Abstract
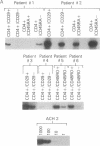
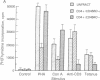
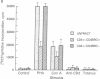
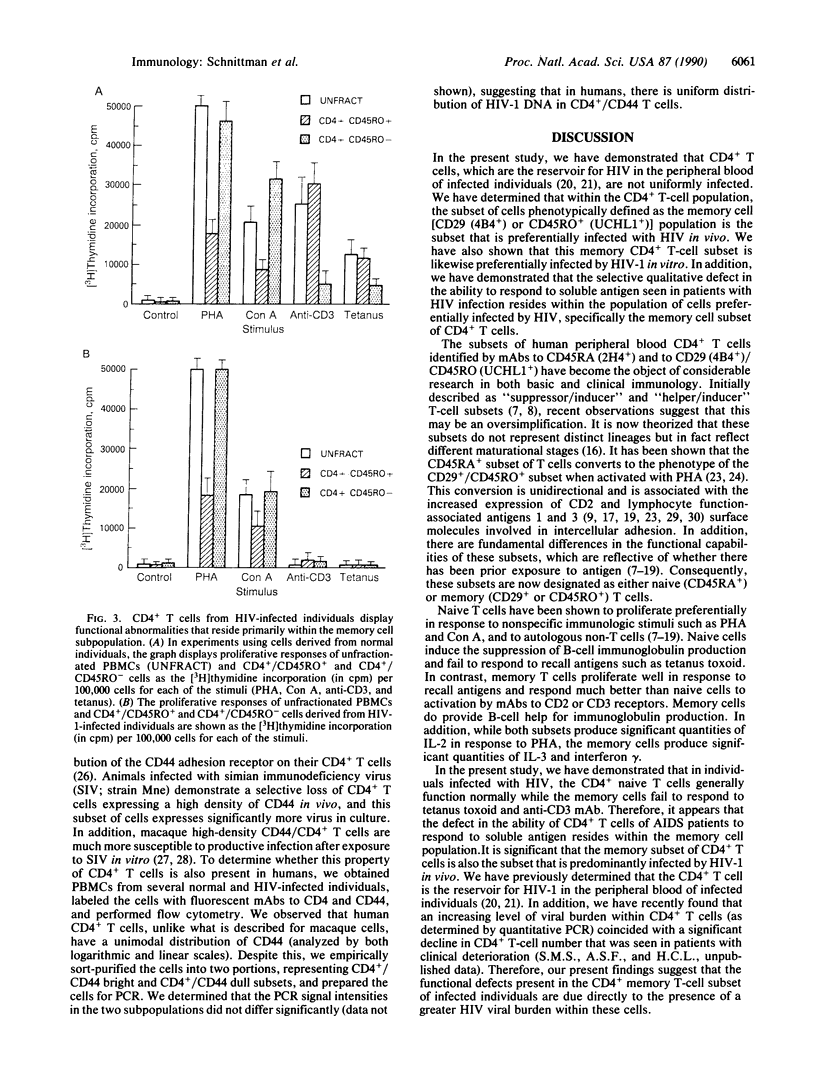
CD4+ T cells of patients with AIDS exhibit a qualitative defect in their ability to respond to soluble antigen while their responses to mitogens remain normal. CD4+ T cells can be broadly divided phenotypically into "naive" [CD45RA+ (2H4+)] and "memory" [CD29+ (4B4+) or CD45RO+ (UCHL1+)] cell subpopulations, which represent distinct maturation stages. To determine the human immunodeficiency virus type 1 (HIV-1) infectability of memory and naive CD4+ T-cell subsets in vitro and to determine the in vivo preference of HIV-1 in these subpopulations, we obtained highly purified CD4+ T-cell subsets from normal and HIV-1-infected individuals and studied them by viral cultivation, quantitative polymerase chain reaction, and functional assays. Polymerase chain reaction studies demonstrated that the memory cell subset of CD4+ T cells is preferentially infected (4- to 10-fold more than naive T cells) by HIV-1 in vitro, and these memory cells are the principal reservoir for HIV-1 within CD4+ T cells obtained from infected individuals. Functional abnormalities attributable to CD4+ T cells in HIV-infected individuals (failure to respond in vitro to soluble antigen or to anti-CD3 monoclonal antibodies) were shown to reside primarily within these memory cells. Thus, the present study suggests that the selective functional defects present in the memory CD4+ T-cell subset of HIV-infected individuals may be a direct result of the preferential infection and consequently greater viral burden within these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Beverley P. C. Human T cell subsets. Immunol Lett. 1987 Apr;14(4):263–267. doi: 10.1016/0165-2478(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., Horvath C., Bron C., Pedrazzini T., Howe R. C., MacDonald H. R. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987 May 15;138(10):3120–3129. [PubMed] [Google Scholar]
- Budd R. C., Cerottini J. C., MacDonald H. R. Selectively increased production of interferon-gamma by subsets of Lyt-2+ and L3T4+ T cells identified by expression of Pgp-1. J Immunol. 1987 Jun 1;138(11):3583–3586. [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Leukocyte cell surface enzymology: CD45 (LCA, T200) is a protein tyrosine phosphatase. Immunol Today. 1989 Jul;10(7):225–228. doi: 10.1016/0167-5699(89)90257-0. [DOI] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Damle N. K., Childs A. L., Doyle L. V. Immunoregulatory T lymphocytes in man. Soluble antigen-specific suppressor-inducer T lymphocytes are derived from the CD4+CD45R-p80+ subpopulation. J Immunol. 1987 Sep 1;139(5):1501–1508. [PubMed] [Google Scholar]
- Deans J. P., Boyd A. W., Pilarski L. M. Transitions from high to low molecular weight isoforms of CD45 (T200) involve rapid activation of alternate mRNA splicing and slow turnover of surface CD45R. J Immunol. 1989 Aug 15;143(4):1233–1238. [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Gale M. J., Jr, Hoffman P. A., Willerford D. M., Draves K. E., Benveniste R. E., Morton W. R., Clark E. A. Selective replication of simian immunodeficiency virus in a subset of CD4+ lymphocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3301–3305. doi: 10.1073/pnas.86.9.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Subpopulations of CD4+ (T4+) cells in homosexual/bisexual men with persistent generalized lymphadenopathy. Clin Exp Immunol. 1987 Apr;68(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Fauci A. S. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu Rev Immunol. 1985;3:477–500. doi: 10.1146/annurev.iy.03.040185.002401. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S., Spira T. J., Cross G. D., Jones B. M., Reinherz E. L. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J Clin Invest. 1984 Jan;73(1):191–201. doi: 10.1172/JCI111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Koyanagi Y., Miles S., Wiley C., Vinters H. V., Chen I. S. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990 Jan 4;343(6253):85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., De Maria A., Koenig S., Butini L., Moss B., Baseler M., Lane H. C., Fauci A. S. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psallidopoulos M. C., Schnittman S. M., Thompson L. M., 3rd, Baseler M., Fauci A. S., Lane H. C., Salzman N. P. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989 Nov;63(11):4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. E., Morimoto C., Wong L. L., Schlossman S. F. The subdivision of the T4 (CD4) subset on the basis of the differential expression of L-C/T200 antigens. J Exp Med. 1987 Dec 1;166(6):1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., June C. H., Young H. A., Shaw S. Enhanced responsiveness of human memory T cells to CD2 and CD3 receptor-mediated activation. Eur J Immunol. 1989 May;19(5):803–808. doi: 10.1002/eji.1830190504. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Psallidopoulos M. C., Lane H. C., Thompson L., Baseler M., Massari F., Fox C. H., Salzman N. P., Fauci A. S. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989 Jul 21;245(4915):305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Bernstein D. C., Tung K. S., Via C. S., Redfield R., Salahuddin S. Z., Gallo R. C. A model for the selective loss of major histocompatibility complex self-restricted T cell immune responses during the development of acquired immune deficiency syndrome (AIDS). J Immunol. 1986 Oct 15;137(8):2514–2521. [PubMed] [Google Scholar]
- Singer A., Munitz T. I., Golding H., Rosenberg A. S., Mizuochi T. Recognition requirements for the activation, differentiation and function of T-helper cells specific for class I MHC alloantigens. Immunol Rev. 1987 Aug;98:143–170. doi: 10.1111/j.1600-065x.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Cooper M. D., Clement L. T. Human lymphocyte differentiation antigens HB-10 and HB-11. II. Differential production of B cell growth and differentiation factors by distinct helper T cell subpopulations. J Immunol. 1985 May;134(5):2989–2994. [PubMed] [Google Scholar]
- Vuillier F., Lapresle C., Dighiero G. Comparative analysis of CD4-4B4 and CD4-2H4 lymphocyte subpopulations in HIV negative homosexual, HIV seropositive and healthy subjects. Clin Exp Immunol. 1988 Jan;71(1):8–12. [PMC free article] [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Gale M. J., Jr, Benveniste R. E., Clark E. A., Gallatin W. M. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J Immunol. 1990 May 15;144(10):3779–3783. [PubMed] [Google Scholar]
- Willerford D. M., Hoffman P. A., Gallatin W. M. Expression of lymphocyte adhesion receptors for high endothelium in primates. Anatomic partitioning and linkage to activation. J Immunol. 1989 May 15;142(10):3416–3422. [PubMed] [Google Scholar]