Abstract
Purpose
The nature of allergic rhinitis (AR) in preschool aged children remains incompletely characterized. This study aimed to investigate the prevalence of AR and its associated risk factors in preschool-aged children and to assess the clinical utility of fractional exhaled nitric oxide (FeNO).
Methods
This general population-based, cross-sectional survey included 933 preschool-aged (3- to 7-year-old) children from Korea. Current AR was defined as having nasal symptoms within the last 12 months and physician-diagnosed AR.
Results
The prevalence of current AR in preschool children was 17.0% (156/919). Mold exposure (adjusted odds ratio [aOR], 1.67; 95% confidence interval [CI], 1.15-2.43) and the use of antibiotics (aOR, 1.97; 95% CI, 1.33-2.90) during infancy were associated with an increased risk of current AR, whereas having an older sibling (aOR, 0.52; 95% CI, 0.35-0.75) reduced the risk. Children with current atopic AR had significantly higher geometric mean levels of FeNO compared to those with non-atopic rhinitis (12.43; range of 1standard deviation [SD], 7.31-21.14 vs 8.25; range of 1SD, 5.62-12.10, P=0.001) or non-atopic healthy children (8.58; range of 1SD, 5.51-13.38, P<0.001). The FeNO levels were higher in children with current atopic AR compared with atopic healthy children (9.78; range of 1SD, 5.97-16.02, P=0.083).
Conclusions
Mold exposure and use of antibiotics during infancy increases the risk of current AR, whereas having an older sibling reduces it. Children with current atopic AR exhibit higher levels of FeNO compared with non-atopic rhinitis cases, suggesting that FeNO levels may be a useful discriminatory marker for subtypes of AR in preschool children.
Keywords: Rhinitis, allergic, children, preschool, prevalence, risk factor, nitric oxide
INTRODUCTION
Allergic rhinitis (AR) is a common chronic disease in childhood and adolescence.1,2 AR negatively impacts physical, social, and psychological well-being, and these effects can also extend to other family members.1,3 Children with AR complain of disruptive sneezing, itching, watery rhinorrhea, and nasal blockage; these symptoms can indirectly cause sleep disturbances and daily fatigue, thereby resulting in impaired school performance.4
The prevalence of AR is increasing worldwide.5 The International Study of Asthma and Allergies in Childhood (ISAAC) I studies revealed that the median average prevalence of rhinoconjunctivitis in the past year for 6- to 7-year-old children was 6.9% (range 0.8%-14.9%) in 1997.6 ISSAC III studies (1999-2004) reported an average prevalence of current rhinoconjunctivitis symptoms of 8.5% (range 2.8%-21.8%) in 6- to 7-year-old children and 14.6% (1.0%-45.0%) in 13- to 14-year-old children.7 The prevalence of AR in early childhood has continually increased from 1995 to 2008 in Seoul, Korea. Indeed, the prevalence of current AR was 11.9% in 1995, 14.2% in 2000, 19.4% in 2005, and 22.2% in 2008.8
By contrast, limited data on the prevalence of AR are available because of difficulties in the diagnosis or definition of AR, especially in preschool-aged children. In a study from central China, the prevalence of AR in 3- to 6-year-old children was reported to be 10.8% based on the diagnostic criteria of nasal symptoms and positive skin prick test (SPT) results.9 The prevalence of rhinitis symptoms ever in life time was reported in another study to be 43.4%, whereas the prevalence of physician-diagnosed AR was 26.4% and 26.1% of 3- to 5-year old children were treated because of AR.5 Most studies of the prevalence of AR in preschool children have been performed using a questionnaire; therefore, the wide range of reports of the prevalence of AR may be partially attributed to an overestimation of AR. Furthermore, the prevalence of AR can be varied and depend upon the definition of AR.
Rhinitis is defined as inflammation of the nasal epithelium and characterized by at least 2 of the following nasal symptoms: rhinorrhea, blockage, sneezing, or itching. AR is caused by exposure to an allergen to which an individual is sensitized.10 In preschool-aged children, it is challenging to distinguish AR from infectious rhinitis and to define AR because of low rates of allergen sensitization.3,11
The fractional concentration of exhaled nitric oxide (FeNO) is a noninvasive parameter used to measure airway inflammation by analyzing gas collected from a single breath.12 Up-regulation of inducible nitric oxide synthase during intranasal and airway inflammation can increase FeNO levels.13 FeNO is correlated with other markers of inflammation, such as total serum immunoglobulin E (IgE) and eosinophil counts in the airway mucosa, and its levels are elevated in asthmatic patients.12 Because allergic diseases, such as AR and atopic dermatitis (AD), can confound the relationship between FeNO and asthma, AR should be considered in the assessments of FeNO levels in patients with asthma.14 Additionally, subjects with AR who do not exhibit asthmatic symptoms can have significantly higher levels of FeNO than those in healthy school-aged children (15.3 vs 5.9 ppb).15 However, the levels of FeNO in preschool children with AR have not yet been reported and little is known about the epidemiology of AR in preschool-aged children.
This study aimed to investigate the prevalence and associated risk factors of AR in preschool-aged children (3- to 7-years-old). Additionally, we compared FeNO levels in children with AR according to the presence or absence of potential confounding factors, such as comorbidities related to asthma.
MATERIALS AND METHODS
Study participants
The present study was designed as a cross-sectional and community-based study as a part of a standardization study for the diagnosis and treatment of pediatric allergic diseases. It included 16 randomly selected childcare centers from Seoul and Gyeonggi province in Korea, between July and August of 2010. A modified ISAAC questionnaire2 was used to evaluate the prevalence of allergic diseases.
The study population included 480 boys and 453 girls (mean age, 4.87±1.05 years). Patient demographic profiles and general information are presented in Table 1. A parental history of any allergic disease, including asthma, AR, or AD, was reported for 49.0% of individuals, while a parental history of AR was noted for 44.0%. Exposure to environmental tobacco smoke was reported in 45.2% of the study population.
Table 1. Subject characteristics.
| Characteristics | No./total No. (%)* |
|---|---|
| No. of subject | 933 |
| Responded to questionnaire | 919/933 (98.5) |
| Age, mean±SD (year) | 4.87±1.05 |
| Sex (M/F) | 480/453 |
| BMI, mean±SD (kg/m2) | 15.9±1.65 |
| Parental history of allergic disease | 449/916 (49.0) |
| Parental history of asthma | 46/914 (5.0) |
| Parental history of AR | 403/915 (44.0) |
| Parental history of AD | 105/915 (11.5) |
| Environmental tobacco smoking | 412/911 (45.2) |
| Maternal education level | |
| < College degree | 240/905 (26.5) |
| ≥ College degree | 665/905 (73.5) |
| Economic status (monthly income, USD) | |
| Low (< 3,000) | 328/890 (36.9) |
| Middle (3,000–5,000) | 341/890 (38.3) |
| High (≥5,000) | 221/890 (24.8) |
| Biomarker | |
| Eosinophil, mean±SD (%) | 4.17±3.20 |
| Total IgE, mean±SD (IU/mL) | 194.35±376.47 |
| Atopy† | 147/659 (22.3) |
| FeNO, mean±SD (ppb) | 10.31±6.51 |
SD, standard deviation; M, male; F, female; BMI, body mass index; AR, allergic rhinitis; AD, atopic dermatitis; USD, US dollar; IgE, immunoglobulin E; FeNO, fractional exhaled nitric oxide; SPT, skin prick test.
*Data are presented as number/total number (percentage) of patients, unless otherwise indicated; †Defined as at least 1 positive SPT result (an allergen wheal diameter greater than a histamine wheal diameter and a histamine wheal diameter ≥3 mm).
The Institutional Review Board (2010-02 CON-14-P) of Asan Medical Center approved the study protocol. Written consent was obtained from all parents and guardians following a detailed explanation of the study.
ISAAC questionnaire
A modified Korean version of the ISAAC was previously validated as a tool for the assessment and diagnosis of allergic symptoms in Korean children.16 The questionnaire includes 3 main sections: (1) general patient characteristics, including name, sex, date of birth, height, and weight; (2) a history of symptoms related to asthma, AR, AD, allergic conjunctivitis, and food allergy; and (3) exposure to environmental factors associated with allergic disease. The Korean version of the ISAAC questionnaire was completed by the parents or guardians of the preschool-aged children.
Prevalence of AR
We determined the prevalence of the following factors: (1) sneezing or a runny/blocked nose at any point in life (i.e., AR symptoms ever); (2) sneezing or runny/blocked nose and AR symptoms within the 12 months prior to survey completion (i.e., AR symptoms in the last 12 months; (3) a diagnosis of AR at any point in a lifetime (i.e., AR diagnosis ever); (4) treatment for AR within 12 months prior to survey completion (i.e., AR treatment in the last 12 months); (5) exhibiting AR symptoms within the last 12 months and a diagnosis of AR by clinicians at any point in life (i.e., current AR); (6) exhibiting AR symptoms within the last 12 months and a diagnosis of AR by clinicians at any point in life along with atopy (i.e., current atopic AR); and (7) AR symptoms in the last 12 months who had 1 or more positive reactions on SPTs (i.e., atopic AR).
White blood cells (WBCs), eosinophils, total IgE, and specific IgE
WBCs and the percentage of blood eosinophils were measured. The levels of total serum IgE and specific IgE were measured by fluorescent enzyme immunoassay using the ImmunoCAP system (Phadia AB, Uppsala, Sweden). Titers of specific IgE were measured for 7 major inhaled allergens: Dermatophagoides farina (Der f), cat, dog, cockroach, Alternaria alternate, mugwort, and alder. The titers of specific IgE were considered to be positive if they were greater than 0.35 kUA/L.
SPTs
SPTs were performed against the following 18 common aeroallergens and food allergens (Allergopharma, Reinbek, Germany): Dermatophagoides pteronyssinus (Der p), Der f, cat, dog, mugwort, grass, birch, ragweed, alder, oak, Japanese hop, Aspergillus fumigatus, Alternaria alternate, cockroach, milk, soybean, egg white, and peanut. As positive and negative controls, histamine, and isotonic saline, respectively, were used. Maximum wheal diameters that were greater than both of those caused by histamine and 3 mm in the absence of any reaction to saline were considered positive responses on SPTs after 15 minutes. Atopy was defined as exhibiting a positive response against at least 1 allergen on SPTs.
FeNO
The Levels of FeNO were measured using a Niox Mino analyzer (Aerocrine, Solna, Sweden) according to American Thoracic Society/European Respiratory Society (ATS/ERS) recommendations by a single experienced operator.17 Measurements were made at an expiratory flow rate of 50 mL/sec and the duration of exhalation was at least 6 seconds to ensure that NO levels were stable. A total of 3 recorded FeNO measurements with repeated measurements within 10% of the mean were obtained for each subject. The mean value of the 3 measurements was recorded as the final FeNO.17
Statistical analysis
Statistical analyses were performed using SAS version 9.0 (SAS Institute, Cary, NC, USA). The prevalence of allergic diseases is presented as mean values with the 95% confidence interval (CI). To identify potential risk factors for current AR, χ2 tests and logistic regression analyses were conducted. For multivariate analysis, age, sex, and body mass index (BMI) were adjusted as personal factors, a parental history of AD was adjusted as a genetic factor, and the level of maternal education was considered as a socioeconomic factor. Sensitization to Der p or Der f indicated a positive response on SPTs or ImmunoCAP for house dust mite antigen. A P value of <0.05 was used to define statistically significant differences.
RESULTS
Prevalence of AR
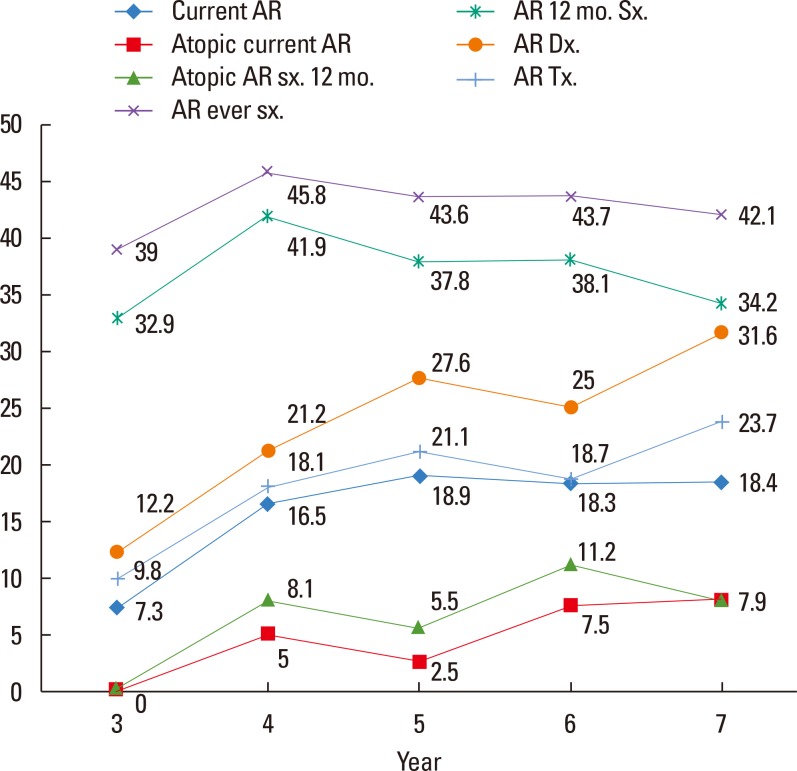
The prevalence of allergic diseases, including current AR, is shown in Fig. 1. Generally, an increasing trend was observed for the prevalence of AR with age in preschool-aged children. The rates of prevalence of AR symptoms ever in life, AR symptoms in the last 12 months, AR diagnosis in the life time, and AR treatment in the life time in 3- to 7-year-old children were 43.8%, 38.5%, 23.8%, and 18.6%, respectively. The prevalence of current AR, current atopic AR, and atopic AR symptoms within 12 months in 3- to 7-year-old children was 17.0%, 4.7%, and 7.5%, respectively (Fig. 1).
Fig. 1. The prevalence of all related AR questionnaires in 3- to 7-year-old children. AR, allergic rhinitis; sx., symptom; 12 mo., within 12 months; Dx., diagnosis; Tx., treatment.
Risk factors for current AR
The independent risk factors for current AR included a history of asthma diagnosis (adjusted odds ratio [aOR], 2.96; 95% CI, 1.73-5.08), a history of wheezing in the last 12 months (aOR, 2.09; 95% CI, 1.21-3.59), a history of AD diagnosis (aOR, 1.61; 95% CI, 1.11-2.34), and a parental history of asthma (aOR, 2.10; 95% CI, 1.08-4.08). We also identified independent environmental risk factors for AR, which included the use of antibiotics in infancy for more than 3 days (aOR, 1.97; 95% CI, 1.33-2.90) and exposure to indoor mold during infancy (aOR, 1.67; 95% CI, 1.15-2.43). Additionally, having an older sibling reduced the risk of current AR (aOR, 0.52; 95% CI, 0.35-0.75) (Table 2).
Table 2. Risk factors for current AR in preschool children.
| Risk factors | OR (95% CI) | P value | aOR* (95% CI) | P value |
|---|---|---|---|---|
| Genetic factors | ||||
| Parental asthma | 3.22 (1.71–6.07) | <0.001 | 2.10 (1.08–4.08) | 0.028 |
| Parental AR | 2.88 (2.01–4.14) | <0.001 | 2.25 (0.92–5.52) | 0.077 |
| Parental AD | 1.49 (0.91–2.43) | 0.115 | 0.93 (0.54–1.61) | 0.800 |
| Past history | ||||
| Past history of asthma diagnosis | 3.44 (2.10–5.63) | <0.001 | 2.96 (1.73–5.08) | <0.001 |
| Wheezing, last 12 months | 1.89 (1.13–3.16) | 0.015 | 2.09 (1.21–3.59) | 0.008 |
| Past history of AD diagnosis | 1.74 (1.23–2.47) | 0.002 | 1.61 (1.11–2.34) | 0.012 |
| History of bronchiolitis before 2 years of age | 1.39 (0.91–2.12) | 0.132 | 1.18 (0.74–1.89) | 0.479 |
| Breast milk feeding >6 months | 1.27 (0.84–1.95) | 0.262 | 1.24 (0.80–1.94) | 0.338 |
| Use of antibiotics in infancy (>3 days) | 1.95 (1.36–2.80) | <0.001 | 1.97 (1.33–2.90) | <0.001 |
| Cesarean-section delivery | 1.35 (0.95–1.92) | 0.100 | 1.37 (0.94–2.00) | 0.099 |
| Environmental tobacco smoking | 1.09 (0.77–1.53) | 0.127 | 1.15 (0.79–1.67) | 0.460 |
| Current pet ownership | 0.63 (0.19–2.12) | 0.451 | 0.66 (0.19–2.29) | 0.508 |
| Pet ownership in infancy | 0.85 (0.37–1.94) | 0.323 | 0.80 (0.34–1.86) | 0.600 |
| Dog ownership in infancy | 0.96 (0.42–2.21) | 0.928 | 0.90 (0.38–2.12) | 0.810 |
| Cat ownership in infancy | 1.17 (0.13–10.51) | 0.891 | 1.00 (0.11–9.30) | 0.996 |
| Day care attendance before 1-year-old | 0.84 (0.48–1.48) | 0.551 | 0.97 (0.53–1.76) | 0.908 |
| Having an older sibling | 0.56 (0.39–0.79) | <0.001 | 0.52 (0.35–0.75) | 0.001 |
| Having a younger sibling | 1.09 (0.76–1.57) | 0.633 | 1.11 (0.77–1.64) | 0.561 |
| History of remodeling, currently | 0.92 (0.57–1.48) | 0.736 | 0.82 (0.48–1.39) | 0.452 |
| Indoor mold exposure during infancy | 1.69 (1.18–2.40) | 0.004 | 1.67 (1.15–2.43) | 0.007 |
| Indoor mold exposure during previous last 12 months | 1.18 (0.84–1.67) | 0.347 | 1.09 (0.75–1.58) | 0.646 |
AR, allergic rhinitis; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; AD, atopic dermatitis; BMI, body mass index.
*Adjusted by age, sex, BMI, parental history of allergic diseases, and maternal education levels.
Sensitization for current AR
Table 3 shows analyses of associations between positive sensitization on SPTs and current AR. The independent risk factors for current AR included any any sensitization on SPTs (aOR, 1.99; 95% CI, 1.26-3.14), Der p (aOR, 2.23; 95% CI, 1.37-3.54), Der f (aOR, 2.23; 95% CI, 1.27-3.91), and Japanese Hop (aOR, 6.59; 95% CI, 1.09-39.85). The independent risk factors for current AR on immunoCAP included more than 1 sensitization (aOR, 1.84; 95% CI, 1.20-2.82) and sensitization to Der f (aOR, 1.96; 95% CI, 1.27-3.03) or mugwort (aOR, 4.63; 95% CI, 1.51-14.23) (Table 4).
Table 3. Associated positive sensitization on SPTs for the development of current AR.
| Risk factors | OR (95% CI) | P value | aOR* (95% CI) | P value |
|---|---|---|---|---|
| SPT any positive | 2.34 (1.52–3.61) | <0.001 | 1.99 (1.26–3.14) | 0.003 |
| Der p | 2.56 (1.63–4.02) | <0.001 | 2.23 (1.37–3.54) | 0.001 |
| Der f | 2.62 (1.54–4.46) | <0.001 | 2.23 (1.27–3.91) | 0.005 |
| Alder | 0.86 (0.10–7.42) | 0.890 | 0.77 (0.09–6.84) | 0.815 |
| Birch | 1.44 (0.15–13.93) | 0.755 | 1.14 (0.11–11.4) | 0.913 |
| Mugwort | 2.90 (0.48–17.53) | 0.247 | 0.81 (0.08–7.97) | 0.856 |
| Japanese Hop | 6.59 (1.09–39.85) | 0.040 | 3.57 (0.47–27.37) | 0.221 |
| Dog | 2.19 (0.65–7.39) | 0.207 | 2.14 (0.53–8.60) | 0.285 |
| Cat | 4.35 (0.61–31.22) | 0.143 | 4.56 (0.61–34.30) | 0.140 |
| Egg white | 8.72 (0.78–96.99) | 0.078 | 10.31 (0.86–123.00) | 0.065 |
| Alternaria | 4.35 (0.61–31.22) | 0.143 | 4.49 (0.58–34.77) | 0.151 |
| Aspergillus | 4.32 (0.27–69.64) | 0.302 | 2.73 (0.16–45.29) | 0.485 |
| Ragweed | 4.33 (0.27–69.64) | 0.302 | 5.83 (0.35–96.80) | 0.219 |
| Oak | 1.08 (0.12–9.71) | 0.865 | 0.81 (0.09–7.58) | 0.852 |
SPT, skin prick test; AR, allergic rhinitis; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Der p, Dermatophagoides pteronyssinus; Der f, Dermatophagoides farina; BMI, body mass index.
*Adjusted by age, sex, BMI, parental history of allergic diseases, and maternal education levels.
Table 4. Associated positive serum-specific IgE test results for the development of current AR.
| Risk factors | OR (95% CI) | P value | aOR* (95% CI) | P value |
|---|---|---|---|---|
| Sensitization ≥1 allergens | 2.01 (1.39–3.13) | <0.001 | 1.84 (1.20–2.82) | 0.006 |
| Der f | 2.25 (1.49–3.38) | <0.001 | 1.96 (1.27–3.03) | 0.002 |
| Cat | 3.15 (0.83–11.92) | 0.091 | 3.47 (0.89–13.50) | 0.073 |
| Dog | 2.36 (0.78–7.19) | 0.130 | 2.97 (0.90–9.80) | 0.074 |
| Cockroach | 1.03 (0.29–3.72) | 0.961 | 1.03 (0.28–3.82) | 0.965 |
| Alternaria | 2.56 (0.91–7.18) | 0.075 | 2.72 (0.92–8.06) | 0.071 |
| Mugwort | 5.47 (1.86–16.10) | 0.002 | 4.63 (1.51–14.23) | 0.007 |
| Alder | 2.33 (0.84–6.43) | 0.103 | 1.97 (0.69–5.64) | 0.206 |
Positive specific IgE levels are defined as ≥ 0.35 kU/L for each allergen.
IgE, immunoglobulin E; AR, allergic rhinitis; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Der f, Dermatophagoides farinae; BMI, body mass index.
*Adjusted by age, sex, BMI, parental history of allergic diseases, and maternal education levels.
Levels of FeNO and total serum IgE, and blood eosinophil counts in current atopic AR patients without asthma or atopy
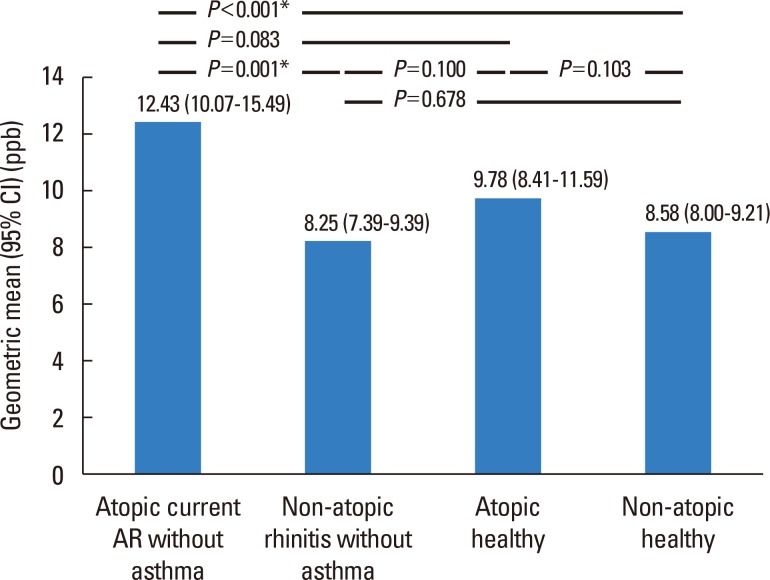
The geometric mean levels of FeNO were significantly higher in children with current atopic AR without asthma than in either control individuals or those with non-atopic rhinitis without asthma groups (12.43; 95% CI, 10.07-15.49 vs 8.25; 95% CI, 7.39-9.39, P=0.001). Furthermore, children with current atopic AR without asthma had significantly higher geometric mean levels of FeNO compared with non-atopic healthy children (8.58; 95% CI, 8.00-9.21, P<0.001). The levels of FeNO were higher in children with current atopic AR without asthma compared with those in atopic healthy children (12.43; 95% CI, 10.07-15.49 vs 9.78; 95% CI, 8.41-11.59, P=0.083) (Fig. 2).
Fig. 2. FeNO levels among children having current atopic AR without asthma along with those in atopic and non-atopic healthy children. FeNO, fractional exhaled nitric oxide; AR, allergic rhinitis; CI, confidence interval. *P<0.01.
Levels of total serum IgE (210.61; 95% CI, 134.29-333.62 vs 55.70; 95% CI, 44.70-70.10, P<0.001) and the percentages of blood eosinophils (5.58; 95% CI, 4.39-6.82 vs 3.16; 95% CI, 2.72-3.63, P<0.001) from children having current atopic AR without asthma were higher than those with non-atopic rhiinitis without asthma (Supplementary Figs. 1 and 2). The total serum IgE levels tended to be higher in children with current atopic AR without asthma compared with those in the atopic healthy children (210.61; 95% CI, 134.29-333.62 vs 149.90; 95% CI, 114.43-200.34, P=0.193). Children with current atopic AR without asthma had a significantly higher geometric mean blood eosinophil percentage compared with those from the atopic healthy children (3.93; 95% CI, 3.35-4.62, P=0.015).
Cutoff values for FeNO, total serum IgE, and blood eosinophil percentages to define atopic current AR
To define cutoff values for FeNO to differentiate children atopic current AR from atopic healthy children, we constructed a receiver operation characteristic (ROC) curve for the diagnosis of current atopic AR. A FeNO >7.5 ppb yielded a sensitivity of 86.4%, a specificity of 47.7%, and the area under the ROC curve (AUC) of 0.714 (95% CI, 0.600-0.829). Additionally, we calculated optimal cutoff values for serum IgE of >86.1 kU/L, with a sensitivity of 83.2%, a specificity of 71.5%, and an AUC of 0.835 (95% CI, 0.811-0.858), as well as a blood eosinophil frequency of >2.5%, sensitivity of 66.1%, specificity of 68.1%, and an AUC of 0.706 (95% CI, 0.674-0.738; Table 5). A significant linear correlation between FeNO, total serum IgE levels, and blood eosinophil percentage was observed (r=0.244, P<0.001 for serum IgE and r=0.296, P<0.001 for blood eosinophilia; Supplementary Figs. 3A and B).
Table 5. Cutoff values for FeNO, serum IgE, and blood eosinophil levels in children with current atopic AR without asthma.
| Cut-off value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|
| FeNO (ppb) | >7.5 | 86.4 | 47.7 |
| Serum IgE (kU/L) | >86.1 | 83.2 | 71.5 |
| Blood eosinophil (%) | >2.5 | 66.1 | 68.1 |
FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E; AR, allergic rhinitis.
DISCUSSION
Our current general population-based study demonstrated the prevalence of AR and the discriminative value of FeNO for the diagnosis of AR in preschool-aged children in Seoul, Korea. In the present study, the prevalence of current AR, atopic current AR, AR symptoms ever in lifetime, and atopic AR symptoms ever in lifetime in preschool-aged children were 17.0%, 4.7%, 43.8%, and 8.4%, respectively, which indicates that AR is not uncommon in preschool-aged children. A parental history of asthma, a past history of asthma, AD, indoor mold exposure, antibiotic use during infancy, and atopy increased the risk of current AR, whereas the presence of an older sibling reduced the risk of current AR. Sensitization to Der p, Der f, and Japanese hop was associated with an increased risk of current AR in preschool-aged children. In this study, we excluded children with asthma and divided the current AR group into 2 subgroups based on the presence of atopy. The levels of FeNO were significantly higher in children with current atopic AR without asthma (12.43 ppb; 95% CI, 10.07-15.49) compared those with non-atopic rhinitis without asthma (8.25 ppb; 95% CI, 7.39-9.39; P=0.001) or atopic healthy children (9.78 ppb; 95% CI, 8.41-11.59), although the latter difference was not statistically significant (P=0.083). Therefore, we should suspect AR when atopic preschool children complain of AR symptoms and present with high levels of FeNO.
Some children may only exhibit significant symptoms with a combination of both allergic inflammation and coexisting viral infection. Additionally, it can be difficult to differentiate AR from other common diseases, such as non-allergic rhinitis (NAR) and chronic rhinosinusitis, as these diseases share similar symptoms with AR in young children. These similarities are particularly common in preschool-aged children.18 We diagnosed current AR as nasal symptoms and also used physician diagnoses to reduce potential errors in the misdiagnosis of AR. Furthermore, the findings of our study are important, as they combine a questionnaire survey and clinical diagnoses with the results of SPT in a large randomly selected preschool children with AR.
The reported prevalence of AR in preschool-aged children is diverse and varies by region.19 This finding might be partially attributed to misdiagnoses and be related to the physician's experience in the diagnosis of AR. However, the prevalence of AR has been progressively increasing worldwide, while the age of onset is decreasing.19 In a birth cohort study carried out in Sweden in 2003, the frequency of 4.5-year-old children who reported symptoms that were compatible with AR during the previous year was 5.5% (246/4,465), and the frequency of doctor-diagnosed AR based on symptoms during the last year was 1.7% (75/4,465).20 In a study conducted in China using an ISAAC questionnaire, the prevalence of AR, which was defined as nasal symptoms and atopy on SPTs, was 10.8% in 3- to 6-year-old children,9 a rate that was similar to the prevalence of atopic AR symptoms in an individual's lifetime (8.4%) observed in our study. In a study carried out in Seoul, Korea using an ISAAC questionnaire, the prevalences of AR ever, AR in the last 12 months, AR diagnosis ever, and treatment in the last 12 months were 31.8%, 26.2%, 16.2%, and 12.9%, respectively, in children who were 2-7 years old in 2009.21 Furthermore, in a study of 3- to 6-year-old preschool children in the Gyeonggi Province of Korea in 2009, the prevalence of AR ever was 44.2%, while that of AR symptoms in the last 12 months was 40.7%, that of AR diagnosis ever was 34.5%, and that of treatment in the last 12 months was 28.0%.22
The findings of the aforementioned studies were similar to those of our study, which used the ISAAC questionnaire, as the prevalences of AR symptoms ever in life, AR symptoms in the 12 months, AR diagnosis in the life time and AR treatment in the life time were 43.8%, 38.5%, 23.8%, and 18.6% respectively. Differences in the prevalence of AR between our report and earlier studies from China might be in part attributed to differences in the definition of AR combined with atopy in SPTs. Additionally, differences in environmental factors according to regions may affect the prevalence of AR. However, the use of a questionnaire method may result in a the high probability of overestimation. The prevalence of atopy for SPTs is low, especially in preschool children; therefore, application results based on SPTs could result in an underestimated diagnosis of AR. Thus, further studies will be required to accurately determine the prevalence of AR in a large study using a unified definition of AR. Moreover, considering that conducting SPTs on preschool children was particularly difficult in this study, a non-invasive FeNO test may aid in diagnoses of AR in preschool children. However, the prevalence of AR is not uncommon in preschool children. Hence, we must further characterize when AR develops, determine how to diagnose AR, and assess whether cases of AR in preschool children are increasing, so that follow-up studies about the pathogenesis of AR are needed.
In our study, environmental factors that we identified as independent risk factors for AR included the use of antibiotics in infancy and exposure to household mold during infancy. A protective effect of having an older sibling on AR in preschool-aged children was observed, which was consistent with an inverse association between the number of siblings and hay fever.23,24 The use of antibiotics in early life is known to lead to alterations in the intestinal flora and increase the risk of childhood atopy and asthma.23,24,25 Therefore, our data support the hygiene hypothesis that microbiome naturally colonizing in our body may affect the development of human immune system and the development of allergic disease. Especially during pregnancy and within 3 years after birth, the composition of the microorganisms in the intestinal microbiome is affected by the delivery method of the infant as well as differences in diet composition.26 Antibiotic use during infancy induces changes in the composition of the intestinal microbial strain, and this is associated with suppressed T helper 1 (Th1)-type immunity after normal birth as well as progressively increased serum IgE levels and regulatory T cells, which are known to suppress T helper 2 (Th2)-type immune responses.27,28
In our study, Der f and Der p were found to be independent risk factors for allergic sensitization on SPT and CAP in preschool-aged children with current AR. Exposure to indoor allergens at home is a common cause of perennial AR, especially in young children who spend most of their time indoors. Therefore, in children with allergic sensitization to house dust mite allergens, avoidance of house dust mites is always a critical component of a management strategy for AR in preschool-aged children.10 Additionally, mugwort was identified as a major independent risk factor for AR in preschool-aged children.
A key issue is the relationship between AR and FeNO levels. FeNO analysis is often used to assess eosinophilic airway inflammation in bronchial asthmatic patients. Many studies have shown increased levels of FeNO in adults with AR.19 Although the literature on FeNO measurements in children with AR is less extensive, FeNO has been reported to be increased in children with AR.29 In China, in a case-control study with age- and gender-matched children based on a lager cross-sectional general population survey on asthma, AR and AD among 9- to 11-year-old school-aged children with physician-diagnosed AR without asthma had significantly elevated levels of FeNO (25.8±2.1 ppb) compared with the control group (13.3±1.6 ppb), which included children without physician-diagnosed asthma, AR, or AD (P=0.008). Although they did not control whether the patients had atopy or not, they showed that AR could independently cause an increase in FeNO levels in Chinese school-aged children.30 Although several studies have assessed FeNO levels in school-children with asthma or AR,30,31 limited data are available about FeNO levels in preschool-aged children. The FeNO level is low at younger age32 and a study that suggested FeNO level of recurrent wheezers in preschool children was approximately 10-15 ppb, which is lower than school-aged children's levels.33 Our study focused on the presentation of FeNO levels in preschool children with AR who were 3-7 years old and controlled for the confounding factor of asthma, which is one of the most powerful determinants of increased FeNO levels. We believe that further studies on FeNO with preschool children will be required to compare the results with our study, rather than using the FeNO level of the older children's reference values.
Most previous reports of the prevalence of AR have been based on a questionnaire survey alone, which may have resulted in an overestimation of the prevalence (up to 50%) of AR.34 Similar to other studies, our findings were based on a questionnaire survey. Therefore, as we diagnosed current AR based on a questionnaire, both recall and selection bias may exist. Additionally, reporting bias might have exist because clinicians did not directly examine and follow up the current AR patients. To evaluate the actual prevalence of AR, we classified current atopic AR and non-atopic rhinitis to differentiate between rhinitis with and without sensitization to allergens. Another limitation of our study was its cross-sectional design, which limited our ability to determine causation. However, our study was population-based, which is a major strength that allows for an estimation of the population attributable risk of multiple factors. Furthermore, it is difficult to compare the prevalence of current AR calculated herein with previous studies because of differences between the study population groups, which were from different areas. Despite these limitations, our current findings merit attention because limited data are available on the prevalence of AR in preschool-aged children. An additional strength of our study was that we performed the FeNO test among preschool children who were suspected AR cases using a non-invasive biomarker.
In conclusion, the prevalence of current AR in preschool-aged children was 17.0%, indicating that the onset of AR can be significant at younger age. Furthermore, levels of FeNO were higher in children who had current atopic AR, which suggests that FeNO levels can be a useful diagnostic marker of current AR in preschool-aged children. Future surveillance efforts that follow a well-designed large patient cohort will be required to quantitatively assess the prevalence of AR and its associated risk factors in preschool-aged children. Furthermore, prospective, large-scale, randomized control trials will be needed to better characterize and understand the role of FeNO in the clinical care of young children with AR.
ACKNOWLEDGMENTS
This study was supported by grant of the Korean Health Technology R & D Project, Ministry of Health and Welfare, Republic of Korea (A092076 and HI13C1674).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTAL MATERIALS
A significant linear correlation between fractional exhaled nitric oxide (FeNO), total serum immunoglobulin E (IgE) levels, and blood eosinophil percentage was observed (r=0.244, P<0.001 for serum IgE and r=0.296, P<0.001 for blood eosinophilia; Supplementary Figs. 3A and B).
IgE was expressed as ku/L, eosinophilia was expressed as cells/uL and reported on the abscissa, whereas FeNO levels were expressed as ppb and reported on the ordinate (Supplementary Fig. 3A). Blood eosinophilia was expressed as a percentage (%) and reported on the abscissa, whereas FeNO levels were expressed as ppb and reported on the ordinate (Supplementary Fig. 3B). Statistical analysis was performed using Spearman's correlation.
Comparisons of total serum IgE levels children having current atopic AR without asthma and atopic and non-atopic healthy children. AR, allergic rhinitis; CI, confidence interval. *P<0.01.
Comparisons of blood eosinophil percentage between children having current atopic AR without asthma and atopic and non-atopic healthy children. AR, allergic rhinitis; CI, confidence interval. *P<0.01.
(A) Correlation between FeNO and IgE levels. (B) Correlation between FeNO levels and blood eosinophilia. FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E.
References
- 1.Chen Y, Wong GW, Li J. Environmental exposure and genetic predisposition as risk factors for asthma in China. Allergy Asthma Immunol Res. 2016;8:92–100. doi: 10.4168/aair.2016.8.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon HI. Respiratory review of 2014: asthma. Tuberc Respir Dis (Seoul) 2014;77:237–242. doi: 10.4046/trd.2014.77.6.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers DA, Bleecker ER, Holloway JW, Holgate ST. Asthma genetics and personalised medicine. Lancet Respir Med. 2014;2:405–415. doi: 10.1016/S2213-2600(14)70012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vahedi G, Richard AC, O'Shea JJ. Enhancing the understanding of asthma. Nat Immunol. 2014;15:701–703. doi: 10.1038/ni.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tizaoui K, Kaabachi W, Hamzaoui K, Hamzaoui A. Association of single nucleotide polymorphisms in toll-like receptor genes with asthma risk: a systematic review and meta-analysis. Allergy Asthma Immunol Res. 2015;7:130–140. doi: 10.4168/aair.2015.7.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao CN, Fan Y, Huang JJ, Zhang HX, Gao T, Wang C, et al. The association of GSDMB and ORMDL3 gene polymorphisms with asthma: a meta-analysis. Allergy Asthma Immunol Res. 2015;7:175–185. doi: 10.4168/aair.2015.7.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Wu S, Barrera J, Matthews K, Pan D. The hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Angus L, Moleirinho S, Herron L, Sinha A, Zhang X, Niestrata M, et al. Willin/FRMD6 expression activates the hippo signaling pathway kinases in mammals and antagonizes oncogenic YAP. Oncogene. 2012;31:238–250. doi: 10.1038/onc.2011.224. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alarcón C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGFβ pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 12.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol. 2015;7:35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungvári I, Hullám G, Antal P, Kiszel PS, Gézsi A, Hadadi É, et al. Evaluation of a partial genome screening of two asthma susceptibility regions using bayesian network based bayesian multilevel analysis of relevance. PLoS One. 2012;7:e33573. doi: 10.1371/journal.pone.0033573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungvári I, Hadadi E, Virág V, Bikov A, Nagy A, Semsei ÁF, et al. Implication of BIRC5 in asthma pathogenesis. Int Immunol. 2012;24:293–301. doi: 10.1093/intimm/dxs007. [DOI] [PubMed] [Google Scholar]
- 17.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 18.Antal P, Millinghoffer A, Hullám G, Hajós G, Sárközy P, Gézsi A, et al. Bayesian, systems-based, multilevel analysis of associations for complex phenotypes: from interpretation to decisions. In: Sinoquet C, Mourad R, editors. Probabilistic graphical models for genetics, genomics, and postgenomics. Oxford: Oxford University Press; 2014. pp. 318–362. [Google Scholar]
- 19.Antal P, Millinghoffer A, Hullám G, Hajós G, Szalai C, Falus A. A bioinformatic platform for a bayesian, multiphased, multilevel analysis in immunogenomics. In: Flower DR, Davies M, Ranganathan S, editors. Bioinformatics for immunomics. New York (NY): Springer; 2010. pp. 157–185. [Google Scholar]
- 20.Hullám G, Antal P, Szalai C, Falus A. Evaluation of a Bayesian model-based approach in GA studies. JMLR Workshop Conf Proc. 2010;8:30–43. [Google Scholar]
- 21.Antal P, Millinghoffer A, Hullám G, Szalai C, Falus AA. Bayesian view of challenges in feature selection: multilevel analysis, feature aggregation, multiple targets, redundancy and interaction. JMLR Workshop Conf Proc. 2008;4:74–89. [Google Scholar]
- 22.Gézsi A, Lautner-Csorba O, Erdélyi DJ, Hullám G, Antal P, Semsei ÁF, et al. In interaction with gender a common CYP3A4 polymorphism may influence the survival rate of chemotherapy for childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2015;15:241–247. doi: 10.1038/tpj.2014.60. [DOI] [PubMed] [Google Scholar]
- 23.Lautner-Csorba O, Gézsi A, Erdélyi DJ, Hullám G, Antal P, Semsei ÁF, et al. Roles of genetic polymorphisms in the folate pathway in childhood acute lymphoblastic leukemia evaluated by Bayesian relevance and effect size analysis. PLoS One. 2013;8:e69843. doi: 10.1371/journal.pone.0069843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lautner-Csorba O, Gézsi A, Semsei AF, Antal P, Erdélyi DJ, Schermann G, et al. Candidate gene association study in pediatric acute lymphoblastic leukemia evaluated by Bayesian network based Bayesian multilevel analysis of relevance. BMC Med Genomics. 2012;5:42. doi: 10.1186/1755-8794-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan TK, Loh XY, Peh HY, Tan WN, Tan WS, Li N, et al. House dust mite-induced asthma causes oxidative damage and DNA double-strand breaks in the lungs. J Allergy Clin Immunol. 2016;138:84–96. doi: 10.1016/j.jaci.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Mao B, Gao Y, Bai Y, Yuan Z. Hippo signaling in stress response and homeostasis maintenance. Acta Biochim Biophys Sin (Shanghai) 2015;47:2–9. doi: 10.1093/abbs/gmu109. [DOI] [PubMed] [Google Scholar]
- 27.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 28.Yin MX, Zhang L. Hippo signaling in epithelial stem cells. Acta Biochim Biophys Sin (Shanghai) 2015;47:39–45. doi: 10.1093/abbs/gmu111. [DOI] [PubMed] [Google Scholar]
- 29.Moleirinho S, Patrick C, Tilston-Lünel AM, Higginson JR, Angus L, Antkowiak M, et al. Willin, an upstream component of the hippo signaling pathway, orchestrates mammalian peripheral nerve fibroblasts. PLoS One. 2013;8:e60028. doi: 10.1371/journal.pone.0060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- 31.Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989;139:806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- 32.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131:599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 33.Boulet LP, O'Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med. 2015;372:641–648. doi: 10.1056/NEJMra1407552. [DOI] [PubMed] [Google Scholar]
- 34.Weiler JM, Anderson SD, Randolph C, Bonini S, Craig TJ, Pearlman DS, et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105:S1–S47. doi: 10.1016/j.anai.2010.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A significant linear correlation between fractional exhaled nitric oxide (FeNO), total serum immunoglobulin E (IgE) levels, and blood eosinophil percentage was observed (r=0.244, P<0.001 for serum IgE and r=0.296, P<0.001 for blood eosinophilia; Supplementary Figs. 3A and B).
IgE was expressed as ku/L, eosinophilia was expressed as cells/uL and reported on the abscissa, whereas FeNO levels were expressed as ppb and reported on the ordinate (Supplementary Fig. 3A). Blood eosinophilia was expressed as a percentage (%) and reported on the abscissa, whereas FeNO levels were expressed as ppb and reported on the ordinate (Supplementary Fig. 3B). Statistical analysis was performed using Spearman's correlation.
Comparisons of total serum IgE levels children having current atopic AR without asthma and atopic and non-atopic healthy children. AR, allergic rhinitis; CI, confidence interval. *P<0.01.
Comparisons of blood eosinophil percentage between children having current atopic AR without asthma and atopic and non-atopic healthy children. AR, allergic rhinitis; CI, confidence interval. *P<0.01.
(A) Correlation between FeNO and IgE levels. (B) Correlation between FeNO levels and blood eosinophilia. FeNO, fractional exhaled nitric oxide; IgE, immunoglobulin E.