Abstract
Purpose
Transarterial chemoembolization (TACE) is indicated for Barcelona Clinic Liver Cancer (BCLC) B hepatocellular carcinoma (HCC). Whether TACE provides any long-term survival benefits remains unclear. We aimed to investigate micrometastases predictors with which to identify patients who would benefit from surgical resection (SR).
Materials and Methods
First, we analyzed risk factors of micrometastases, microvascular invasion, and poor histologic grade in 38 patients with newly diagnosed resectable BCLC stage B HCC limited to one or two segments with well-preserved liver function and who underwent SR between January 2006 and December 2013. Second, we validated identified risk factors in 54 newly diagnosed resectable BCLC B HCC patients with well-preserved liver function who underwent TACE during the same period to determine their influence on survival.
Results
Risk factors of micrometastases in SR patients were α-fetoprotein (AFP) ≥110 [hazard ratio (HR)=5.166; 95% confidence interval (CI), 1.031–25.897; p=0.046] and prothrombin induced by vitamin K absence-II (PIVKA-II) ≥800 (HR=5.166; 95% CI, 1.031–25.897; p=0.046). The cumulative probability of tumor recurrence (p=0.009) after SR differed according to levels of AFP and PIVKA-II. After validation of these risk factors in the TACE group, patients with SR and AFP <110 and PIVKA-II <800 had superior survival outcomes than other patients (HR=0.116; 95% CI, 0.027–0.497; p=0.004).
Conclusion
AFP and PIVKA-II levels predict micrometastases and survival. Therefore, they should be considered when selecting SR for BCLC B HCC.
Keywords: Predictors, micrometastases, Barcelona Clinic Liver Cancer classification B hepatocellular carcinoma, surgical resection, transarterial chemoembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is a devastating disease with poor prognosis and is one of the most common causes of cancer-related deaths worldwide.1,2 The prognosis for HCC patients is determined by tumor status, liver function reserve, general performance, and treatment efficacy.3 To improve patient survival, several staging systems have been described for HCC including the Barcelona Clinic Liver Cancer (BCLC) classification, Okuda et al., Cancer of the Liver Italian Program, and French scores.4,5,6,7 Among them, only the BCLC classification has been approved by the European Association for Study of the Liver and the American Association for the Study of Liver Disease for the prognostic classification and treatment selection of HCC.4,8,9 This staging system, which has been validated in regards to its clinical usefulness in several studies, is associated with treatment indications and aspects of estimated life expectancy.10,11
BCLC stage B HCC includes Child-Pugh scores A and B, as well as preserved performance status, with asymptomatic multifocal tumors without vascular invasion or extrahepatic spread. For this group of patients, the BCLC staging system indicates transarterial chemoembolization (TACE) as the main treatment option.4 A previous study showed that TACE provided a better 2-year survival rate than supportive treatment;12 however, it is unclear whether TACE provides any long-term survival benefit. BCLC stage B is a heterogeneous category that includes patients who vary widely in tumor stage, liver function, and disease etiology. Owing to this heterogeneity, TACE may not be the optimal therapy for all patients with BCLC stage B HCC. Recent studies have verified that surgical resection (SR) can be safely performed on a subset of patients with BCLC stage B HCC who exhibit good liver function, resulting in a long-term survival rate comparable to that of patients treated with TACE.1,3,13,14 However, the indication of SR for BCLC stage B HCC remains controversial and needs further assessment.
If HCC progresses, venous outflow might be obstructed and portal backflow could develop. This can be a route of micrometastases, one of the major causes of multinodular HCC.15 Micrometastases, which cannot often be detected by various imaging modalities, can remain in remnant liver, holding a strong possibility of causing local recurrence after SR. Therefore, we hypothesized that if a patient has few risk factors for the presence of micrometastases, such as microvascular invasion or poor histologic differentiation,16 the intrahepatic spread of the tumor might be limited. In the event of limited intrahepatic tumor spread, SR may confer a greater chance of curative treatment and may represent a better treatment option. Additionally, determining preoperative risk factors for intrahepatic tumor spread in patients with multinodular HCC would allow for a more accurate prediction of the possibility of curative SR. SR could, therefore, be recommended in patients with no risk factors who are predicted to have a good prognosis.
The aims of this study were to identify preoperative risk factors for the micrometastases of tumors in patients who underwent SR for BCLC stage B HCC and to validate these identified risk factors in terms of overall survival in a group of patients who underwent TACE.
MATERIALS AND METHODS
Patients
This retrospective cohort study was approved by the Institutional Review Board of Chung-Ang University Hospital (Seoul, Korea) and was exempted from the requirement to obtain informed consent. The first step of this study was to identify preoperative risk factors in 38 patients with well-preserved liver function, indicated as Child-Turcotte-Pugh (CTP) A liver cirrhosis, and newly diagnosed resectable BCLC stage B HCC limited to one or two segments, and who underwent SR between January 2001 and December 2013 at Chung-Ang University Hospital. Patients with a previous treatment history of HCC or insufficient clinical data were excluded from analysis. The risk for micrometastases, which represents the possibility of intrahepatic spread of the tumor, was defined as the presence of Edmonson and Steiner histologic grade 3 or 4, and/or microvascular invasion. The following data were collected for all patients to determine risk factors for tumor aggressiveness: age, sex, body mass index, presence of hepatitis B virus and hepatitis C virus, preoperative laboratory results (levels of total bilirubin, albumin, and the international normalized ratio), tumor marker levels [α-fetoprotein (AFP) and prothrombin induced by vitamin K absence-II (PIVKA-II)], and tumor characteristics (tumor size, histologic grade, and presence of microvascular invasion).
The second step of the study was to validate identified risk factors in 52 patients who met the same criteria as the SR group, including well-preserved liver function and resectable BCLC stage B HCC limited to one or two segments initially treated with TACE during the same period as the first step of the study. We validated the identified risk factors after adjusting for several parameters that might have influenced the overall survival of patients in the TACE group, such as demographics, laboratory results, and radiologic findings. Clinical variables were the same as those outlined for the first step of the study, with the following exceptions: pathologic results were collected from patients who underwent TACE, and risk factors for overall survival after TACE were identified. The probability of overall survival was analyzed according to the type of treatment and the presence of identified risk factors.
All patients were followed-up at 1, 3, and 6 months post-treatment and every 3 to 6 months thereafter, as necessary. At each visit, imagining studies, such as computed tomography, magnetic resonance imaging, and ultrasonography, were performed. Serologic tests, such as tumor marker analyses and biochemical liver function tests, were performed at each visit. If tumor recurrence was found after SR, multiple modalities, such as re-SR, radiofrequency ablation, or TACE, in consideration of tumor characteristics were performed as early as possible before progression of the recurrent tumor. Also, TACE was repeatedly preformed until the disappearance of viable tumor was noted, and if the tumor progressed, TACE was continued.
Procedure
The SR was performed under low central venous pressure at less than 5 mm Hg. Anatomic partial hepatectomy was performed in a standardized manner; however, if the patients had poor liver function, non-anatomic partial hepatectomy was also performed. All SRs were performed based on a curative aim by ligating the feeding vessels, and securing at least 2 cm of resection margin using ultrasonographic guidance and after resection, the frozen section was performed to confirm the minimal length of resection margin.
The TACE was performed under local anesthesia: a 2.4F highly flexible microcatheter (Progreat, Terumo Corporation, Tokyo, Japan) was introduced into the abdominal aorta via the superficial femoral artery using the Seldinger technique. Hepatic arterial angiography was performed using fluoroscopy to guide the catheter into the celiac artery. Then, the feeding arteries, tumor stain, and vascular anatomy surrounding the tumor were identified. A microcatheter was introduced through the catheter and directed to the feeding arteries. A mixture of doxorubicin hydrochloride (Adriamycin; Ildong Pharm., Seoul, Korea) and iodized oil (Lipiodol; Laboratoire Guerbet, Aulnay-Sous-Bois, France) was injected under fluoroscopic control, followed by embolization of the feeding arteries using 1-mm diameter absorbable gelatin sponge particles (Gelform; Upjohn, Kalamazoo, MI, USA). The volume of ionized oil ranged from 2 to 10 mL, and the amount of Adriamycin ranged from 10 to 70 mg.
Statistical analysis
For intergroup comparisons, the distribution of the data was first evaluated for normality using the Shapiro-Wilk test. Normally distributed data are presented herein as mean±standard deviation. Groups were compared using Student's t-test. Descriptive variables were subjected to χ2 analysis or Fisher's exact test, as appropriate. The optimal cut-off values for the levels of AFP and PIVKA-II were determined by the area under the receiver operating characteristic analysis. Multivariate analysis using an ordinary logistic regression model was performed to investigate risk factors for intrahepatic spread of the tumor in SR patients, as well as the influence of these factors on overall survival in TACE patients. Survival and recurrence after each type of procedure, as well as the presence of identified risk factors, were plotted using the Kaplan-Meier method and compared using the log-rank test. A multivariate analysis using the Cox proportional hazard regression method was performed to investigate the risk factors for recurrence-free survival and overall survival. A p-value of <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
A comparison of patient demographics in each group (low risk/SR, high risk/SR, low risk/TACE, high risk/TACE) is shown in Table 1. Mean age was significant highest in the TACE/low risk group (p=0.003). Laboratory results indicative of liver function showed slight differences in each group, although all laboratory results were within normal limits, representing well-preserved liver function as CTP A liver cirrhosis. Expression of the tumor markers, such as AFP and PIVKA-II, showed significant differences in each group, and high risk groups had elevated serum levels, compared with low risk groups (Table 1).
Table 1. Demographics of Patients in the SR and TACE Groups.
| Variable | SR group | TACE group | p value | ||
|---|---|---|---|---|---|
| Low risk (n=14) | High risk (n=24) | Low risk (n=34) | High risk (n=18) | ||
| Age | 54.4 (±6.4) | 56.1 (±11.0) | 64.2 (±10.1) | 62.7 (±11.2) | 0.003 |
| Sex (male) | 14 (100) | 20 (83.3) | 25 (73.5) | 12 (66.7) | 0.101 |
| Presence of HBV | 14 (100) | 21 (87.5) | 29 (85.3) | 17 (94.4) | 0.399 |
| Presence of HCV | 0 (0) | 1 (4.2) | 1 (2.9) | 1 (5.6) | 0.843 |
| BMI | 24.1 (±3.7) | 22.8 (±2.6) | 22.5 (±3.2) | 22.8 (±2.6) | 0.414 |
| Total bilirubin | 0.9 (±0.3) | 0.9 (±0.5) | 1.3 (±0.6) | 0.9 (±0.6) | 0.040 |
| Albumin | 4.2 (±0.3) | 3.9 (±0.4) | 3.5 (±0.4) | 3.5 (±0.3) | 0.000 |
| PT-INR | 1.1 (±0.1) | 1.0 (±0.2) | 1.2 (±0.1) | 1.1 (±0.1) | 0.007 |
| Tumor size (sum) | 7.3 (±3.0) | 11.7 (±5.9) | 7.1 (±4.7) | 10.1 (±4.7) | 0.002 |
| Tumor number | 2.9 (±1.5) | 2.7 (±1.3) | 3.4 (±1.6) | 3.0 (±1.1) | 0.271 |
| AFP (ng/mL) | 16.3 (±28.0) | 8390 (±20422.8) | 24.0 (±27.1) | 6477.0 (±15005.8) | 0.044 |
| AFP ≥110 (ng/mL) | 0 (0) | 15 (62.5) | 0 (0) | 14 (77.8) | 0.000 |
| PIVKA-II (nAU/mL) | 164.2 (±188.3) | 6658.8 (±10994.5) | 120.5 (±158.2) | 612.8 (±608.4) | 0.000 |
| PIVKA-II ≥800 (nAU/mL) | 0 (0) | 15 (62.5) | 0 (0) | 8 (44.4) | 0.000 |
HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; PT-INR, prothrombin time-international normalized ratio; AFP, α-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II; SR, surgical resection; TACE, transarterial chemoembolization.
Data are presented as the mean±standard deviations or numbers with percentages in parentheses unless otherwise indicated.
In the first step of the study, 60.5% (23/38) of patients who underwent SR were designated as being at high risk for micrometastases, indicating the possibility of intrahepatic spread. These patients displayed the presence of either poor histologic grade (34.2%) or microvascular invasion (50.0%). AFP ≥110 [hazard ratio (HR)=5.166; 95% confidence interval (CI), 1.031–25.897; p=0.046] and PIVKA-II ≥800 (HR=5.166; 95% CI, 1.031–25.897; p=0.046) were the only significant risk factors identified using multivariate analysis (Table 2).
Table 2. Risk Factor Analysis for Tumor Aggressiveness in the SR Group.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Low risk group (n=15) | High risk group (n=23) | p value | Relative risk (95% CI) | p value | |
| Age | 54.1 (±10.1) | 56.4 (±9.2) | 0.491 | ||
| Sex (male) | 14 (93.3) | 20 (87.0) | 0.531 | ||
| Presence of HBV | 14 (93.3) | 21 (91.3) | 0.821 | ||
| Presence of HCV | 0 (0) | 1 (4.3) | 0.413 | ||
| BMI | 24.4 (±3.7) | 22.5 (±2.5) | 0.068 | ||
| Total bilirubin | 1.0 (±0.3) | 0.9 (±0.4) | 0.245 | ||
| Albumin | 4.1 (±0.3) | 3.9 (±0.4) | 0.192 | ||
| PT-INR | 1.1 (±0.1) | 1.0 (±0.2) | 0.497 | ||
| Tumor size (sum) | 8.1 (±3.5) | 11.4 (±6.1) | 0.064 | ||
| Tumor number | 2.9 (±1.4) | 2.7 (±1.3) | 0.703 | ||
| AFP ≥110 (ng/mL) | 3 (20.0) | 12 (52.2) | 0.047 | 5.166 (1.031–25.897) | 0.046 |
| PIVKA-II ≥800 (nAU/mL) | 3 (20.0) | 12 (52.2) | 0.047 | 5.166 (1.031–25.897) | 0.046 |
SR, surgical resection; HBV, hepatitis B virus; HCV, hepatitis C virus; BMI, body mass index; PT-INR, prothrombin time-international normalized ratio; AFP, α-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II.
Data are presented as the mean±standard deviations or numbers with percentages in parentheses unless otherwise indicated.
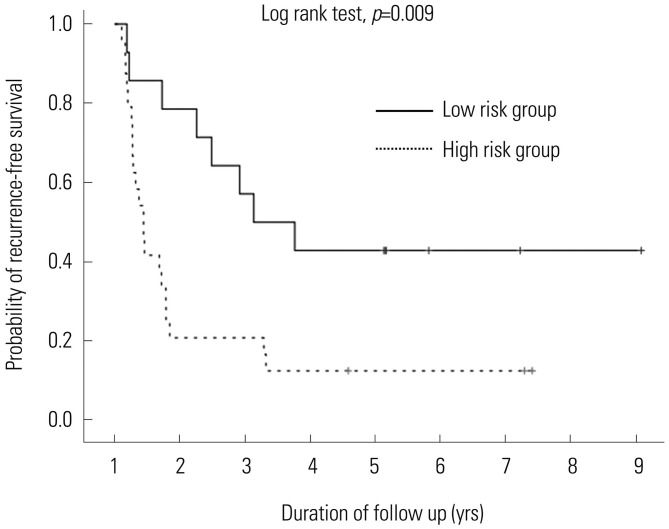
Patients with AFP <110 and PIVKA-II <800 had lower tumor recurrence (57.1%) and long disease-free survival (36.7±28.5 months), compared with the patients who had AFP ≥110 or PIVKA-II ≥800 (87.5% and 14.5±21.8 months). Multiple (33.3%) or extrahepatic tumor recurrence (19.0%) were observed more often in patients with AFP ≥110 or PIVKA-II ≥800; however, most patients who had AFP <110 and PIVKA-II <800 showed only single tumor recurrence of less than 2 cm (Table 3). The cumulative probability of tumor recurrence (p=0.009) after SR differed significantly according to AFP and PIVKA-II levels (Fig. 1).
Table 3. Recurrence Patterns of Hepatocellular Carcinoma in the SR Group According to AFP and PIVKA-II Levels.
| AFP <110 and PIVKA-II <800 (n=14) | AFP ≥110 or PIVKA-II ≥800 (n=24) | p value | |
|---|---|---|---|
| Recurrence | 8 (57.1) | 21 (87.5) | 0.034 |
| Disease free survival (month) | 36.7±28.5 | 14.5±21.8 | 0.011 |
| Pattern of tumor recurrence | |||
| Intrahepatic | |||
| Single tumor | |||
| <1 cm | 1 (12.5) | 2 (9.5) | |
| ≥1 and <2 cm | 4 (50.0) | 5 (23.8) | |
| ≥2 and <3 cm | 0 (0) | 3 (14.3) | |
| Multiple tumor | 2 (25.0) | 7 (33.3) | |
| Extrahepatic | 1 (12.5) | 4 (19.0) |
SR, surgical resection; AFP, α-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II.
Data are presented as the mean±standard deviations or numbers with percentages in parentheses unless otherwise indicated.
Fig. 1. Recurrence-free survival and overall survival in surgical resection patients. The cumulative probability of tumor recurrence. The frequency of recurrence was significantly different according to the levels of AFP and PIVKA-II, especially during the first year (1-year recurrence-free survival: AFP <110 and PIVKA-II <800, 78.6%; AFP ≥110 or PIVKA-II ≥800, 20.8%). The curves continued to diverge during 2 years of follow-up, but then continued parallel (p=0.009). AFP, α-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II.
In the second step of the study, we validated the identified risk factors stated above in the TACE group. Overall survival rates at 1, 3, and 5 years, respectively, were as follows: 78.6, 42.9, and 42.9% for Group 1 (SR, AFP <110 and PIVKA-II <800); 20.8, 12.5, and 12.5% for Group 2 (SR, AFP ≥110 or PIVKA-II ≥800); 73.5, 41.2, and 8.8% for Group 3 (TACE, AFP <110 and PIVKA-II <800); and 83.3, 50.0, and 16.7% for Group 4 (TACE, AFP ≥110 or PIVKA-II ≥800). The survival curve of Group 1 differed significantly from those of the other groups (p=0.001) (Fig. 2). Multivariate analysis of overall survival showed a significant difference between Group 1 and the other groups (HR=0.116; 95% CI, 0.027–0.497; p=0.004).
Fig. 2. The cumulative probability of overall survival in all patients. A significant difference was found in overall survival according to the type of procedure and combined levels of AFP and PIVKA-II (p=0.001). The curve of Group 1 significantly diverged from that of the other groups (HR=0.116; 95% CI, 0.027–0.497; p=0.004). SR, surgical resection; TACE, transarterial chemoembolization; AFP, α-fetoprotein; PIVKA-II, prothrombin induced by vitamin K absence-II; HR, hazard ratio; CI, confidence interval.
DISCUSSION
The BCLC staging system is considered the standard of care for patients with HCC.16,17 TACE is the first line treatment option of BCLC stage B HCC, because it confers a median survival of 20 months, which represents an improvement when compared with supportive treatment.12,18 It remains unknown, however, whether TACE confers a survival advantage over other treatment options, such as SR. The indications for SR have expanded with advances in surgical techniques and preoperative preparation in recent decades. Several studies have reported improved overall survival after SR for BCLC B HCC as compared with TACE.19,20,21 Our results were consistent with the results of these reports, demonstrating that patients treated with SR had superior overall survival than those treated with TACE (57.4±6.4 months vs. 27.2±2.8 months; p=0.000). Many studies have examined prognostic factors for survival of BCLC stage B HCC patients after SR, and patients with select risk factors are predicted to have lower overall survival. Therefore, the identification of patients suitable for SR is of critical importance.7,22,23 Based on the clinical observation of a marked heterogeneity in the clinical benefit of TACE, one study has proposed a subclassification of the BCLC stage B score into four stages (B1–B4) to facilitate therapeutic decisions, and patients who classified as the B1 subgroup showed acceptable survival rates after SR.24
Among the patients who underwent SR, patients who had an identified risk factor had significant earlier tumor recurrence, compared with other patients (14.5±21.8 months vs. 36.7±28.5 months; p=0.011). Early recurrence of HCC is the likely result of local tumor recurrence, which may have arisen either from micrometastases that were left untreated by SR or from the intrahepatic spread of the primary tumor via the portal venous system. Micrometastases are common in patients with HCC and are noted more often in HCC patients with either microvascular invasion or poor histologic differentiation.16,25 The possibility remains that some micrometastases will persist, increasing the possibility of intrahepatic tumor spread and thereby presenting difficulty in achieving curative SR. Our study showed that patients in the SR group with an identifiable risk factor of AFP ≥110 or PIVKA-II ≥800 had higher rates of multiple tumor recurrence (33.3%) and extrahepatic metastasis (19.0%). These features represents increased tumor aggressiveness, as defined by the possibility of micrometastases or intrahepatic tumor spread, when compared with SR patients who had AFP <110 and PIVKA-II <800.
Several studies have demonstrated the clinical significance of serum AFP or PIVKA-II in patients with HCC. However, these two markers involve different synthetic pathways, such that the levels of these two markers show independent patterns.26,27,28,29 Therefore, it appears that AFP and PIVKA-II are complementary markers for clinical usefulness. Accordingly, we defined the high-risk group as AFP ≥110 or PIVKA-II ≥800 to reflect levels of both markers. Despite high recurrence rates of HCC, Group 1 had significantly superior survival outcomes than the other groups. Patients with AFP <110 and PIVKA-II <800 usually had long disease-free survival (36.7±28.5 months) and single tumor recurrence (62.5%), the latter of which we suspect arose as a result of de novo tumorigenesis due to underlying cirrhosis.
There are some limitations to this study. It is a retrospective study, forcing us to rely on the integrity of completed medical records for our analysis. In addition, the background characteristics of the SR and TACE groups were significantly different; selection bias, therefore, cannot be eliminated. The study population was relatively small, and further studies with prospectively randomized large populations are warranted to confirm these promising results.
In conclusion, serum levels of AFP and PIVKA-II are useful predictors of tumor aggressiveness and micrometastasis in BCLC B HCC. AFP and PIVKA-II levels can also predict early tumor recurrence after SR. The type of treatment and the combined levels of AFP and PIVKA-II carry significant prognostic value in relation to overall survival rates. The findings in the current study may be helpful to determining optimal treatment strategies in BCLC B HCC. We propose that if a patient has no risk of micrometastasis as defined by AFP and PIVKA-II levels, SR should be considered for first-line therapy.
ACKNOWLEDGEMENTS
This work was supported by Samjin Pharm Co., Ltd., Seoul, Korea.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. doi: 10.1371/journal.pone.0068193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137–145. doi: 10.1002/hep.22312. [DOI] [PubMed] [Google Scholar]
- 3.Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 7.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 8.European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Livraghi T, Brambilla G, Carnaghi C, Tommasini MA, Torzilli G. Is it time to reconsider the BCLC/AASLD therapeutic flow-chart? J Surg Oncol. 2010;102:868–876. doi: 10.1002/jso.21733. [DOI] [PubMed] [Google Scholar]
- 10.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 11.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 13.Jianyong L, Lunan Y, Wentao W, Yong Z, Bo L, Tianfu W, et al. Barcelona clinic liver cancer stage B hepatocellular carcinoma: transarterial chemoembolization or hepatic resection? Medicine (Baltimore) 2014;93:e180. doi: 10.1097/MD.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol. 2016;22:7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 16.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Sala M, Forner A, Varela M, Bruix J. Prognostic prediction in patients with hepatocellular carcinoma. Semin Liver Dis. 2005;25:171–180. doi: 10.1055/s-2005-871197. [DOI] [PubMed] [Google Scholar]
- 19.Delis SG, Bakoyiannis A, Tassopoulos N, Athanassiou K, Kelekis D, Madariaga J, et al. Hepatic resection for hepatocellular carcinoma exceeding Milan criteria. Surg Oncol. 2010;19:200–207. doi: 10.1016/j.suronc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Yang T, Lin C, Zhai J, Shi S, Zhu M, Zhu N, et al. Surgical resection for advanced hepatocellular carcinoma according to Barcelona Clinic Liver Cancer (BCLC) staging. J Cancer Res Clin Oncol. 2012;138:1121–1129. doi: 10.1007/s00432-012-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 22.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–452. doi: 10.1200/JCO.2008.18.8151. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Zhou JG, Sun Y, Zhang L, Xing BC. Hepatic resection improved the long-term survival of patients with BCLC stage B hepatocellular carcinoma in Asia: a systematic review and meta-analysis. J Gastrointest Surg. 2015;19:1271–1280. doi: 10.1007/s11605-015-2811-6. [DOI] [PubMed] [Google Scholar]
- 24.Weinmann A, Koch S, Sprinzl M, Kloeckner R, Schulze-Bergkamen H, Düber C, et al. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int. 2015;35:591–600. doi: 10.1111/liv.12696. [DOI] [PubMed] [Google Scholar]
- 25.Plessier A, Codes L, Consigny Y, Sommacale D, Dondero F, Cortes A, et al. Underestimation of the influence of satellite nodules as a risk factor for post-transplantation recurrence in patients with small hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):S86–S90. doi: 10.1002/lt.20039. [DOI] [PubMed] [Google Scholar]
- 26.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, et al. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–1040. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]
- 27.Kang SH, Kim DY, Jeon SM, Ahn SH, Park JY, Kim SU, et al. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur J Gastroenterol Hepatol. 2012;24:849–856. doi: 10.1097/MEG.0b013e3283535c34. [DOI] [PubMed] [Google Scholar]
- 28.Fujiyama S, Tanaka M, Maeda S, Ashihara H, Hirata R, Tomita K. Tumor markers in early diagnosis, follow-up and management of patients with hepatocellular carcinoma. Oncology. 2002;62(Suppl 1):57–63. doi: 10.1159/000048277. [DOI] [PubMed] [Google Scholar]
- 29.Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:111–117. doi: 10.1016/s1542-3565(05)00855-4. [DOI] [PubMed] [Google Scholar]