Abstract
Background
Haemophilus influenzae is commonly isolated from the airways of COPD patients. Antibiotic treatment may cause the emergence of resistant H. influenzae strains, particularly ampicillin-resistant strains, including β-lactamase-negative ampicillin resistance (BLNAR) strains. Genetic identification using ftsI sequencing is the optimum method for identifying mutations within BLNAR strains. The prevalence of BLNAR in COPD patients during the stable state has not been reported. We investigated the antibiotic resistance patterns of H. influenzae present in the sputum of stable COPD patients, focusing on ampicillin resistance; the prevalence of enzyme and non-enzyme-mediated ampicillin resistance was determined. A subset of patients was followed up longitudinally to study H. influenzae strain switching and antibiotic sensitivity changes.
Patients and methods
Sputum sampling was performed in 61 COPD patients, with 42 samples obtained at baseline; H. influenzae was detected by polymerase chain reaction in 28 samples. In all, 45 patients completed the follow-up for 2 years; 24 H. influenzae isolates were obtained.
Results
Disk diffusion showed the highest antibiotic resistance in the penicillin antibiotic group (eg, 67% for ampicillin) and macrolides (eg, 46% for erythromycin), whereas all isolates were susceptible to quinolones. Of the 16 isolates resistant to ampicillin, 9 (56%) were β-lactamase positive. The β-lactamase-negative isolates were further investigated; none of these fulfilled the phenotypic BLNAR classification criteria of ampicillin minimum inhibitory concentration >1 µg/mL, and only one demonstrated an ftsI mutation. Frequent H. influenzae strain switching was confirmed using multilocus sequence typing and was associated with changes in the antibiotic sensitivity pattern.
Conclusion
We observed an overidentification of ampicillin resistance by disk diffusion. The majority of ampicillin resistance was due to enzyme production. H. influenzae strain changes during the stable state may be associated with a change in antibiotic sensitivity; this has implications for empirical antibiotic prescribing.
Keywords: COPD, BLNAR, ampicillin resistance, MLST
Introduction
COPD is characterized by airflow limitation and the presence of airway inflammation.1 Many COPD patients suffer with exacerbations, defined as an acute deterioration in symptoms greater than the usual day-to-day variation1 These events are often caused by bacterial infection.2 COPD patients appear to have reduced ability to clear bacteria from the airways,3 predisposing to bacterial colonization both in the stable state and at exacerbation. Bacterial infection increases the levels of airway inflammation.2
Haemophilus influenzae is one of the most commonly isolated bacterial pathogens from COPD patients.2,4 It can be capsulated or noncapsulated based on the presence or absence of a polysaccharide capsule. Noncapsulated H. influenzae (NTHi) is frequently isolated from COPD patients both in the stable state and during exacerbations.2,4 COPD exacerbations may be caused by either new or persisting H. influenzae strains,5,6 with antigenic variation5,7 allowing evasion of the host immune response.5 Simultaneous colonization with multiple strains of H. influenzae both in the stable state and during exacerbations may also occur.5,8
Antibiotics are often used to treat COPD exacerbations. They may also be administered in the stable state to clear infection from colonizing bacteria. Frequent antibiotic treatment predisposes to the development of antibiotic-resistant pathogens,7 which can occur by mutation.6 There are reports of antibiotic resistance trends in COPD patients,6,9,10 with geographic variations in the types of bacteria in the lungs and antibiotic resistance patterns.
H. influenzae resistance to β-lactams is either enzyme (β-lactamase) or non-enzyme mediated. Non-enzyme-mediated resistance occurs through mutations in penicillin-binding protein 3 (PBP3), with ftsI gene mutations identified as a cause of decreased β-lactam-binding affinity. This is termed β-lactamase-negative ampicillin resistance (BLNAR).11 Disk diffusion and gradient tests are routinely used to evaluate the antibiotic sensitivity. BLNAR identification by gradient tests using ampicillin minimum inhibitory concentration (MIC) breakpoints12 is called phenotypic BLNAR. However, results using these arbitrary breakpoints can poorly be associated with genetic confirmation, called genetic BLNAR (gBLNAR). Nevertheless, it has been reported that 31.6% of H. influenzae isolates from adults with pneumonia were resistant to ampicillin using MIC criteria and that two-thirds of these were due to gBLNAR.13 Furthermore, BLNAR is reported to be the most common cause of the reduced susceptibility of H. influenzae to ampicillin in some regions.14 To our knowledge, the prevalence of BLNAR in COPD patients in the stable state has not been reported.
We have investigated the antibiotic resistance patterns of H. influenzae present in the sputum of a cohort of COPD patients in the stable state, focusing on ampicillin resistance. The prevalence of enzyme- and non-enzyme-mediated ampicillin resistance was determined, with confirmation of BLNAR by genetic analysis of ftsI. A subset of patients was followed up longitudinally to investigate the relationship between H. influenzae strain switching and antibiotic sensitivity.
Patients and methods
Subjects
Sixty-one COPD patients aged ≥40 years were recruited. All patients had a physician diagnosis of COPD, post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio <0.7, ≥10-pack year smoking history, and no previous asthma diagnosis. All subjects provided written informed consent using a protocol approved by the Greater Manchester South Ethics Committees (10/H/1003/108).
Study design
Demographic details, lung function, symptoms, health status, and previous exacerbation history were recorded at the base-line visit. Sputum samples were collected at this visit, and the patients were asked to return at 6, 12, 18, and 24 months for the collection of sputum samples in the stable state.
Sputum analysis
Spontaneous or induced sputum was processed for quantitative polymerase chain reaction (qPCR) detection of the common respiratory potentially pathogenic microorganisms H. influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in the baseline and 6-month samples. Briefly, selected sputum plugs were weighed and suspended in eight times volume of phosphate-buffered saline. Glass beads were added to this suspension, and it underwent homogenization by vortexing for 15 s, rocking for 15 min, and vortexing for an additional 15 s. Aliquots of 500 µL were stored at −80°C for subsequent qPCR processing.
Quantitative culture
Sputum quantitative culture was performed according to standard practice.15 Briefly, sputum was cultured onto suitable agar plates, that is, Columbia blood agar (a general growth media), Chocolate agar (for fastidious bacteria), MacConkey agar (for gram-negative bacteria), and Columbia Blood Agar Base (COBA) agar (Streptococcus selective supplement). The plates were incubated for 24–48 h at 37°C and 5% CO2 and identi-fied accordingly to standard practice using X and V tests.15 All identified H. influenzae isolates were stored in frozen broth (brain heart infusion broth + 15% glycerol) at −80°C.
Antibiotic sensitivity testing
The antibiotic sensitivity testing by disk diffusion was performed and interpreted according to national guidelines16 with antibiotics amoxicillin (2 µg), ampicillin (2 µg), amoxicillin-clavulanic acid (2:1 µg), cefaclor (30 µg), cefotaxime (5 µg), ceftazidime (30 µg), ceftriaxone (30 µg), imipenem (10 µg), azithromycin (15 µg), erythromycin (5 µg), clarithromycin (2 µg), moxifloxacin (5 µg), ciprofloxacin (1 µg), levofloxacin (1 µg), chloramphenicol (10 µg), trimethoprim-sulfamethoxazole (25 µg), and tetracycline (10 µg). Ampicillin results were interpreted as resistant if the zone diameter is ≤17 mm and interpreted as sensitive if the zone diameter is ≥18 mm.16
Enzyme-mediated β-lactamase detection
Enzyme-mediated resistance was detected with β-lactamase production test by chromogenic assay using nitrocefin as the substrate (Sigma-Aldrich, Poole, UK).
DNA extractions
From homogenized sputum samples, DNA was extracted using the MagNA Pure 96 DNA and Viral NA Small Volume Kit (Roche Diagnostics, Forenstrasse, Switzerland), as per the manufacturer’s instructions. From pure bacterial isolates, DNA was extracted with chelex-100 using InstaGene Matrix (Bio-Rad, Hertfordshire, UK). The extracted DNAs were stored at 4°C for short term and at −80°C for long term.
qPCR analysis
A triplex quantitative real-time PCR was used for detection and quantification of three potentially pathogenic microorganisms; targeting the lipo-oligosaccharide glycosyltransferase-encoding gene (lgtC) of H. influenzae, the CopB outer membrane protein-encoding gene (copB) of M. catarrhalis, and the autolysin-encoding gene (lytA) of S. pneumoniae. The sequences of the primers and probe for the lytA were described elsewhere.17 The presence of potential pathogenic microorganisms was defined as detection above the lower limit of detection for each pathogen corresponding to 2,000, 15,000, and 12,875 copies/mL, respectively, for H. influenzae, M. catarrhalis, and S. pneumoniae.
PCR and DNA sequencing
All PCR primers were obtained from Eurofins Genomics, Germany. All end point PCR reactions and sample preparation for DNA sequencing were carried out in-house, and all sequencing work was outsourced to Eurofins Genomics.
Phenotypic BLNAR
The MICs were performed using E-strip (Oxoid-Thermo Scientific, Basingstoke, UK) gradient method. The measured MIC corresponds to 90% of inhibition of bacterial growth. For phenotypic classification of BLNAR, a MIC breakpoints of >1 µg/mL was used.12
Genetic BLNAR
For genotypic resistance determination of BLNAR (gBLNAR), PCR amplifications and sequencing of the PBP3 locus of ftsI were carried out as described previously.18 The DNA sequences were analyzed using Clustal Omega – a multiple sequence alignment program for both DNA and proteins (www.ebi.ac.uk/Tools/msa/clustalo/). The DNA sequences were translated to their corresponding peptide sequences using EMBOSS Transeq (www.ebi.ac.uk/Tools/st/emboss_transeq/). The translated amino acid sequences were compared with the corresponding fragment of PBP3 from H. influenzae reference strain RdKW20 for identifying specific amino acid substitutions and their positions. There are three classes of gBLNAR: class I has substitutions of arginine 517 for histidine (Arg517His), class II has substitutions of asparagine 526 for lysine (Asn526Lys) near the Lys–Thr–Gly (KTG) motif, and class III have the substitutions methionine for isoleucine (Met377Ile), serine for threonine (Ser385Thr), and leucine for phenylalanine (Leu389Phe) near the Ser–Ser–Asn (SSN) motif in addition to asparagine 526 for lysine (Asn526Lys).
Biotyping
Biotyping was performed using three biochemical reactions: urease and indole production and ornithine decarboxylase reaction.19 The eight biotypes of H. influenzae (I–VIII) can be identified based on the biochemical reactions.
Multilocus sequence typing (MLST)
The MLST was carried out as described elsewhere.20 Briefly, it included amplifying seven housekeeping genes (adk, atpG, frdB, fucK, mdh, pgi, and recA) and sequencing to determine the allele numbers of each gene for subsequently identifying the sequence type (ST). Allele numbers and ST were assigned using H. influenzae MLST website (http://pubmlst.org/hinfluenzae/). The database was analyzed using eBURST v3 to define groups from the available 1036 STs representing entire NTHi population in the database (accessed on July 15, 2016) for identifying related genotypes. The STs were included in a group if they shared alleles at a minimum of six of the seven loci, that is, single-locus variants (SLVs).
Results
The baseline demography of the 61 subjects at the start of the study is shown in Table 1. The mean FEV1 predicted was 48.9%, and the mean St George’s Respiratory Questionnaire was 54.7, suggesting a high burden of symptoms. Forty-two subjects produced sufficient sputum samples for qPCR analysis at the baseline visit; H. influenzae was present in 28 (67%) of these samples, either as the only pathogen or with other pathogens (Table 1). Of these 42 subjects, 37 also had a quantitative culture sample available at baseline. Comparison of the techniques showed that qPCR had increased sensitivity for detecting bacteria compared to culture (Figure 1); bacteria were not detected in 73% of samples using culture compared to 35% using qPCR. Of the 61 patients, 2 were on long-term azithromycin: neither patient had H. influenzae present in sputum samples.
Table 1.
Baseline demographics and qPCR detection of bacterial pathogens
| Demographic | n=61 |
|---|---|
| Age (years) | 64.5 (5.4) |
| Gender, male (%) | 69 |
| Current smoker (%) | 38 |
| Pack years | 45.0 (11.1–172.0) |
| BMI (kg/m2) | 26.7 (5.2) |
| Exacerbations (12 months prior) | 2 (0–6) |
| Frequent exacerbations (≥2 in 12 months prior) (%) | 56 |
| Chronic bronchitis (%) | 69 |
| Inhaled steroid use (%) | 84 |
| LAMA only (%) | 8 |
| LABA + LAMA use (%) | 3 |
| ICS + LABA use (%) | 15 |
| LABA + LAMA + ICS (%) | 69 |
| CAT | 23 (2–37) |
| mMRC | 2 (0–4) |
| SGRQ total | 50.6 (22.6) |
| Post-bronchodilator FEV1 (L) | 1.3 (0.5) |
| Post-bronchodilator FEV1 (%) | 48.9 (16.2) |
| Post-bronchodilator FEV1, <50 (%) | 57 |
| Post-bronchodilator FEV1/FVC (%) | 41.5 (10.1) |
|
| |
| qPCR detection of bacteria | |
|
| |
| 42 qPCR samples | n (%) |
|
| |
| No pathogens detecteda | 14 (33) |
| H. influenzae | 15 (36) |
| H. influenzae + M. catarrhalis | 7 (17) |
| H. influenzae + M. catarrhalis + S. pneumoniae | 4 (10) |
| H. influenzae + S. pneumonia | 2 (5) |
Notes: Summaries are presented as percentage, mean (SD), or median (range) as appropriate.
No pathogens detected = if count was below the limit of detection for each pathogen.
Abbreviations: qPCR, quantitative polymerase chain reaction; BMI, body mass index; LAMA, long-acting muscarinic antagonist; LABA, long-acting beta agonist; ICS, inhaled corticosteroid; FEV1, forced expired volume in 1 s; FVC, forced vital capacity; H. influenzae, Haemophilus influenzae; M. catarrhalis, Moraxella catarrhalis; S. pneumoniae, Streptococcus pneumoniae; CAT, COPD Assessment Test; mMRC, Modified Medical Research Council.
Figure 1.
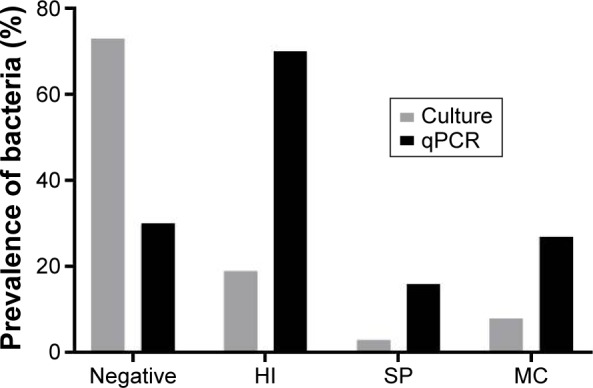
Detection of bacteria by culture and qPCR (n=37 paired samples).
Notes: qPCR: negative is defined if the count was below the limit of detection for each pathogen. Culture: negative is defined as no bacteria detected. No lower limit of detection was applied.
Abbreviations: qPCR, quantitative polymerase chain reaction; HI, Haemophilus influenzae; SP, Streptococcus pneumoniae; MC, Moraxella catarrhalis.
Follow-up for 2 years was completed in 45 subjects. There were 90 samples for quantitative culture, from which 24 H. influenzae isolates were obtained; 9 subjects provided one isolate and 6 subjects provided an isolate on more than one visit.
Disk diffusion antibiotic sensitivity
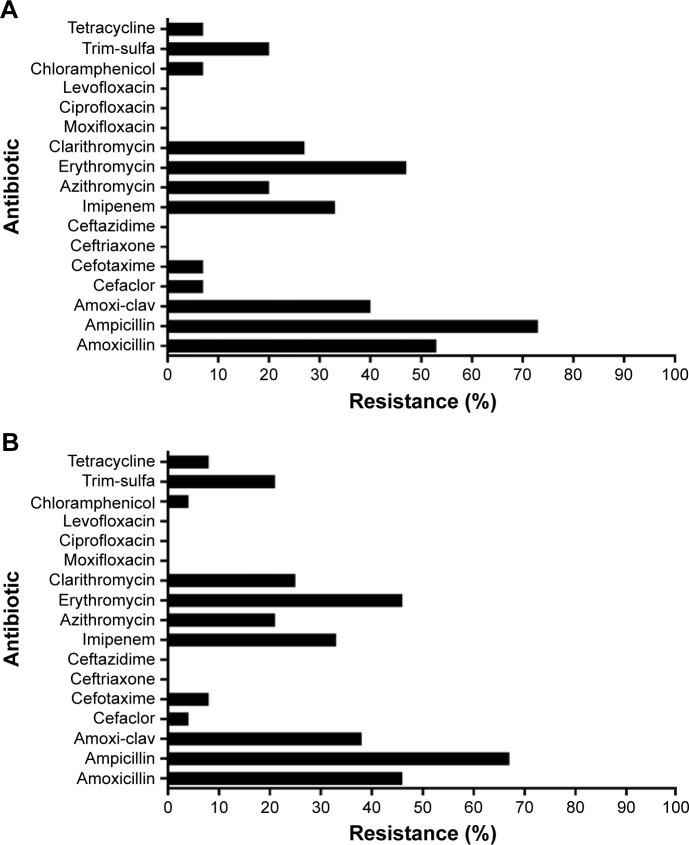
The antibiotic sensitivity testing results for all 24 H. influenzae isolates are presented in detail in Table S1. Figure 2A shows the resistance in the first available H. influenzae isolate (n=15), and Figure 2B shows the resistance in all the collected H. influenzae isolates (n=24). The highest percentage of resistance was observed in the penicillin antibiotic group (67% for ampicillin and 38% for amoxicillin-clavulanic acid) followed by the macrolides (46% for erythromycin, 21% for azithromycin, and 25% for clarithromycin). All 24 isolates were susceptible to quinolones.
Figure 2.
Antibiotic sensitivity of H. influenzae isolates from (A) the first stable visit (n=15); and (B) all H. influenzae isolates (n=24).
Abbreviation: H. influenzae, Haemophilus influenzae.
Of the eight possible H. influenzae biotypes, the collected isolates belonged to biotypes I–IV, which is characteristic of respiratory isolates. The most common biotypes were III (46%) and II (42%). Biotype II showed a high prevalence of ampicillin resistance (80%) (Figure 3).
Figure 3.
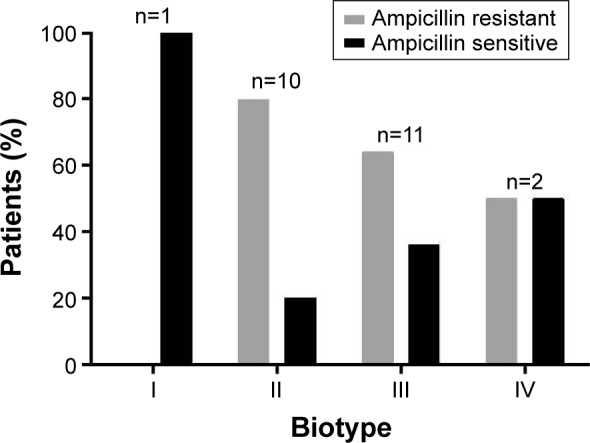
Biotype distribution among ampicillin-resistant and ampicillin-sensitive H. influenzae isolates (n=24).
Abbreviation: H. influenzae, Haemophilus influenzae.
From the six patients with H. influenzae isolates available on more than one visit, two subjects had ampicillin-resistant isolates at all visits, one subject had ampicillin-sensitive strains at all visits, and the remaining three subjects had H. influenzae isolates that changed between resistant and sensitive (Table S1).
β-Lactamase detection and BLNAR
The 16 isolates resistant to ampicillin were selected for detecting β-lactamase enzyme production; 56% (9/16) were β-lactamase positive and 44% (7/16) were β-lactamase negative. The β-lactamase-negative isolates were further investigated for phenotypic and genotypic characterization of BLNAR (Table S2). None of the seven BLNAR isolates fulfilled the phenotypic BLNAR classification criteria of ampicillin MIC >1 µg/mL. Only one of the seven β-lactamase-negative isolates was classified as gBLNAR due to a PBP3 mutation (Table S3). Three isolates had no amino acid substitutions in the PBP3 transpeptidase domain and three had mutations outside the KTG or SSN motif termed miscellaneous (M) mutations.21
Strain switching–MLST analysis
MLST was carried out on 15 isolates from six subjects with a sample available on more than one visit (Table 2). These 15 isolates were assigned to 13 different STs: 11 were known STs (ie, STs already present in the database) and 2 were new STs (ST1608 and ST1562). The allelic profiles of each ST are presented in Table S4.
Table 2.
MLST in multiple stable isolates available within a patient
| Patient | Visit | Biotype | MLST |
|---|---|---|---|
| Pt 1 | Baseline | III | 11 |
| 6 M | III | 1,608a | |
| 12 M | II | 436 | |
| 24 M | II | 263 | |
| Pt 2 | 18 M | IV | 986 |
| 24 M | IV | 986 | |
| Pt 3 | 6 M | III | 46 |
| 12 M | II | 367 | |
| 18 M | II | 1,562a | |
| Pt 4 | Baseline | II | 57 |
| 18 M | II | 3 | |
| Pt 5 | 12 M | III | 388 |
| 18 M | III | 388 | |
| Pt 6 | 6 M | III | 411 |
| 18 M | II | 349 |
Notes: Gray shading represents patient reporting an exacerbation had occurred since the previous visit.
Assigned new ST in this study.
Abbreviations: MLST, multilocus sequence typing; pt, patient; M, months; ST, sequence type.
Two patients showed stable ST and biotype over 6 months. For the other four patients, ST changed at each visit, which was associated with either no biotype change or a biotype switch, with no predictable pattern observable. The previously identified genetically determined BLNAR isolate was identified as ST367, which is a known BLNAR strain.22 There was no clear association between the occurrence of an exacerbation between visits and changes in either biotype or MLST.
For the two patients with no ST change, there was no change in antibiotic sensitivity by disk diffusion in one subject, whereas ampicillin and clarithromycin sensitivity changed in the other subject. For the remaining four patients with ST changes, there were changes in antibiotic sensitivity in three subjects, but no change in one subject despite ST switch.
The eBURST analysis using SLV revealed 10 groups (Table 3). Three STs were classified into eBURST group 2, which contains 39 possible NTHi isolates belonging to 37 STs deposited onto the MLST database. The predicted founder of this clonal group 2 is ST3, which was observed in one sample in this study.
Table 3.
eBURST group numbers and the number of isolates in each group
| eBURST group number | No of STs in each group |
|---|---|
| 1 | 2 |
| 2 | 3 |
| 5 | 1 |
| 9 | 1 |
| 11 | 1 |
| 15 | 1 |
| 36 | 1 |
| 54 | 1 |
| 94 | 1 |
| 97 | 1 |
Abbreviation: STs, sequence types.
All of the observed STs were related to other NTHi STs in the database with none existing as singletons. Five STs (ST3, ST11, ST57, ST349, and ST486) are predicted founders of their respective groups (Figure S1).
In the entire H. influenzae MLST database, there were 88 records from COPD and 24 records from the UK. There was only one ST present in our study that is also present in the MLST database, namely, ST57 (Figure S2).
Discussion
Cross-sectional analysis of sputum samples obtained at the baseline visit in a cohort of stable COPD patients identified that approximately two-thirds of the subjects had H. influenzae present in the airways measured by qPCR. Disk diffusion, which is commonly used in clinical practice, demonstrated a relatively high rate of H. influenzae penicillin resistance (eg, 16 of 24 isolates for ampicillin), in contrast to quinolones, where all isolates were susceptible. β-Lactamase production was confirmed in 9/24 H. influenzae isolates, whereas gBLNAR was found in one isolate by ftsI sequencing. These results indicate the overidentification of ampicillin resistance by disk diffusion, and that the majority of confirmed resistance is due to enzyme production. Longitudinal follow-up showed that a high proportion of H. influenzae isolates demonstrated ST change, which was often associated with a change in antibiotic sensitivity.
Penicillin and macrolide antibiotics are often used as an empirical therapy for upper respiratory tract infections in COPD patients. Furthermore, some COPD patients are prescribed long-term macrolide treatment due to its antibacterial and anti-inflammatory properties. We show that many H. influenzae isolates are resistant to either one or both of these antibiotics. Long-term prescribing of macrolides can encourage the rapid emergence of antibiotic-resistant strains. In contrast, all H. influenzae isolates were susceptible to quinolones, suggesting that this class of drug can be used in preference to eradicate H. influenzae infection in COPD patients. A recent report from a cohort of COPD patients in the US has also demonstrated a high level of susceptibility of H. influenzae to quinolones.10
The confirmed ampicillin resistance identified here either by enzyme production or by ftsI mutation was 41.6% (10/24), which is higher than the UK national average of 21.4%.23 This apparent high prevalence is likely to be due to our data received from COPD patients rather than a general population, with increased antibiotic use in COPD patients selectively allowing proliferation of resistant strains. The β-lactamase production was confirmed in 9 of 24 (37.5%) H. influenzae iso-lates. The UK has relatively higher H. influenzae β-lactamase prevalence compared with most other European countries.24
Six isolates classified as ampicillin resistant by disk diffusion did not produce β-lactamase and did not show evidence of meeting either phenotypic BLNAR definition criteria (ampicillin MIC >1 µg/mL) or gBLNAR definition due to a PBP3 mutation. Such major errors in classification between methods have been previously reported where disk diffusion suggests the presence of a BLNAR mutation, but sequencing shows no mutations.25 The optimum methods for documenting ampicillin resistance are through measuring β-lactamase production and genetic sequencing; of the 16 isolates classified as ampicillin resistant by disk diffusion, 10 were confirmed by one of these methods. This gives a true-positive and a false-positive rate for disk diffusion in this context of 62.5% and 37.5%, respectively. It should be noted that mechanisms other than PBP3 mutations or β-lactamase production were not investigated in our study such as mutations in the acrR gene.26
We used European Committee on Antimicrobial Suscep-tibility Testing MIC cutoff value of >1 µg/mL (in contrast to Clinical and Laboratory Standards Institute cutoff values of >2 µg/mL) to identify phenotypic BLNAR but found disagreement between this result and sequencing for the one gBLNAR isolate discovered. The clinical relevance of current breakpoints has been debated.11,26–28 The MIC of gBLNAR has been previously reported between 0.5 and 16 µg/mL,11,26,27 and the reduced reliability of gradient tests for H. influenzae sensitivity to aminopenicillins has been documented.29
The low prevalence of H. influenzae gBLNAR in our population is consistent with the reported prevalence of 1.5% in the UK, whereas in European countries, the range is between 15% and 30%.13,30,31 Our BLNAR isolate had a characteristic substitution pattern (D350N, M377I, A502V, N526K, V547I, and N569S) that has been previously reported.22 This genotype confirmed H. influenzae isolate belonged to ST367; it has previously been reported that ST367 and ST14 have increased prevalence of PBP3 substitutions.13,22,32 The dissemination of resistant clones of H. influenzae isolates from the upper respiratory tract has been demonstrated,33 and it is possible that the gBLNAR ST367 that we observed could clonally disseminate to other individuals including COPD patients. In addition, continuous, unnecessary antibiotic prescription may cause a shift from low to high level penicillin binding protein resistance; such a shift has been noted in respiratory isolates of H. influenzae.34 Miscellaneous PBP3 mutations were identified21 outside the SSN and KTG motifs; their significance toward contributing to β-lactam resistance is unknown.
The appearance of different H. influenzae strains during longitudinal follow-up is known to occur in stable COPD patients.4–6 A strain may be present, then absent, and then reappear.4,5,8 This reappearance of the same strain could be due to host clearance and then reinfection or lack of methodological sensitivity in bacterial detection. In order to investigate whether the same or different strains appear over time, we have used the most sensitive method for detecting ST change, namely, MLST. Despite the relatively small number of patients in this particular analysis (n=6), we observed strain switching in four patients and the same strain was observed in two patients. Most strain switches were associated with at least one exacerbation between sample collections, which clearly could be key events causing changes in lung microbiome.
There were some changes in antibiotic sensitivity in one of the two patients with no ST change. Given the issues already discussed concerning overidentification of antibiotic resistance using disk diffusion, it is possible that method variability may cause an apparent change in sensitivity in the same strain. Most of the instances of strain change were associated with changes in antibiotic sensitivity. In clinical practice, empirical antibiotic treatment in COPD often follows the pattern of antibiotic sensitivity from historical experience, but our results highlight the frequent changes in antibiotic sensitivity that occur due to H. influenzae strain switching.
Biotypes II and III were the most prevalent in our H. influenzae isolates. Biotyping provides a limited amount of information regarding H. influenzae strain stability or change over time, and we show that ST change can cause either a change or no change in biotype.
Analysis using eBURST revealed a large number of singletons present with little clustering, signifying diversity present among NTHi. Comparison of our isolates to the database did not show that any particular group or groups of STs were present in COPD patients; this means that any ST can potentially be present in COPD patients. The heterogeneity in our H. influenzae population indicates that there are no H. influenzae strains with an increased predilection to colonize COPD lungs.
A limitation of our study is that the sample size for some analysis was relatively small. However, the detailed molecular description of BLNAR by PBP3 sequencing and of strain switching by MLST provides robust data, albeit in limited samples. In additional, we were not able to record the types of antibiotic used between sputum sampling, so we could not identify antibiotics that may have caused resistance.
Conclusion
We show a significant prevalence of penicillin resistance in H. influenzae isolated from COPD patients, mainly due to β-lactamase production. We also demonstrate that H. influenzae present in the lungs of COPD patients show frequent ST change over time, which can change antibiotic resistance. These results highlight the changes in antibiotic sensitivity that occurs due to H. influenzae strain switching in COPD patients.
Supplementary materials
eBURST analysis for defining groups.
Notes: Analysis of NTHi isolates allelic profile to a list of all NTHi isolates profile in the database using eBURST. STs differing from another ST by only one of the seven loci are connected by lines and form a group. Pink spots represent the known 11 STs. Green spots represent the new STs found in our study. Blue spots represent the founding ST for each cluster. Yellow spots represent the subgroup founder for each cluster.
Abbreviation: ST, sequence type.
A comparison of the 13 STs from our study with COPD STs in the database.
Notes: The ST57 (pink) was present in the database of COPD STs, while, the remaining 12 STs (green) were not present in the database as STs from COPD. The blue spot represents the founding ST for each cluster. The yellow spot represents the subgroup founder.
Table S1.
Antibiotic sensitivity from the H. influenzae isolates in stable COPD patients
| Patient | Visit | Antibiotic sensitivity | Biotype | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | Baseline | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 6 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III | |
| 12 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| 24 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 2 | 18 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | IV |
| 24 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | IV | |
| Pt 3 | 6 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 12 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| 18 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 4 | Baseline | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| 18 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 5 | 12 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 18 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III | |
| Pt 6 | 6 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 18 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 7 | 18 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 8 | Baseline | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 9 | 6 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 10 | 24 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| Pt 11 | 6 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 12 | 6 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| Pt 13 | 12 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| Pt 14 | 18 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | I |
| Pt 15 | Baseline | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
Notes: Red color = resistance to antibiotic. Green color = sensitive to antibiotic. Gray color = patient reporting an exacerbation that had occurred since the previous visit. AML, amoxicillin (2 µg); AMP, ampicillin (2 µg); AMC, amoxi-clavulanic acid (3 µg 2:1 ratio); CEC, cefaclor (30 µg); CRO, ceftriaxone (30 µg); IMP, imipenem (10 µg); AZM, azithromycin (15 µg); E, erythromycin (5 µg); CLR, clarithromycin (2 µg); MXF, moxifloxacin (1 µg); CIP, ciprofloxacin (1 µg); LEV, levofloxacin (1 µg); C, chloramphenicol (10 µg); SXT, trimethoprim-sulfamethoxazole (25 µg); TE, tetracycline (10 µg).
Abbreviations: H. influenzae, Haemophilus influenzae; pt, patient.
Table S2.
Non-enzyme-mediated detection of resistance in ampicillin-resistant isolates that were negative for β-lactamase detection
| Patient | Visit | Non-enzyme mediated
|
Biotype | |
|---|---|---|---|---|
| Phenotypic BLNAR: MIC >1 μg/mL | gBLNAR | |||
| Pt 1 | Baseline | <0.015 | No | III |
| 6 M | 0.25 | No | III | |
| 24 M | 0.5 | No | II | |
| Pt 3 | 6 M | 0.50 | No | III |
| 12 M | 1.00 | Class II | II | |
| Pt 6 | 18 M | 1.00 | No | II |
| Pt 10 | 24 M | 0.25 | No | II |
Abbreviations: BLNAR, β-lactamase-negative ampicillin resistance; MIC, minimum inhibitory concentration; gBLNAR, genetic BLNAR; pt, patient; M, months.
Table S3.
Amino acid substitutions in the transpeptidase domain of PBP3 (amino acids from 338 to 573) identified in seven H. influenzae isolates
| PBP3 amino acid substitutions
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rd_KW20a | gBLNAR | Asp350 | Met377b | Ser385b | Leu389b | Arg517c | Ala437 | Ala502 | Asn526d | Val547 | Asn569 | |
|
|
|
|
||||||||||
| Patient | Visit | SSN motif | KTG motif | |||||||||
| Pt 1 | Baseline | Not gBLNAR | ||||||||||
| 6 M | Not gBLNAR | Ile | ||||||||||
| 24 M | Not gBLNAR | Asn | Ser | Ile | Ser | |||||||
| Pt 3 | 6 M | Not gBLNAR | ||||||||||
| 12 M | Class II | Asn | Ile | Val | Lys | Ile | Ser | |||||
| Pt 6 | 18 M | Not gBLNAR | Asn | Ser | Ile | Ser | ||||||
| Pt 10 | 24 M | Not gBLNAR | ||||||||||
Notes:
The amino acid sequence of PBP3 from H. influenzae reference strain RdKW20 used for identifying specific amino acid substitutions and their positions.
Substitutions of methionine for isoleucine (Met377Ile), serine for threonine (Ser385Thr), and leucine for phenylalanine (Leu389Phe) near the Ser–Ser–Asn (SSN) motif in addition to asparagine 526 for lysine (Asn526Lys) equals class III.
Substitution of arginine 517 for histidine (Arg517His) equals class I gBLNAR.
Substitutions of asparagine 526 for lysine (Asn526Lys) near the Lys–Thr–Gly (KTG) motif equals class II gBLNAR.
Abbreviations: PBP3, penicillin-binding protein 3; H. influenzae, Haemophilus influenzae; gBLNAR, genetic β-lactamase-negative ampicillin resistance; pt, patient; M, months.
Table S4.
Allelic profiles of H. influenzae isolates in this study
| Patient | Visit | ST | Allelic profiles
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| adk | atpG | frdB | fucK | mdh | pgi | recA | |||
| Pt 1 | Baseline | 11 | 1 | 8 | 1 | 14 | 9 | 14 | 13 |
| 6 M | 1,608a | 16 | 8 | 160 | 48 | 10 | 1 | 3 | |
| 12 M | 436 | 40 | 1 | 1 | 10 | 15 | 1 | 5 | |
| 24 M | 263 | 28 | 14 | 1 | 1 | 16 | 55 | 1 | |
| Pt 2 | 18 M | 986 | 35 | 1 | 22 | 36 | 7 | 58 | 29 |
| 24 M | 986 | 35 | 1 | 22 | 36 | 7 | 58 | 29 | |
| Pt 3 | 6 M | 46 | 1 | 25 | 1 | 14 | 15 | 1 | 5 |
| 12 M | 367 | 1 | 1 | 1 | 1 | 67 | 1 | 5 | |
| 18 M | 1,562a | 1 | 7 | 1 | 1 | 67 | 1 | 5 | |
| Pt 4 | Baseline | 57 | 14 | 7 | 13 | 7 | 17 | 13 | 17 |
| 18 M | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | |
| Pt 5 | 12 M | 388 | 60 | 51 | 16 | 48 | 15 | 1 | 31 |
| 18 M | 388 | 60 | 51 | 16 | 48 | 15 | 1 | 31 | |
| Pt 6 | 6 M | 411 | 14 | 7 | 13 | 30 | 1 | 21 | 1 |
| 18 M | 349 | 28 | 14 | 13 | 1 | 62 | 13 | 1 | |
Note:
A new ST identified in this study.
Abbreviations: H. influenzae, Haemophilus influenzae; ST, sequence type; pt, patient; M, months.
Acknowledgments
The authors thank the laboratory personnel of GlaxoSmith-Kline Vaccines who performed the qPCR testing: Séverine Roland, Stéphanie Fannoy, and Sophie Boffé.
This report is independent research supported by National Institute for Health Research (NIHR) South Manchester Respiratory and Allergy Clinical Research Facility at University Hospital of South Manchester NHS Foundation Trust.
Footnotes
Disclosure
BM, KDS, TGP, SD, and EMH are employees of the Glaxo-SmithKline group of companies. BM, KDS, TGP, and EMH hold shares/restricted shares in the GlaxoSmithKline group of companies. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards, and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Merck, NAPP, Novartis, Pfizer, Respivert, Skypharma, Takeda, Teva, Therevance, and Verona. The authors report no other conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Finney LJ, Ritchie A, Pollard E, Johnston SL, Mallia P. Lower airway colonization and inflammatory response in COPD: a focus on Haemophilus influenzae. Int J Chron Obstruct Pulmon Dis. 2014;9:1119–1132. doi: 10.2147/COPD.S54477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor AE, Finney-Hayward TK, Quint JK, et al. Defective macrophage phagocytosis of bacteria in COPD. Eur Respir J. 2010;35(5):1039–1047. doi: 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- 4.Garmendia J, Viadas C, Calatayud L, et al. Characterization of nontypable Haemophilus influenzae isolates recovered from adult patients with underlying chronic lung disease reveals genotypic and phenotypic traits associated with persistent infection. PLoS One. 2014;9(5):e97020. doi: 10.1371/journal.pone.0097020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 6.Glogovska PT, Ivanova ZI, Popova TP, et al. Sputum isolates from ambulatory patients with chronic obstructive pulmonary disease – frequent pathogens and antibiotic resistance. J IMAB. 2015;21(2):770–774. [Google Scholar]
- 7.Miravitlles M, Anzueto A. Antibiotic prophylaxis in COPD: why, when, and for whom? Pulm Pharmacol Ther. 2015;32:119–123. doi: 10.1016/j.pupt.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TF, Sethi S, Klingman KL, Brueggemann AB, Doern GV. Simultaneous respiratory tract colonization by multiple strains of nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease: implications for antibiotic therapy. J Infect Dis. 1999;180(2):404–409. doi: 10.1086/314870. [DOI] [PubMed] [Google Scholar]
- 9.Ye F, He LX, Cai BQ, et al. Spectrum and antimicrobial resistance of common pathogenic bacteria isolated from patients with acute exacerbation of chronic obstructive pulmonary disease in mainland of China. Chin Med J (Engl) 2013;126(12):2207–2214. [PubMed] [Google Scholar]
- 10.Pettigrew MM, Tsuji BT, Gent JF, et al. Effect of fluoroquinolones and macrolides on eradication and resistance of Haemophilus influenzae in chronic obstructive pulmonary disease. Antimicrob Agents Chemother. 2016;60(7):4151–4158. doi: 10.1128/AAC.00301-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaare D, Lia A, Hannisdal A, et al. Haemophilus influenzae with non-beta-lactamase-mediated beta-lactam resistance: easy to find but hard to categorize. J Clin Microbiol. 2015;53(11):3589–3595. doi: 10.1128/JCM.01630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EUCAST TECoAST [webpage on the Internet] Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2015. [Accessed March, 2016]. Available from: http://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/
- 13.Puig C, Calatayud L, Marti S, et al. Molecular epidemiology of nontypeable Haemophilus influenzae causing community-acquired pneumonia in adults. PLoS One. 2013;8(12):e82515. doi: 10.1371/journal.pone.0082515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagiwara E, Baba T, Shinohara T, Nishihira R, Komatsu S, Ogura T. Antimicrobial resistance genotype trend and its association with host clinical characteristics in respiratory isolates of Haemophilus influenzae. Chemotherapy. 2012;58(5):352–357. doi: 10.1159/000343973. [DOI] [PubMed] [Google Scholar]
- 15.HPA . UK Standards for Microbiology Investigations Investigation of Bronchoalveolar Lavage, Sputum and Associated Specimens. B57. London: Health Protection Agency; 2012. pp. 1–36. [Google Scholar]
- 16.BSAC Methods for Antimicrobial Susceptibility Testing Version 12The British Society for Antimicrobial Chemotherapy; 2013Birmingham, UK. [Google Scholar]
- 17.Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol. 2007;45(8):2460–2466. doi: 10.1128/JCM.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaare D, Allum AG, Anthonisen IL, et al. Mutant ftsI genes in the emergence of penicillin-binding protein-mediated beta-lactam resistance in Haemophilus influenzae in Norway. Clin Microbiol Infect. 2010;16(8):1117–1124. doi: 10.1111/j.1469-0691.2009.03052.x. [DOI] [PubMed] [Google Scholar]
- 19.Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- 20.Meats E, Feil EJ, Stringer S, et al. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol. 2003;41(4):1623–1636. doi: 10.1128/JCM.41.4.1623-1636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherkaoui A, Diene SM, Emonet S, Renzi G, Francois P, Schrenzel J. Ampicillin-resistant Haemophilus influenzae isolates in Geneva: serotype, antimicrobial susceptibility, and beta-lactam resistance mechanisms. Eur J Clin Microbiol Infect Dis. 2015;34(10):1937–1945. doi: 10.1007/s10096-015-2435-5. [DOI] [PubMed] [Google Scholar]
- 22.Skaare D, Anthonisen IL, Caugant DA, et al. Multilocus sequence typing and ftsI sequencing: a powerful tool for surveillance of penicillin-binding protein 3-mediated beta-lactam resistance in nontypeable Haemophilus influenzae. BMC Microbiol. 2014;14:131. doi: 10.1186/1471-2180-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.BSAC [database on the Internet] Respiratory Resistance Surveillance Programme. 2016. [Accessed April 8, 2016]. Available from: http://www.bsacsurv.org/reports/respiratory#results.
- 24.Fluit AC, Florijn A, Verhoef J, Milatovic D. Susceptibility of European β-lactamase-positive and -negative Haemophilus influenzae isolates from the periods 1997/1998 and 2002/3. J Antimicrob Chemother. 2005;56(1):133–138. doi: 10.1093/jac/dki167. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Cobos S, Arroyo M, Perez-Vazquez M, Aracil B, Oteo J, Campos J. Evaluation of the EUCAST disc diffusion susceptibility testing method for Haemophilus influenzae based on the resistance mechanism to beta-lactam antibiotics. J Antimicrob Chemother. 2013;68(1):159–163. doi: 10.1093/jac/dks374. [DOI] [PubMed] [Google Scholar]
- 26.Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20(2):368–389. doi: 10.1128/CMR.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Eldere J, Slack MP, Ladhani S, Cripps AW. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect Dis. 2014;14(12):1281–1292. doi: 10.1016/S1473-3099(14)70734-0. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs PC, Barry AL. Interpretive criteria for susceptibilities of Haemophilus influenzae to ampicillin, amoxicillin, and amoxicillin-clavulanic acid. J Clin Microbiol. 1994;32(11):2846–2850. doi: 10.1128/jcm.32.11.2846-2850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennie RP, Turnbull L, Brosnikoff C, Cloke J. First comprehensive evaluation of the M.I.C. evaluator device compared to Etest and CLSI reference dilution methods for antimicrobial susceptibility testing of clinical strains of anaerobes and other fastidious bacterial species. J Clin Microbiol. 2012;50(4):1153–1157. doi: 10.1128/JCM.05397-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witherden EA, Bajanca-Lavado MP, Tristram SG, Nunes A. Role of inter-species recombination of the ftsI gene in the dissemination of altered penicillin-binding-protein-3-mediated resistance in Haemophilus influenzae and Haemophilus haemolyticus. J Antimicrob Chemother. 2014;69(6):1501–1509. doi: 10.1093/jac/dku022. [DOI] [PubMed] [Google Scholar]
- 31.Jansen WT, Verel A, Beitsma M, Verhoef J, Milatovic D. Longitudinal European surveillance study of antibiotic resistance of Haemophilus influenzae. J Antimicrob Chemother. 2006;58(4):873–877. doi: 10.1093/jac/dkl310. [DOI] [PubMed] [Google Scholar]
- 32.Resman F, Ristovski M, Forsgren A, et al. Increase of beta-lactam-resistant invasive Haemophilus influenzae in Sweden, 1997 to 2010. Antimicrob Agents Chemother. 2012;56(8):4408–4415. doi: 10.1128/AAC.00415-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotomi M, Fujihara K, Billal DS, et al. Genetic characteristics and clonal dissemination of beta-lactamase-negative ampicillin-resistant Haemophilus influenzae strains isolated from the upper respiratory tract of patients in Japan. Antimicrob Agents Chemother. 2007;51(11):3969–3976. doi: 10.1128/AAC.00422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bae S, Lee J, Lee J, et al. Antimicrobial resistance in Haemophilus influenzae respiratory tract isolates in Korea: results of a nationwide acute respiratory infections surveillance. Antimicrob Agents Chemother. 2010;54(1):65–71. doi: 10.1128/AAC.00966-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eBURST analysis for defining groups.
Notes: Analysis of NTHi isolates allelic profile to a list of all NTHi isolates profile in the database using eBURST. STs differing from another ST by only one of the seven loci are connected by lines and form a group. Pink spots represent the known 11 STs. Green spots represent the new STs found in our study. Blue spots represent the founding ST for each cluster. Yellow spots represent the subgroup founder for each cluster.
Abbreviation: ST, sequence type.
A comparison of the 13 STs from our study with COPD STs in the database.
Notes: The ST57 (pink) was present in the database of COPD STs, while, the remaining 12 STs (green) were not present in the database as STs from COPD. The blue spot represents the founding ST for each cluster. The yellow spot represents the subgroup founder.
Table S1.
Antibiotic sensitivity from the H. influenzae isolates in stable COPD patients
| Patient | Visit | Antibiotic sensitivity | Biotype | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt 1 | Baseline | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 6 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III | |
| 12 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| 24 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 2 | 18 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | IV |
| 24 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | IV | |
| Pt 3 | 6 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 12 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| 18 months | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 4 | Baseline | AML | AMP | AMC | CEC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| 18 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 5 | 12 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 18 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III | |
| Pt 6 | 6 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| 18 months | AML | AMP | AMC | EC | CTX | CRO | CAZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II | |
| Pt 7 | 18 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 8 | Baseline | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 9 | 6 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 10 | 24 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| Pt 11 | 6 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
| Pt 12 | 6 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| Pt 13 | 12 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | II |
| Pt 14 | 18 months | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | I |
| Pt 15 | Baseline | AML | AMP | AMC | EC | CTX | CRO | AZ | IPM | AZM | E | CLR | MXF | CIP | LEV | C | SXT | TE | III |
Notes: Red color = resistance to antibiotic. Green color = sensitive to antibiotic. Gray color = patient reporting an exacerbation that had occurred since the previous visit. AML, amoxicillin (2 µg); AMP, ampicillin (2 µg); AMC, amoxi-clavulanic acid (3 µg 2:1 ratio); CEC, cefaclor (30 µg); CRO, ceftriaxone (30 µg); IMP, imipenem (10 µg); AZM, azithromycin (15 µg); E, erythromycin (5 µg); CLR, clarithromycin (2 µg); MXF, moxifloxacin (1 µg); CIP, ciprofloxacin (1 µg); LEV, levofloxacin (1 µg); C, chloramphenicol (10 µg); SXT, trimethoprim-sulfamethoxazole (25 µg); TE, tetracycline (10 µg).
Abbreviations: H. influenzae, Haemophilus influenzae; pt, patient.
Table S2.
Non-enzyme-mediated detection of resistance in ampicillin-resistant isolates that were negative for β-lactamase detection
| Patient | Visit | Non-enzyme mediated
|
Biotype | |
|---|---|---|---|---|
| Phenotypic BLNAR: MIC >1 μg/mL | gBLNAR | |||
| Pt 1 | Baseline | <0.015 | No | III |
| 6 M | 0.25 | No | III | |
| 24 M | 0.5 | No | II | |
| Pt 3 | 6 M | 0.50 | No | III |
| 12 M | 1.00 | Class II | II | |
| Pt 6 | 18 M | 1.00 | No | II |
| Pt 10 | 24 M | 0.25 | No | II |
Abbreviations: BLNAR, β-lactamase-negative ampicillin resistance; MIC, minimum inhibitory concentration; gBLNAR, genetic BLNAR; pt, patient; M, months.
Table S3.
Amino acid substitutions in the transpeptidase domain of PBP3 (amino acids from 338 to 573) identified in seven H. influenzae isolates
| PBP3 amino acid substitutions
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rd_KW20a | gBLNAR | Asp350 | Met377b | Ser385b | Leu389b | Arg517c | Ala437 | Ala502 | Asn526d | Val547 | Asn569 | |
|
|
|
|
||||||||||
| Patient | Visit | SSN motif | KTG motif | |||||||||
| Pt 1 | Baseline | Not gBLNAR | ||||||||||
| 6 M | Not gBLNAR | Ile | ||||||||||
| 24 M | Not gBLNAR | Asn | Ser | Ile | Ser | |||||||
| Pt 3 | 6 M | Not gBLNAR | ||||||||||
| 12 M | Class II | Asn | Ile | Val | Lys | Ile | Ser | |||||
| Pt 6 | 18 M | Not gBLNAR | Asn | Ser | Ile | Ser | ||||||
| Pt 10 | 24 M | Not gBLNAR | ||||||||||
Notes:
The amino acid sequence of PBP3 from H. influenzae reference strain RdKW20 used for identifying specific amino acid substitutions and their positions.
Substitutions of methionine for isoleucine (Met377Ile), serine for threonine (Ser385Thr), and leucine for phenylalanine (Leu389Phe) near the Ser–Ser–Asn (SSN) motif in addition to asparagine 526 for lysine (Asn526Lys) equals class III.
Substitution of arginine 517 for histidine (Arg517His) equals class I gBLNAR.
Substitutions of asparagine 526 for lysine (Asn526Lys) near the Lys–Thr–Gly (KTG) motif equals class II gBLNAR.
Abbreviations: PBP3, penicillin-binding protein 3; H. influenzae, Haemophilus influenzae; gBLNAR, genetic β-lactamase-negative ampicillin resistance; pt, patient; M, months.
Table S4.
Allelic profiles of H. influenzae isolates in this study
| Patient | Visit | ST | Allelic profiles
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| adk | atpG | frdB | fucK | mdh | pgi | recA | |||
| Pt 1 | Baseline | 11 | 1 | 8 | 1 | 14 | 9 | 14 | 13 |
| 6 M | 1,608a | 16 | 8 | 160 | 48 | 10 | 1 | 3 | |
| 12 M | 436 | 40 | 1 | 1 | 10 | 15 | 1 | 5 | |
| 24 M | 263 | 28 | 14 | 1 | 1 | 16 | 55 | 1 | |
| Pt 2 | 18 M | 986 | 35 | 1 | 22 | 36 | 7 | 58 | 29 |
| 24 M | 986 | 35 | 1 | 22 | 36 | 7 | 58 | 29 | |
| Pt 3 | 6 M | 46 | 1 | 25 | 1 | 14 | 15 | 1 | 5 |
| 12 M | 367 | 1 | 1 | 1 | 1 | 67 | 1 | 5 | |
| 18 M | 1,562a | 1 | 7 | 1 | 1 | 67 | 1 | 5 | |
| Pt 4 | Baseline | 57 | 14 | 7 | 13 | 7 | 17 | 13 | 17 |
| 18 M | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 5 | |
| Pt 5 | 12 M | 388 | 60 | 51 | 16 | 48 | 15 | 1 | 31 |
| 18 M | 388 | 60 | 51 | 16 | 48 | 15 | 1 | 31 | |
| Pt 6 | 6 M | 411 | 14 | 7 | 13 | 30 | 1 | 21 | 1 |
| 18 M | 349 | 28 | 14 | 13 | 1 | 62 | 13 | 1 | |
Note:
A new ST identified in this study.
Abbreviations: H. influenzae, Haemophilus influenzae; ST, sequence type; pt, patient; M, months.