Abstract
The tools for asthma control assessment recommended by the current guideline are cognitive function- and effort-dependent, which is substantially impaired in the elderly. The aim of this study is to investigate objective assessment tools of asthma control status and previous asthma exacerbation (AE) in elderly subjects. Asthmatics aged >60 years who were treated with step 2 or 3 by the Global Initiative for Asthma (GINA) guideline were enrolled. During the 12-week study period, the subjects used either 400 µg of budesonide plus 10 mg of montelukast or 800 µg of inhaled budesonide. The occurrence of AE during the 4-week run-in and 12-week treatment period was monitored. After 12-week of treatment, sputum eosinophil count, peripheral eosinophil count, the plasma leukotriene E4 (LTE4), and prostaglandin F2α (PGF2α) metabolite levels were measured using the UHPLC/Q-ToF MS system. The study subjects were divided into group 1 (asthmatics who experienced AE during the study period) and group 2 (those who did not). A total of 101 patients aged 60-85 years were enrolled. Twenty-three patients (22.8%) had experienced AE. The plasma LTE4 level, LTE4/PGF2α ratio, and peripheral eosinophil count were significantly higher in group 1 than in group 2 (P=0.023, P=0.010, P=0.033, respectively). The plasma LTE4/PGF2α ratio and peripheral eosinophil count at week 12 were significantly associated with previous AE (odds ratio [OR]=1.748, P=0.013; OR=1.256, P=0.027). Receiver operating characteristic (ROC) curves to discriminate the subjects with previous AE, including these 2 parameters, showed that the area under the curve was 0.700 (P=0.004), with 73.9% sensitivity and 47.9% specificity. In conclusion, a combination of plasma LTE4/PGF2α ratio and peripheral eosinophil count can be an objective assessment tool which is significantly associated with asthma control status in elderly asthmatics.
Keywords: Elderly asthma, exacerbation, leukotriene
INTRODUCTION
The geriatric population is increasing worldwide and the burden of asthma is more significant in elderly asthmatics compared to younger ones. The number of elderly people is projected to reach 20% and 36% of total populations of the United States and China, respectively.1,2 The prevalence of asthma is increasing at all ages, and it is reported to be 5% to 10% in elderly people, similar to younger ones.1,3 A main burden of elderly asthma is attributed to asthma severity, hospitalization, near-fatal asthma related-events, and medical costs.2,4,5 To reduce the severity, mortality, and burden of asthma in elderly asthmatics, assessment of asthma control is mandatory.
Asthma control is assessed in terms of symptom control and future risk for asthma exacerbation (AE).6 Global Initiative for Asthma (GINA) symptom control tool, Asthma Control Questionnaire, and Asthma Control Test (ACT) are validated methods for assessing symptom control. To perform these tools for symptom control, patients have to recall their symptoms, including day/night time symptoms, reliever use, and activity limitation during the previous 1 to 4 weeks. One of the most important risk factors for AE is the history of ≥1 exacerbation in the previous year; therefore, patients should recall their AE history to evaluate the future risk for AE.6 Spirometry which is recommended in assessing the future risk for AE requires proper coordination, and cognitive function and effort.1 The tools for asthma control recommended by the current guideline6 are dependent on cognitive function and effort which is substantially impaired in elderly. In addition, assessment of asthma symptoms, especially dyspnea, may be inappropriate for elderly because of multiple comorbidities that can cause dyspnea in elderly. Therefore, objective assessment tool which is significantly associated with asthma control status and AE in elderly subjects are needed.
MATERIALS AND METHODS
Asthmatics aged >60 years who were diagnosed with asthma for more than 6 months and treated with step 2 or 3 by the GINA guidelines were enrolled. Subjects were recruited between August 2011 and February 2012 from 4 university hospitals in Korea.7 This study was approved by Ajou University Institutional Review Board (IRB No. GEN-CT4-10-095), and the other 4 hospitals approved the study. Informed consent was obtained from each patient. During the 12-week study period, the subjects used either 400 µg of inhaled budesonide plus 10 mg of montelukast or 800 µg of inhaled budesonide. The occurrence of AE during the 4-week run-in (maintaining with inhaled budesonide at 400 µg per a day) and 12-week treatment period was monthly monitored, which was defined when one of the following criteria was satisfied: use of systemic oral corticosteroids, asthma-related unscheduled visit/emergency department visit/hospitalization, and use of reliever medication. For the overall study period, all study subjects wrote a diary and the completeness was calculated as a compliance. After 12 weeks of antiasthmatic medication (maintaining same dose), the plasma levels of the metabolites leukotriene E4 (LTE4) and prostaglandin F2α (PGF2α) were measured. ACT score calculation, asthma quality of life (AQoL) assessment, sputum eosinophil counts, peripheral eosinophil counts, and pulmonary function tests were performed at week 12. The study subjects were divided into group 1 (asthmatics who experienced AE during the study period) and group 2 (those who did not). The LTE4 and PGF2α levels were quantified as previously reported.8 In brief, Mass Hunter Quantitative Analysis B.07.00 (Agilent Technologies, Santa Clara, CA, USA) was used to quantify the LTE4 and PGF2 levels in plasma samples. The following deuterated internal standards were used: LTE4-d5 for LTE4 and 8-iso PGF2α-d4 for PGF2α (Cayman Chemical Company, Ann Arbor, MI, USA). The concentration of each metabolite was determined from calibration curves using linear regression analysis. Correlation coefficients were >0.99 for all metabolites. All the chemicals were of high-performance liquid chromatograph (HPLC) grade. General linear regression analysis was performed to adjust for confounding factors in order to compare the metabolite levels between group 1 and 2 subjects. Receiver operating characteristic (ROC) curve analysis was used to discriminate the groups of patients who experienced AE.
RESULTS
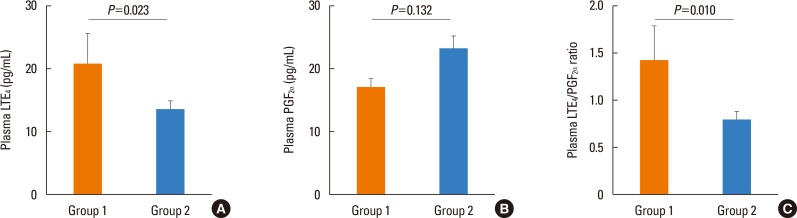
A total of 101 patients aged 60-85 years were enrolled. Twenty-three patients (22.8%) had experienced AE over the study period. Among the group 1 patients, 5 (21.74%), 1 (4.35%), 14 (60.87%), and 3 (13.04%) had experienced the last AE in the first, second, third, and fourth months, respectively. Baseline clinical characteristics are summarized in Table 1. Group 1 subjects had a significantly higher plasma LTE4 level and a LTE4/PGF2α ratio at week 12 than group 2 subjects (LTE4 level: 20.83±23.37 vs 13.49±11.04 pg/mL, P=0.023; LTE4/PGF2α ratio: 1.43±1.76 vs 0.79±0.82, P=0.010) (Figure). The plasma PGF2α level showed no significant difference between group 1 and group 2 subjects (17.17±6.40 vs 23.24±19.13, P=0.132). The compliance was not significantly different between group 1 and 2 subjects. The plasma LTE4 level and LTE4/PGF2α ratio were not significantly different according to the time that subjects experienced the last AE. The plasma LTE4 level and LTE4/PGF2α ratio showed no significant difference between the patients who had used 400 µg of budesonide plus 10 mg of montelukast and those who had used 800 µg of inhaled budesonide after adjusting for age and gender (LTE4 level: 15.92±12.04 vs 14.55±17.02 pg/mL, P=0.436; LTE4/PGF2α ratio: 1.00±0.96 vs 0.89±1.26, P=0.476).
Table 1. Clinical characteristics of the study subjects.
| Characteristics | Total study subjects (n=101) | Group 1 (n=23) | Group 2 (n=78) | P value |
|---|---|---|---|---|
| Age (year) | 67.82±5.46 | 67.48±5.15 | 67.92±5.57 | 0.82 |
| Gender (M:F) | 46:55 | 9:14 | 37:41 | 0.482 |
| FEV1 (% predicted) | 97.93±24.55 | 96.43±25.46 | 98.38±24.42 | 0.702 |
| Sputum eosinophil count (%) | 3.65±13.14 | 5.10±19.67 | 3.23±10.70 | 0.575 |
| Peripheral eosinophil count (%) | 2.89±2.37 | 3.74±3.21 | 2.63±2.01 | 0.033 |
| ACT score | 19.02±4.50 | 17.48±3.75 | 19.48±4.62 | 0.064 |
| AQoL | 27.87±23.04 | 32.48±24.18 | 26.49±22.68 | 0.293 |
| Type of anti-asthmatic medication | 0.551 | |||
| Low dose ICS+montelukast | 45 | 9 | 36 | |
| High dose ICS | 56 | 14 | 42 |
General linear regression analysis adjusted by age, gender was performed. Continuous data are presented as mean±SD. Group 1: subjects that experienced AE during the study period, Group 2: subjects that did not experienced AE during the study period.
FEV1, forced expiratory volume in 1 second; ACT, asthma control test; AE, asthma exacerbation; AQoL, asthma quality of life; ICS, inhaled corticosteroid; SD, standard deviation.
Figure. Comparison of the plasma levels of LTE4 (A) and PGF2α, (B) and LTE4/PGF2α ratio according to the presence of previous AE (C). The plasma LTE4 level and LTE4/PGF2α ratio were significantly higher in group 1 than in group 2 (P=0.023 and P=0.010, respectively). Data were analyzed by general linear regression analysis adjusted for age, gender, and type of anti-asthmatics treatment. Group 1: patients who experienced AE for previous 12 weeks, Group 2: patients who that did not experience AE for previous 12 weeks. AE, asthma exacerbation; LTE4, leukotriene E4; PGF2α, prostaglandin F2α.
By a binary logistic regression model, the plasma LTE4/PGF2α ratio and peripheral eosinophil count (%) at week 12 were significantly associated with AE during the study period (odds ratio [OR]=1.748, P=0.013; OR=1.256, P=0.027, respectively) (Table 2). ROC curves were generated to discriminate the asthmatics with previous AE from those without previous AE using a logistic regression model for age, gender, plasma LTE4/PGF2α ratio, and peripheral eosinophil count (%) at week 12. The area under the curve was 0.700 (P=0.004), with 73.9% sensitivity and 47.9% specificity. When ROC curves are generated from the LTE4 level, LTE4/PGF2α ratio or peripheral eosinophil count (%) at week 12, the area under the curve was 0.608, 0.622, and 0.619, respectively.
Table 2. Factors associated with AE during the previous 16-week period.
| Characteristics | Group 1 | Group 2 | OR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age (year) | 67.48±5.15 | 67.92±5.57 | 1.029 | 0.930 | 1.138 | 0.578 |
| Gender (M:F) | 9:14 | 37:41 | 0.385 | 0.123 | 1.201 | 0.100 |
| Plasma levels of LTE4/PGF2α ratio | 1.43±1.76 | 0.78±0.82 | 1.748 | 1.009 | 1.082 | 0.013 |
| Peripheral eosinophil count (%) | 3.74±3.21 | 2.63±2.01 | 1.256 | 1.027 | 1.536 | 0.027 |
| FEV1 (% predicted) | 96.43±25.46 | 98.38±24.42 | 0.996 | 0.975 | 1.017 | 0.707 |
| Type of medication (Low dose ICS + montelukast) | 9 | 36 | 0.863 | 0.308 | 2.422 | 0.780 |
Data were analyzed by binary logistic regression analysis. Group 1: subjects that experienced AE during the study period, Group 2: subjects that did not experienced AE during the study period. Type of medication: Patients using either 400 µg of inhaled budesonide plus 10 mg of montelukast or 800 µg of inhaled budesonide.
AE, asthma exacerbation; LTE4, leukotriene E4; PGF2α, prostaglandin F2α; FEV1, forced expiratory volume in 1 second; OR, odds ratio; CI, confidence interval.
DISCUSSION
This is the first study to suggest objective assessment tools which is significantly associated with previous AE in elderly asthmatics. A combination of plasma LTE4/PGF2α ratio and peripheral eosinophil count is reliable for assessing previous AE.
Biomarkers including fractional exhaled nitric oxide (FeNO), sputum eosinophilia, and urinary LTE4, related with future AE have been widely studied.9 However, objective assessment tools related with previous AE have been rarely studied. The current guideline recommends to treat asthmatics according to asthma control status.6 The validated tools for assessing asthma control status are symptom control status by GINA, ACT score, asthma control questionnaire, spirometry, and presence of previous AE. Questionnaire items of GINA symptom control, ACT, and asthma control questionnaire all includes reliever usage for previous 1 to 4 weeks. Therefore, we included reliever use in the definition of AE in the present study which is more relevant to assess asthma control status on the basis of real life in clinical practice. These tools are recommended to be applied to elderly asthmatics as in younger asthmatics, though they are substantially cognitive function- and effort-dependent. Taking into account the characteristics of elderly,1 objective tools for assessing asthma control status, such as, previous AE are needed. In the present study, although the ACT score and AQoL were not significantly different whether the patients experienced AE or not, the plasma LTE4/PGF2α ratio and peripheral eosinophil count at week 12 were significantly associated with AE using a binary logistic regression model. With the ROC curve to discriminate asthmatics with previous AE, a combination of 2 parameters had 73.9% sensitivity and 47.9% specificity. Taken together, the plasma LTE4/PGF2α ratio and peripheral eosinophil count may be potential tools for assessing asthma control status along with GINA symptom control, ACT, and asthma control questionnaire, especially for elderly asthmatics.
LTE4 mediates inflammatory cell infiltration, mucus secretion, vascular permeability, and smooth muscle contraction, and plays an important role in asthma pathogenesis.10 Previous studies demonstrated that the increase in the LTE4 level during acute AE were reversed after the resolution of AE.11,12 In a previous study,11 urinary LTE4 was measured in asthmatic children during an acute exacerbation and 1 month later and the levels were compared. The authors demonstrated that the production of urinary LTE4 was significantly higher than normal controls not only during AE, but also 1 month later after AE. In the present study, increased plasma LTE4 level and LTE4/PGF2α ratio were noted in patients with previous AE during the previous 16-week period. Similar to the previous study,11 our study showed no significant difference in the plasma LTE4 level according to the time point of the last AE, suggesting that plasma LTE4 level may represent an ongoing inflammatory process rather than acute inflammatory response and can be applied to assess asthma control status. Considering that plasma collection is convenient and that metabolite measurement is an objective method, plasma LTE4/PGF2α ratio representing dysregulation of arachidonic acid metabolism can be useful for assessing an episode of previous AE.
The plasma LTE4 level and LTE4/PGF2α ratio showed no significant difference between the patients who used 400 µg of budesonide plus 10 mg of montelukast and those who used 800 µg of inhaled budesonide. Cysteinyl LTs are known to be not completely suppressed by treatment with corticosteroids alone. However, previous studies13,14 demonstrated that not only systemic steroids, but also inhaled corticosteroids (ICSs) decrease cysteinyl LTs concentration. Therefore, it is difficult to directly compare the plasma LTE4 level and LTE4/PGF2α ratio between patients treated with LT receptor antagonists and double dose of ICSs and to evaluate the effect of LT receptor antagonists on the LTE4 level.
In conclusion, a combination of plasma LTE4/PGF2α ratio and peripheral eosinophil count can be an objective assessment tool which is significantly associated with asthma control status in elderly asthmatics. Further studies in a larger number of patients are needed to validate our results.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0992) and Dong-A ST Co., Ltd.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, et al. Asthma in the elderly: current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128:S4–S24. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014;7:8–25. doi: 10.1186/1939-4551-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park J, Kim TB, Joo H, Lee JS, Lee SD, Oh YM. Diseases concomitant with asthma in middle-aged and elderly subjects in Korea: a population-based study. Allergy Asthma Immunol Res. 2013;5:16–25. doi: 10.4168/aair.2013.5.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song WJ, Cho SH. Challenges in the management of asthma in the elderly. Allergy Asthma Immunol Res. 2015;7:431–439. doi: 10.4168/aair.2015.7.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song WJ, Jee YK. More effective strategies are needed for elderly asthmatics in real-world practice. Allergy Asthma Immunol Res. 2015;7:419–420. doi: 10.4168/aair.2015.7.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma. Global strategy for asthma management and prevention: 2016 (revision) [Internet] [place unknown]: Global Initiative for Asthma; 2016. [cited 2016 Oct 6]. Available from: http://ginasthma.org/gina-reports. [Google Scholar]
- 7.Ye YM, Kim SH, Hur GY, Kim JH, Park JW, Shim JJ, et al. Addition of montelukast to low-dose inhaled corticosteroid leads to fewer exacerbations in older patients than medium-dose inhaled corticosteroid monotherapy. Allergy Asthma Immunol Res. 2015;7:440–448. doi: 10.4168/aair.2015.7.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ban GY, Cho K, Kim SH, Yoon MK, Kim JH, Lee HY, et al. Metabolomic analysis identifies potential diagnostic biomarkers for aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2017;47:37–47. doi: 10.1111/cea.12797. [DOI] [PubMed] [Google Scholar]
- 9.Puranik S, Forno E, Bush A, Celedón JC. Predicting severe asthma exacerbations in children. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201606-1213PP. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res. 2014;6:288–295. doi: 10.4168/aair.2014.6.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson AP, Castling DP, Green CP, Price JF. Persistent increase in plasma and urinary leukotrienes after acute asthma. Arch Dis Child. 1995;73:221–225. doi: 10.1136/adc.73.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation. Thorax. 2004;59:100–104. doi: 10.1136/thorax.2003.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baraldi E, Carraro S, Alinovi R, Pesci A, Ghiro L, Bodini A, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58:505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondino C, Ciabattoni G, Koch P, Pistelli R, Trové A, Barnes PJ, et al. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J Allergy Clin Immunol. 2004;114:761–767. doi: 10.1016/j.jaci.2004.06.054. [DOI] [PubMed] [Google Scholar]