Abstract
Background
We investigated the frequency, clinical presentation, risk factors, and outcome after the first deep vein thrombosis (DVT) event.
Material/Methods
A retrospective study was conducted for patients with DVT between 2008 and 2012 with a 1-year follow-up. Patients were divided into 2 groups: single vs. recurrent DVT (RDVT).
Results
Of the 6420 patients screened for DVT, 662 (10.3%) had DVT. RDVT constituted 22% of cases. A single event was more frequent in left lower limb DVT (p=0.01), while RDVT cases had more bilateral DVT (p=0.01). Recurrent pulmonary embolism (PE) and comorbidities were significantly higher in the RDVT group (P<0.05). Protein C, protein S, and anti-thrombin III deficiency were higher in patients with RDVT (P<0.05). Post-thrombotic syndrome was significantly higher among RDVT cases (p=0.01). In addition, obesity, abnormal coagulation, and prior history of PE and bilateral DVT were found to be independent predictors of RDVT. The PE rate was greater with RDVT than those with single events (22% vs. 9%, p=0.001); however, during follow-up and after adjustment for age and sex, this effect was statistically insignificant (adjusted HR 1.23, 95% CI 0.43–3.57, p=0.68). The age- and sex-adjusted mortality rate was higher in patients with single events with a HR 2.3; 95%CI 1.18–4.54 (p=0.01); however, this effect disappeared after adjusting for the duration of warfarin therapy (p=0.22).
Conclusions
Patients with RDVT are common and have characteristic features that required more attention and further evaluation. These findings should help identifying high-risk patients and set effective preventive measures for RDVT that may revise the duration of warfarin therapy.
MeSH Keywords: Pulmonary Embolism, Recurrence, Risk Factors, Venous Thrombosis
Background
Venous thromboembolism (VTE) is a potentially severe disease that possesses a higher risk of recurrence (30%) at 10 years or more after an initial episode [1]. The treatment of acute VTE with anticoagulants is primarily confined to an initial period of 3–6 months, which helps to prevent short-term thromboembolic complications [2]. It has been shown that residual venous thrombosis is positively correlated with recurrent VTE; therefore, ultrasonographic follow-up is needed to guide long-term use of anticoagulation [3]. Continuation of thromboprophylaxis remains challenging for clinicians due to the associated risk of hemorrhage [4–7]. Therefore, the use of long-term anticoagulation treatment to minimize VTE recurrence requires careful risk assessment [4,8].
The reported rate of recurrent VTE varies widely from 0.6–15% at 90 days to 13–25% at 5 years [9]. Earlier studies have suggested various clinical and biochemical predisposing factors associated with single DVT [10]. However, only few of these risk factors have been identified as potential predictors of recurrence [11]. It has been observed that patients with persistent risk factors (malignancy, thrombophilic disorders, and idiopathic DVT) had a 2- to 3-fold higher risk of recurrence than those with transient risk factors (major surgery and lower-limb fracture) [6]. Therefore, it is imperative to identify the factors involved in the pathogenesis of RDVT. The prevalence and risk factors of RDVT in the Arab Middle-East are not well investigated. Herein, we aimed to determine the frequency, diagnosis, clinical presentation, potential risk factors, management, and outcome of patients with RDVT in Qatar.
Material and Methods
A retrospective analysis was conducted for all patients who presented with clinical suspicion of DVT (pain, tenderness, and swelling in the lower extremity) that was confirmed by Doppler ultrasonography at the Hamad General Hospital in Qatar between January 2008 and December 2012. Records of all DVT cases were identified from outpatient clinics, inpatient wards, and emergency departments, with 1-year clinical follow-up. DVT in our study was diagnosed based on clinical characteristics, laboratory findings, and color Doppler ultrasonography. The procedure for Doppler ultrasonography was primarily based on examination of all patients with GE Logic 9, Logic E9, or Philips IU22 devices using high-frequency or multi-frequency probes. Deep veins were examined from the common femoral vein (CFV) up to the distal posterior tibial vein (PTV) for compressibility, spontaneous flow, and augmentation by distal compression and Valsalva maneuver using grayscale imaging, color flow imaging and pulsed wave Doppler. The examination was also extended to both sapheno-femoral junction (SFJ) and sapheno-popliteal junction (SPJ). The occurrence of pulmonary embolism was identified by Computed Tomography Pulmonary Angiography (CTPA) examination. Recurrent VTE was defined as thrombosis of a venous site that was either previously uninvolved or had interval documentation of incident thrombus (DVT or PE) resolution [12].
We reviewed data on demographics, baseline characteristics (clinical signs and symptoms), risk factors and comorbidities, laboratory investigations (routine tests, D-dimer, thrombophilic disorder), radiological findings (presence of DVT, site of thrombi, location, and anatomic segments involved), occurrence of PE, and single and RDVT. We also analyzed VTE treatment (low molecular-weight heparin, warfarin, antiplatelet, and thrombolytic therapy), length of hospital stay, duration of follow-up, post-thrombotic syndrome (leg pain, swelling, redness, and ulcers), and mortality. This study was approved by the Medical Research Center (IRB# 15002/15) at Hamad Medical Corporation (HMC), Doha, Qatar.
Statistical analysis
Data are presented as proportions, medians (range), or mean [± standard deviation (SD)], as appropriate. We compared variables such as baseline characteristics, risk factors, comorbidities, diagnostic testing, complications, and outcome between patients with single vs. RDVT. Comparison between respective groups was performed using the Student t test for continuous variables and the Pearson chi-square test for categorical variables. Multivariate logistic regression analysis was performed to determine the predictors of RDVT, and data are presented as odds ratio (OR) and 95% confidence interval (CI). A significant difference was considered as a 2-tailed P value less than 0.05. Kaplan-Meier curve was constructed to display survival for the first vs. recurrent DVT. A multivariate Cox regression analysis was performed to assess the hazard ratio (HR) and 95% confidence interval (CI) for the risk of mortality as well as PE during the follow-up based on RDVT. Patients were censored at the time of death or last follow-up. Data analysis was carried out using SPSS version 18 (SPSS Inc. Chicago, IL, USA).
Results
We screened 6420 patients over a 5-year period, of which 662 (10.3%) had confirmed diagnosis of DVT. A total of 146 of the 662 (22%) patients had RDVT (56% were females). Tables 1 and 2 compare the clinical characteristics, risk factors, comorbidities, treatment, and outcome in patients with single vs. RDVT. The 2 groups were comparable for age and sex. RDVT was found to be significantly higher than single DVT (32.9% vs. 23.1%, p=0.01) in the Arab population in Qatar. In-hospital diagnosis of DVT was confirmed in 241 (36.4%), of which 63 cases were admitted to the ICU. Notably, a higher proportion of RDVT was diagnosed at the outpatient clinics (80.8% vs. 58.7%, p=0.001), whereas in-hospital patients were more likely to present with single DVT (41.3% vs. 19.2%, p=0.001).
Table 1.
Comparison of demographics, risk factors and comorbidities by single versus recurrent DVT.
| All DVT (n=662) | Single DVT (n=516) | Recurrent DVT (n=146) | P value | |
|---|---|---|---|---|
| Females | 337 (51%) | 255 (49.3%) | 82 (56.2%) | 0.14 |
| Age (mean ±SD) | 50±17 | 50.1±17.4 | 50.7±16.8 | 0.72 |
| Qatari (Arabs) | 167 (25.2%) | 119 (23.1%) | 48 (32.9%) | 0.01 |
| Locations of diagnosis | ||||
| Out-Patient clinics | 421 (63.6%) | 303 (58.7%) | 118 (80.8%) | 0.001 |
| In-hospital wards | 241 (36.4%) | 213 (41.3%) | 28 (19.2%) | |
| Risk factors | ||||
| Abnormal coagulation | 157 (23.8%) | 86 (16.7%) | 71 (48.6%) | 0.001 |
| Malignancy | 106 (16%) | 94 (18.2%) | 12 (8.3%) | 0.004 |
| Pregnancy | 57 (9%) | 41 (7.9%) | 16 (11%) | 0.25 |
| Oral contraceptives | 13 (2%) | 12 (2.3%) | 1 (0.7%) | 0.20 |
| History of pulmonary embolism | 33 (5%) | 7 (1.4%) | 26 (17.8%) | 0.001 |
| History of surgery (>24hrs) | 82 (12.4%) | 66 (12.8%) | 16 (11%) | 0.55 |
| Paraplegia | 17 (2.6%) | 15 (2.9%) | 2 (1.4%) | 0.30 |
| Bedridden/immobilization | 57 (8.6%) | 43 (8.35) | 14 (9.6%) | 0.63 |
| Neck central line | 23 (3.5%) | 19 (3.7%) | 4 (2.7%) | 0.58 |
| Femoral central line | 16 (2.4%) | 15 (2.9%) | 1 (0.7%) | 0.12 |
| Polytrauma | 50 (8%) | 38 (7.4%) | 12 (8.2%) | 0.73 |
| History of travel | 29 (4.4%) | 23 (4.5%) | 6 (4.1%) | 0.85 |
| Leg fracture | 29 (4.4%) | 20 (3.9%) | 9 (6.2%) | 0.23 |
| Pelvic fracture | 8 (1.2%) | 7 (1.4%) | 1 (0.7%) | 0.51 |
| Comorbidities | ||||
| Diabetes mellitus | 187 (28%) | 136 (26.4%) | 51 (35%) | 0.04 |
| Hypertension | 243 (37%) | 187 (36.2%) | 56 (38.4%) | 0.64 |
| Dyslipidemia | 184 (28%) | 133 (25.8%) | 51 (34.9%) | 0.02 |
| Coronary artery disease | 65 (10%) | 54 (10.5%) | 11 (7.5%) | 0.28 |
| Congestive heart failure | 15 (2.3%) | 9 (1.7%) | 6 (4.1%) | 0.09 |
| Obesity | 257 (47%) | 168 (40.3%) | 89 (67.4%) | 0.001 |
Table 2.
Thrombophilic disorders, treatment and outcome of single versus recurrent DVT cases.
| All DVT (n=662) | Single DVT (n=516) | Recurrent DVT (n=146) | P value | |
|---|---|---|---|---|
| Thrombophilic disorders | ||||
| Protein C deficiency | 57 (9%) | 30 (5.8%) | 27 (18.5%) | 0.001 |
| Protein S deficiency | 43 (7%) | 21 (4.1%) | 22 (15.1%) | 0.001 |
| Hyperhomocysteinemia | 46 (7%) | 32 (6.2%) | 14 (9.6%) | 0.15 |
| Anti thrombin III deficiency | 26 (4%) | 14 (2.7%) | 12 (8.2%) | 0.002 |
| Factor V Leiden | 25 (3.8%) | 21 (4.1%) | 4 (2.7%) | 0.45 |
| Antiphospholipid syndrome | 15 (2.3%) | 4 (0.8%) | 11 (7.5%) | 0.001 |
| Homocysteine level | 14.3 (4–68) | 14.3 (3.5–68) | 14.3 (8.2–40.3) | 0.94 |
| Treatment | ||||
| Enoxaparin | 522 (78.9%) | 408 (79.1%) | 114 (78.1%) | 0.79 |
| Warfarin | 487 (73.6%) | 358 (69.4%) | 129 (88.4%) | 0.001 |
| Antiplatelet | 248 (37.5%) | 184 (35.7%) | 64 (43.8%) | 0.07 |
| Dalteparin | 108 (16.3) | 79 (15.3%) | 29 (19.9%) | 0.18 |
| Heparin | 67 (10.1) | 51 (9.9%) | 16 (11%) | 0.70 |
| Warfarin for life | 107 (16.2) | 41 (7.9%) | 66 (45.2%) | 0.001 |
| Thrombolytic therapy | 10 (1.5%) | 5 (1.0%) | 5 (3.4%) | 0.03 |
| Post-thrombotic syndrome | 328 (50%) | 243 (47.1%) | 85 (58.2%) | 0.01 |
| Calf pain | 441 (67%) | 336 (65.1%) | 105 (72%) | 0.12 |
| Leg edema | 355 (54%) | 263 (51%) | 92 (63%) | 0.01 |
| Leg ulcer | 21 (3.2%) | 11 (2.1%) | 10 (6.8%) | 0.004 |
| Pulmonary embolism | 81 (12.2%) | 52 (10.1%) | 29 (19.9%) | 0.001 |
| Mortality | 100 (15%) | 85 (16.5%) | 15 (10.3%) | 0.06 |
Coagulation abnormality, recurrent PE, and comorbidities (diabetes mellitus, obesity, and dyslipidemia) were significantly higher in RDVT cases as compared to patients with single DVT (P<0.05 for all). On the other hand, patients with a single DVT event had higher association with malignancy (18.2%, vs. 8.3%, P=0.004) than did RDVT cases.
The most common site of RDVT was the left lower limb (LL) (49.3%) followed by right (36.8%) and bilateral (13.9%) LL DVT (Figure 1). A single DVT event was frequently associated with left LL DVT (56.6% vs. 49.3%, p=0.01), while cases with RDVT had more bilateral LL involvement (13.9% vs. 6.5%, p=0.01). The most frequently thrombosed anatomic segments were the popliteal vein (74.7%), posterior tibial vein (71.2%), and profunda femoris vein (70.5%) in the entire cohort. There was no significant difference between single and RDVT with regard to the frequency of thrombosed anatomic segments and location of thrombi.
Figure 1.
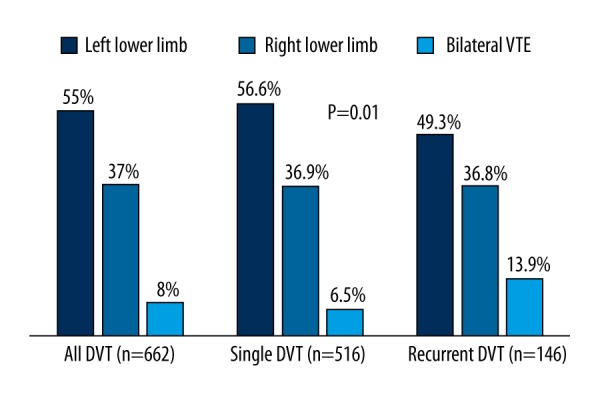
Single vs. recurrent DVT by lower limb involvement.
Moreover, the deficiency of protein C (18.5% vs. 5.8%, p=0.001), protein S (15% vs. 4%, p=0.001), and anti-thrombin III (8.2% vs. 2.7%, p=0.002) were significantly higher in patients with RDVT as compared to single DVT (Table 2).
Management
Patients with RDVT were frequently treated with warfarin (88.4% vs. 69.4%, p=0.001) and thrombolytic therapy (3.4% vs. 1%, p=0.03). Also, long-term warfarin treatment (45.2% vs. 7.9%, p=0.001) was prescribed more for RDVT cases. The mean duration of warfarin treatment was longer in those who had RDVT than those who had single DVT [(16, 95% CI 11.64–21.17) vs. (8.1, 95% CI 6.86–9.38), p=0.001].
Outcomes
Post-thrombotic syndrome was identified in 58.2% of RDVT cases, with characteristic presentation of calf pain (72%), leg edema (63%), and leg ulcer (6.8%). Twenty percent of the RDVT cases developed PE. Also, the frequency of post-thrombotic syndrome and PE was significantly higher in cases with RDVT (p=0.01) when compared to single DVT. Fifteen deaths occurred in patients with RDVT. The overall mortality rate was non-significantly higher in cases with single DVT (16.5% vs. 10.3%, p=0.06) than those with recurrence. In the multivariate analysis to determine factors associated with RDVT, obesity, abnormal coagulation, prior history of PE, and bilateral DVT were found to be independent predictors of RDVT (Table 3).
Table 3.
Predictors of DVT recurrence.
| Predictors | Odd ratio | 95% Confidence interval | P value | |
|---|---|---|---|---|
| Age | 0.99 | 0.97 | 1.00 | 0.27 |
| Sex | 0.68 | 0.42 | 1.07 | 0.10 |
| Abnormal coagulation | 3.62 | 2.25 | 5.83 | 0.001 |
| Malignancy | 0.38 | 0.17 | 0.84 | 0.01 |
| History of prior PE | 20.9 | 7.01 | 62.7 | 0.001 |
| Diabetes mellitus | 1.40 | 0.81 | 2.42 | 0.21 |
| Obesity | 2.20 | 1.37 | 3.53 | 0.001 |
| Bilateral DVT | 2.24 | 1.02 | 4.92 | 0.04 |
Kaplan-Meier survival curves and Cox regression models
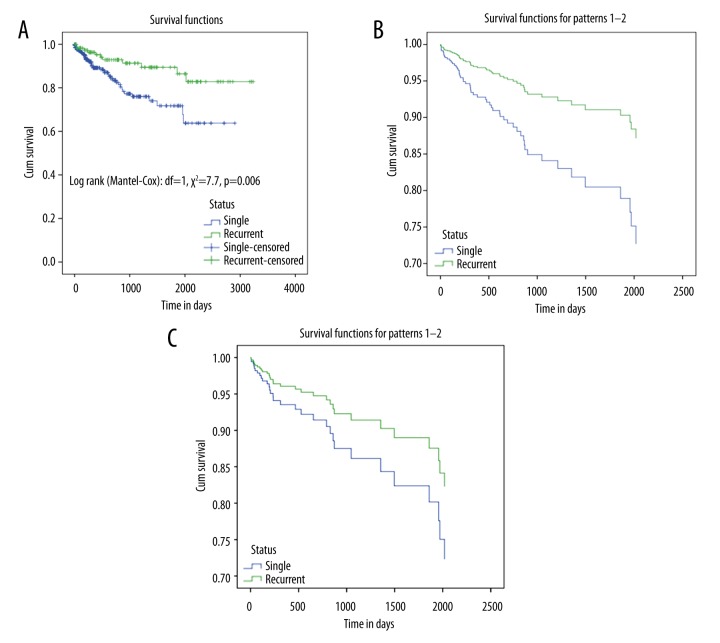
On univariate analysis, the rate of pulmonary embolism was greater with RDVT than those with a single event (20% vs. 10%, p=0.001); however, during follow-up and after adjustment for age and sex, this effect on PE was statistically insignificant (adjusted HR 1.23, 95% CI 0.43–3.57, p=0.68) (Figure 2). The age- and sex-adjusted mortality was higher in patients with a single event, with an HR 2.3; 95% CI 1.18–4.54 (p=0.01). However, this effect disappeared after adjusting for the duration of warfarin therapy (aHR 1.67, 95% CI 0.69–4.00, p=0.22) (Figure 3A–3C).
Figure 2.
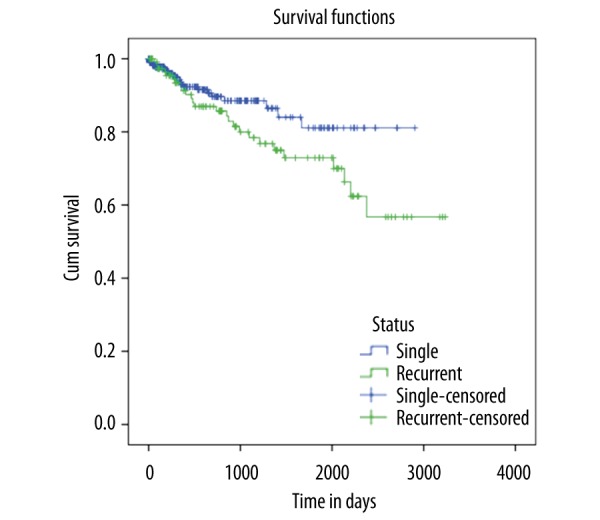
Cox regression analysis for the predictor of age- and sex-adjusted pulmonary embolism in DVT cases.
Figure 3.
(A) Kaplan-Meier survival curve for the first vs. recurrent VTE; (B) Cox regression analysis for the prediction of age- and sex-adjusted mortality in DVT cases; (C) Cox regression analysis for the prediction of age-, sex-, and warfarin duration-adjusted mortality in DVT cases.
Discussion
Several epidemiological studies have identified risk factors involved in the clinico-pathological manifestations of recurrent VTE [13,14]. In our series, 662 DVT cases were identified from a single hospital. The rate of RDVT was found to be 22%, which is consistent with an earlier report of 20% recurrence in symptomatic DVT patients [7]. The present study shows that the PE rate was 2-fold greater in RDVT than in those with a single event; however, during follow-up and after adjustment for age and sex, this effect was statistically insignificant. The GRIP-VTE study reported a recurrence rate of almost 21% among 242 VTE cases from 5 Gulf Cooperation Council (GCC) countries [15]. The authors reported prior history of a VTE event to be a strong risk factor for recurrence. Hansson et al. [7] reported a 5-year cumulative incidence of recurrent VTE of 21.5% and 27.9% after the first and second DVT, respectively.
Earlier reports have suggested a sex predilection towards VTE with a high-risk of recurrence among males. A meta-analysis by Douketis et al. [16] demonstrated 2.2-fold increased risk of recurrence in males with unprovoked VTE. However, such an association was not observed in patients with first-provoked VTE. Another meta-analysis of 15 studies observed a 1.6-fold higher relative risk of recurrence in males [17]. Increased risk of VTE could be attributed to the sex-specific predispositions and clinical risk factors. It has been suggested that males with recurrent VTE are older than females and thus advanced age could be a risk factor for recurrence [16]. The present study found no differences in risk of recurrence between men and women, which is in agreement with previous reports [7,9].
Notably, Riddle and Wells reported frequent diagnosis of DVT among inpatients as compared to outpatients [18]. Similarly, a prospective study from Saudi Arabia observed that patients attending outpatient clinics are less likely to develop RDVT (9.7%) when compared to hospitalized patients (13.3%) [19]. However, in our series a higher proportion of RDVT cases were diagnosed from outpatient clinics, which necessitates development of an institutional protocol for the risk assessment and treatment of DVT to prevent recurrence among high-risk patients.
The rate of recurrence also depends upon the site of thrombosis and involvement of anatomic segments. Ouriel et al. [20] demonstrated a higher association of left LL venous thrombosis in patients with single DVT. Similarly, in our study patients with single DVT showed significant involvement of the left LL. Also, RDVT cases were more likely to involve bilateral LL thrombosis, which is in agreement with a recent study that reported a 2-fold higher rate of recurrence in bilateral LL DVT than that of multiple unilateral thrombosis [21]. Palareti et al. [4] reported similar rates of recurrence in patients with either unilateral or bilateral proximal DVT, but cases with bilateral distal DVT showed higher involvement with RDVT. In our study, the most frequently involved anatomic segments were popliteal vein, posterior tibial vein, and profunda femoris vein, which correlates well with earlier reports [22].
The association between thrombophilic disorders and risk of VTE recurrence is well recognized [23]. Swiatkiewicz et al. [24] demonstrated an association between recurrent DVT and deficiency of anti-thrombin III, protein C, protein S activity, and other thrombophilic disorders. Similarly, in the present study, RDVT patients had higher frequencies of protein C, protein S, and anti-thrombin III deficiency. However, the clinical relevance of thrombophilia screening for prevention of RDVT remains uncertain [5,11,25].
Some studies have demonstrated a higher risk of recurrence in patients with malignancy [9,26]. Similarly, Ren et al. [27] identified that patients with malignancy had a 3.5-fold higher independent risk of RDVT as compared to those without malignancy. In contrast, a higher association of malignancy was observed among single DVT cases in our series, which is in agreement with a previous report [9]. It has been suggested that RDVT could be related to the extent or aggressiveness of cancer and associated thrombogenicity.
Tripodi et al. [28] reviewed the effect of coagulopathy in patients with chronic liver disease, finding a higher risk of DVT in patients with liver cirrhosis than in those without cirrhosis. Development of malignancy, prolonged hospitalization, and interventions are associated with the risk of VTE in patients with cirrhotic liver. Similarly, a systematic review and meta-analysis identified that the 1% of cirrhotic patients developed or presented with VTE during hospitalization [29]. Recently, Zhang et al. [30] observed a 0.4% prevalence rate of VTE in liver cirrhosis patients who required hospitalization. In our series, we lacked information on liver diseases in patients with DVT.
Advanced age, obesity, smoking, diabetes mellitus, and dyslipidemia were the most frequent comorbidities associated with the pathogenesis of single as well as recurrent VTE [31–34]. Piazza et al. [34] identified diabetes to be an independent predictor of recurrence, with an adjusted odds ratio of 1.74. Consistent with these reports, we observed diabetes, obesity, and dyslipidemia to be associated with RDVT. Since these risk factors could be controlled through lifestyle modification and physical fitness, these findings could have important implications for prevention of the disease. Among comorbidities, only obesity was identified as an independent predictor of RDVT in our series.
PTS remains a frequently observed complication in patients with a single DVT event. Moreover, the risk of developing PTS significantly correlates with the ipsilateral RDVT (hazard ratio of 6.4) [35], which is consistent with our findings of a higher correlation of PTS with RDVT. Therefore, appropriate thromboprophylaxis treatment for recurrence could eventually minimize the incidence of PTS and thereby improve the quality of life and clinical outcomes of high-risk patients [36]. A previous study showed that male sex, PE, and elevated D-dimer levels are important predictors of VTE recurrence in patients who discontinued oral anticoagulation treatment [37]. However, Khaladkar et al. [22] observed no independent risk of RDVT with respect to the anatomic location of DVT, diabetes mellitus, nephrotic syndrome, systemic lupus erythematosus, and non-compliance with treatment after discharge from the hospital.
A systematic review of patients treated for VTE demonstrated that the risk of mortality was higher in major bleeding patients on continued anticoagulation therapy as compared to RDVT cases after discontinuation of therapy [38]. Young et al. [39] reported 2- and 5-year cumulative mortality to be 12% and 27%, respectively, in cases with recurrent VTE. The majority of deaths were attributed to malignancy (68%) and vascular events (18%). Therefore, appropriate and timely anticoagulation treatment is warranted [40]. Heit reported advanced age, male sex; obesity, active cancer, and thrombophilic disorders to be independent predictors of VTE recurrence [1]. In our study, obesity, abnormal coagulation, bilateral DVT, and history of pulmonary embolism were the independent predictors of DVT recurrence.
Several strategies are available for the primary prevention of VTE. These strategies will be successful with a tailored prophylactic approach based on risk assessment by healthcare workers. Warfarin is the most frequently prescribed oral anticoagulant for the prophylaxis of recurrent events among high-risk patients with a first VTE event [41]. The age- and sex-adjusted mortality rate in our study was higher in patients with a single event; however, this effect disappeared after adjusting for the duration of warfarin therapy. The mean duration of therapy was longer in those who had RDVT. The latter finding may raise the issue of revising the duration of therapy after the first DVT event. Therefore, to improve outcome, a systematic approach should be used for the management of oral anticoagulant therapy, together with regular follow-up and effective communication with patients for treatment compliance [2].
Our retrospective study design has several limitations inherent with potential to overlook certain risk factors and past DVT events. Moreover, there is the possibility of incomplete information on risk factors such as liver cirrhosis, which might lead to underestimation of predictors of recurrent DVT. Information on the clinical prediction rules prior to definitive diagnosis and the use of thromboprophylaxis in high-risk patients are not complete. We lack data on the adherence to management therapy during follow-up, which strongly affects the rate of recurrence. Follow-up residual venous thrombosis measurements were not available in our database.
Conclusions
In conclusion, patients with RDVT are common and have characteristic features that require more attention and further evaluation. These findings may help to identify high-risk patients and set effective preventive measures for RDVT, which may revise the duration of warfarin therapy.
Acknowledgement
We thank all the vascular surgery nursing staff at HGH, particularly Mrs. Pushpalatha Pillai, Sara John, and Jini Paul, for their contribution.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Source of support: This study was approved by the Medical Research Center at Hamad Medical Corporation, Doha, Qatar (IRB#15002/15)
Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–72. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan M, Mos IC, Klok FA, Huisman MV. Residual venous thrombosis as predictive factor for recurrent venous thromboembolim in patients with proximal deep vein thrombosis: A sytematic review. Br J Haematol. 2011;153:168–78. doi: 10.1111/j.1365-2141.2011.08578.x. [DOI] [PubMed] [Google Scholar]
- 4.Palareti G. Recurrent venous thromboembolism: What is the risk and how to prevent it. Scientifica. 2012 doi: 10.6064/2012/391734. Article ID 391734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnelli G, Becattini C. Risk assessment for recurrence and optimal agents for extended treatment of venous thromboembolism. Hematology Am Soc Hematol Educ Program. 2013;2013:471–77. doi: 10.1182/asheducation-2013.1.471. [DOI] [PubMed] [Google Scholar]
- 6.Hansson PO, Sorbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis. Arch Intern Med. 2000;160:769–74. doi: 10.1001/archinte.160.6.769. [DOI] [PubMed] [Google Scholar]
- 7.Fahrni J, Husmann M, Gretener SB, Keo HH. Assessing the risk of recurrent venous thromboembolism – a practical approach. Vasc Health Risk Manag. 2015;11:451–59. doi: 10.2147/VHRM.S83718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannegieter SC, van Hylckama Vlieg A. Venous thrombosis: understanding the paradoxes of recurrence. J Thromb Haemost. 2013;11:161–69. doi: 10.1111/jth.12263. [DOI] [PubMed] [Google Scholar]
- 9.Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: A population-based cohort study. Arch Intern Med. 2000;160:761–68. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 10.Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 11.Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. 2010;376:2032–39. doi: 10.1016/S0140-6736(10)60962-2. [DOI] [PubMed] [Google Scholar]
- 12.Heit JA, Lahr BD, Petterson TM, et al. Heparin and warfarin anticoagulation intensity as predictors of recurrence after deep vein thrombosis or pulmonary embolism: a population-based cohort study. Blood. 2011;118:4992–99. doi: 10.1182/blood-2011-05-357343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreidy R. Influence of acquired and genetic risk factors on the prevention, management, and treatment of thromboembolic disease. Int J Vas Med. 2014 doi: 10.1155/2014/859726. Article ID 859726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montagnana M, Favaloro EJ, Franchini M, et al. The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis. 2010;29:489–96. doi: 10.1007/s11239-009-0365-8. [DOI] [PubMed] [Google Scholar]
- 15.Al Sayegh F, Almahmeed W, Al Humood S, et al. Global risk profile verification in patients with venous thromboembolism (GRIP VTE) in 5 Gulf countries. Clin Appl Thromb Hemost. 2009;15:289–96. doi: 10.1177/1076029608315168. [DOI] [PubMed] [Google Scholar]
- 16.Douketis J, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: Patient level meta-analysis. BMJ. 2011;342:d813. doi: 10.1136/bmj.d813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McRae S, Tran H, Schulman S, et al. Effect of patient’s sex on risk of recurrent venous thromboembolism: A meta-analysis. Lancet. 2006;368:371–78. doi: 10.1016/S0140-6736(06)69110-1. [DOI] [PubMed] [Google Scholar]
- 18.Riddle DL, Wells PS. Diagnosis of lower-extremity deep vein thrombosis in outpatients. Phys Ther. 2004;84:729–35. [PubMed] [Google Scholar]
- 19.Algahtani F, Aseri ZA, Aldiab A, Aleem A. Hospital versus home treatment of deep vein thrombosis in a tertiary care hospital in Saudi Arabia: Are we ready? Saudi Pharm J. 2013;21:165–68. doi: 10.1016/j.jsps.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouriel K, Green RM, Greenberg RK, Clair DG. The anatomy of deep venous thrombosis of the lower extremity. J Vasc Surg. 2000;31:895–900. doi: 10.1067/mva.2000.105956. [DOI] [PubMed] [Google Scholar]
- 21.Galanaud JP, Sevestre MA, Genty C, et al. OPTIMEV-SFMV investigators. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost. 2014;12:436–43. doi: 10.1111/jth.12512. [DOI] [PubMed] [Google Scholar]
- 22.Khaladkar SM, Thakkar DK, Shinde K, et al. Deep vein thrombosis of the lower limbs: A retrospective analysis of doppler ultrasound findings. Med J DY Patil Univ. 2014;7:612–19. [Google Scholar]
- 23.Brouwer JL, Lijfering WM, Ten Kate MK, et al. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93–99. [PubMed] [Google Scholar]
- 24.Swiatkiewicz A, Jurkowski P, Kotschy M, et al. Level of antithrombin III, protein C, protein S and other selected parameters of coagulation and fibrinolysis in the blood of the patients with recurrent deep venous thrombosis. Med Sci Monit. 2002;8(4):CR263–68. [PubMed] [Google Scholar]
- 25.Ribeiro DD, Lijfering WM, Barreto SM, et al. Epidemiology of recurrent venous thrombosis. Braz J Med Biol Res. 2012;45:1–7. doi: 10.1590/S0100-879X2011007500166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. doi: 10.1182/blood-2013-04-460121. [DOI] [PubMed] [Google Scholar]
- 27.Ren W, Li Z, Fu Z, Fu Q. Analysis of risk factors for recurrence of deep venous thrombosis in lower extremities. Med Sci Monit. 2014;20:199–204. doi: 10.12659/MSM.889819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–56. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 29.Qi X, Ren W, Guo X, Fan D. Epidemiology of venous thromboembolism in patients with liver diseases: A systematic review and meta-analysis. Intern Emerg Med. 2015;10:205–17. doi: 10.1007/s11739-014-1163-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Qi X, De Stefano V, et al. Epidemiology, risk factors, and in-hospital mortality of venous thromboembolism in liver cirrhosis: A single-center retrospective observational study. Med Sci Monit. 2016;22:969–76. doi: 10.12659/MSM.896153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ensor J, Riley RD, Jowett S, et al. PIT-STOP collaborative group. Prediction of risk of recurrence of venous thromboembolism following treatment for a first unprovoked venous thromboembolism: systematic review, prognostic model and clinical decision rule, and economic evaluation. Health Technol Assess. 2016;20:i–xxxiii. 1–190. doi: 10.3310/hta20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mi Y, Yan S, Lu Y, et al. Venous thromboembolism has the same risk factors as atherosclerosis: A PRISMA-compliant systemic review and meta-analysis. Medicine (Baltimore) 2016;95:e4495. doi: 10.1097/MD.0000000000004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Goldberg RJ, Anderson FA, et al. Occurrence and predictors of recurrence after a first episode of acute venous thromboembolism: Population-based Worcester Venous Thromboembolism Study. J Thromb Thrombolysis. 2016;41:525–38. doi: 10.1007/s11239-015-1301-8. [DOI] [PubMed] [Google Scholar]
- 34.Piazza G, Goldhaber SZ, Kroll A, et al. Venous thromboembolism in patients with diabetes mellitus. Am J Med. 2012;125:709–16. doi: 10.1016/j.amjmed.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labropoulos N, Waggoner T, Sammis W, et al. The effect of venous thrombus location and extent on the development of post-thrombotic signs and symptoms. J Vasc Surg. 2008;48(2):407–12. doi: 10.1016/j.jvs.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Kearon C, Kahn SR, Agnelli G, et al. American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 37.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: The Vienna Prediction Model. Circulation. 2010;121:1630–36. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 38.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: Case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152:578–89. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 39.Young L, Ockelford P, Milne D, et al. Post-treatment residual thrombus increases the risk of recurrent deep vein thrombosis and mortality. J Thromb Haemost. 2006;4:1919–24. doi: 10.1111/j.1538-7836.2006.02120.x. [DOI] [PubMed] [Google Scholar]
- 40.Streiff MB, Agnelli G, Connors JM, et al. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis. 2016;41(1):32–67. doi: 10.1007/s11239-015-1317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen SY, Wu N, Gulseth M, et al. One-year adherence to warfarin treatment for venous thromboembolism in high-risk patients and its association with long-term risk of recurrent events. J Manag Care Pharm. 2013;19:291–301. doi: 10.18553/jmcp.2013.19.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]