Abstract
Purpose
Differences in the utility of routine angiographic follow-up (RAF) and clinical follow-up (CF) after percutaneous coronary intervention (PCI) in acute myocardial infarction (AMI) are not well understood. The present study aimed to compare the 3-year clinical outcomes of RAF and CF in AMI patients who underwent PCI with drug-eluting stents (DES).
Materials and Methods
A total of 774 consecutive AMI patients who underwent PCI with DES were enrolled. RAF was performed at 6 to 9 months after index PCI (n=425). The remaining patients were medically managed and clinically followed (n=349); symptom-driven events were captured. To adjust for any potential confounders, a propensity score matched analysis was performed using a logistic regression model, and two propensity-matched groups (248 pairs, n=496, C-statistic=0.739) were generated. Cumulative clinical outcomes up to 3 years were compared between RAF and CF groups.
Results
During the 3-year follow-up period, the cumulative incidences of revascularization [target lesion revascularization: hazard ratio (HR), 2.40; 95% confidence interval (CI), 1.18–4.85; p=0.015, target vessel revascularization (TVR): HR, 3.33; 95% CI, 1.69–6.58; p=0.001, non-TVR: HR, 5.64; 95% CI, 1.90–16.6; p=0.002] and major adverse cardiac events (MACE; HR, 3.32; 95% CI, 1.92–5.73; p<0.001) were significantly higher in the RAF group than the CF group. However, the 3-year incidences of death and myocardial infarction were not different between the two groups.
Conclusion
RAF following index PCI with DES in AMI patients was associated with increased incidences of revascularization and MACE. Therefore, CF seems warranted for asymptomatic patients after PCI for AMI.
Keywords: Acute myocardial infarction, coronary angiography, outcomes
INTRODUCTION
The annual volume of coronary revascularizations has continuously increased between 2006 and 2010 in Korea, although this trend differs for individual procedure types. Nevertheless, a high percentage of drug-eluting stent (DES) procedures and a high rate of in-hospital mortality at low-volume hospitals have been noted.1 One comparative study between second-generation DES in Korea2 showed target lesion revascularization (TLR) rates of 1.1% and 0.7%.2
One of the most important problems after a percutaneous coronary intervention (PCI) is restenosis. It occurs in 10–30% of patients treated with bare metal stents (BMS) and 5–15% of patients treated with DES.3,4 Restenosis can cause recurrence of symptoms, rehospitalization, and/or TLR or target vessel revascularization (TVR).5 Clinical restesnosis occurs less than angiographic restenosis, and repeat revascularization is performed in only 50–70% of patients in whom angiographic restenosis has been confirmed.5 This discrepancy may be due to the fact that restenotic lesions do not always compromise enough of the lumen area to cause ischemic symptoms. Current U.S. and European guidelines recommend clinical follow-up (CF) after PCI, and angiography is reserved for evaluating patients who have recurrent symptoms or objective evidence of myocardial ischemia.6,7 PCI of functionally insignificant stenosis shows little added benefit above continued optical medical therapy.8 According to recent reports,9 routine follow-up coronary angiography (CAG) after primary PCI in ST-segment elevation myocardial infarction (STEMI) is associated with doubling the rate of revascularization without an improvement in death or myocardial infarction (MI), and therefore, cannot be recommended. However, there is limited data on whether routine follow-up CAG, regardless of patient symptoms after a successful PCI with DES, in overall acute myocardial infarction (AMI) patients is beneficial or not, particularly in Koreans. We aimed to compare 3-year clinical outcomes of routine angiographic follow-up (RAF) and CF in AMI patients who underwent PCI with DESs.
MATERIALS AND METHODS
This study was designed as a single-center, prospective, all-comer registry study to reflect “real world” practice since 2004. Data were collected by a trained study-coordinator with a standardized case report form. This study was approved by the local Ethics Committee, and all subjects provided informed written consent. This study was performed in accordance with the ethical standards stipulated in the 1964 Declaration of Helsinki.
Study population
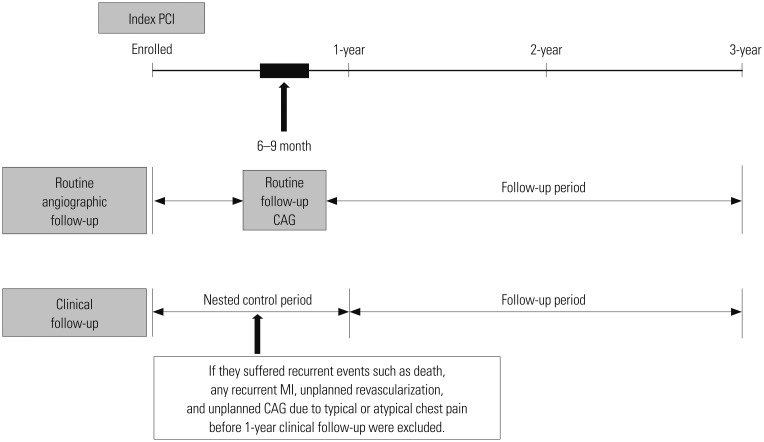
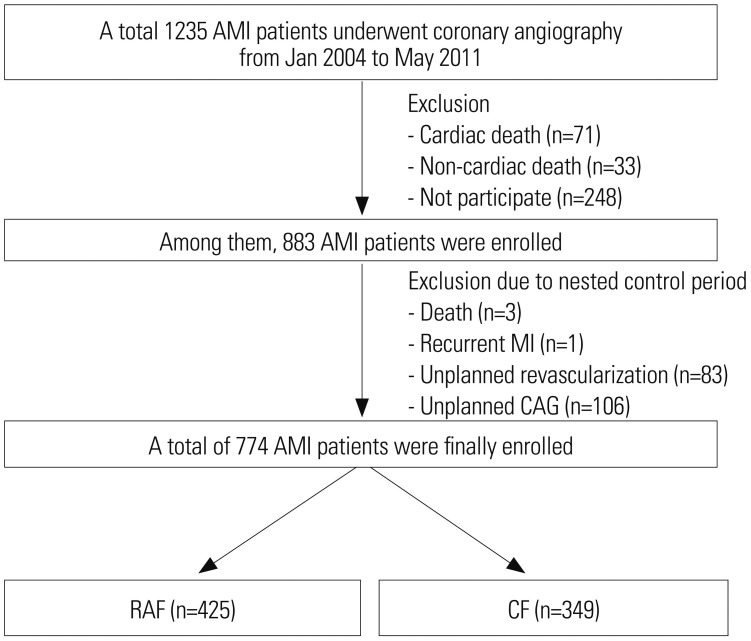
From January 2004 to May 2011, a total of 883 AMI patients were enrolled. Patients who suffered from recurrent events, such as death, any recurrent MI, unplanned revascularization, or unplanned CAG, due to typical or atypical chest pain before CF, especially during 1-year after index PCI, were excluded due to the nested control period (Fig. 1). In general, most of the procedure related complications occurred within 1 year after index PCI. Because we wanted to investigate the beneficial effects of RAF and CF on major adverse cardiac events (MACE) during the follow-up period, these patients were excluded. During the nested control period, our study strictly defined patients who underwent CAG as scheduled (6–9 months after index PCI) without having any of the above conditions as the RAF group. Follow-up strategies were planned at the day of index PCI, and RAF was scheduled 6–9 months after index PCI, at the operator's discretion. Finally, a total of 774 eligible AMI patients who successfully underwent PCI with DES without clinical events within 1 year after the index PCI were enrolled in our study. We classified them into either the RAF group (n=425) or CF group (n=349) according to the two different follow-up strategies (Fig. 2). The choice of follow-up modality after index PCI was decided in accordance with physician's preference. A total eight interventional cardiologists at our single center participated in our registry. Coincidentally, four physicians insisted on RAF, while the other four physicians insisted on CF. None of the physicians changed their choice of follow-up modality until the end of study, such that the enrolled patients never crossed-over. Although this may introduce some inherent limitations, we feel this better reflects real and routine hospital clinical practice. After propensity score-matching (PSM) analysis, two PSM groups (248 pairs, n=496, C-statistic=0.739) were generated, and the baseline characteristics of the two groups were balanced.
Fig. 1. Schematic presentation of flow sheet of this study. PCI, percutaneous coronary intervention; CAG, coronary angiography; MI, myocardial infarction.
Fig. 2. Flow chart of study number of patients. AMI, acute myocardial infarction; MI, myocardial infarction; CAG, coronary angiography; RAF, routine angiographic follow-up; CF, clinical follow-up.
PCI procedure and medical treatment
Diagnostic CAG and PCI were performed through either the femoral or radial artery after administration of unfractionated heparin (70–100 IU/kg). Each patient's activated clotting time was maintained above 250 seconds during the procedure. Revascularization was considered clinically indicated when the patient had angina and/or signs of ischemia and restenosis ≥50% in diameter by angiography or restenosis ≥70% in diameter, even in the absence of signs and symptoms. The use of cilostazol (Pletaal®, Otsuka Pharmaceutical Co., Tokyo, Japan) or platelet glycoprotein IIb/IIIa receptor blockers was left to the discretion of the individual operators. A successful PCI was defined as the achievement of an angiographic residual stenosis less than 30% and a final thrombolysis in myocardial infarction blood flow grade of 3. During hospitalization, enrolled patients were to take cardiovascular beneficial medications, including beta-blockers (BB), angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), calcium channel blockers (CCB), and lipid lowering agents. After discharge, the patients were encouraged to stay on the same medications they received during hospitalization. Dual antiplatelet therapy, which comprised a combination of aspirin (100 mg/day) and clopidogrel (75 mg/day), was recommended for at least 12 months to patients who underwent PCI.
Study definitions and clinical follow-up
The recording of cardiovascular risk factors and past medical histories were based on patient self reports. We defined the occurrences of MACE as total death, recurrent non-fatal myocardial infarction, TLR, TVR, or non-TVR. The primary endpoint was composite patient-based outcomes. All deaths were classified cardiac in origin unless a non-cardiac cause could be documented. Re-AMI was defined as the presence of clinical symptoms, electrocardiographic changes, or abnormal imaging findings of MI in combination with an increase in creatine kinase myocardial band fraction above the upper normal limits or an increase in troponin-T/troponin-I to greater than the 99th percentile of the upper normal limit. TLR was defined as a revascularization of the target lesion due to restenosis or reocclusion within the stent or 5 mm in and adjacent to the distal or proximal segment. TVR was defined as a revascularization of the target vessel or any segment of the coronary artery containing the target lesion. Non-TVR was defined as a revascularization of any segment of the non-target coronary artery. TLR-MACE was defined as the composite of cardiac death, recurrent Q-wave MI, and TLR. TVR-MACE was defined as the composite of total death, recurrent any MI (Q-wave MI and non-Q wave MI), and TVR. Total MACE was defined as the composite of TVR-MACE and non-TVR. TLR-MACE was considered a lesion-based clinical outcome, while total MACE and TVR-MACE were deemed patient-based clinical outcomes. We attempted to compare the cumulative incidences of TLR, TVR, and non-TVR by the Kaplan-Meier analysis in order to evaluate their contributions to MACE. All participants were required to visit the outpatient clinic of the cardiology department at the end of the first month and then every 3 to 6 months after the index PCI procedure, as well as whenever angina-like symptoms occurred. Clinical restenosis was suspected when the patients showed new development of one of the following symptoms: recurrent resting or exertional chest pain, electrocardiographic ST-segment changes during resting or provocation test, elevation of cardiac enzyme [troponin-I, troponin-T, or creatine kinase-MB (CK-MB) level], and abnormal result of imaging study.7 The cumulative incidences of various MACE during the 3-year follow-up period were compared between the two groups.
Statistical analysis
For continuous variables, differences between groups were evaluated with the unpaired t-test or Mann-Whitney rank test. Data are expressed as mean±standard deviations. For discrete variables, differences are expressed as counts and percentages, and were analyzed with χ2 or Fisher's exact test between the groups as appropriate. To adjust for potential confounders, propensity score marching (PSM) analysis was performed using a logistic regression model. We tested all available variables that could be of potential relevance: sex (men), age, left ventricular ejection fraction, STEMI, cardiogenic shock, cardiovascular diseases risk factors [hypertension, diabetes, dyslipidemia, chronic kidney disease, cerebrovascular accident (CVA), peripheral vascular disease (PVD), history of coronary artery disease, current smokers, and current alcoholics], laboratory findings [hemoglobin, CK-MB, troponin-T, high sensitivity C-reactive protein (hs-CRP), lipid profiles, fasting serum glucose, hemoglobin A1c and serum creatinine], number of target vessels, number of diseased vessels, total number of stent per patient, American College of Cardiology (ACC)/American Heart Association (AHA) B1/B2/C lesions, type of DES, and post-PCI medications (aspirin, clopidogrel, cilostazol, prasugrel, BB, CCB, ACEI, ARB, diuretics, lipid lowering agents, and proton pump inhibitors). The logistic model by which the propensity scores were estimated showed good predictive value (C-statistic=0.739). Patients in the RAF group were then one-to-one matched to those in the CF group according to propensity scores with the nearest available pair matching method. Subjects were matched with a caliper width equal to 0.01. The procedure yielded 248 well-matched pairs. Cox-proportional hazard models were used to assess the adjusted hazard ratio (HR) comparing the two groups in PSM population. For all analyses, a two-sided p<0.05 was considered statistically significant. All data were processed with Statistical Package for the Social Sciences version 20.0 (IBM SPSS, Inc., Chicago, IL, USA).
RESULTS
The final study population included 774 eligible AMI patients who successfully underwent PCI with DESs. After PSM analysis, two PSM groups (248 pairs, n=496, C-statistic=0.739) were generated. The patient's baseline clinical characteristics, laboratory findings, and angiographic characteristics are summarized in Tables 1 and 2. In the unmatched study population, the mean age (mean±SD) of the RAF group was 59.8±11.5 years, and that of the CF group was 63.6±12.7 years (p<0.001). The RAF group had a higher number of smokers and higher levels of serum hemoglobin and hematocrit, compared with the CF group, while the CF group was more likely to have hypertension, chronic kidney disease, and renal dysfunction. There was no difference in the proportion of patients with STEMI, non-STEMI, cardiogenic shock, dyslipidemia, prior CVA, PVD, prior MI, prior PCI, prior coronary artery bypass graft, lipid profile, hs-CRP, fasting blood glucose, and hemoglobin A1c (Table 1). Angiographic characteristics in the unmatched population were similar between the two groups, including treated vessels, ACC/AHA lesion type, bifurcation type (Lefevre), left main disease, multi-vessel disease, ostial lesion, diffuse long lesion, and small vessel disease. Types of DES deployed between the two groups were different. Sirolimus-eluting (Cypher™, Corporation, Johnson and Johnson, Warren County, NJ, USA) stent and paclitaxel-eluting (Taxus™, Boston Scientific, Natick, MA, USA) stents were more frequently deployed in the RAF group; Zotarolimus-eluting (Resolute™, Medtronic Inc., Santa Rosa, CA, USA) stents and everolimus-eluting stents (Xience V™/Promus™, Boston Scientific) were more common in the CF group (Table 2). The unmatched CF group were more likely than the RAF group to have received aspirin after PCI (96.2% vs. 92.7%, p=0.033). The use of other medications, including clopidogrel, cilostazol (Pletaal®, Otsuka Pharmaceutical Co., Tokyo, Japan), prasugrel (Effient®, Daiichi Sankyo Company Ltd. UK/Eli Lilly and Company Ltd., Tokyo, Japan), BB, CCB, ACEI, ARB, diuretics, and lipid lowering agents, were similar between the two groups (Table 3). Table 4 shows the cumulative clinical outcomes between one to three years in patients with RAF and CF. The incidences of total death and MI were not significantly different between the two groups. However, the incidence of repeat revascularization (TLR, TVR, non-TVR) in the RAF group was significantly higher than that in the CF group regardless of PSM [TLR: HR, 2.40; 95% confidence interval (CI), 1.18–4.85; p=0.015, TVR: HR, 3.33; 95% CI, 1.69–6.58; p=0.001, and non-TVR: HR, 5.64; 95% CI, 1.90–16.6; p=0.002].
Table 1. Clinical Characteristics and Laboratory Findings.
| Variables | Entire patients | Propensity score-matched patients | ||||
|---|---|---|---|---|---|---|
| RAF (n=425) | CF (n=349) | p value | RAF (n=248) | CF (n=248) | p value | |
| Men, n (%) | 318 (74.8) | 237 (67.9) | 0.034 | 170 (68.5) | 182 (73.3) | 0.235 |
| Age (yr) | 59.8±11.5 | 63.6±12.7 | <0.001 | 61.8±11.1 | 61.7±12.9 | 0.912 |
| LVEF (%) | 48.1±10.5 | 47.1±11.6 | 0.255 | 48.5±10.4 | 48.1±10.8 | 0.638 |
| Myocardial infarction (MI), n (%) | ||||||
| ST-segment elevation | 228 (53.6) | 187 (53.6) | 0.986 | 127 (51.2) | 130 (52.4) | 0.787 |
| Non-ST-segment elevation | 197 (46.4) | 162 (46.4) | 0.986 | 121 (48.8) | 118 (47.6) | 0.787 |
| Cardiogenic shock, n (%) | 17 (4.0) | 16 (4.5) | 0.689 | 11 (4.4) | 11 (4.4) | NS |
| Hypertension, n (%) | 225 (52.9) | 227 (65.0) | 0.001 | 147 (59.2) | 141 (56.8) | 0.585 |
| Diabetes, n (%) | 129 (30.3) | 132 (37.8) | 0.029 | 88 (35.4) | 80 (32.2) | 0.448 |
| Insulin | 47 (11.1) | 60 (17.2) | 34 (13.7) | 35 (14.1) | ||
| Medication | 76 (17.8) | 69 (19.8) | 49 (19.7) | 44 (17.7) | ||
| Dietary | 6 (1.4)0 | 3 (0.8)0 | 5 (2.0)0 | 1 (0.4)0 | ||
| Dyslipidemia, n (%) | 94 (22.1) | 62 (17.7) | 0.133 | 43 (17.3) | 51 (20.5) | 0.359 |
| Chronic kidney disease, n (%) | 9 (2.1)0 | 27 (7.7)0 | <0.001 | 8 (3.2)0 | 2 (0.8)0 | 0.055 |
| Renal dysfunction, n (%) | 9 (2.1) | 24 (6.8) | 0.001 | 8 (3.2) | 2 (0.8) | 0.055 |
| Cerebrovascular accident, n (%) | 20 (4.7)0 | 16 (4.5)0 | 0.936 | 11 (4.4)0 | 11 (4.4)0 | NS |
| Ischemic | 15 (3.6) | 13 (3.7) | 0.885 | 7 (2.8) | 8 (3.2) | 0.793 |
| Hemorrhagic | 5 (1.1)0 | 3 (0.8)0 | 0.736 | 4 (1.6)0 | 3 (1.2)0 | NS |
| Peripheral vessel disease, n (%) | 5 (1.1) | 9 (2.5) | 0.145 | 3 (1.2) | 2 (0.8) | NS |
| History of CAD, n (%) | 19 (4.5)0 | 14 (4.0)0 | 0.788 | 12 (4.8)0 | 9 (3.6)0 | 0.426 |
| Prior MI | 6 (1.4) | 2 (0.6) | 0.335 | 3 (1.2) | 2 (0.8) | NS |
| Prior PTCA | 13 (3.1)0 | 10 (2.9)0 | 0.869 | 9 (3.6)0 | 5 (2.0)0 | 0.278 |
| Prior CABG | 0 (0.0) | 2 (0.5) | 0.203 | 0 (0.0) | 2 (0.8) | 0.499 |
| Smokers, n (%) | 243 (57.1) | 165 (47.2) | 0.006 | 125 (50.4) | 132 (53.2) | 0.529 |
| Current smokers | 186 (43.7) | 127 (36.3) | 0.038 | 96 (38.7) | 103 (41.5) | 0.521 |
| Alcoholics, n (%) | 136 (32.0) | 107 (30.6) | 0.689 | 74 (29.8) | 81 (32.6) | 0.498 |
| Current alcoholics | 122 (28.7) | 97 (27.7) | 0.779 | 69 (27.8) | 75 (30.2) | 0.553 |
| Hemoglobin (g/dL) | 13.3±1.7 | 12.9±2.0 | 0.002 | 13.1±1.6 | 13.0±1.8 | 0.632 |
| Hematocrit (%) | 39.3±4.9 | 38.0±5.6 | 0.001 | 38.8±4.6 | 38.6±5.3 | 0.599 |
| CK-MB (mg/dL) | 111±152 | 98±147 | 0.212 | 112±149 | 100±148 | 0.344 |
| Troponin-T (ng/dL) | 1.13±2.41 | 1.28±3.07 | 0.450 | 1.12±2.16 | 1.33±3.26 | 0.409 |
| High sensitivity CRP (mg/L) | 17.0±32.1 | 17.0±33.2 | 0.917 | 16.0±28.3 | 16.0±34.0 | 0.830 |
| Total cholesterol (mg/dL) | 186±41 | 183±43 | 0.373 | 184±42 | 184±44 | 0.999 |
| Triglyceride (mg/dL) | 133±85 | 123±92 | 0.260 | 127±78 | 130±96 | 0.722 |
| HDL cholesterol (mg/dL) | 44±10 | 43±11 | 0.502 | 44±11 | 43±11 | 0.205 |
| LDL cholesterol (mg/dL) | 124±36 | 119±38 | 0.213 | 121±38 | 120±39 | 0.735 |
| Apolipoprotein A-I (mg/dL) | 122±21 | 118±27 | 0.205 | 125±22 | 118±27 | 0.050 |
| Apolipoprotein B (mg/dL) | 75±25 | 80±29 | 0.104 | 76±25 | 81±29 | 0.193 |
| Lipoprotein A (mg/dL) | 30±27 | 27±27 | 0.240 | 29±26 | 27±27 | 0.676 |
| Hemoglobin A1c (%) | 6.4±1.3 | 6.6±1.6 | 0.091 | 6.5±1.3 | 6.5±1.6 | 0.941 |
| Serum creatinine (mg/dL) | 0.9±0.7 | 1.1±1.0 | 0.063 | 0.9±0.8 | 0.9±0.7 | 0.815 |
RAF, routine angiographic follow-up; CF, clinical follow-up; LVEF, left ventricular ejection fraction; CAD, coronary artery disease; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass graft; CK, creatine kinase; CRP, C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NS, not significant (>0.999).
Values are mean±SD or n (%). The p value for continuous data from analysis of variance. The p value for categorical data from chi-square test.
Table 2. Angiographic Characteristics.
| Variables | Entire patients | Propensity score-matched patients | ||||
|---|---|---|---|---|---|---|
| RAF (n=425) | CF (n=349) | p value | RAF (n=248) | CF (n=248) | p value | |
| Treated vessels, n (%) | ||||||
| Left main | 14 (3.2)0 | 8 (2.2)0 | 0.404 | 5 (2.0)0 | 5 (2.0)0 | NS |
| Left artery descending | 222 (52.2) | 205 (58.7) | 0.070 | 131 (52.8) | 129 (52.0) | 0.857 |
| Left circumflex | 131 (30.8) | 97 (27.7) | 0.358 | 74 (29.8) | 75 (30.2) | 0.922 |
| Right coronary artery | 172 (40.4) | 123 (35.2) | 0.136 | 95 (38.3) | 99 (39.9) | 0.713 |
| ACC/AHA lesion type, n (%) | ||||||
| Type B1 | 6 (1.4) | 10 (2.8) | 0.157 | 5 (2.0) | 5 (2.0) | NS |
| Type B2 | 71 (16.7) | 58 (16.6) | 0.974 | 41 (16.5) | 43 (17.3) | 0.811 |
| Type C | 348 (81.8) | 281 (80.5) | 0.628 | 202 (81.4) | 200 (80.6) | 0.819 |
| Bifurcation type (Lefevre), n (%) | 162 (38.1) | 130 (37.2) | 0.804 | 93 (37.5) | 99 (39.9) | 0.580 |
| Type 1 | 77 (18.1) | 64 (18.3) | 43 (17.3) | 53 (21.4) | ||
| Type 2 | 38 (8.9)0 | 22 (6.3)0 | 22 (8.9)0 | 15 (6.1)0 | ||
| Type 3 | 11 (2.6) | 11 (3.2) | 7 (2.8) | 10 (4.0) | ||
| Type 4 | 8 (1.9)0 | 3 (0.9)0 | 4 (1.6)0 | 1 (0.4)0 | ||
| Type 5 | 20 (4.7) | 18 (5.1) | 11 (4.4) | 12 (4.8) | ||
| Type 6 | 8 (1.9)0 | 12 (3.4)0 | 6 (2.5)0 | 8 (3.2)0 | ||
| Left main disease, n (%) | 21 (4.9) | 20 (5.7) | 0.626 | 10 (4.0) | 12 (4.8) | 0.663 |
| Multi-vessel disease, n (%) | 103 (24.2) | 72 (20.6) | 0.233 | 49 (19.7) | 53 (21.3) | 0.657 |
| 1 vessel disease | 322 (75.7) | 277 (79.3) | 199 (80.2) | 195 (78.6) | ||
| 2 vessel disease | 90 (21.1) | 58 (16.6) | 39 (15.7) | 44 (17.7) | ||
| 3 vessel disease | 13 (3.0) | 14 (4.0) | 10 (4.0) | 9 (3.6) | ||
| Number of vessels | 1.2±0.5 | 1.2±0.5 | 0.475 | 1.2±0.5 | 1.2±0.5 | 0.793 |
| Number of lesions | 1.5±0.8 | 1.5±0.8 | 0.635 | 1.5±0.8 | 1.5±0.8 | 0.959 |
| IABP, n (%) | 58 (13.6) | 41 (11.7) | 0.106 | 28 (11.3) | 33 (13.3) | 0.564 |
| Ostial (≤5 mm) lesion, n (%) | 77 (18.1) | 66 (18.9) | 0.777 | 43 (17.3) | 44 (17.7) | 0.906 |
| Diffuse long lesion (>3 cm), n (%) | 191 (44.9) | 160 (45.8) | 0.802 | 117 (47.1) | 114 (45.9) | 0.787 |
| Small vessel (≤2.25 mm), n (%) | 23 (5.4) | 18 (5.1) | 0.875 | 10 (4.0) | 13 (5.2) | 0.522 |
| Calcified lesion, n (%) | 47 (11.0) | 62 (17.7) | 0.008 | 30 (12.0) | 32 (12.9) | 0.786 |
| Type of DES, n (%) | ||||||
| Sirolimus-eluting | 124 (29.1) | 63 (18.0) | <0.001 | 58 (23.3) | 51 (20.5) | 0.448 |
| Paclitaxel-eluting | 149 (35.0) | 68 (19.4) | <0.001 | 66 (26.6) | 58 (23.3) | 0.407 |
| Zotarolimus-eluting | 108 (25.4) | 135 (38.6) | <0.001 | 84 (33.8) | 83 (33.4) | 0.924 |
| Endeavor Sprint | 55 (12.9) | 49 (14.0) | 0.656 | 36 (14.5) | 37 (14.9) | 0.899 |
| Endeavor Resolute | 53 (12.4) | 88 (25.2) | <0.001 | 48 (19.3) | 47 (18.9) | 0.909 |
| Everolimus-eluting | 68 (16.0) | 104 (29.7) | <0.001 | 51 (20.5) | 64 (25.8) | 0.167 |
| Xience V/Promus | 47 (11.0) | 80 (22.9) | <0.001 | 37 (14.9) | 48 (19.3) | 0.190 |
| Promus element | 21 (4.9) | 23 (6.5) | 0.324 | 14 (5.6) | 17 (6.8) | 0.578 |
| Xience prime | 1 (0.2)0 | 3 (0.8)0 | 0.332 | 1 (0.4)0 | 1 (0.4)0 | NS |
| Biodegradable-polymer-biolimus-eluting | 10 (2.3) | 5 (1.4) | 0.355 | 6 (2.4) | 5 (2.0) | 0.760 |
| Others | 7 (1.6)0 | 1 (0.2)0 | 0.079 | 1 (0.4)0 | 1 (0.4)0 | NS |
| Procedure time (min) | 44±34 | 39±24 | 0.058 | 41±32 | 40±25 | 0.755 |
| Total doses of unfractionated heparin (international units) | 4114±1605 | 3952±1767 | 0.205 | 3860±1287 | 4110±1908 | 0.099 |
| Final activated clotting time | 230±73 | 237±85 | 0.268 | 234±71 | 235±85 | 0.889 |
RAF, routine angiographic follow-up; CF, clinical follow-up; ACC/AHA, American College of Cardiology/American Heart Association; IABP, intra-aortic balloon pump; DES, drug-eluting stent; NS, not significant (>0.999).
Values are mean±SD or n (%). The p value for continuous data from analysis of variance. The p value for categorical data from chi-square test.
Table 3. Post-Percutaneous Coronary Intervention Medications.
| Variables | Entire patients | Propensity score-matched patients | ||||
|---|---|---|---|---|---|---|
| RAF (n=425) | CF (n=349) | p value | RAF (n=248) | CF (n=248) | p value | |
| Aspirin, n (%) | 394 (92.7) | 336 (96.2) | 0.033 | 234 (94.3) | 235 (94.7) | 0.843 |
| Clopidogrel, n (%) | 395 (92.9) | 328 (93.9) | 0.561 | 231 (93.1) | 232 (93.5) | 0.857 |
| Cilostazol, n (%) | 110 (25.8) | 80 (22.9) | 0.341 | 57 (22.9) | 61 (24.5) | 0.673 |
| Prasugrel, n (%) | 2 (0.4)0 | 4 (1.1)0 | 0.417 | 2 (0.8)0 | 2 (0.8)0 | NS |
| Beta blockers, n (%) | 244 (57.4) | 218 (62.4) | 0.154 | 146 (58.8) | 149 (60.0) | 0.784 |
| Calcium channel blockers, n (%) | 155 (36.4) | 105 (30.0) | 0.061 | 85 (34.2) | 78 (31.4) | 0.503 |
| ACEI, n (%) | 129 (30.3) | 108 (30.9) | 0.859 | 76 (30.6) | 72 (29.0) | 0.695 |
| ARBs, n (%) | 166 (39.0) | 144 (41.2) | 0.534 | 94 (37.9) | 103 (41.5) | 0.409 |
| Diuretics, n (%) | 91 (21.4) | 76 (21.7) | 0.902 | 55 (22.1) | 57 (22.9) | 0.830 |
| Lipid lowering agents, n (%) | 378 (88.9) | 315 (90.2) | 0.552 | 224 (90.3) | 226 (91.1) | 0.757 |
| Proton pump inhibitors, n (%) | 31 (7.2) | 39 (11.1) | 0.061 | 24 (9.6) | 22 (8.8) | 0.757 |
RAF, routine angiographic follow-up; CF, clinical follow-up; ACEI, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; NS, not significant (>0.999).
Values are numbers and percentages. The p value for categorical data from chi-square test.
Table 4. Cumulative Events Up to Three Years after the Nested Control Period.
| Variables | Entire patients | Propensity score-matched patients | Hazard ratio (95% CI) | p value | ||||
|---|---|---|---|---|---|---|---|---|
| RAF (n=425) | CF (n=349) | p value | RAF (n=248) | CF (n=248) | p value | |||
| Total death, n (%) | 7 (1.6) | 13 (3.7) | 0.070 | 4 (1.6) | 6 (2.4) | 0.523 | 0.66 (0.18–2.37) | 0.526 |
| Cardiac death | 03 (0.7) | 06 (1.7) | 0.313 | 02 (0.8) | 03 (1.2) | NS | 0.66 (0.10–4.00) | 0.655 |
| Non-cardiac death | 4 (0.9) | 7 (2.0) | 0.237 | 2 (0.8) | 3 (1.2) | NS | 0.66 (0.11–4.00) | 0.664 |
| Myocardial infarction (MI) , n (%) | 10 (2.3) | 12 (3.4) | 0.366 | 04 (1.6) | 08 (3.2) | 0.242 | 0.49 (0.14–1.65) | 0.252 |
| ST-segment elevation MI | 4 (0.9) | 5 (1.4) | 0.738 | 1 (0.4) | 3 (1.2) | 0.623 | 0.33 (0.03–3.20) | 0.339 |
| Non-ST-segment elevation MI | 06 (1.4) | 07 (2.0) | 0.522 | 03 (1.2) | 05 (2.0) | 0.724 | 0.59 (0.14–2.51) | 0.722 |
| Revascularizations, n (%) | 80 (18.8) | 17 (4.8) | <0.001 | 53 (21.3) | 14 (5.6) | <0.001 | 4.54 (2.44–8.43) | <0.001 |
| TLR | 44 (10.3) | 15 (4.2) | 0.002 | 27 (10.8) | 12 (4.8) | 0.012 | 2.40 (1.18–4.85) | 0.015 |
| TVR | 57 (13.4) | 15 (4.2) | <0.001 | 36 (14.5) | 12 (4.8) | <0.001 | 3.33 (1.69–6.58) | 0.001 |
| Non-TVR | 29 (6.8) | 04 (1.1) | <0.001 | 21 (8.4) | 04 (1.6) | <0.001 | 5.64 (1.90–16.6) | 0.002 |
| Stent thrombosis, n (%) | 6 (1.4) | 4 (1.1) | NS | 2 (0.8) | 4 (1.6) | 0.686 | 0.49 (0.09–2.73) | 0.421 |
| Late | 03 (0.7) | 00 (0.0) | 02 (0.8) | 00 (0.0) | ||||
| Very late | 3 (0.7) | 4 (1.1) | 0 (0.0) | 4 (1.6) | ||||
| TLR MACE, n (%) | 47 (11.0) | 22 (6.3) | 0.021 | 29 (11.6) | 15 (6.0) | 0.027 | 2.05 (1.07–3.94) | 0.030 |
| TVR MACE, n (%) | 65 (15.2) | 29 (8.3) | 0.003 | 40 (16.1) | 18 (7.2) | 0.002 | 2.45 (1.36–4.41) | 0.003 |
| Total MACE, n (%) | 86 (20.2) | 31 (8.8) | <0.001 | 56 (22.5) | 20 (8.0) | <0.001 | 3.32 (1.92–5.73) | <0.001 |
| Follow-up, day | 1088±55 | 1076±10 | 0.039 | 1087±63 | 1082±89 | 0.442 | ||
RAF, routine angiographic follow-up; CF, clinical follow-up; MACE, major adverse cardiovascular events; TLR, target lesion revascularization; TVR, target vessel revascularization; CI, confidence interval; NS, not significant (>0.999).
Values are numbers and percentages. The p value for categorical data from chi-square test or Cox-proportional hazard models.
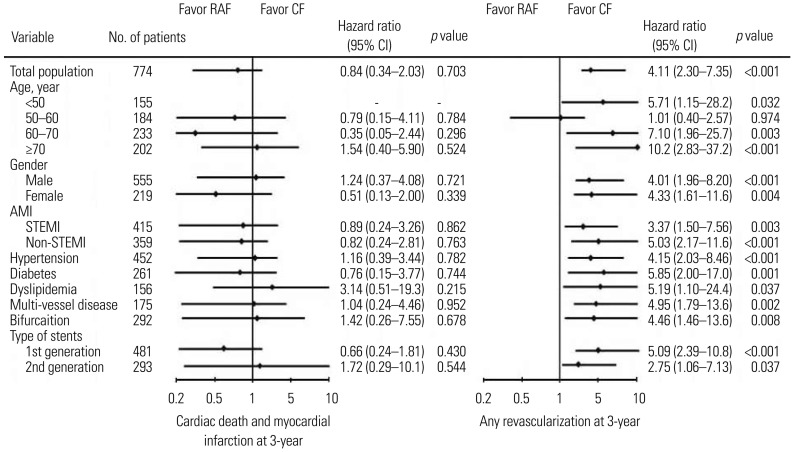
The incidence of MACE was also higher in the RAF group than the CF group unmatched and after PSM (HR, 3.32; 95% CI, 1.92–5.73; p<0.001). However, the incidence of death (HR, 0.66; 95% CI, 0.18–2.37; p=0.526) or MI (HR, 0.49; 95% CI, 0.14–1.65; p=0.252) was not significantly different between the two groups. Results of propensity score adjusted Cox-regression analysis for MACE up to 3 years in various subgroups are shown in Fig. 3. Subgroup analysis for the two different follow-up methods revealed that CF group had more favorable results in view of revascularization rates during the 3 years among all subgroups.
Fig. 3. Propensity score adjusted Cox-regression analysis for cardiac death and myocardial infarction, and any revascularization up to 3-year in various subgroups. RAF, routine angiographic follow-up; CF, clinical follow-up; STEMI, ST-segment elevation myocardial infarction; CI, confidence interval.
DISCUSSION
Despite expected beneficial effects, RAF following index PCI with DES in AMI patients was associated with higher incidences of repeat revascularizations, including TLR, TVR, and non-TVR, without significant differences in the incidence of death or recurrent AMI during a 3-year CF period. These increased revascularization incidences, due to possible ‘oculo-stenotic reflex’ in the RAF group, resulted in a higher MACE incidence in our study. The RAF group's total incidence of MACE was more than three times higher than that for the CF group. We deemed that RAF may have no beneficial effects and could potentially be harmful.
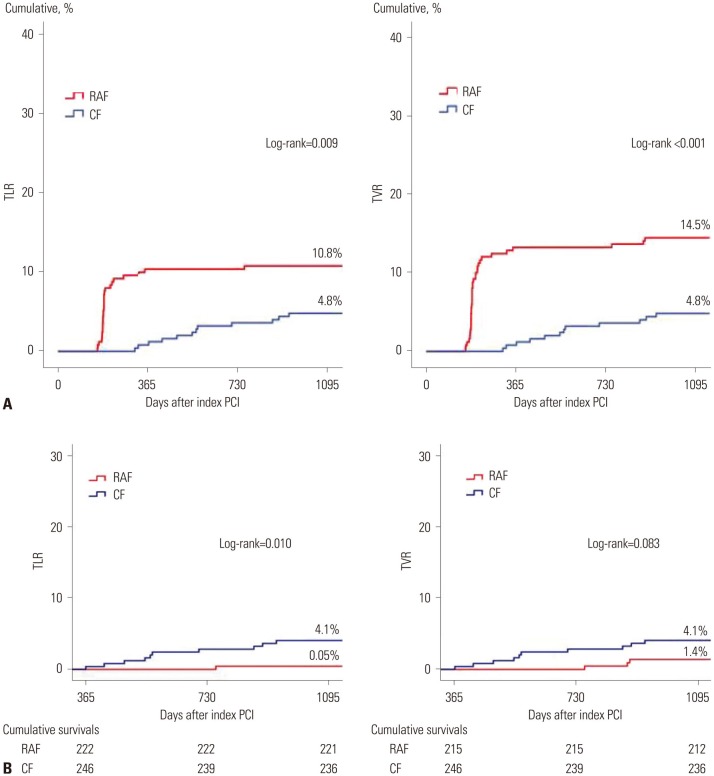
In our study, we excluded patients who suffered recurrent events, such as death, any recurrent MI, unplanned revascularization, or unplanned CAG due to typical or atypical chest pain before CF at 1 year. During the nested control period (Fig. 1), our study strictly defined patients who underwent CAG as scheduled (6–9 months after index PCI) absent any of the above conditions as the RAF group. Fig. 4A shows that the total cumulative incidences of TLR and TVR in the RAF group were significantly higher than those in the CF group. Fig. 4B shows that, after the nested control period, the cumulative incidence of TLR was higher and TVR tended to be higher in the CF group than the RAF group.
Fig. 4. Kaplan-Meier curved analysis for TLR and TVR. (A) Total cumulative events curve of TLR and TVR. (B) Cumulative events curve up to 3-year after the nested control period. TLR, target lesion revascularization; TVR, target vessel revascularization; RAF, routine angiographic follow-up; CF, clinical follow-up; PCI, percutaneous coronary intervention.
We think several possible factors may have influenced these results. As mentioned above, RAF was scheduled at 6–9 months after index PCI. Meanwhile, repeated PCIs that were performed at 10–12 months after index PCI, after the scheduled RAF, were counted as TLRs or TVRs and contributing to the cumulative incidences of TLR or TVR in the RAF group, because these PCIs might be related to RAF strategy. In the RAF group, 96.3% (26 cases/total 27 cases) of TLRs and 91.7% (33 cases/total 36 cases) of TVRs occurred days to within one year (6–12 months after index PCI) after RAF. In the CF group, most of TLR and TVR cases occurred after one year (after the nested control period). The effects thereof are described further in the limitations section. As shown in Fig. 4B, after the nested control period, the cumulative incidence of TLR and TVR in the CF group was higher than that in the RAF group. After the nested control period, patients included in the RAF group were relatively stable and not progressive, while patients in the CF group may have been relatively unstable and more likely to be symptomatic due to progression of underlying stenotic CAD. Although present study showed TVR rates were not significantly different between the two groups, if we can follow up for a longer time and investigate much larger-scaled populations, TVR rates may become similar to the cumulative incidence of TLR. Accordingly, the authors suggest that longer-term and larger-scaled follow-up study is warranted.
Stenotic coronary artery lesions that do not produce ischemic symptoms receive little benefit from revascularization, compared with effective optimal medical therapy alone.8 Despite its cost and periprocedural risk, RAF is still performed at select centers to identify angiographically significant stenosis that is not related to ischemic symptoms. The present study showed that total death or MI during the 3 years of follow-up was not reduced in the routine RAF group, compared with the CF group, in AMI patients after index PCI. Although DES can reduce the incidence of clinical and angiographic restenosis rates significantly, compared with BMS,10 all of the major DES clinical trials have required angiographic follow-up.11,12,13 Protocol-mediated angiographic follow-up in DES may negatively affect safety outcomes,5 and there was substantial controversy regarding the need for and impact of, protocol-mandated angiographic follow-up in PCI trials. Follow-up angiography after PCI has been shown to be associated with accentuated rates of revascularization, resulting from the “oculostenotic reflex,” a term describing revascularization with PCI due to anatomic lesion severity, regardless of clinical or physiologic evidence of ischemia.14 Only a minority (22%) of patients with angiographic restenosis show severe (diameter stenosis >70%) stenosis, which is primarily associated with demonstrable myocardial ischemia.3 In the HORIZONS-AMI study,15 RAF magnified the benefit of paclitaxel-eluting stents (PES) over BMS with respect to TVR beyond 1 year, although RAF showed a relatively higher incidence of TLR, compared with the natural event rates before RAF at 13 months after index PCI. A meta-analysis of 11 randomized trials16 between DES and BMS in STEMI patients revealed a TVR reduction rate of 7.6% in the DES group at 12 months (5.0% vs. 12.6%, p<0.0001). In eight of these 11 studies, RAF was performed before comparison of major clinical outcomes.
In the cases of stable coronary artery disease, TVR was significantly higher at 5 years for patients in whom protocol-mandated angiographic follow-up was planned versus not planned (18.3% vs. 11.1%, p<0.001), although there were no significant differences in death or reinfarction (8.9% vs. 8.8%, p=0.93).14 These increased rates of TVR were due to greater treatment of intermediate stenotic lesions (40–70% stenosis by quantitative angiography), not to severe “pre-infarction” stenosis. In addition, intermediate lesions tend to regress over time (2 to 5 years).17,18 In the Clinical Evaluation of the XIENCE V™ Everolimus Eluting Coronary Stent System in the Treatment of Subjects With de Novo Native Coronary Artery Lesions (SPIRIT) III trial, there was four times as much TVR in the scheduled angiographic follow-up (SAF) group as in the no SAF group, predominantly related to treatment of lesions without documented ischemia (4.5% vs. 1.0%, p=0.002).17 A substantial proportion of restenosis episodes can present as acute coronary syndrome or MI, and whether RAF or SAF can identify such culprits lesions before they become symptomatic, enabling preventive revascularization, is unsettled. Mindrescu, et al.9 also reported that SAF leads to increased rates of revascularization without impacting the occurrences of death, MI, and stent thrombosis. The SPIRIT III trial19 also had indicated that RAF follow-up tended to overestimate decreases in TVR, compared to routine CF. This suggest that RAF does not appear to adversely affect the long-term safety of patients. At 3 years, rates of death or MI were similar between the RAF and CF groups in this study.
The important causes of recurrent disease at the target lesion site are restenosis and stent thrombosis. Fifty-three patients (21.3%) had revascularization procedure during RAF in our study after PSM analysis (Table 4). Among these patients, 40 patients (75.5%) had revascularization procedures within 2 weeks during a routine follow-up angiography (p<0.001). Thirteen patients (25.5%) had a revascularization procedure thereafter. When we considered restenosis rates in patients treated with DES in the general population,4 these high incidences of revascularization, especially within 2 weeks in RAF group, may include restenotic lesions that do not compromise the lumen area sufficiently enough to cause ischemic symptom. The rates of stent thrombosis were relatively low in our study (1.4% vs. 1.1%, p=NS) during the 3-year follow up period. This may be due to the limitations of a single center study and relatively large proportion of one vessel disease. Moreover, 1st generation DES [Sirolimus-eluting stent (Cypher™)] and PES (Taxus™) were much more frequently deployed in the unmatched RAF group in our study. This bias regarding stent type, however, was disregarded after PSM analysis.
In our study, the CF group was more likely to be elderly, hypertensive, and have chronic kidney disease and renal dysfunction. Therefore, we can consider that the CF group faced relatively higher angiographic risk (treated vessels, ACC/AHA lesion type, bifurcation type, left main disease, multi-vessel disease, ostial lesion, diffuse long lesion, and small vessel disease). However, revascularization rates and MACE of the CF group were lower than those in the RAF group before and after PSM analysis. These results were sustained during the subgroup analysis. The CF group showed favorable results in view of any revascularization type at 3 years regardless of the subgroup. Subgroup analysis also showed cardiac death and MI at 3-years to not significantly differ between the two groups (Fig. 3).
This study has some limitations, because it is a non-randomized design and a single center study. In the aspect of the choice in follow-up modality after index PCI, physician preferences may act as a selection bias, although we feel this better reflects real and routine hospital clinical practice. Although we planned to compare 3-year long-term clinical outcomes between RAF and CF in AMI patients undergoing PCI, those who suffered recurrent events, such as death, any recurrent MI, unplanned revascularization, and unplanned CAG due to typical or atypical chest pain, within 1 year of CF were excluded from our study due to nested control period. It is possible that some proportion of MACEs in the CF group could have been included in this nested control period, leading to potential underestimation of MACE in the CF group. Additionally, RAF group patients may have more chances to undergo early detection and early management for their coronary lesions, regardless of the presence of symptoms, than symptom-driven CF patients. In the cases of RAF, the treatment strategies for angiographic stenotic lesions were left to the judgement of the operators. Unfortunately, in this study, functional studies were done only for a small number of patients (<10%). Practically, we cannot perform routine functional studies including fractional flow reserve (FFR) due to cost issues. In Korea, currently there is no reimbursement program for FFR, IVUS, or Cardiac CT and MRI, in addition to CAG. We should depend on angiographic findings and clinical decision in real world clinical practice. Although relatively lower rates of functional studies, non-randomized design, and single center study, this study may be meaningful because we tried to reflect “real world” clinical practice. Thus, the patients included in the RAF group underwent CAG as scheduled, regardless of the results of functional study. Also, the patients included in the CF group underwent CAG whenever angina-like symptoms occurred (symptom-driven CAG), regardless of the results of functional study. Therefore, we think that clinical milieu in terms of function study support in both groups would not be significantly different and would not impact clinical events differently. In the current study, nested control period was defined as 1 year after PCI. However, follow-up CAG was performed 6–9 months after PCI. Therefore, there was a gap of 3 months between “during 1 year after PCI” and the window of the follow-up period. The authors considered that this 3-month gap period may also be related with vulnerable periods, during which procedure-related late complications can happen frequently. During the first 6 months after index PCI, procedure-related acute complications can also occur. Accordingly, we thought 1 year after index PCI was more reasonable as a nested control period than 6 months after index PCI. However, in real-world practice, some patients undergo RAF after the scheduled date. Delayed RAF in the present study was due to personal schedule conflicts and other barrier factors to later than 9 months. Delayed RAF was performed in 16 patients between 10–12 months after index PCI in our study. Exceptionally, in these special cases, these patients were included in the RAF group. However, in these patients, TLR or TVR was not recorded up to 3 years, and they did not contribute to the cumulative incidences of TLR or TVR. In addition, when repeated PCIs were done in these periods (10–12 months after index PCI) after scheduled RAF, these PCIs were counted as TLR or TVR rates and presented in the cumulative incidence of TLR or TVR rates in the RAF group, since these PCIs may be related with RAF strategy. That is to say, these 10–12 months after index PCI included both above exceptionally delayed RAF and the cases of TLR or TVR after RAF in the RAF group. Finally, the index PCI day was considered as the day of enrollment.
Furthermore, like every “real-world” registry, there may have been some under-reporting and/or missing data. Also, we could not obtain exact information concerning the relationship between angina symptom and the degree of angiographic stenosis due to some missing values. Therefore, we could not describe the differences in TLR rates between the two follow-up groups and intermediate stenotic lesions.
In conclusion, given these limitations, our results do not indicate any clinical benefit of RAF in clinical practice. The results of our study confirm that patients who are assigned to RAF undergo more revascularization procedures than CF alone without an improvement from death or reinfarction. Therefore, CF seems warranted for asymptomatic patients after PCI for AMI.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Choi YJ, Kim JB, Cho SJ, Cho J, Sohn J, Cho SK, et al. Changes in the practice of coronary revascularization between 2006 and 2010 in the Republic of Korea. Yonsei Med J. 2015;56:895–903. doi: 10.3349/ymj.2015.56.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tantawy A, Ahn CM, Shin DH, Kim JS, Kim BK, Ko YG, et al. Nobori-biolimus-eluting stents versus resolute zotarolimus-eluting stents in patients undergoing coronary intervention: a propensity score matching. Yonsei Med J. 2017;58:290–295. doi: 10.3349/ymj.2017.58.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(10 Suppl):S1–S42. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198–3206. doi: 10.1161/CIRCULATIONAHA.108.826479. [DOI] [PubMed] [Google Scholar]
- 5.Cutlip DE, Chauhan MS, Baim DS, Ho KK, Popma JJ, Carrozza JP, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol. 2002;40:2082–2089. doi: 10.1016/s0735-1097(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 6.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51:172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Silber S, Albertsson P, Avilés FF, Camici PG, Colombo A, Hamm C, et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur Heart J. 2005;26:804–847. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 8.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 9.Mindrescu C, Brener SJ, Guerchicoff A, Fahy M, Parise H, Mehran R, et al. Impact of scheduled angiographic follow-up in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Interv Cardiol. 2013;26:319–324. doi: 10.1111/joic.12038. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 11.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 12.Pinto DS, Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, et al. Impact of routine angiographic follow-up on the clinical benefits of paclitaxel-eluting stents: results from the TAXUS-IV trial. J Am Coll Cardiol. 2006;48:32–36. doi: 10.1016/j.jacc.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 13.Lasala JM, Cox DA, Dobies D, Muhlestein JB, Katopodis JN, Revtyak G, et al. Usage patterns and 2-year outcomes with the TAXUS express stent: results of the US ARRIVE 1 registry. Catheter Cardiovasc Interv. 2008;72:433–445. doi: 10.1002/ccd.21618. [DOI] [PubMed] [Google Scholar]
- 14.Uchida T, Popma J, Stone GW, Ellis SG, Turco MA, Ormiston JA, et al. The clinical impact of routine angiographic follow-up in randomized trials of drug-eluting stents: a critical assessment of “oculostenotic” reintervention in patients with intermediate lesions. JACC Cardiovasc Interv. 2010;3:403–411. doi: 10.1016/j.jcin.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Stone GW, Parise H, Witzenbichler B, Kirtane A, Guagliumi G, Peruga JZ, et al. Selection criteria for drug-eluting versus bare-metal stents and the impact of routine angiographic follow-up: 2-year insights from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol. 2010;56:1597–1604. doi: 10.1016/j.jacc.2010.08.608. [DOI] [PubMed] [Google Scholar]
- 16.De Luca G, Stone GW, Suryapranata H, Laarman GJ, Menichelli M, Kaiser C, et al. Efficacy and safety of drug-eluting stents in ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. Int J Cardiol. 2009;133:213–222. doi: 10.1016/j.ijcard.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Asakura M, Ueda Y, Nanto S, Hirayama A, Adachi T, Kitakaze M, et al. Remodeling of in-stent neointima, which became thinner and transparent over 3 years: serial angiographic and angioscopic follow-up. Circulation. 1998;97:2003–2006. doi: 10.1161/01.cir.97.20.2003. [DOI] [PubMed] [Google Scholar]
- 18.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 19.Lansky AJ, Brar SS, Yaqub M, Sood P, Applegate RJ, Lazar D, et al. Impact of routine angiographic follow-up after percutaneous coronary intervention with drug-eluting stents in the SPIRIT III randomized trial at three years. Am J Cardiol. 2012;110:21–29. doi: 10.1016/j.amjcard.2012.02.040. [DOI] [PubMed] [Google Scholar]