi: 10.1016/j.pscychresns.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, Harrison BJ, Dandash O, Choi EJ, Kim SC, Kim HH, Shim DH, Kim CD, Kim JW, Yi SH. A selective involvement of putamen functional connectivity in youth with internet gaming disorder. Brain Research. 2015;1602:85–95. doi: 10.1016/j.brainres.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired Functional Connectivity Within and Between Frontostriatal Circuits and Its Association With Compulsive Drug Use and Trait Impulsivity in Cocaine Addiction. JAMA psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Brains online: structural and functional correlates of habitual Internet use. Addiction biology. 2015;20:415–422. doi: 10.1111/adb.12128. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Liu G-C, Hsiao S, Yen J-Y, Yang M-J, Lin W-C, Yen C-F, Chen C-S. Brain activities associated with gaming urge of online gaming addiction. Journal of psychiatric research. 2009a;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Yen J-Y, Chen S-H, Yang M-J, Lin H-C, Yen C-F. Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Comprehensive psychiatry. 2009b;50:378–384. doi: 10.1016/j.comppsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Yen JY, Chen CY, Yen CF, Chen CS. Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addiction biology. 2013;18:559–569. doi: 10.1111/j.1369-1600.2011.00405.x. [DOI] [PubMed] [Google Scholar]
- Koehler S, Ovadia-Caro S, van der Meer E, Villringer A, Heinz A, Romanczuk-Seiferth N, Margulies DS. Increased functional connectivity between prefrontal cortex and reward system in pathological gambling. PLoS ONE. 2013;8:e84565. doi: 10.1371/journal.pone.0084565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Goldstein RZ. Common and distinct neural targets of treatment: changing brain function in substance addiction. Neuroscience & Biobehavioral Reviews. 2013;37:2806–2817. doi: 10.1016/j.neubiorev.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos IL, Abreu CNd, Sougey EB. Internet and video game addictions: a cognitive behavioral approach. Revista de Psiquiatria Clínica. 2014;41:82–88. [Google Scholar]
- Lin F, Zhou Y, Du Y, Zhao Z, Qin L, Xu J, Lei H. Aberrant corticostriatal functional circuits in adolescents with Internet addiction disorder. Frontiers in human neuroscience. 2015a;9 doi: 10.3389/fnhum.2015.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Dong G, Wang Q, Du X. Abnormal gray matter and white matter volume in ‘Internet gaming addicts’. Addictive behaviors. 2015b;40:137–143. doi: 10.1016/j.addbeh.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Lorenz RC, Krüger JK, Neumann B, Schott BH, Kaufmann C, Heinz A, Wüstenberg T. Cue reactivity and its inhibition in pathological computer game players. Addiction biology. 2013;18:134–146. doi: 10.1111/j.1369-1600.2012.00491.x. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Curtin JJ, Piper ME, Baker TB. Negative reinforcement: Possible clinical implications of an integrative model. 2010 [Google Scholar]
- Peters J, Miedl SF, Buchel C. Elevated Functional Connectivity in a Striatal-Amygdala Circuit in Pathological Gamblers. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0074353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan A, Joos L, Dom G, Pattij T, van den Brink W, Veltman D. Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychological medicine. 2014;44:2787–2798. doi: 10.1017/S0033291714000312. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Denier N, Magon S, Radue EW, Huber CG, Riecher-Rossler A, Wiesbeck GA, Lang UE, Borgwardt S, Walter M. Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Translational Psychiatry. 2015;5 doi: 10.1038/tp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MD, Aubin HJ. Craving's place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34:606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. Plos One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suler JR. To get what you need: healthy and pathological Internet use. CyberPsychology & Behavior. 1999;2:385–393. doi: 10.1089/cpb.1999.2.385. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. Annals of the New York Academy of Sciences. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, Chase HW, Clark L. Striatal connectivity changes following gambling wins and near-misses: Associations with gambling severity. Neuroimage-Clinical. 2014;5:232–239. doi: 10.1016/j.nicl.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biological psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug and alcohol dependence. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A, Dörsing B, Rief W, Shen Y, Glombiewski JA. Treatment of internet addiction: a meta-analysis. Clinical Psychology Review. 2013;33:317–329. doi: 10.1016/j.cpr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Donovan DM. Moderating Effects of a Craving Intervention on the Relation Between Negative Mood and Heavy Drinking Following Treatment for Alcohol Dependence. Journal of Consulting and Clinical Psychology. 2011;79:54–63. doi: 10.1037/a0022282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Görres A, Seehaus A, Naumer MJ. Sensory modality of smoking cues modulates neural cue reactivity. Psychopharmacology. 2013;225:461–471. doi: 10.1007/s00213-012-2830-x. [DOI] [PubMed] [Google Scholar]
- Yan C, Zang Y. DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Frontiers in systems neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JT, Yao YW, Li CSR, Zang YF, Shen ZJ, Liu L, Wang LJ, Liu B, Fang XY. Altered resting-state functional connectivity of the insula in young adults with Internet gaming disorder. Addiction biology. 2015 doi: 10.1111/adb.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JT, Yao YW, Potenza MN, Xia CC, Lan J, Liu L, Wang LJ, Liu B, Ma SS, Fang XY. Altered resting-state neural activity and changes following a craving behavioral intervention for Internet gaming disorder. Sci Rep. 2016;6:28109. doi: 10.1038/srep28109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.